Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Material and Methods
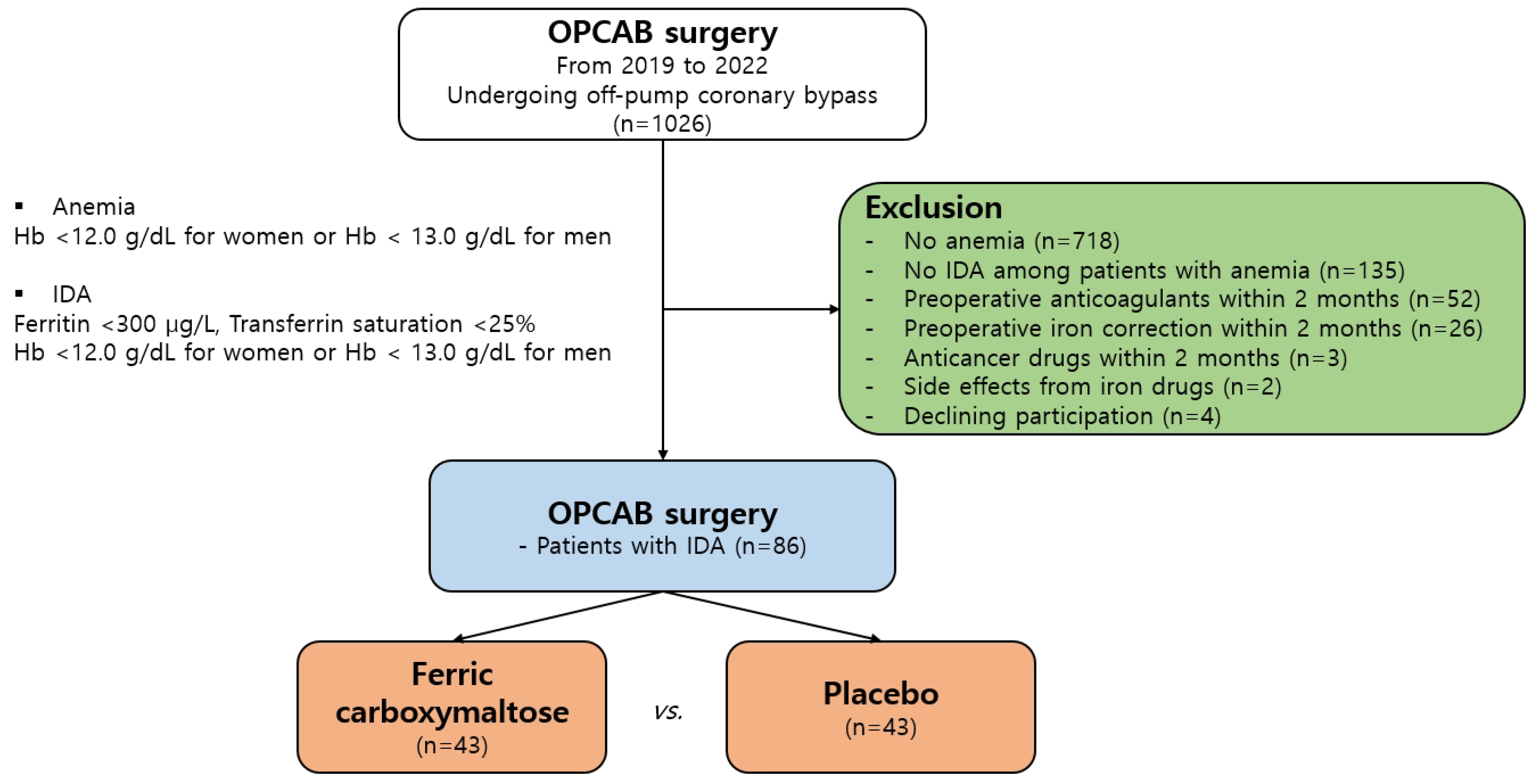
2.1. Patient Selection and Definition of Groups
2.2. Ethics Statements
2.3. Surgical Technique
2.4. Iron Administration
2.5. Endpoints
2.6. Sample Size Calculation
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Prevalence of Anemia
3.2. Operative Data
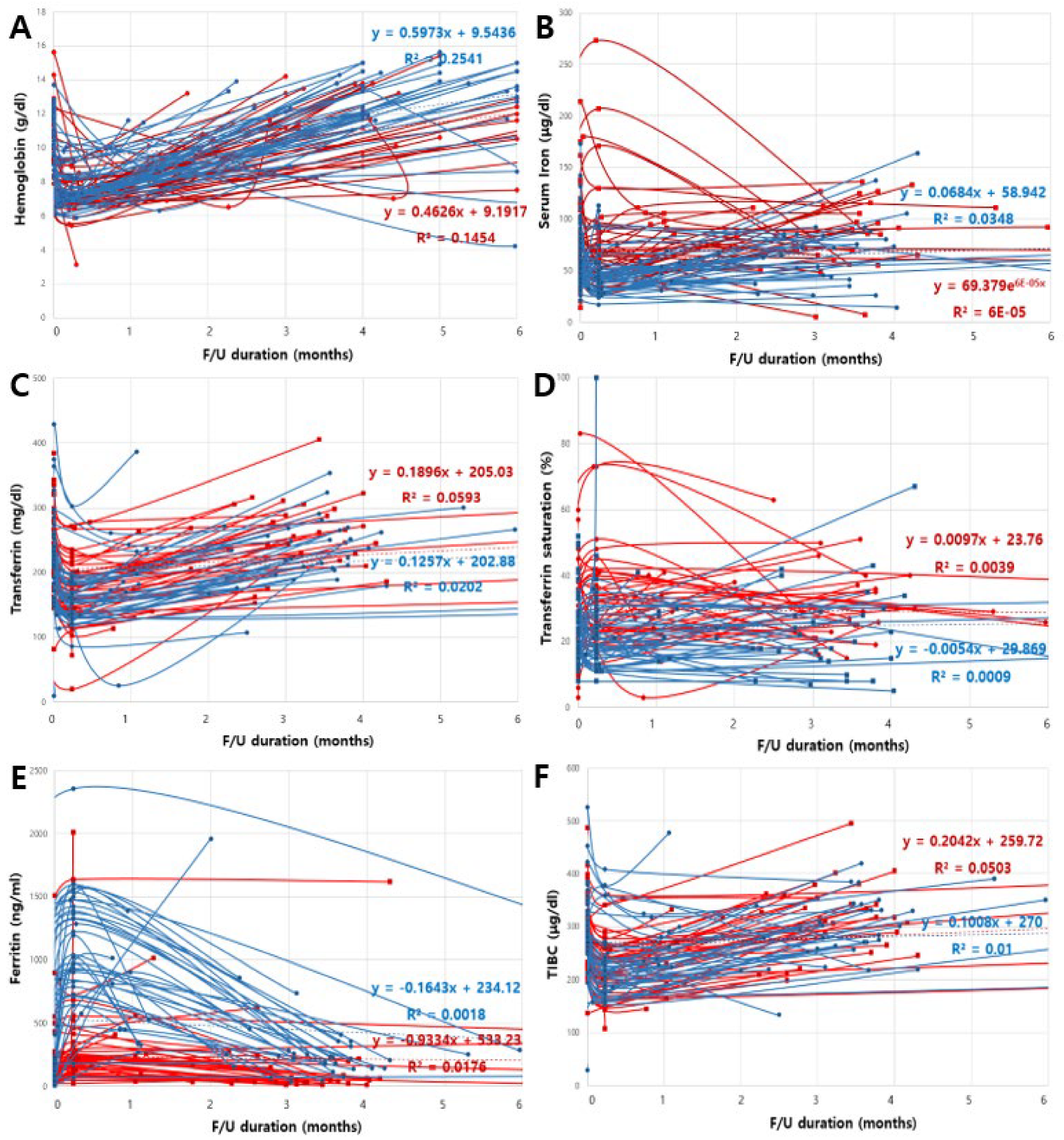
3.3. Changes in Hematologic Parameters
3.4. Linear Mixed-Effects Model for Changes in Hematologic Parameters
3.5. Transfusions and Early Clinical Outcomes
4. Discussion
4.1. Prevalence of Preoperative Anemia in Patients Undergoing OPCAB
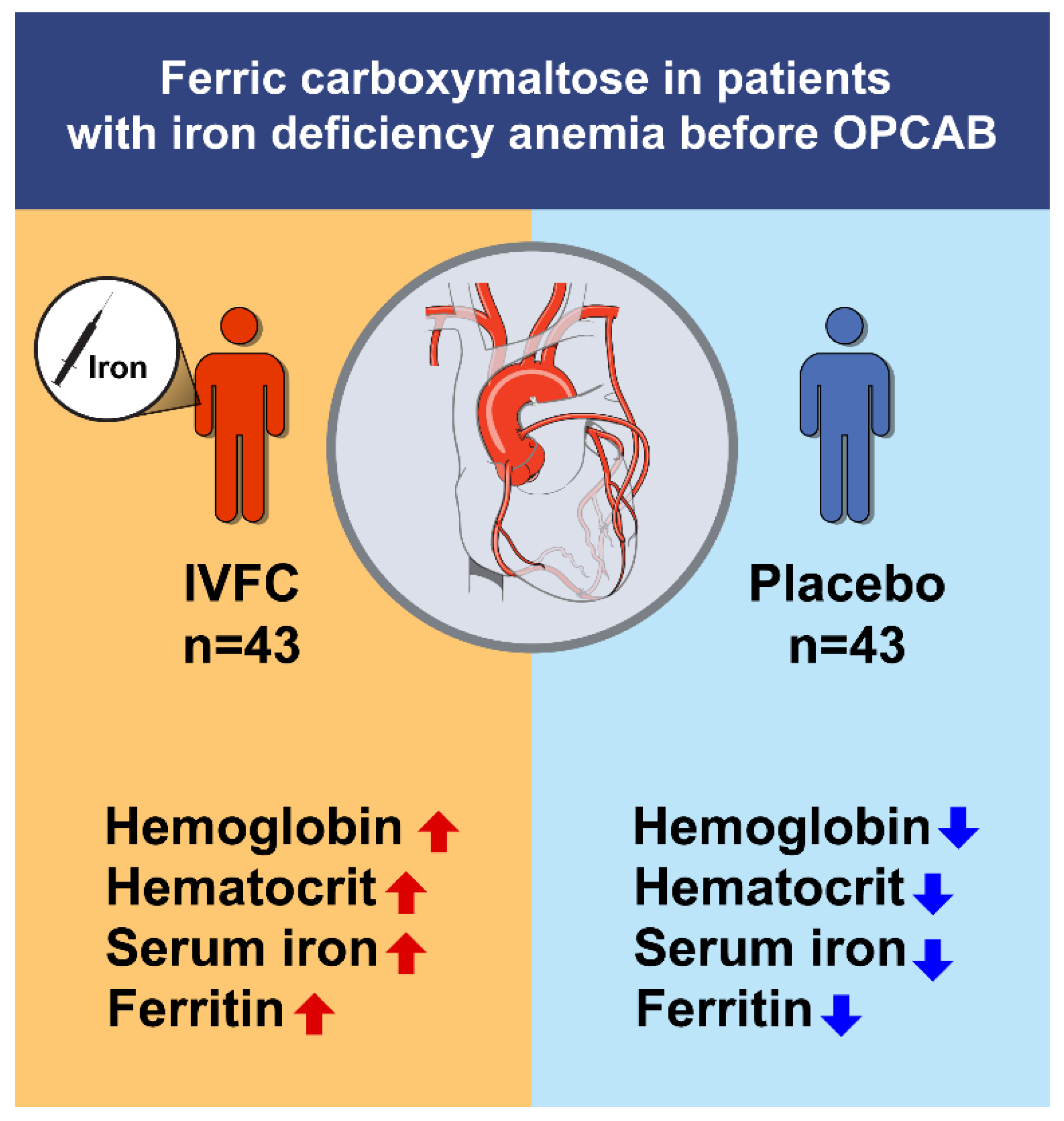
4.2. Effect of IV Iron Treatment on Hematologic Parameters
4.3. Benefit of IV Iron on Hb Levels and Need for RBC Transfusion
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G199–G203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, M.M.; Remoortel, H.V.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R.; Carson, J.L.; Cichutek, K.; De Buck, E.; Devine, D.; et al. Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019, 321, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padhi, S.; Kemmis-Betty, S.; Rajesh, S.; Hill, J.; Murphy, M.F.; Guideline Development Group. Blood transfusion: Summary of NICE guidance. BMJ 2015, 351, h5832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalafallah, A.A.; Yan, C.; Al-Badri, R.; Robinson, E.; Kirkby, B.E.; Ingram, E.; Gray, Z.; Khelgi, V.; Robertson, I.K.; Kirkby, B.P. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anemia: A prospective, open-label, randomised controlled trial. Lancet Haematol. 2016, 3, e415–e425. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Hochrieser, H.; Posch, M.; Metnitz, B.; Rhodes, A.; Moreno, R.P.; Pearse, R.M.; Metnitz, P.; European Surgical Outcomes Study (EuSOS) group for Trials Groups of European Society of Intensive Care Medicine; European Society of Anaesthesiology. Preoperative anemia is associated with poor clinical outcome in non-cardiac surgery patients. Br. J. Anaesth. 2014, 113, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, M.; Besser, M.; Sharples, L.D.; Nair, S.K.; Klein, A.A. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients. Anaesthesia 2011, 66, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.A.; Collier, T.J.; Brar, M.S.; Evans, C.; Hallward, G.; Fletcher, S.N.; Richards, T.; Association of Cardiothoracic Anesthetists. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK—The first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia 2016, 71, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lameson, J.L.; Fauci, A.; Kasper, D.; Hauser, S.; Longo, D.; Joseph, L. Harrison’s Principles of Internal Medicine, 19th ed.; McGraw Hill Education Medical: New York, NY, USA, 2015; pp. 393–399. [Google Scholar]
- IRONMAN Investigators; Litton, E.; Baker, S.; Erber, W.N.; Farmer, S.; Ferrier, J.; French, C.; Gummer, J.; Hawkins, D.; Higgins, A.; et al. Intravenous iron or placebo for anaemia in intensive care: The IRONMAN multicentre randomized blinded trial: A randomized trial of IV iron in critical illness. Intensive Care Med. 2016, 42, 1715–1722. [Google Scholar] [PubMed]
| Variables | IVFC (n = 43) | Placebo (n = 43) | p-Value |
|---|---|---|---|
| Sex | 0.812 | ||
| Male, n (%) | 31 (72.1) | 30 (69.8) | |
| Female, n (%) | 12 (27.9) | 13 (30.2) | |
| Age, mean ± SD, years | 70.6 ± 8.3 | 72.2 ± 9.2 | 0.383 |
| BMI, mean ± SD, kg/m2 | 23.5 ± 2.6 | 22.8 ± 5.8 | 0.503 |
| Preoperative Hb, mean ± SD, g/dL | 11.3 ± 1.3 | 11.1 ± 1.7 | 0.586 |
| Preoperative Hct, mean ± SD, % | 42.0 ± 48.5 | 33.0 ± 5.2 | 0.229 |
| Hypertension, n (%) | 32 (74.4) | 37 (86.0) | 0.279 |
| Diabetes mellitus, n (%) | 31 (72.1) | 20 (46.5) | 0.028 |
| CKD (grades III–V), n (%) | 4 (9.3) | 4 (9.3) | 0.999 |
| CVA history, n (%) | 5 (11.6) | 4 (9.3) | 0.999 |
| Atrial fibrillation, n (%) | 2 (4.7) | 2 (4.7) | 0.999 |
| Preoperative LVEF, mean ± SD, % | 54.6 ± 9.6 | 56.8 ± 11.2 | 0.493 |
| Previous MI, n (%) | 3 (7.0) | 2 (4.7) | 0.828 |
| Previous PCI, n (%) | 4 (9.3) | 2 (4.7) | 0.714 |
| History of peripheral arterial disease, n (%) | 4 (9.3) | 7 (16.3) | 0.520 |
| Type of operation, n (%) | 0.433 | ||
| OPCAB | 38 (88.4) | 41 (95.3) | |
| MIDCAB | 5 (11.6) | 2 (4.7) | |
| Preoperative ACT, mean ± SD, s | 133.1 ± 15.5 | 141.1 ± 15.4 | 0.160 |
| Peak ACT, mean ± SD, s | 232.8 ± 33.5 | 255.8 ± 37.3 | 0.237 |
| Heparin, mean ± SD, U | 6046.3 ± 4994.2 | 5482.9 ± 1236.7 | 0.485 |
| Protamine, mean ± SD, mg | 22.5 ± 3.7 | 21.5 ± 4.2 | 0.462 |
| Total operative time, mean ± SD, min | 249.8 ± 33.3 | 252.1 ± 43.0 | 0.583 |
| EBL during surgery, n (%) | 0.239 | ||
| <100 mL | 13 (30.2) | 12 (27.9) | |
| 100–500 mL | 15 (34.9) | 9 (20.9) | |
| >500 mL | 15 (34.9) | 22 (51.2) | |
| Re-operation for mediastinal bleeding, n (%) | 1 (2.3) | 1 (2.3) | 0.999 |
| Variables | IVFC (n = 43) | Placebo (n = 43) | p-Value | IVFC (n = 43) | Placebo (n = 43) | p-Value | IVFC (n = 43) | Placebo (n = 43) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| 1 Day | 1 Week | 12 Weeks | |||||||
| Hemoglobin (g/dL) | 11.3 ± 1.3 | 11.1 ± 1.7 | 0.993 | 8.3 ± 6.2 | 7.0 ± 0.8 | 0.025 | 13.0 ± 1.6 | 11.4 ± 2.2 | <0.001 |
| Hematocrit (%) | 42.0 ± 48.5 | 33.0 ± 5.2 | 0.278 | 23.5 ± 11.1 | 20.8 ± 2.5 | 0.085 | 41.2 ± 4.4 | 30.6 ± 9.9 | 0.032 |
| Platelet counts (103/μL) | 272.0 ± 33.6 | 217.4 ± 61.3 | 0.987 | 166.4 ± 74.1 | 167.0 ± 50.0 | 0.920 | 218.5 ± 63.5 | 203.1 ± 70.3 | 0.195 |
| Serum iron (μg/dL) | 71.8 ± 43.3 | 70.7 ± 33.2 | 0.861 | 81.4 ± 51.7 | 43.8 ± 16.8 | 0.035 | 81.6 ± 30.9 | 69.0 ± 30.6 | 0.048 |
| TIBC (μg/dL) | 300.6 ± 75.5 | 287.8 ± 71.2 | 0.667 | 229.7 ± 65.3 | 276.2 ± 36.3 | 0.125 | 289.8 ± 65.5 | 268.6 ± 50.8 | 0.215 |
| Transferrin saturation (%) | 25.7 ± 13.2 | 25.2 ± 11.1 | 0.991 | 33.4 ± 15.9 | 25.7 ± 35.8 | 0.049 | 30.2 ± 10.4 | 26.3 ± 17.1 | 0.296 |
| Transferrin (mg/dL) | 231.9 ± 68.0 | 228.0 ± 61.5 | 0.447 | 571.1 ± 260.5 | 273.6 ± 49.8 | <0.001 | 230.1 ± 55.4 | 236.4 ± 65.3 | 0.824 |
| Ferritin (ng/mL) | 97.9 ± 79.0 | 172.4 ± 273.0 | 0.092 | 1173.2 ± 858.4 | 169.9 ± 266.5 | <0.001 | 381.0 ± 393.1 | 133.0 ± 413.2 | 0.035 |
| Variables | IVFC (n = 43) | Placebo (n = 43) | p-Value |
|---|---|---|---|
| Mediastinal drainage 24 h postoperatively, mean ± SD, mL | 413.2 ± 236.5 | 421.9 ± 186.5 | 0.851 |
| Mediastinal drainage 24–48 h postoperatively, mean ± SD, mL | 300.6 ± 115.0 | 327.4 ± 151.5 | 0.358 |
| Postoperative blood transfusion rates, n (%) | 25 (58.1) | 31 (72.1) | 0.258 |
| Packed RBC, n (%) | 8 (18.6) | 12 (27.9) | 0.046 |
| Packed RBC, mean ± SD, mL | 115.9 ± 55.3 | 153.6 ± 88.2 | 0.037 |
| FFP, n (%) | 23 (53.5) | 24 (55.8) | 0.829 |
| FFP, mean ± SD, U | 1.2 ± 1.2 | 1.4 ± 1.5 | 0.474 |
| Platelets, n (%) | 4 (9.3) | 10 (23.3) | 0.049 |
| Platelets, mean ± SD, U | 0.6 ± 1.8 | 1.7 ± 3.3 | 0.043 |
| ICU stay, mean ± SD, days | 2.8 ± 1.4 | 2.3 ± 1.6 | 0.093 |
| Hospital stay, mean ± SD, days | 8.2 ± 13.8 | 7.5 ± 10.3 | 0.520 |
| Adverse drug events †, n (%) | 0 (0) | 0 (0) | 0.999 |
| In-hospital mortality, n (%) | 0 (0) | 2 (4.7) | 0.135 |
| Overall mortality, n (%) | 1 (2.3) | 2 (4.7) | 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-H.; Park, E.H.; Lee, S.H.; Yoo, K.-J.; Youn, Y.-N. Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 1737. https://doi.org/10.3390/jcm12051737
Kim H-H, Park EH, Lee SH, Yoo K-J, Youn Y-N. Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(5):1737. https://doi.org/10.3390/jcm12051737
Chicago/Turabian StyleKim, Hyo-Hyun, Eun Hye Park, Seung Hyun Lee, Kyung-Jong Yoo, and Young-Nam Youn. 2023. "Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 5: 1737. https://doi.org/10.3390/jcm12051737
APA StyleKim, H.-H., Park, E. H., Lee, S. H., Yoo, K.-J., & Youn, Y.-N. (2023). Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(5), 1737. https://doi.org/10.3390/jcm12051737






