Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria and Definitions
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
3. Results
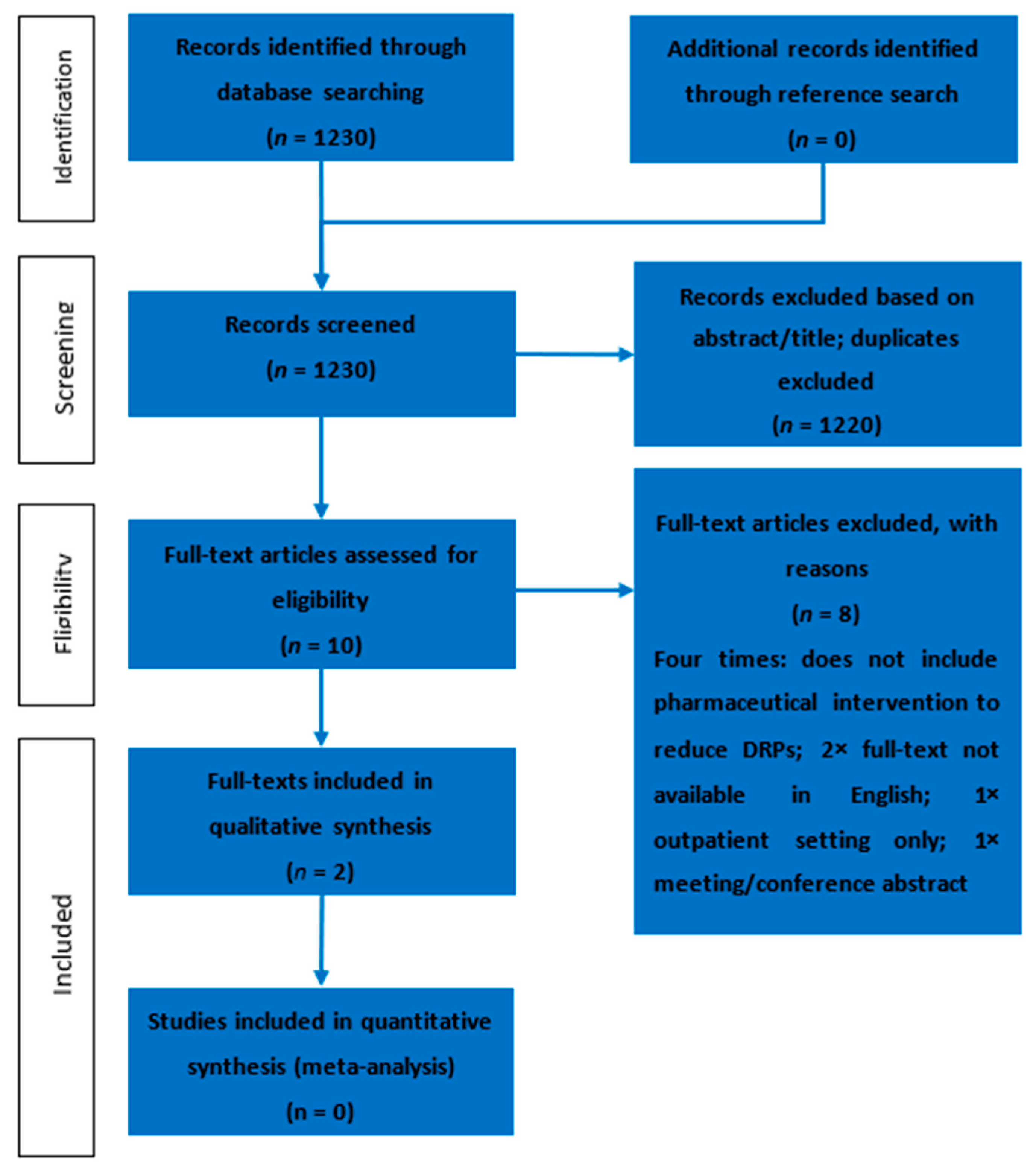
3.1. Study Selection
3.2. Study Characteristics
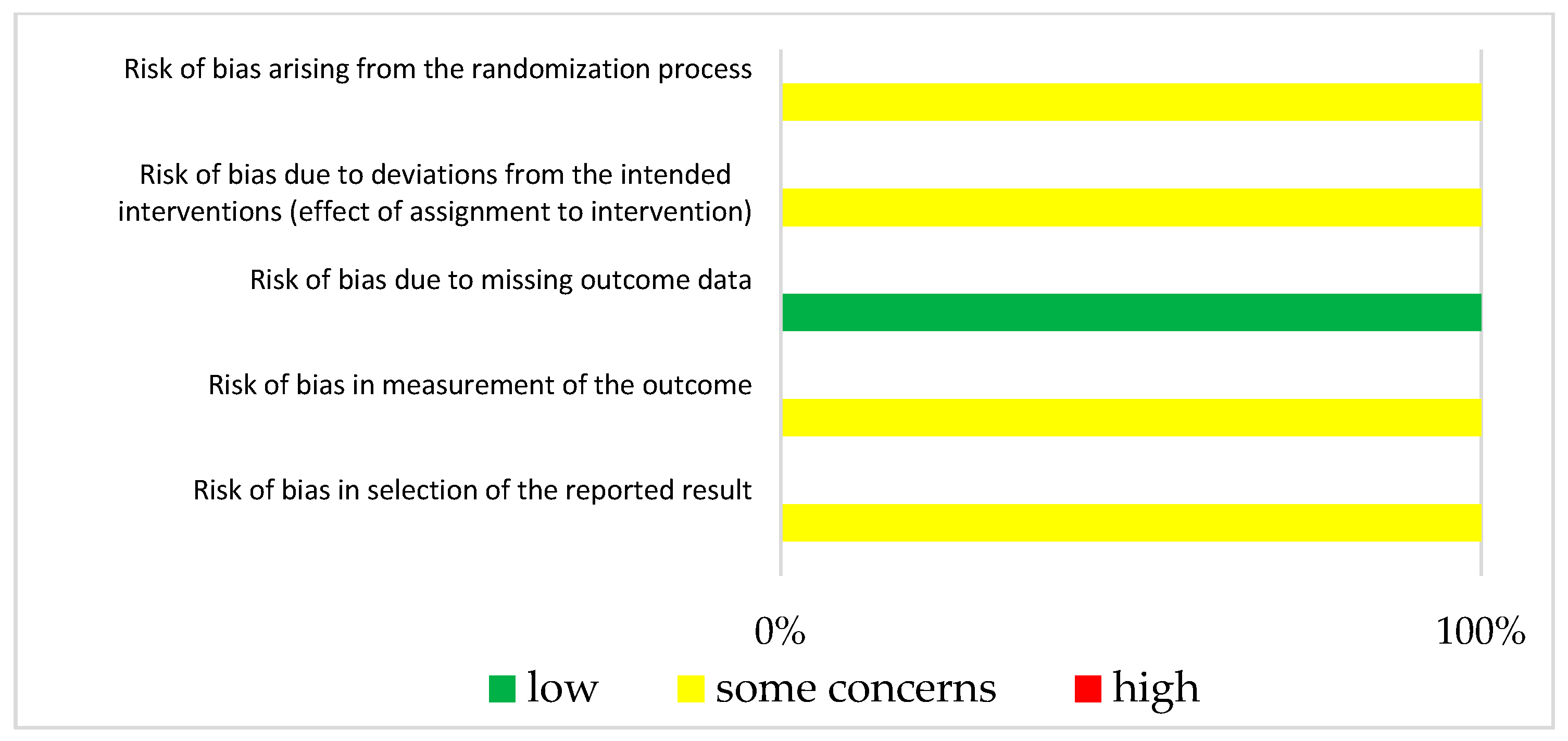
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.5. Synthesis of Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, T.Y.; Hung, T.H.; Livneh, H.; Lin, I.H.; Lu, M.C.; Yeh, C.C. Chinese herbal medicine therapy and the risk of mortality for chronic hepatitis B patients with concurrent liver cirrhosis: A nationwide population-based cohort study. Oncotarget 2018, 9, 18214–18223. [Google Scholar] [CrossRef]
- European Association for The Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [CrossRef] [PubMed]
- Nusrat, S.; Khan, M.S.; Fazili, J.; Madhoun, M.F. Cirrhosis and its complications: Evidence based treatment. World J. Gastroenterol. 2014, 20, 5442–5460. [Google Scholar] [CrossRef]
- Martin Blachier, H.L.; Peck-Radosavljevic, M.; Valla, D.-C.; Roudot-Thoraval, F. The Burden of Liver Disease in Europe A Review of Available Epidemiological Data; EASL: Geneva, Switzerland, 2013. [Google Scholar]
- (WHO), W.H.O. Alcohol in the European Union: Consumption, Harm and Policy Approaches. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/160680/e96457.pdf (accessed on 27 September 2023).
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Weersink, R.A.; Taxis, K.; Drenth, J.P.H.; Houben, E.; Metselaar, H.J.; Borgsteede, S.D. Prevalence of Drug Prescriptions and Potential Safety in Patients with Cirrhosis: A Retrospective Real-World Study. Drug Saf. 2019, 42, 539–546. [Google Scholar] [CrossRef] [PubMed]
- PCNE Classification for Drug Related Problems. Available online: https://www.pcne.org/working-groups/2/drug-related-problems (accessed on 28 November 2022).
- Kaufmann, C.P.; Stämpfli, D.; Hersberger, K.E.; Lampert, M.L. Determination of risk factors for drug-related problems: A multidisciplinary triangulation process. BMJ Open 2015, 5, e006376. [Google Scholar] [CrossRef] [PubMed]
- Viktil, K.K.; Blix, H.S. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin. Pharmacol. Toxicol. 2008, 102, 275–280. [Google Scholar] [CrossRef]
- Hayward, K.L.; Weersink, R.A. Improving Medication-Related Outcomes in Chronic Liver Disease. Hepatol. Commun. 2020, 4, 1562–1577. [Google Scholar] [CrossRef]
- Franz, C.C.; Egger, S.; Born, C.; Rätz Bravo, A.E.; Krähenbühl, S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur. J. Clin. Pharmacol. 2012, 68, 179–188. [Google Scholar] [CrossRef]
- Franz, C.C.; Hildbrand, C.; Born, C.; Egger, S.; Rätz Bravo, A.E.; Krähenbühl, S. Dose adjustment in patients with liver cirrhosis: Impact on adverse drug reactions and hospitalizations. Eur. J. Clin. Pharmacol. 2013, 69, 1565–1573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aghili, M.; Neelathahalli Kasturirangan, M. Identifying characteristics of drug-related problems in critically ill patients with decompensated liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1569–1576. [Google Scholar] [CrossRef]
- Shawaqfeh, M.S.; Alangari, D.; Aldamegh, G.; Almotairi, J.; Bin Orayer, L.; Albekairy, N.A.; Abdel-Razaq, W.; Mardawi, G.; Almuqbil, F.; Aldebasi, T.M.; et al. Unveiling medication errors in liver transplant patients towards enhancing the imperative patient safety. Saudi Pharm. J. 2023, 31, 101789. [Google Scholar] [CrossRef] [PubMed]
- Maes, K.A.; Tremp, R.M.; Hersberger, K.E.; Lampert, M.L. Demonstrating the clinical pharmacist’s activity: Validation of an intervention oriented classification system. Int. J. Clin. Pharm. 2015, 37, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.B.; Doga, B.; Borgsteede, S.D.; van den Burg, A.M.; Metselaar, H.J.; den Hoed, C.M.; Hunfeld, N.G.M. Evaluation of medication-related problems in liver transplant recipients with and without an outpatient medication consultation by a clinical pharmacist: A cohort study. Int. J. Clin. Pharm. 2022, 44, 1114–1122. [Google Scholar] [CrossRef]
- Hierarchy of Controls. The National Institute for Occupational Safety and Health (NIOSH). 2015. Available online: https://www.cdc.gov/niosh/topics/hierarchy/default.html (accessed on 5 July 2023).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Cochrane Effective Practice and Organisation of Care. What Study Designs Should be Included in an EPOC Review and what Should They be Called? Available online: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/EPOC%20Study%20Designs%20About.pdf (accessed on 14 November 2022).
- Pharmaceutical Society of Australia. Guidelines for Pharmacists Performing Clinical Interventions. Available online: https://www.ppaonline.com.au/wp-content/uploads/2019/01/PSA-Clinical-Interventions-Guidelines.pdf (accessed on 30 June 2022).
- Koeck, J.A.; Young, N.J.; Kontny, U.; Orlikowsky, T.; Bassler, D.; Eisert, A. Interventions to Reduce Pediatric Prescribing Errors in Professional Healthcare Settings: A Systematic Review of the Last Decade. Paediatr. Drugs 2021, 23, 223–240. [Google Scholar] [CrossRef]
- Card, A.J.; Ward, J.; Clarkson, P.J. Successful risk assessment may not always lead to successful risk control: A systematic literature review of risk control after root cause analysis. J. Healthc. Risk Manag. 2012, 31, 6–12. [Google Scholar] [CrossRef]
- Koeck, J.A.; Young, N.J.; Kontny, U.; Orlikowsky, T.; Bassler, D.; Eisert, A. Interventions to Reduce Medication Dispensing, Administration, and Monitoring Errors in Pediatric Professional Healthcare Settings: A Systematic Review. Front. Pediatr. 2021, 9, 633064. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 1994. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Klein, A.; Otto, G.; Krämer, I. Impact of a pharmaceutical care program on liver transplant patients’ compliance with immunosuppressive medication: A prospective, randomized, controlled trial using electronic monitoring. Transplantation 2009, 87, 839–847. [Google Scholar] [CrossRef]
- Schuh, M.J.; Massoglia, G. Pharmacist impact on tacrolimus serum concentrations in liver transplant patients. Consult. Pharm. 2018, 33, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Manuele, F.A. Risk Assessment and Hierarchies if Control. Prof. Saf. 2005, 50, 33–39. [Google Scholar]
- Jozefczyk, K.G.; Kennedy, W.K.; Lin, M.J.; Achatz, J.; Glass, M.D.; Eidam, W.S.; Melroy, M.J. Computerized prescriber order entry and opportunities for medication errors: Comparison to tradition paper-based order entry. J. Pharm. Pract. 2013, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef]
- Weersink, R.A.; Bouma, M.; Burger, D.M.; Drenth, J.P.; Hunfeld, N.G.; Kranenborg, M.; Monster-Simons, M.H.; van Putten, S.A.; Metselaar, H.J.; Taxis, K.; et al. Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion. BMJ Open 2016, 6, e012991. [Google Scholar] [CrossRef]
- Sam, S.; Guérin, A.; Rieutord, A.; Belaiche, S.; Bussières, J.F. Roles and Impacts of the Transplant Pharmacist: A Systematic Review. Can. J. Hosp. Pharm. 2018, 71, 324–337. [Google Scholar] [CrossRef]
- Lieber, S.R.; Volk, M.L. Non-adherence and graft failure in adult liver transplant recipients. Dig. Dis. Sci. 2013, 58, 824–834. [Google Scholar] [CrossRef]
- O’Carroll, R.E.; McGregor, L.M.; Swanson, V.; Masterton, G.; Hayes, P.C. Adherence to medication after liver transplantation in Scotland: A pilot study. Liver Transpl. 2006, 12, 1862–1868. [Google Scholar] [CrossRef]
- Asavakarn, S.; Sirivatanauksorn, Y.; Promraj, R.; Ruenrom, A.; Limsrichamrern, S.; Kositamongkol, P.; Mahawithitwong, P.; Tovikkai, C.; Dumronggittigule, W. Systematic Pharmaceutical Educational Approach to Enhance Drug Adherence in Liver Transplant Recipients. Transplant. Proc. 2016, 48, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- The British Hepatology Pharmacy Group (BHPG). Available online: https://www.basl.org.uk/index.cfm/content/page/cid/20 (accessed on 22 September 2023).
- Maldonado, A.Q.; Hall, R.C.; Pilch, N.A.; Ensor, C.R.; Anders, S.; Gilarde, J.A.; Tichy, E.M. ASHP Guidelines on Pharmacy Services in Solid Organ Transplantation. Am. J. Health Syst. Pharm. 2020, 77, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Duncan, N.; Moreno-Martinez, M.E.; Pires, V.; Domingos, V.; Bonnin, A.; Nezvalova-Henriksen, K.; Admiraal, R.; Bauters, T.; Langebrake, C. Role and competencies of the EBMT clinical pharmacists and clinical pharmacologists: A pan-European survey. Bone Marrow Transplant. 2023, 58, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Rathert, C.; Wyrwich, M.D.; Boren, S.A. Patient-centered care and outcomes: A systematic review of the literature. Med. Care Res. Rev. 2013, 70, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant Annual Report. Available online: https://www.eurotransplant.org/wp-content/uploads/2023/09/Annual-Report-ET-2022.pdf (accessed on 24 October 2023).
- Eurotransplant Factsheet. Available online: https://www.eurotransplant.org/wp-content/uploads/2023/03/ET-Factsheet-2022_11.pdf (accessed on 24 October 2023).
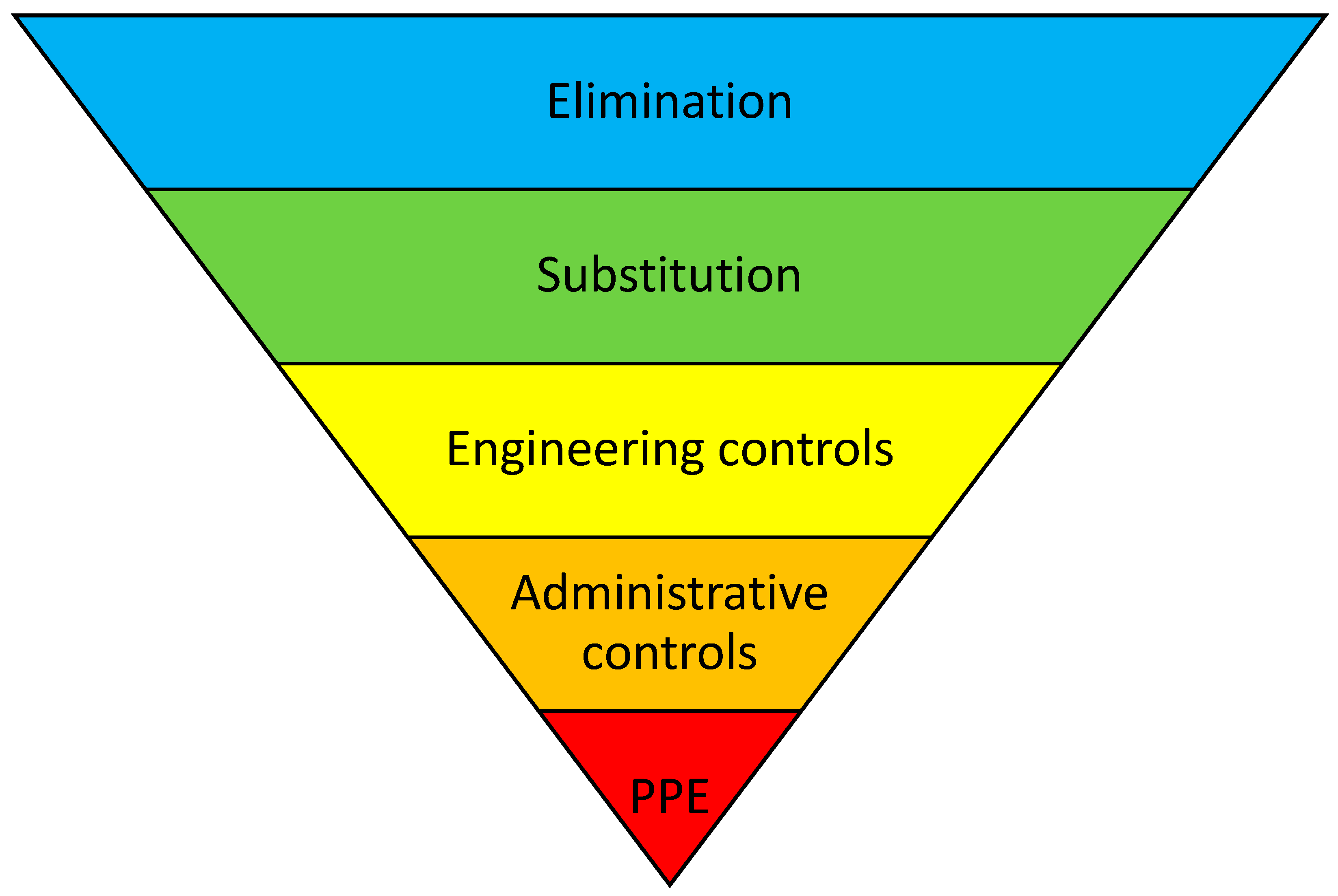

| First Author (Year of Publication) | Country | Study Type | Study and Patient Setting | Population and Age Group | Gender | Standard Care | Number of Intervention(s), Description of Intervention(s) | “Hierarchy of Controls” | Results | Effect of Primary Outcome [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| Klein, A. (2009) [28] | Germany | RCT | University hospital, inpatient, and follow-up care. | Liver transplant patients. CG—24 patients; IG—26 patients. Age: CG mean, 50.1 years; IG mean, 52.8 years. | CG: Female: 11 (46%) Male: 13 (54%) IG: Female: 12 (46%) Male: 14 (54%) | Routine clinical care. | Single intervention. Pharmaceutical care program:
| Administrative Controls | Primary outcome: patients’ compliance with immunosuppressive therapy, defined as the number of correct MEMS bottle openings per all monitored days; ≥80% was seen as compliant. CG: data from 21 patients were available and 12 patients were compliant (57%). IG: data from 20 patients were available and 18 patients were compliant (90%). Secondary outcomes:
| ARR = 33% |
| Schuh, MJ (2018) [29] | USA | UBA | Tertiary care and inpatients. | Liver transplant patients. 74 patients. Age: mean, 59.7 years | Female: 29 Male: 45 | Routine clinical care with pre-transplant pharmacist consultation. | Single intervention. Post-transplant face-to-face pharmacist consultation (medication adherence monitoring; screening for CYP3A interacting foods, medications, and supplements; and education of patients/caregivers). | Administrative Controls | Primary outcome: the percentage of tacrolimus drug levels in range. CG (tacrolimus drug levels in range three weeks before the post-transplant pharmacist consultation): 25% of drug levels in range (5–10 ng/mL). IG (tacrolimus drug levels in range four months after the post-transplant pharmacist consultation): 49% of drug levels in range. | ARR = 24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jibai, N.; Koch, A.; Ulmer, T.F.; Erdmann, P.; Koeck, J.A.; Eisert, A. Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies. J. Clin. Med. 2023, 12, 7030. https://doi.org/10.3390/jcm12227030
Jibai N, Koch A, Ulmer TF, Erdmann P, Koeck JA, Eisert A. Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies. Journal of Clinical Medicine. 2023; 12(22):7030. https://doi.org/10.3390/jcm12227030
Chicago/Turabian StyleJibai, Nagham, Alexander Koch, Tom Florian Ulmer, Pia Erdmann, Joachim Andreas Koeck, and Albrecht Eisert. 2023. "Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies" Journal of Clinical Medicine 12, no. 22: 7030. https://doi.org/10.3390/jcm12227030
APA StyleJibai, N., Koch, A., Ulmer, T. F., Erdmann, P., Koeck, J. A., & Eisert, A. (2023). Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies. Journal of Clinical Medicine, 12(22), 7030. https://doi.org/10.3390/jcm12227030







