Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
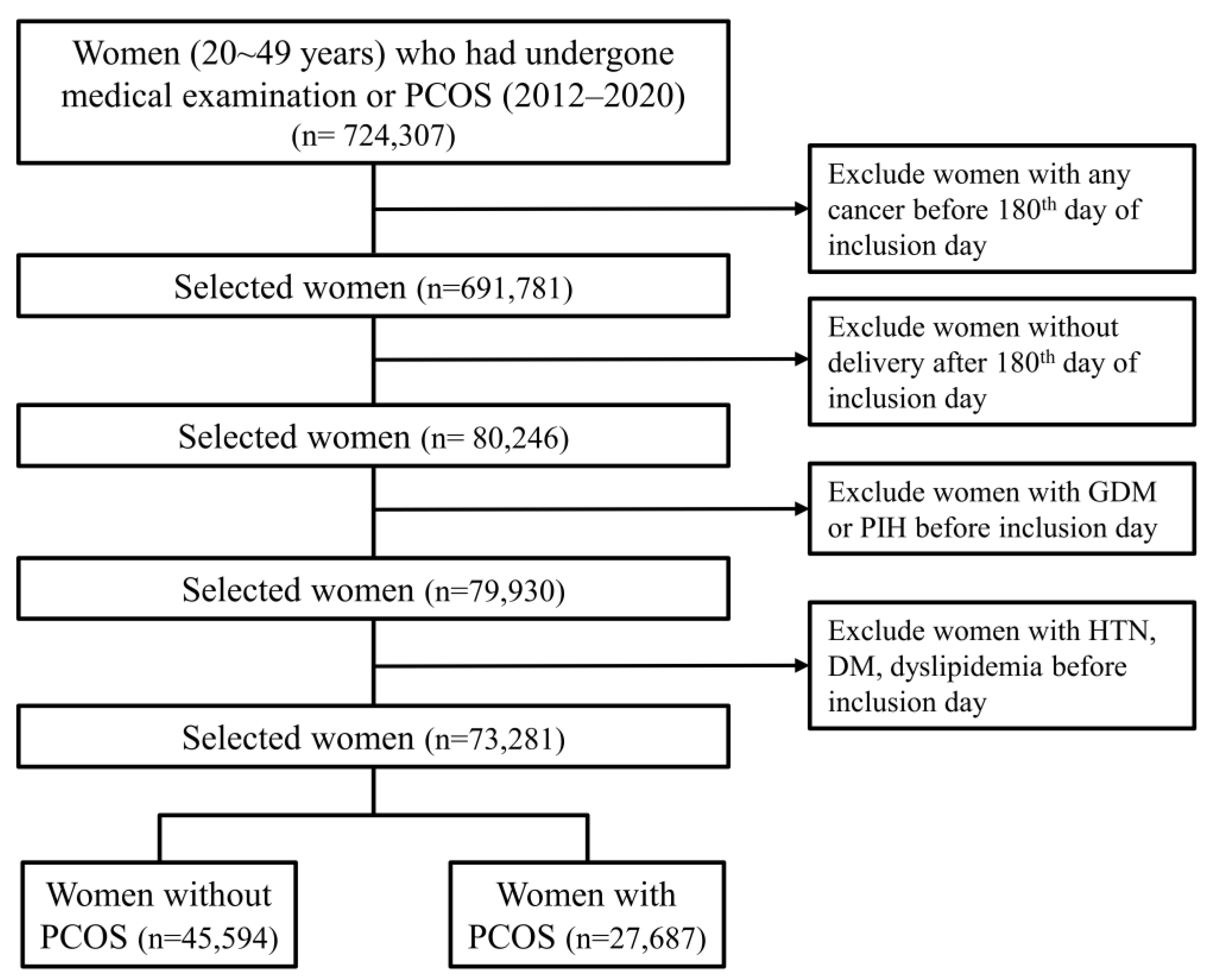
2.2. Selection of Participants
2.3. Outcome
2.4. Variables
2.5. Statistical Analyses
2.6. Ethics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Laughlin, G.A.; Morales, A.J.; Yen, S.S. Inappropriate gonadotropin secretion in polycystic ovary syndrome: Influence of adiposity. J. Clin. Endocrinol. Metab. 1997, 82, 3728–3733. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chun, S. Influence of combined oral contraceptives on polycystic ovarian morphology-related parameters in Korean women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 2020, 63, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef] [PubMed]
- Kjerulff, L.E.; Sanchez-Ramos, L.; Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A meta-analysis. Am. J. Obstet. Gynecol. 2011, 204, 585.e1–585.e6. [Google Scholar] [CrossRef]
- Boutzios, G.; Livadas, S.; Piperi, C.; Vitoratos, N.; Adamopoulos, C.; Hassiakos, D.; Iavazzo, C.; Diamanti-Kandarakis, E. Polycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspring. Fertil. Steril. 2013, 99, 943–950. [Google Scholar] [CrossRef]
- Sivan, E.; Boden, G. Free fatty acids, insulin resistance, and pregnancy. Curr. Diab. Rep. 2003, 3, 319–322. [Google Scholar] [CrossRef]
- Legro, R.S.; Castracane, V.D.; Kauffman, R.P. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obstet. Gynecol. Surv. 2004, 59, 141–154. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Eijkemans, M.J.; Hughes, E.G.; Visser, G.H.; Fauser, B.C.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2013, 11, 56. [Google Scholar] [CrossRef]
- Bahri Khomami, M.; Joham, A.E.; Boyle, J.A.; Piltonen, T.; Silagy, M.; Arora, C.; Misso, M.L.; Teede, H.J.; Moran, L.J. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—A systematic review, meta-analysis, and meta-regression. Obes. Rev. 2019, 20, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Badeghiesh, A.; Suarthana, E.; Baghlaf, H.; Dahan, M.H. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: A population-based study on 9.1 million pregnancies. Hum. Reprod. 2020, 35, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lim, N.K.; Choi, Y.M.; Kim, J.J.; Hwang, K.R.; Chae, S.J.; Park, C.W.; Choi, D.S.; Bang, B.M.; Lee, B.S.; et al. Prevalence of metabolic syndrome is higher among non-obese PCOS women with hyperandrogenism and menstrual irregularity in Korea. PLoS ONE 2014, 9, e99252. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Franks, S. Polycystic ovary syndrome. N. Engl. J. Med. 1995, 333, 853–861. [Google Scholar] [CrossRef]
- Kuhl, C.; Holst, J.J. Plasma glucagon and the insulin:glucagon ratio in gestational diabetes. Diabetes 1976, 25, 16–23. [Google Scholar] [CrossRef]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.; La Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Palomba, S.; Russo, T.; Falbo, A.; Di Cello, A.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Falbo, A.; Chiossi, G.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Early trophoblast invasion and placentation in women with different polycystic ovary syndrome phenotypes. Reprod. Biomed. Online 2014, 29, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Snell-Bergeon, J.K.; Hartsfield, C.L.; Bischoff, K.J.; Hamman, R.F.; McDuffie, R.S. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005, 28, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C. Gestational diabetes in Korea: Incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab. J. 2011, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Farland, L.V.; Stern, J.E.; Liu, C.L.; Cabral, H.J.; Coddington, C.C., 3rd; Diop, H.; Dukhovny, D.; Hwang, S.; Missmer, S.A. Pregnancy outcomes among women with endometriosis and fibroids: Registry linkage study in Massachusetts. Am. J. Obstet. Gynecol. 2022, 226, 829.e1–829.e14. [Google Scholar] [CrossRef]
- Mu, F.; Harris, H.R.; Rich-Edwards, J.W.; Hankinson, S.E.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. A Prospective Study of Inflammatory Markers and Risk of Endometriosis. Am. J. Epidemiol. 2018, 187, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Ness, R.B.; Harger, G.F.; Roberts, J.M. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am. J. Epidemiol. 2005, 162, 1198–1206. [Google Scholar] [CrossRef]
- Ryu, K.J.; Kim, M.S.; Kim, H.K.; Kim, Y.J.; Yi, K.W.; Shin, J.H.; Hur, J.Y.; Kim, T.; Park, H. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: The National Health Insurance Service-National Sample Cohort Study. Fertil. Steril. 2021, 115, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Gynecologists’ Committee on Practice B-G. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef] [PubMed]

| Non-PCOS | PCOS | Total | p-Value | |
|---|---|---|---|---|
| Number of women | 45,594 | 27,687 | 73,281 | |
| Median age (years) | 30 (28–33) | 29 (27–32) | 30 (27–33) | <0.001 |
| Median follow-up period (days) | 616 (343–1107) | 672 (416–1184) | 638 (373–1136) | <0.001 |
| Age at inclusion (years) | <0.001 | |||
| 20~24 | 3855 (8.5) | 3205 (11.6) | 7060 (9.6) | |
| 25~29 | 15,224 (33.4) | 10,962 (39.6) | 26,186 (35.7) | |
| 30~34 | 19,463 (42.7) | 11,223 (40.5) | 30,686 (41.9) | |
| 35~39 | 6274 (13.8) | 2169 (7.8) | 8443 (11.5) | |
| 40~44 | 764 (1.7) | 126 (0.5) | 890 (1.2) | |
| 45~49 | 14 (0) | 2 (0) | 16 (0) | |
| SES | <0.001 | |||
| Mid–high SES | 45,336 (99.4) | 27,591 (99.7) | 72,927 (99.5) | |
| Low SES | 258 (0.6) | 96 (0.3) | 354 (0.5) | |
| Region | <0.001 | |||
| Urban area | 28,915 (63.4) | 14,548 (52.5) | 43,463 (59.3) | |
| Rural area | 16,679 (36.6) | 13,139 (47.5) | 29,818 (40.7) | |
| CCI | <0.001 | |||
| 0 | 37,719 (82.7) | 23,590 (85.2) | 61,309 (83.7) | |
| 1 | 5569 (12.2) | 3138 (11.3) | 8707 (11.9) | |
| ≥2 | 2306 (5.1) | 959 (3.5) | 3265 (4.5) | |
| Parity | <0.001 | |||
| Primi | 38,389 (84.2) | 25,693 (92.8) | 64,082 (87.4) | |
| Multi | 7205 (15.8) | 1994 (7.2) | 9199 (12.6) | |
| Multiple pregnancy | <0.001 | |||
| Singleton | 44,022 (96.6) | 25,075 (90.6) | 69,097 (94.3) | |
| Multiple | 1572 (3.4) | 2612 (9.4) | 4184 (5.7) | |
| Adnexal surgery | <0.001 | |||
| Absent | 44,160 (96.9) | 27,267 (98.5) | 71,427 (97.5) | |
| Present | 1434 (3.1) | 420 (1.5) | 1854 (2.5) | |
| Uterine leiomyoma | <0.001 | |||
| Absent | 42,296 (92.8) | 26,669 (96.3) | 68,965 (94.1) | |
| Present | 3298 (7.2) | 1018 (3.7) | 4316 (5.9) | |
| Endometriosis | <0.001 | |||
| Absent | 43,925 (96.3) | 27,174 (98.1) | 71,099 (97) | |
| Present | 1669 (3.7) | 513 (1.9) | 2182 (3) | |
| Obesity | 0.25 | |||
| Absent | 45,532 (99.9) | 27,640 (99.8) | 73,172 (99.9) | |
| Present | 62 (0.1) | 47 (0.2) | 109 (0.1) |
| Non-PCOS | PCOS | Total | p-Value | RR (95% CI) | |
|---|---|---|---|---|---|
| Number of women | 45,594 | 27,687 | 73,281 | ||
| GDM | <0.001 | 1.709 (1.61–1.814) | |||
| Absent | 43,273 (94.9) | 25,362 (91.6) | 68,635 (93.7) | ||
| Present | 2321 (5.1) | 2325 (8.4) | 4646 (6.3) | ||
| PIH | 0.016 | 1.385 (1.062–1.808) | |||
| Absent | 45,475 (99.7) | 27,587 (99.6) | 73,062 (99.7) | ||
| Present | 119 (0.3) | 100 (0.4) | 219 (0.3) |
| GDM | PIH | |||
|---|---|---|---|---|
| RR (95% CI) a | p-Value | RR (95% CI) a | p-Value | |
| Adjusted a | ||||
| PCOS | 1.719 (1.616–1.828) | <0.001 | 1.243 (0.94–1.644) | 0.127 |
| Age at inclusion (years) (reference = 20~24) | ||||
| 25~29 | 1.585 (1.391–1.806) | <0.001 | 1.209 (0.686–2.13) | 0.511 |
| 30~34 | 1.896 (1.667–2.157) | <0.001 | 1.56 (0.897–2.714) | 0.115 |
| 35~39 | 2.551 (2.204–2.953) | <0.001 | 1.642 (0.861–3.131) | 0.132 |
| 40~44 | 3.431 (2.646–4.45) | <0.001 | 4.677 (1.854–11.8) | 0.001 |
| 44~49 | 7.681 (2.153–27.408) | 0.002 | 0 (0-Infinite) | 0.969 |
| Low SES | 1.131 (0.709–1.804) | 0.607 | 2.727 (0.661–11.246) | 0.165 |
| Rural area | 1.117 (1.051–1.188) | <0.001 | 0.868 (0.656–1.149) | 0.322 |
| CCI (reference = 0) | ||||
| 1 | 1.1 (1.005–1.205) | 0.119 | 1.866 (1.328–2.622) | 0.008 |
| ≥2 | 1.012 (0.872–1.174) | 0.643 | 1.022 (0.521–2.005) | 0.401 |
| Primiparity | 1.447 (1.304–1.605) | <0.001 | 2.293 (1.292–4.082) | 0.005 |
| Multiple pregnancy | 1.127 (1.004–1.265) | 0.043 | 3.668 (2.605–5.165) | <0.001 |
| Adnexal surgery before inclusion | 0.995 (0.803–1.232) | 0.96 | 0.719 (0.296–1.744) | 0.466 |
| Uterine leiomyoma | 0.965 (0.846–1.1) | 0.592 | 0.751 (0.417–1.353) | 0.34 |
| Endometriosis | 0.841 (0.686–1.031) | 0.096 | 2.399 (1.31–4.395) | 0.005 |
| Obesity | 0.958 (0.444–2.07) | 0.913 | 2.626 (0.354–19.478) | 0.345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-W.; Yoon, S.-H.; Kim, M.; Seo, Y.-S.; Yuk, J.-S. Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2023, 12, 1738. https://doi.org/10.3390/jcm12051738
Yang S-W, Yoon S-H, Kim M, Seo Y-S, Yuk J-S. Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. Journal of Clinical Medicine. 2023; 12(5):1738. https://doi.org/10.3390/jcm12051738
Chicago/Turabian StyleYang, Seung-Woo, Sang-Hee Yoon, Myounghwan Kim, Yong-Soo Seo, and Jin-Sung Yuk. 2023. "Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study" Journal of Clinical Medicine 12, no. 5: 1738. https://doi.org/10.3390/jcm12051738
APA StyleYang, S.-W., Yoon, S.-H., Kim, M., Seo, Y.-S., & Yuk, J.-S. (2023). Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. Journal of Clinical Medicine, 12(5), 1738. https://doi.org/10.3390/jcm12051738






