Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Evaluation of the Hemoglobin Level and Its Oxygen Saturation in the Intraoperative Period
3.2. Evaluation of the Levels of Hemoglobin and Hematocrit, Venous Blood Saturation, Blood Lactate Level, WBC, Blood Creatinine and Urea Level in the Postoperative Period
3.3. Evaluation of Brain Injury Marker Levels
3.4. Evaluation of the SIRS Markers Level
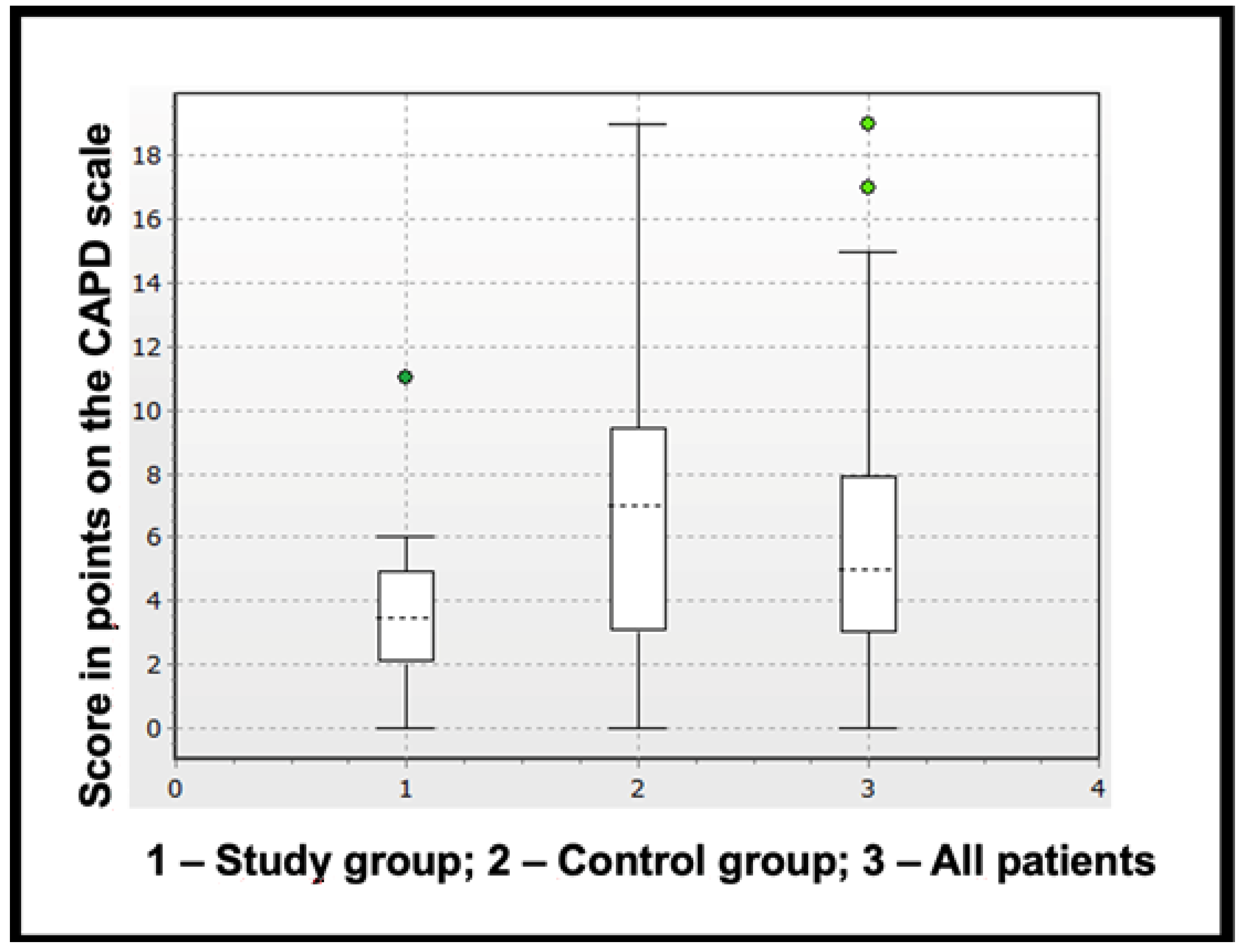
3.5. Diagnosis of Delirium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHD | congenital heart defects |
| CPB | cardiopulmonary bypass |
| S100β | S100 calcium-binding protein β |
| NSE | neuron-specific enolase |
| GFAP | glial fibrillary acidic protein |
| PRBCs | packed red blood cells |
| w/oRBC | without PRBCs |
| wRBC | with PRBCs |
| SIRS | systemic inflammatory response syndrome |
| NVU | neurovascular unit |
| POD | postoperative delirium |
References
- Kaushal, V.; Schlichter, L.C. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008, 28, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Torbett, B.E.; Baird, A.; Eliceiri, B.P. Understanding the rules of the road: Proteomic approaches to interrogate the blood brain barrier. Front. Neurosci. 2015, 4, 70. [Google Scholar] [CrossRef]
- Shrader, N.I.; Shaybakova, V.L.; Likhvantsev, V.V.; Levikov, D.I.; Levin, O.S. Neurological complications of coronary artery bypass grafting. Nevrol. I Psihiatr. Im. S.S. Korsakova 2012, 3, 76–81. (In Russian) [Google Scholar] [CrossRef]
- Guenther, U.; Theuerkauf, N.; Frommann, I.; Brimmers, K.; Malik, R.; Stori, S.; Scheidemann, M.; Putensen, C.; Popp, J. Predisposing and precipitating factors of delirium after cardiac surgery. A prospective observational cohort study. Ann. Surg. 2013, 257, 1160–1167. [Google Scholar] [CrossRef]
- Ramlawi, B.; Rudolph, J.L.; Mieno, S.; Khabbaz, K.; Sodha, N.R.; Boodhwani, M.; Levkoff, S.E.; Marcantonio, E.R.; Sellke, F.W. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann. Surg. 2006, 244, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, M.C.; Boers, C.; Vroege, R.; Kelder, J.C.; Yilmaz, A.; Bruins, P. Clinical evaluation of the air removal characteristics of an oxygenator with integrated arterial filter in a minimized extracorporeal circuit. Int. J. Artif. Organs 2011, 34, 374–382. [Google Scholar] [CrossRef]
- Grigore, A.M.; Murray, C.F.; Ramakrishna, H.; Djaiani, G. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: Does rewarming rate matter? Anesth. Analg. 2009, 109, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Jirschik, M.; Keyl, C.; Beyersdorf, F. A clinical comparison of bubble elimination in Quadrox and Polystan oxygenators. Perfusion 2009, 24, 423–427. [Google Scholar] [CrossRef]
- Wahba, A.; Milojevic, M.; Boer, C.; De Somer, F.M.; Gudbjartsson, T.; Van Den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; Ranucci, M.; et al. EACTS/EACTA/EBCP Committee Reviewers, 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2020, 57, 210–251. [Google Scholar]
- Hirata, Y. Cardiopulmonary bypass for pediatric cardiac surgery. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 65–70. [Google Scholar] [CrossRef]
- Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children*. Pediatr Crit Care Med. 2014, 15, e111-9. [CrossRef]
- Denes, A.; Vidyasagar, R.; Feng, J.; Narvainen, J.; McColl, B.W.; Kauppinen, R.A.; Allan, S.M.J. Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. 2007, 27, 1941–1953. [Google Scholar] [CrossRef]
- Hori, D.; Brown, C.; Ono, M.; Rappold, T.; Sieber, F.; Gottschalk, A.; Neufeld, K.J.; Gottesman, R.; Adachi, H.; Hogue, C.W. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br. J. Anaesth. 2014, 113, 1009–1017. [Google Scholar] [CrossRef]
- Güvener, M.; Korun, O.; Demirtürk, O.S. Risk factors for systemic inflammatory response after congenital cardiac surgery. J. Card Surg. 2015, 30, 92–96. [Google Scholar] [CrossRef]
- Nellis, M.E.; Goel, R.; Feinstein, S.; Shahbaz, S.; Kaur, S.; Traube, C. Association between transfusion of RBCs and subsequent development of delirium in critically ill children. Pediatr. Crit. Care Med. 2018, 19, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Stark, P.C.; Suh, M.; Triulzi, D.J.; Hess, J.R.; Steiner, M.E.; Stowell, C.P.; Sloan, S.R. The Impact of Blood Component Ratios on Clinical Outcomes and Survival. Anesth. Analg. 2017, 124, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A.; Ballert, E.Q.; Mahan, A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome. Am. J. Surg. 2013, 205, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.L.; Wade, J.; Roback, J.D. Transfusion-Transmitted Infections: An Update on Product Screening, Diagnostic Techniques, and the Path Ahead. J. Clin. Microbiol. 2018, 56, e00352-18. [Google Scholar] [CrossRef]
- Frazier, S.K.; Higgins, J.; Bugajski, A.; Jones, A.R.; Brown, M.R. Adverse Reactions to Transfusion of Blood Products and Best Practices for Prevention. Crit. Care Nurs. Clin. North Am. 2017, 29, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Rothoerl, R.D.; Brawanski, A.; Woertgen, C. S100B protein serum levels after controlled cortical impact injury in the rat. Acta Neurochir. 2001, 142, 199–203. [Google Scholar] [CrossRef]
- Beer, C.; Blacker, D.; Bynevelt, M. Systemic markers of inflammation are independently associated with S100B concentration: Results of an observational study in subjects with acute ischaemic stroke. J. Neuroinflammation 2010, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Rabinowicz, A.J.; Correale, J.; Boutros, R.B. Neuronspecific enolase is increased after single seizures during inpatient video/EEG monitoring. Epilepsia 1996, 37, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Botwinski, C.A. Systemic inflammatory response syndrome. Neonatal Netw. 2001, 20, 21–28. [Google Scholar] [CrossRef]
- Smok, B.; Domagalski, K.; Pawłowska, M. Diagnostic and Prognostic Value of IL-6 and sTREM-1 in SIRS and Sepsis in Children. Mediat. Inflamm 2020, 2020, 8201585. [Google Scholar] [CrossRef] [PubMed]
- Silver, G.; Kearney, J.; Traube, C.; Hertzig, M. Delirium screening anchored in child development: The Cornell Assessment for Pediatric Delirium. Palliat. Support. Care 2014, 13, 1005–1011. [Google Scholar] [CrossRef]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Kain, Z.N.; Mayes, L.C.; Cicchetti, D.V.; Bagnall, A.L.; Finley, J.D.; Hofstadter, M.B. The Yale Preoperative Anxiety Scale: How does it compare with a “gold standard”? Anesth. Analg. 1997, 85, 783–788. [Google Scholar] [CrossRef]
- Trukhacheva, N.V. Matematicheskaya statistika v mediko-biologicheskikh issle- dovaniyakh s primeneniem paketa Statistica. Mosc. GEOTAR-Media 2013, 57–60. (In Russian) [Google Scholar]
- Clark, R.K.; Lee, E.V.; Fish, C.J.; White, R.F.; Price, W.J.; Jonak, Z.L.; Feuerstein, G.Z.; Barone, F.C. Development of tissue damage, inflammation and resolution following stroke: An immunohistochemical and quantitative planimetric study. Brain Res. Bull. 1993, 31, 565–572. [Google Scholar] [CrossRef]
- Yao, F.S.F.; Tseng, C.C.A.; Ho, C.Y.A.; Levin, S.K.; Illner, P. Cerebral oxygen desaturation is associated with early post- operative neuropsychological dysfunction in patients undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 552–558. [Google Scholar] [CrossRef]
- Panch, S.R.; Montemayor-Garcia, C.; Klein, H.G. Hemolytic Transfusion Reactions. N. Engl. J. Med. 2019, 381, 150–162. [Google Scholar] [CrossRef]
- Pozhilenkova, E.A.; Lopatina, O.L.; Komleva, Y.K.; Salmin, V.V.; Salmina, A.B. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Rev. Neurosciences. 2017, 28, 397–415. [Google Scholar] [CrossRef]
- Tong G, Krauss A, Mochner J, Wollersheim S, Soltani P, Berger F, Schmitt KRL. Deep hypothermia therapy attenuates LPS-induced microglia neuroinflammation via the STAT3 pathway. Neuroscience 2017, 358, 201–210. [CrossRef]
- Jonsson, H.; Johnsson, P.; Hoglund, P. Elimination of S100B and renal function after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2000, 14, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M. S100 and S100β: Biomarkers of cerebral damage in cardiac surgery with or without the use of cardiopulmonary bypass. Rev Bras Cir Cardiovasc. 2014, 29, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Larionov, M.V.; Trubnikova, O.A.; Plotnikov, G.P.; Grigoryev, E.V.; Shukevich, D.L. Justification of the choice of anesthetics to protect the brain and prevent cognitive decline during coronary bypass surgery. Meditsina V Kuzbasse 2015, 14, 43–51. (In Russian) [Google Scholar]
- Patel, A.K.; Biagas, K.V.; Clarke, E.C. Delirium in Children After Cardiac Bypass Surgery. Pediatr. Crit. Care Med. 2017, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.; Bellinger, D.C. Executive function deficits in congenital heart disease: Why is intervention important? Cardiol. Young 2015, 25, 1238–1246. [Google Scholar] [CrossRef]
- Alvarez, R.V.; Palmer, C.; Czaja, A.S.; Peyton, C.; Silver, G.; Traube, C.; Mourani, P.M.; Kaufman, J. Delirium is a Common and Early Finding in Patients in the Pediatric Cardiac Intensive Care Unit. J. Pediatr. 2018, 195, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Saxena, K.; Verma, M.; Bharosay, A. Correlative study between neuron-specific enolase and blood sugar level in ischemic stroke patients. J. Neurosci. Rural. Pract. 2011, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.; Jónsson, K.; Zetterberg, H.; Blennow, K.; Kolsrud, O.; Ricksten, S.-E.; Dellgren, G.; Björk, K.; Jeppsson, A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol Scand 2022, 66, 447–453. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, M.; Furey, W.; Hajj, J.; Lindekens, J.; Patel, S.; Acker, M.; Bavaria, J.; Szeto, W.Y.; Atluri, P.; Haber, M.; et al. Observational study of long-term persistent elevation of neurodegeneration markers after cardiac surgery. Sci. Rep. 2019, 9, 7177. [Google Scholar] [CrossRef]
- Mille, F.K.; Badheka, A.; Yu, P.; Zhang, X.; Friedman, D.F.; Kheir, J.; van den Bosch, S.; Cabrera, A.G.; Lasa, J.J.; Katcoff, H.; et al. Red Blood Cell Transfusion After Stage I Palliation Is Associated with Worse Clinical Outcomes. J. Am. Heart Assoc. 2020, 9, e015304. [Google Scholar] [CrossRef]
| Investigated Characteristic | Study Group | Control Group | p | |
|---|---|---|---|---|
| Number of patients | 20 (50%) | 20 (50%) | 1 | |
| Male | 7 (35%) | 9 (45%) | 0.52 | |
| Female | 13 (65%) | 11 (55%) | 0.52 | |
| Age (months) | 15 (12–23.3) | 13 (11–21.3) | 0.27 | |
| Height (cm) | 81 (76–86) | 75 (71.3–84.3) | 0.14 | |
| Body weight (kg) | 10.5 (9.2–11.3) | 9.2 (8.7–11.8) | 0.15 | |
| Laboratory indicators of blood before surgery | ||||
| Leukocyte level (×109/L) | 7.4 (6.6–7.9) | 7.5 (7–9) | 0.17 | |
| Erythrocyte level (×1012/L) | 4.6 (4.5–4.75) | 4.6 (3.9–5) | 0.7 | |
| Hemoglobin level (g/L) | 118.5 (115–121.3) | 117 (112.8–119) | 0.29 | |
| Hematocrit level (%) | 36 (34–38) | 35 (33–37) | 0.34 | |
| Direct bilirubin level (μmol/L) | 2.4 (2.1–3.3) | 2.9 (2.1–3.7) | 0.54 | |
| Indirect bilirubin level (μmol/L) | 4.3 (2.5–5.5) | 4.5 (2.4–6.7) | 0.68 | |
| Creatinine level (μmol/L) | 38.5 (30.5–44.3) | 31 (24.3–43.3) | 0.23 | |
| Urea level (mmol/L) | 3.8 (3.4–4.3) | 4 (3–5) | 0.98 | |
| Preoperative NGAL concentration (ng/mL) | 49.19 (24.3–100.1) | 45.98 (34.58–98.98) | 0.3 | |
| Surgical intervention | ||||
| Diagnosis | ASD | 15 (75%) | 15 (75%) | 1 |
| VSD | 5 (25%) | 5 (25%) | 1 | |
| Surgical approach | Median sternotomy | 14 (70%) | 15 (75%) | 0.85 |
| Side sternotomy | 6 (30%) | 5 (25%) | 0.85 | |
| Duration of surgery | 196 (188–203) | 189 (181–200) | 0.3 | |
| CPB duration (min.) | 40.5 (33–47) | 45 (35–49.5) | 0.5 | |
| Duration of aortic clamping (min.) | 27.5 (20.3–33) | 29 (22.3–36.3) | 0.59 | |
| Investigated Characteristic | Study Group | Control Group | p |
|---|---|---|---|
| Laboratory indicators | |||
| Hemoglobin level during the CPB (g/L) | 87 (81.0–91.3) | 92 (87.3–97.3) | 0.008 |
| Hematocrit level during the CPB (%) | 25.5 (24.0–27.0) | 29 (27.8–31.0) | ˂0.001 |
| Hemoglobin level at the end of the operation (g/L) | 106.0 (101.8–110.3) | 130.5 (104.0–125.5) | ˂0.001 |
| Hematocrit level at the end of the operation (%) | 31.5 (30–33.3) | 40.0 (38.8–41.5) | ˂0.001 |
| Venous blood saturation during the CPB (%) | 85.0 (83.8–89.0) | 88.5 (86.0–90.0) | 0.26 |
| Venous blood saturation at the end of the operation (%) | 71.0 (69.8–73.0) | 73.0 (71.8–77.0) | 0.01 |
| Blood lactate during the CPB (mmol/L) | 1.5 (1.3–1.8) | 1.5 (1.2–1.9) | 0.87 |
| Blood lactate at the end of the operation (mmol/L) | 1.5 (1.3–1.7) | 1.5 (1.2–1.7) | 0.46 |
| Preoperative NGAL concentration (ng/mL) | 49.2 (24.3–100.1) | 46.0 (34.6–99.0) | 0.3 |
| Monitoring indicators | |||
| SpO2 indicators before the operation (%) | 97.0 (90.5–98.0) | 98.0 (95.5–98.5) | 0.33 |
| SpO2 indicators at the end of the operation (%) | 99.0 (98.0–99.0) | 99.0 (99.0–100.0) | 0.03 |
| rSO2 indicators before the operation (%) | 65.0 (61.5–73.5) | 67.0 (61.5–70.5) | 0.77 |
| rSO2 indicators during the CPB (%) | 83.0 (80.5–86.5) | 85.0 (81.5–87.0) | 0.40 |
| rSO2 indicators at the end of the operation (%) | 70.5 (69.8–75.0) | 77.0 (74.5–78.0) | 0.008 |
| Inotropic drugs | |||
| Number of patients with nootropic drugs | 4 (20%) | 5 (25%) | 0.7 |
| Hydrobalance indicators | |||
| Intravenous infusion volume (ml/kg) | 15.6 (13.5–16.4) | 15.7 (12.8–17.4) | 0.31 |
| Diuresis volume (ml/kg) | 11.0 (9.0–12.4) | 10.5 (9.3–12.3) | 0.43 |
| Ultrafiltration volume during CPB (ml/kg) | 11.0 (10.1–13.3) | 11.7 (10.2–13.5) | 0.37 |
| Investigated Characteristic | Study Group | Control Group | p |
|---|---|---|---|
| Laboratory indicators | |||
| Hemoglobin level (Γ/л) | 101.0 (98.8–107.0) | 124.0 (113.0–127.0) | ˂0.001 |
| Hematocrit level (%) | 30.0 (29.0–32.0) | 34.0 (33.0–36.0) | ˂0.001 |
| Venous blood saturation (%) | 70.0 (68.8–73.3) | 76.5 (73.0–80.0) | ˂0.001 |
| Blood lactate (mmol/L) | 1.2 (1.1–1.35) | 1.2 (1.08–1.3) | 0.67 |
| Red blood cell level (×1012) | 3.8 (3.6–4.1) | 4.8 (4.5–5.0) | ˂0.001 |
| Leukocyte level (×109) | 8.5 (7.9–11.1) | 10.8 (9.3–12.8) | 0.013 |
| Direct bilirubin level (μmol/L) | 2.9 (2.2–3.2) | 3.3 (2.3–4.4) | 0.29 |
| Indirect bilirubin level (μmol/L) | 3.8 (2.7–4.9) | 9.5 (4.9–13.0) | ˂0.001 |
| Creatinine level (μmol/L) | 26.5 (19.8–31.0) | 32.5 (26.0–40.0) | 0.015 |
| Urea level (mmol/L) | 3.7 (3.1–4.9) | 4.5 (4.0–5.5) | 0.032 |
| Postoperative NGAL concentration (ng/mL) | 87.3 (41.3–159.1) | 74.5 (49.5–136.2) | 0.46 |
| Dynamic observation indicators | |||
| Drainage losses in the first day after surgery (ml/kg) | 54.6 (46.4–84.0) | 68.0 (53.3–82.4) | 0.3 |
| Duration of stay in the intensive care unit (hours) | 23.5 (21.0–29.0) | 23.0 (21.8–41.5) | 0.97 |
| Duration of mechanical ventilation (hours) | 7.0 (6.0–8.0) | 8.0 (6.8–9.0) | 0.34 |
| Inotropic drugs | |||
| Number of patients with nootropic drugs | 4 (20%) | 5 (25 %) | 0.7 |
| Hydrobalance indicators | |||
| The volume of fluid injected during the period of stay in the intensive care unit (ml) | 64.0 (62.70–69.2) | 61.0 (59.4–64.9) | 0.1 |
| The volume of diuresis during the period of being in the intensive care unit (ml) | 24.0 (22.0–26.5) | 28.0 (22.5–30.0) | 0.08 |
| Investigated Characteristic | Study Group | Control Group | p |
|---|---|---|---|
| IL-1b BO, pg/mL | 2.6 (2.2–2.8) | 2.6 (2.5–3.0) | 0.16 |
| IL-1b EO, pg/mL | 2.9 (2.7–3.1) | 3.3 (3.2–3.5) | 0.003 |
| IL-1b 16 h after surgery, pg/mL | 2.7 (2.6–3.1) | 2.8 (2.7–3.1) | 0.46 |
| IL-6 BO, pg/mL | 2.5 (2.4–2.7) | 2.6 (2.4–5.9) | 0.21 |
| IL-6 EO, pg/mL | 29.1 (15.5–40.6) | 27.6 (16.9–48.5) | 0.18 |
| IL-6 16 h after surgery, pg/mL | 31.6 (26.8–48.9) | 48.9 (33.9–57.6) | 0.087 |
| IL-10 BO, pg/mL | 0.6 (0.6–0.7) | 0.6 (0.6–0.9) | 0.39 |
| IL-10 EO, pg/mL | 7.9 (4.5–12.1) | 8.8 (5.6–38.5) | 0.07 |
| IL-10 16 h after surgery, pg/mL | 0.7 (0.6–0.8) | 0.8 (0.8–1.4) | 0.005 |
| TNF-α BO, pg/mL | 1.3 (1.1–1.5) | 1.2 (1.2–1.3) | 0.19 |
| TNF-α EO, pg/mL | 1.3 (1.3–1.8) | 1.81 (1.4–3.3) | 0.034 |
| TNF-α 16 h after surgery, pg/mL | 1.2 (1.1–1.6) | 1.3 (1.2–1.9) | 0.1 |
| S-100-ß BO, ng/m | 185.3 (147.05–230.1) | 244.2 (165.93–360.18) | 0.33 |
| S-100-ß EO, ng/mL | 522.1 (386.65–702.9) | 947.7 (696.93–1378.25) | p ˂ 0.001 |
| S-100-ß 16 h after surgery, ng/mL | 167 (95.7–204.8) | 207.7 (125.23–291.25) | 0.18 |
| NSE BO, ng/m | 16.57 (13.39–19.58) | 14.51 (12.34–18.47) | 0.358 |
| NSE EO, ng/mL | 30.51 (22.8–36.99) | 44.92 (34.1–55.06]) | 0.007 |
| NSE 16 h after surgery, ng/mL | 19.85 (17.04–24.4) | 24.15 (16.67–29.29) | 0.494 |
| GFAP BO, ng/m | 0.1094 (0.1035–0.1115) | 0.1137 (0.1079–0.1242) | 0.06 |
| GFAP EO, ng/mL | 0.1172 (0.1093–0.1198) | 0.1238 (0.1195–0.1348) | 0.004 |
| GFAP 16 h after surgery, ng/mL | 0.11 (0.105–0.1197) | 0.1212 (0.1177–0.1404) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivkin, A.A.; Grigoriev, E.; Sinitskaya, A.V. Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery. J. Clin. Med. 2023, 12, 1465. https://doi.org/10.3390/jcm12041465
Ivkin AA, Grigoriev E, Sinitskaya AV. Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery. Journal of Clinical Medicine. 2023; 12(4):1465. https://doi.org/10.3390/jcm12041465
Chicago/Turabian StyleIvkin, Artem A., Evgeny Grigoriev, and Anna V. Sinitskaya. 2023. "Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery" Journal of Clinical Medicine 12, no. 4: 1465. https://doi.org/10.3390/jcm12041465
APA StyleIvkin, A. A., Grigoriev, E., & Sinitskaya, A. V. (2023). Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery. Journal of Clinical Medicine, 12(4), 1465. https://doi.org/10.3390/jcm12041465





