Renal Perfusion, Oxygenation and Metabolism: The Role of Imaging
Abstract
:1. Introduction
1.1. Renal Perfusion
1.2. Principles of MRI-Measurement of Renal Perfusion (ASL) and Oxygenation (BOLD)
1.3. Renal Perfusion and Renal Oxygenation Using MRI
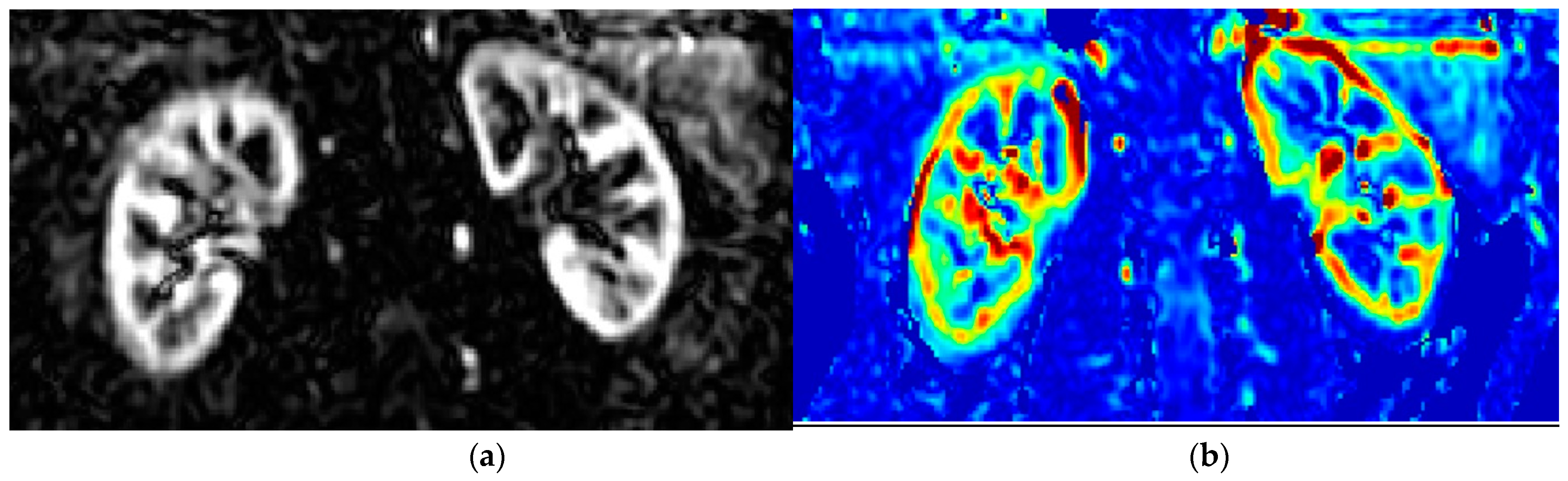
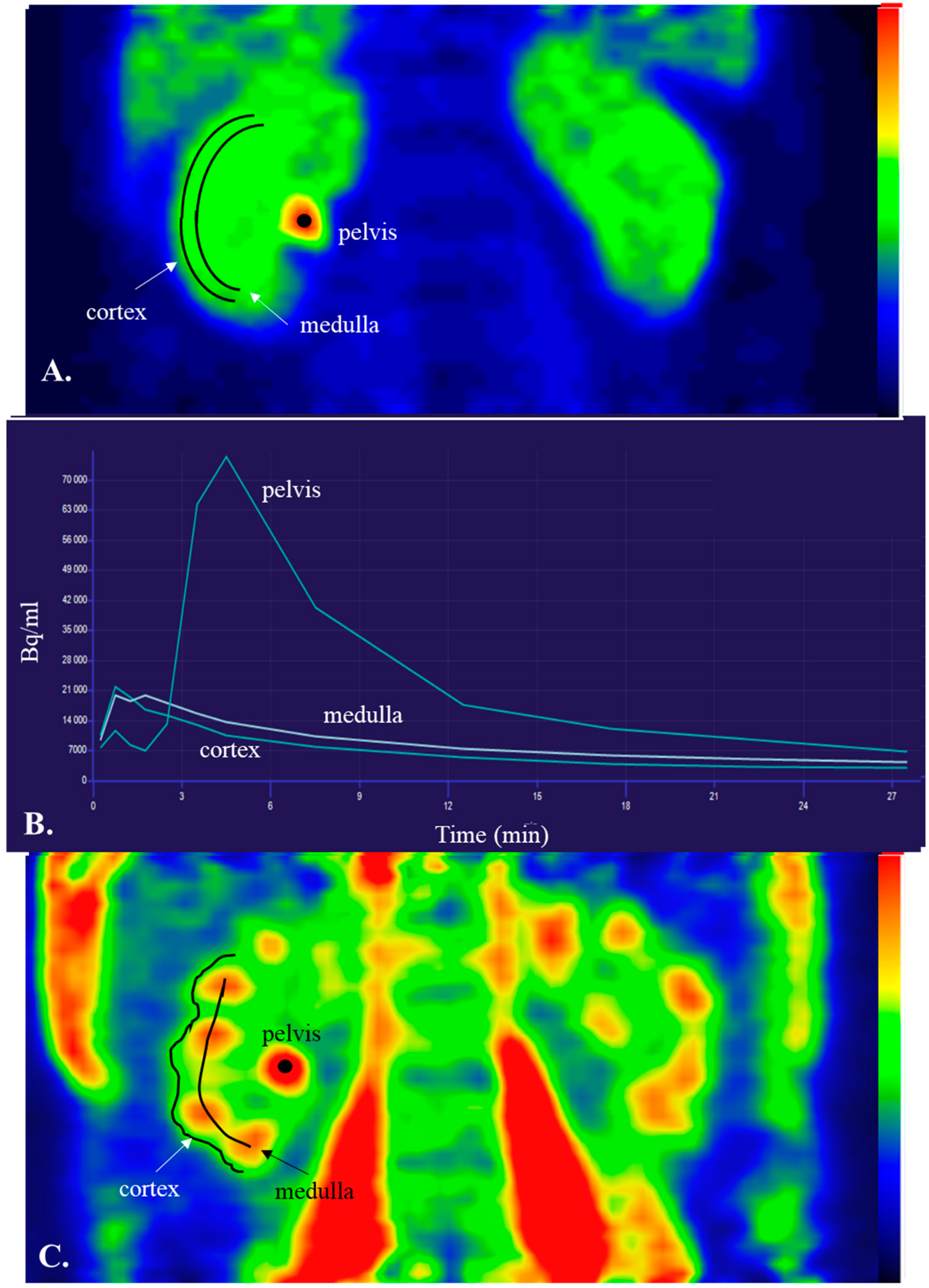
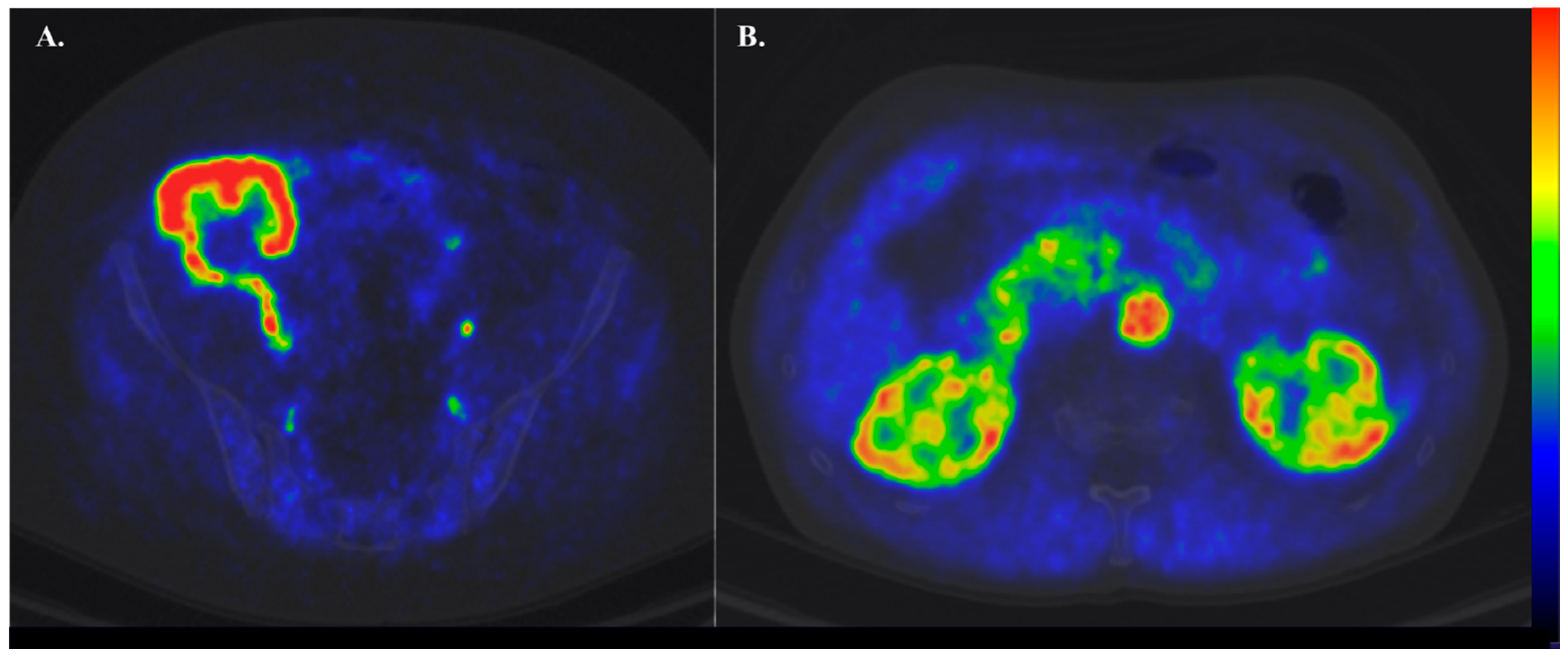
1.4. Basics of PET and Renal Cortical Perfusion
1.5. Tracers in Renal Perfusion PET
1.6. Clinical Settings Where Renal Perfusion Has Been Assessed with PET
| Reference | N and Type of Subjects | Tracer | Cortex Perfusion (mL·g−1·min−1) |
| Nitzsche et al., 1993 [45] | 20 healthy subjects | [15O]H2O [13N]-ammonia | 4.7 (0.3) 4.6 (0.5) |
| Middlekauff et al., 2001 [51] | 29 healthy subjects | [15O]H2O | 4.4 (0.1) |
| Middlekauff et al., 1997 [52] | 19 healthy subjects 19 pts with heart failure | [15O]H2O [15O]H2O | 4.2 (0.1) 3.0 (0.1) |
| Alpert et al., 2002 [43] | 5 healthy subjects 10 pts with renal disease | [15O]H2O [15O]H2O | 3.4 (0.4) 2.1 (1.1) |
| Juillard et al., 2002 [42] | 8 pts with CKD | [15O]H2O | 2.2 (2.0) |
| Kudomi et al., 2009 [53] | 6 healthy subjects | [15O]H2O | 1.6 (0.6) * 3.6 (2.2) |
| Damkjær et al., 2010 [54] | 9 healthy subjects | [15O]H2O | 4.7 (0.3) |
| Damkjær et al., 2012 [55] | 7 healthy subjects | [15O]H2O | 3.6 (0.1) |
| Assersen et al., 2019 [49] | 12 healthy subjects 6 pts with hypertension | [15O]H2O | 4.1 (0.3) |
| Rebelos et al., 2019 [50] | 15 healthy subjects 23 women with obesity | [15O]H2O | 2.7 (104) 2.6 (62) |
| Koivuviita et al., 2012 [48] | 10 healthy subjects 7 pts with CKD 17 pts with RAS and CKD | [15O]H2O | 1.8 (0.3) 1.26 (0.5) 1.43 (0.4) |
| Päivärinta et al., 2019 [44] | 10 healthy subjects 19 pts with kidney Tx | [15O]H2O | 2.7 (2.4–4.0) 2.2 (2.0–3.0) |
| Normand et al., 2019 [46] | 10 healthy subjects | [15O]H2O [11C]-acetate | 3.3 (0.7) 1.7 (0.3) |
1.7. Reproducibility of Renal Cortical PET Perfusion
1.8. Assessment of Renal Metabolism Using PET
2. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of Global Kidney Health Care Status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Casserly, L.F.; Cronin, C.J.; Chernenko, T.; Cullen, W.; Hannigan, A.; Saran, R.; Johnson, H.; Browne, G.; Ferguson, J.P. Prevalence and Variation of Chronic Kidney Disease in the Irish Health System: Initial Findings from the National Kidney Disease Surveillance Programme. BMC Nephrol. 2014, 15, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Johansen, K.; Kasiske, B.; Kutner, N.; Liu, J.; St Peter, W.; et al. United States Renal Data System 2011 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Am. J. Kidney Dis. 2012, 59, e1-420. [Google Scholar]
- Muntner, P.; Coresh, J.; Smith, J.C.; Eckfeldt, J.; Klag, M.J. Plasma Lipids and Risk of Developing Renal Dysfunction: The Atherosclerosis Risk in Communities Study. Kidney Int. 2000, 58, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-Y.; Liu, J.-S.; Hung, S.-C. Obesity and Risk of End-Stage Renal Disease in Patients with Chronic Kidney Disease: A Cohort Study. Am. J. Clin. Nutr. 2018, 108, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Moriconi, D.; Antonioli, L.; Masi, S.; Bellini, R.; Pellegrini, C.; Rebelos, E.; Taddei, S.; Nannipieri, M. Glomerular Hyperfiltration in Morbid Obesity: Role of the Inflammasome Signalling. Nephrology 2022, 27, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef] [Green Version]
- Fine, L.G.; Norman, J.T. Chronic Hypoxia as a Mechanism of Progression of Chronic Kidney Diseases: From Hypothesis to Novel Therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Chade, A.R. Small Vessels, Big Role: Renal Microcirculation and Progression of Renal Injury. Hypertension 2017, 69, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Parving, H.H.; Lehnert, H.; Bröchner-Mortensen, J.; Gomis, R.; Andersen, S.; Arner, P. The Effect of Irbesartan on the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strippoli, G.F.M.; Bonifati, C.; Craig, M.; Navaneethan, S.D.; Craig, J.C. Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Antagonists for Preventing the Progression of Diabetic Kidney Disease. Cochrane Database Syst. Rev. 2006, 2006, CD006257. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients With Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Pitt, B.; Anker, S.D.; Rossing, P.; Kovesdy, C.P.; Pecoits-Filho, R.; Pergola, P.; Joseph, A.; Lage, A.; Mentenich, N.; et al. Kidney Outcomes with Finerenone: An Analysis from the FIGARO-DKD Study. Nephrol. Dial. Transplant. 2023, 38, 372–383. [Google Scholar] [CrossRef]
- Rebelos, E.; Tentolouris, N. Complementary Actions of Finerenone and SGLT2-i on Renal Outcomes?: An Urgent Need for More Information. Nephrology 2022, 27, 915–916. [Google Scholar] [CrossRef]
- Selby, N.M.; Blankestijn, P.J.; Boor, P.; Combe, C.; Eckardt, K.-U.; Eikefjord, E.; Garcia-Fernandez, N.; Golay, X.; Gordon, I.; Grenier, N.; et al. Magnetic Resonance Imaging Biomarkers for Chronic Kidney Disease: A Position Paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol. Dial. Transplant. 2018, 33, ii4–ii14. [Google Scholar] [CrossRef] [Green Version]
- Mendichovszky, I.; Pullens, P.; Dekkers, I.; Nery, F.; Bane, O.; Pohlmann, A.; de Boer, A.; Ljimani, A.; Odudu, A.; Buchanan, C.; et al. Technical Recommendations for Clinical Translation of Renal MRI: A Consensus Project of the Cooperation in Science and Technology Action PARENCHIMA. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grist, J.T.; Hansen, E.S.; Zöllner, F.G.; Laustsen, C. Sodium (23Na) MRI of the Kidney: Basic Concept. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2021; Volume 2216, pp. 257–266. [Google Scholar] [CrossRef]
- Laustsen, C. Hyperpolarized Renal Magnetic Resonance Imaging: Potential and Pitfalls. Front. Physiol. 2016, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal Oxygenation: Unique Challenges and the Biophysical Basis of Homeostasis. Am. J. Physiol. Renal Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, C.-L.; Anderson, W.P.; O’Connor, P.M.; Evans, R.G. Evidence That Renal Arterial-Venous Oxygen Shunting Contributes to Dynamic Regulation of Renal Oxygenation. Am. J. Physiol. Renal Physiol. 2007, 292, F1726–F1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and Potential Imaging Applications of Ferumoxytol for Magnetic Resonance Imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef]
- Odudu, A.; Nery, F.; Harteveld, A.A.; Evans, R.G.; Pendse, D.; Buchanan, C.E.; Francis, S.T.; Fernández-Seara, M.A. Arterial Spin Labelling MRI to Measure Renal Perfusion: A Systematic Review and Statement Paper. Nephrol. Dial. Transplant. 2018, 33, ii15–ii21. [Google Scholar] [CrossRef] [Green Version]
- Nery, F.; Gordon, I.; Thomas, D.L. Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities. Diagnostics 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Nery, F.; Buchanan, C.E.; Harteveld, A.A.; Odudu, A.; Bane, O.; Cox, E.F.; Derlin, K.; Gach, H.M.; Golay, X.; Gutberlet, M.; et al. Consensus-Based Technical Recommendations for Clinical Translation of Renal ASL MRI. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Echeverria-Chasco, R.; Vidorreta, M.; Aramendía-Vidaurreta, V.; Cano, D.; Escalada, J.; Garcia-Fernandez, N.; Bastarrika, G.; Fernández-Seara, M.A. Optimization of Pseudo-Continuous Arterial Spin Labeling for Renal Perfusion Imaging. Magn. Reson. Med. 2021, 85, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Pruijm, M.; Milani, B.; Pivin, E.; Podhajska, A.; Vogt, B.; Stuber, M.; Burnier, M. Reduced Cortical Oxygenation Predicts a Progressive Decline of Renal Function in Patients with Chronic Kidney Disease. Kidney Int. 2018, 93, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Pruijm, M.; Mendichovszky, I.A.; Liss, P.; van der Niepen, P.; Textor, S.C.; Lerman, L.O.; Krediet, C.T.P.; Caroli, A.; Burnier, M.; Prasad, P.V. Renal Blood Oxygenation Level-Dependent Magnetic Resonance Imaging to Measure Renal Tissue Oxygenation: A Statement Paper and Systematic Review. Nephrol. Dial. Transplant. 2018, 33, ii22–ii28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.; Li, L.-P.; Thacker, J.M.; Li, W.; Hack, B.; Kohn, O.; Sprague, S.M. Cortical Perfusion and Tubular Function as Evaluated by Magnetic Resonance Imaging Correlates with Annual Loss in Renal Function in Moderate Chronic Kidney Disease. Am. J. Nephrol. 2019, 49, 114–124. [Google Scholar] [CrossRef]
- Zanchi, A.; Burnier, M.; Muller, M.-E.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Loncle, N.; Milani, B.; Dufour, N.; Bonny, O.; Pruijm, M. Acute and Chronic Effects of SGLT2 Inhibitor Empagliflozin on Renal Oxygenation and Blood Pressure Control in Nondiabetic Normotensive Subjects: A Randomized, Placebo-Controlled Trial. J. Am. Heart Assoc. 2020, 9, e016173. [Google Scholar] [CrossRef]
- Laursen, J.C.; Søndergaard-Heinrich, N.; de Melo, J.M.L.; Haddock, B.; Rasmussen, I.K.B.; Safavimanesh, F.; Hansen, C.S.; Størling, J.; Larsson, H.B.W.; Groop, P.-H.; et al. Acute Effects of Dapagliflozin on Renal Oxygenation and Perfusion in Type 1 Diabetes with Albuminuria: A Randomised, Double-Blind, Placebo-Controlled Crossover Trial. EClinicalMedicine 2021, 37, 100895. [Google Scholar] [CrossRef] [PubMed]
- Gullaksen, S.; Vernstrøm, L.; Sørensen, S.S.; Ringgaard, S.; Laustsen, C.; Funck, K.L.; Poulsen, P.L.; Laugesen, E. Separate and Combined Effects of Semaglutide and Empagliflozin on Kidney Oxygenation and Perfusion in People with Type 2 Diabetes: A Randomised Trial. Diabetologia 2023, 66, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Grenier, N.; Cornelis, F.; Le Bras, Y.; Rigou, G.; Boutault, J.R.; Bouzgarrou, M. Perfusion Imaging in Renal Diseases. Diagn. Interv. Imaging 2013, 94, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A.; Hutchins, G.D. Positron Emission Tomography (PET) Assessment of Renal Perfusion. Semin. Nephrol. 2011, 31, 291–299. [Google Scholar] [CrossRef]
- Juillard, L.; Janier, M.F.; Fouque, D.; Cinotti, L.; Maakel, N.; Le Bars, D.; Barthez, P.Y.; Pozet, N.; Laville, M. Dynamic Renal Blood Flow Measurement by Positron Emission Tomography in Patients with CRF. Am. J. Kidney Dis. 2002, 40, 947–954. [Google Scholar] [CrossRef]
- Alpert, N.M.; Rabito, C.A.; Correia, D.J.A.; Babich, J.W.; Littman, B.H.; Tompkins, R.G.; Rubin, N.T.; Rubin, R.H.; Fischman, A.J. Mapping of Local Renal Blood Flow with PET and H215O. J. Nucl. Med. 2002, 43, 470–475. [Google Scholar]
- Päivärinta, J.; Oikonen, V.; Räisänen-Sokolowski, A.; Tolvanen, T.; Löyttyniemi, E.; Iida, H.; Nuutila, P.; Metsärinne, K.; Koivuviita, N. Renal Vascular Resistance Is Increased in Patients with Kidney Transplant. BMC Nephrol. 2019, 20, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitzsche, E.U.; Choi, Y.; Killion, D.; Hoh, C.K.; Hawkins, R.A.; Rosenthal, J.T.; Buxton, D.B.; Huang, S.C.; Phelps, M.E.; Schelbert, H.R. Quantification and Parametric Imaging of Renal Cortical Blood Flow in Vivo Based on Patlak Graphical Analysis. Kidney Int. 1993, 44, 985–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normand, G.; Lemoine, S.; Le Bars, D.; Merida, I.; Irace, Z.; Troalen, T.; Costes, N.; Juillard, L. PET [11C]Acetate Is Also a Perfusion Tracer for Kidney Evaluation Purposes. Nucl. Med. Biol. 2019, 76–77, 10–14. [Google Scholar] [CrossRef]
- Tahari, A.K.; Bravo, P.E.; Rahmim, A.; Bengel, F.M.; Szabo, Z. Initial human experience with Rubidium-82 renal PET/CT imaging. J. Med. Imaging Radiat. Oncol. 2014, 58, 25–31. [Google Scholar] [CrossRef]
- Koivuviita, N.; Liukko, K.; Kudomi, N.; Oikonen, V.; Tertti, R.; Manner, I.; Vahlberg, T.; Nuutila, P.; Metsärinne, K. The Effect of Revascularization of Renal Artery Stenosis on Renal Perfusion in Patients with Atherosclerotic Renovascular Disease. Nephrol. Dial. Transplant. 2012, 27, 3843–3848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assersen, K.B.; Høilund-Carlsen, P.F.; Olsen, M.H.; Greve, S.V.; Gam-Hadberg, J.C.; Braad, P.-E.; Damkjaer, M.; Bie, P. The Exaggerated Natriuresis of Essential Hypertension Occurs Independently of Changes in Renal Medullary Blood Flow. Acta Physiol. 2019, 226, e13266. [Google Scholar] [CrossRef] [Green Version]
- Rebelos, E.; Dadson, P.; Oikonen, V.; Iida, H.; Hannukainen, J.C.; Iozzo, P.; Ferrannini, E.; Nuutila, P. Renal Hemodynamics and Fatty Acid Uptake: Effects of Obesity and Weight Loss. Am. J. Physiol. -Endocrinol. Metab. 2019, 317, E871–E878. [Google Scholar] [CrossRef]
- Middlekauff, H.R.; Nitzsche, E.U.; Hoh, C.K.; Hamilton, M.A.; Fonarow, G.C.; Hage, A.; Moriguchi, J.D. Exaggerated Muscle Mechanoreflex Control of Reflex Renal Vasoconstriction in Heart Failure. J. Appl. Physiol. 2001, 90, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Middlekauff, H.R.; Nitzsche, E.U.; Nguyen, A.H.; Hoh, C.K.; Gibbs, G.G. Modulation of Renal Cortical Blood Flow during Static Exercise in Humans. Circ. Res. 1997, 80, 62–68. [Google Scholar] [CrossRef]
- Kudomi, N.; Koivuviita, N.; Liukko, K.E.; Oikonen, V.J.; Tolvanen, T.; Iida, H.; Tertti, R.; Metsärinne, K.; Iozzo, P.; Nuutila, P. Parametric Renal Blood Flow Imaging Using [15O]H2O and PET. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 683–691. [Google Scholar] [CrossRef]
- Damkjær, M.; Vafaee, M.; Møller, M.L.; Braad, P.E.; Petersen, H.; Høilund-Carlsen, P.F.; Bie, P. Renal Cortical and Medullary Blood Flow Responses to Altered NO Availability in Humans. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R1449-55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damkjær, M.; Vafaee, M.; Braad, P.E.; Petersen, H.; Høilund-Carlsen, P.F.; Bie, P. Renal Cortical and Medullary Blood Flow during Modest Saline Loading in Humans. Acta Physiol. 2012, 205, 472–483. [Google Scholar] [CrossRef]
- Mather, A.; Pollock, C. Glucose Handling by the Kidney. Kidney Int. 2011, 79, S1–S6. [Google Scholar] [CrossRef] [Green Version]
- Balaban, R.S.; Mandel, L.J. Metabolic Substrate Utilization by Rabbit Proximal Tubule. An NADH Fluorescence Study. Am. J. Physiol. 1988, 254, F407–F416. [Google Scholar] [CrossRef]
- Rebelos, E.; Mari, A.; Oikonen, V.; Iida, H.; Nuutila, P.; Ferrannini, E. Evaluation of Renal Glucose Uptake with [18F]FDG-PET: Methodological Advancements and Metabolic Outcomes. Metabolism 2023, 141, 155382. [Google Scholar] [CrossRef] [PubMed]
- Kessara, A.; Buyukcizmeci, N.; Gedik, G.K. Estimation of patient organ and Whole-Body Doses in [18F-FDG] PET/CT SCAN. Radiat. Prot. Dosim. 2023, 199, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.; Li, L.-P.; Hack, B.; Leloudas, N.; Sprague, S.M. Quantitative Blood Oxygenation Level Dependent Magnetic Resonance Imaging for Estimating Intra-Renal Oxygen Availability Demonstrates Kidneys Are Hypoxemic in Human CKD. Kidney Int. Rep. 2023, 8, 1057–1067. [Google Scholar] [CrossRef]
- Gooding, K.M.; Lienczewski, C.; Papale, M.; Koivuviita, N.; Maziarz, M.; Dutius Andersson, A.-M.; Sharma, K.; Pontrelli, P.; Garcia Hernandez, A.; Bailey, J.; et al. Prognostic Imaging Biomarkers for Diabetic Kidney Disease (IBEAt): Study Protocol. BMC Nephrol. 2020, 21, 242. [Google Scholar] [CrossRef]
- Moriconi, D.; Nannipieri, M.; Dadson, P.; Rosada, J.; Tentolouris, N.; Rebelos, E. The Beneficial Effects of Bariatric-Surgery-Induced Weight Loss on Renal Function. Metabolites 2022, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.; Dadson, P.; Saukko, E.; Honka, M.-J.; Koskensalo, K.; Seppälä, K.; Pekkarinen, L.; Moriconi, D.; Helmiö, M.; Salminen, P.; et al. Renal Sinus Fat Is Expanded in Patients with Obesity and/or Hypertension and Reduced by Bariatric Surgery Associated with Hypertension Remission. Metabolites 2022, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Summers, P.; Faita, F.; Brunetto, M.R.; Callea, F.; de Nicola, A.; Di Lascio, N.; Farinati, F.; Gastaldelli, A.; Gridelli, B.; et al. Digital Liver Biopsy: Bio-Imaging of Fatty Liver for Translational and Clinical Research. World J. Hepatol. 2018, 10, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Pelusi, S.; Margarita, S.; Malvestiti, F.; Dell’Alma, M.; Bianco, C.; Ronzoni, L.; Prati, D.; Targher, G.; Valenti, L. Adverse Effect of PNPLA3 p.I148M Genetic Variant on Kidney Function in Middle-Aged Individuals with Metabolic Dysfunction. Aliment. Pharmacol. Ther. 2023, 57, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Päivärinta, J.; Anastasiou, I.A.; Koivuviita, N.; Sharma, K.; Nuutila, P.; Ferrannini, E.; Solini, A.; Rebelos, E. Renal Perfusion, Oxygenation and Metabolism: The Role of Imaging. J. Clin. Med. 2023, 12, 5141. https://doi.org/10.3390/jcm12155141
Päivärinta J, Anastasiou IA, Koivuviita N, Sharma K, Nuutila P, Ferrannini E, Solini A, Rebelos E. Renal Perfusion, Oxygenation and Metabolism: The Role of Imaging. Journal of Clinical Medicine. 2023; 12(15):5141. https://doi.org/10.3390/jcm12155141
Chicago/Turabian StylePäivärinta, Johanna, Ioanna A. Anastasiou, Niina Koivuviita, Kanishka Sharma, Pirjo Nuutila, Ele Ferrannini, Anna Solini, and Eleni Rebelos. 2023. "Renal Perfusion, Oxygenation and Metabolism: The Role of Imaging" Journal of Clinical Medicine 12, no. 15: 5141. https://doi.org/10.3390/jcm12155141





