Abstract
Objective: For peripheral artery disease (PAD) patients, after endovascular revascularization, it is crucial to manage associated factors that can affect the risk of major adverse events. We aimed to investigate the associated factors of major adverse events in these patients. Materials and Methods: We conducted a retrospective longitudinal analysis using the electronic medical records from a tertiary hospital in Korea and included the data of 1263 patients. Eligible patients were categorized into four groups based on diabetes mellitus (DM) and regular exercise. The major adverse events included major adverse limb events and major adverse cardiovascular events. Major adverse events-free survival was assessed using the Kaplan–Meier method, and associated factors of major adverse events were analyzed using Cox proportional hazards analyses. Results: Kaplan–Meier survival curves showed that patients with DM and non-regular exercise had a shorter major adverse events-free survival. The Cox regression analysis showed that for patients with critical limb ischemia or chronic kidney disease, the risk of major adverse events increased, while group variables were not significant. Conclusion: Target management of patients with DM, critical limb ischemia, and chronic kidney disease is essential to reduce major adverse events after endovascular revascularization in patients with PAD.
1. Introduction
Peripheral artery disease (PAD) is defined as an atherosclerotic arterial disease that reduces blood flow to the affected limb by stenosis or occlusion of the arteries [1,2,3]. The prevalence of PAD was estimated to be 4.6% in the general population in South Korea [4] and affected 236.62 million patients worldwide in 2015, rising steeply with aging [5].
The manifestation of PAD may be asymptomatic, or there may be symptoms such as intermittent claudication, atypical leg pain, and critical limb ischemia [2,6,7]. However, regardless of symptoms, patients with PAD may experience an impaired walking ability and functional status and poor quality of life, which further increase the risk of cardiovascular ischemic events, limb-related events, and mortality [2,7,8]. Endovascular revascularization is the preferred procedure for relieving the symptoms of PAD according to lesion characteristics [2,9].
Major risk factors for PAD are similar to cardiovascular risk factors, such as diabetes mellitus (DM), smoking, dyslipidemia, and hypertension; among these factors, DM and cigarette smoking have the strongest risks [7,10]. DM, a known metabolic disease, promotes local inflammatory reactions on vascular walls and reduces peripheral blood flow, which may result in a disorder of endothelial cell function (nitric oxide mechanism) and vascular control [11]. DM significantly increases adverse clinical outcomes [5,12,13] and induces restenosis after endovascular revascularization [14]. The incidence of DM has quadrupled worldwide over the past 30 years [15], which indicates that the burden of PAD due to DM is likely to increase. Meanwhile, the cigarette-smoking population steadily declined worldwide between 2000 and 2015 [16].
Several studies showed that regular exercise improved the walking ability, functional status, and overall quality of life of patients with PAD [2,17,18]. One study found that regular exercise improved metabolic function, the levels of C-reactive protein, and the ankle–brachial index [18]. Other studies showed that regular exercise positively affected clinical outcomes by increasing oxygen uptake in the lower extremities [17,19]. However, the impact of regular exercise on major adverse events after revascularization of PAD patients remains unknown.
Major adverse events are defined as major adverse limb events (MALE) and major adverse cardiovascular events (MACE), which are indicators of long-term outcomes in PAD [20,21]. Therefore, this study investigated the associated factors of MALE and MACE in patients with PAD after endovascular revascularization using electronic medical records (EMRs).
2. Materials and Methods
2.1. Data Source and Study Patients
We conducted a retrospective longitudinal analysis using EMR data from a tertiary hospital in Seoul, Korea, between January 2009 and December 2018. The EMRs contain inpatient and outpatient clinical records of PAD patients. In this study, we excluded patients diagnosed with PAD before 2009 and considered 3852 patients who were first diagnosed with PAD between 2009 and 2018, of which 1288 underwent endovascular revascularization. Of these, we excluded 25 patients because they had undergone either bypass surgery or major amputation before 2009. The remaining 1263 eligible patients were then categorized into four groups according to the following criteria: whether they were diagnosed with DM and whether they exercised regularly (Group A: non-DM/regular exercise, Group B: non-DM/non-regular exercise, Group C: DM/regular exercise, and Group D: DM/non-regular exercise). The flow of the cohort derivation is shown in Figure 1.
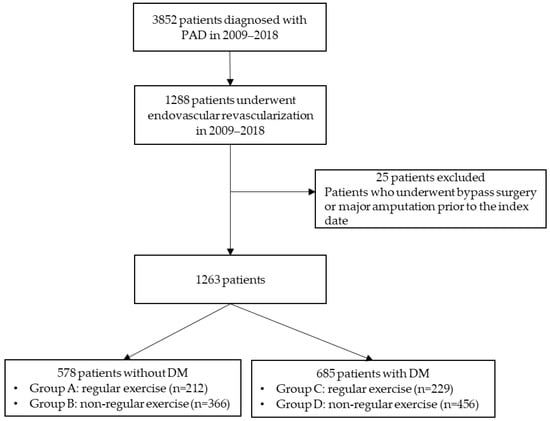
Figure 1.
Flow chart of this study. DM, diabetes mellitus; PAD, peripheral artery disease.
2.2. Baseline Variables
Baseline demographic and clinical data, including comorbidity, disease severity, initial nursing assessment, physician’s progress notes, and procedure record, were extracted from the EMRs. We defined the date of the first endovascular revascularization as the index date for each patient who was hospitalized to undergo endovascular revascularization. The initial nursing assessment included the patient’s past medical history, medications, activities, and psychosocial status on the day of admission [22]. Baseline regular exercise data were extracted from the initial nursing assessment record gathered from a self-reported question regarding whether the patient exercised regularly. Clinical manifestations (asymptomatic, claudication, critical limb ischemia, and abnormal skin color) were recorded as either Rutherford stage or narrative in the physicians’ progress notes, procedure records, and initial nursing assessment. Abnormal skin color recorded in physicians’ progress notes means a color change in toes without symptoms.
We extracted the past medical history (DM, hypertension, dyslipidemia, chronic kidney disease, coronary artery disease, congestive heart failure, and ischemic stroke) from the physicians’ progress notes using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. Additionally, DM was defined by ICD-10-CM codes and by a 6.5% or higher level of glycosylated hemoglobin (HbA1c).
2.3. Major Adverse Events
The major adverse events included MALE and MACE. MALE was defined as a composite of major amputation, bypass surgery, repeat endovascular revascularization, and in-hospital death. The procedure records were used to confirm repeat endovascular revascularization, and death records were used to determine in-hospital death. MACE was defined as a composite of myocardial infarction, ischemic stroke, and in-hospital death [2].
In the hospital used for EMR analysis, the surgical/procedure code lists and disease diagnosis were recorded using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM, respectively. Therefore, ICD-9-CM was used to identify major amputation (8414, 8415, 8417) and bypass surgery (3925, 3929) from the operation record. The ICD-10-CM codes were used to identify myocardial infarction (I21, I22, I23, I241, and I252) and ischemic stroke (I63) from the physicians’ progress notes. Time to events was measured in years from the index date to the first major adverse event’s incidence.
2.4. Statistical Analysis
Descriptive statistics were used for the clinical characteristics of the groups. The chi-square test or Fisher’s exact test and analysis of variance (ANOVA) were used for group differences. The Kaplan–Meier survival analysis was performed for MALE- and MACE-free survival curves, and log-rank tests were carried out for differences between the groups. A Cox proportional hazards regression model determined associated factors of MALE and MACE incidence. The proportional hazards assumption was tested using the Schoenfeld residual analysis. The hazard ratio (HR) was provided with a 95% confidence interval (CI). For all tests, a p-value < 0.05 was considered statistically significant. Data analysis was performed using IBM SPSS Statistics for Window, Version 25.0 (Armonk, NY, USA) and RStudio (version 1.3.1056, RStudio, PBC, Boston, MA, USA).
3. Results
3.1. Characteristics of the Study Patients
A total of 1263 patients were classified into four groups based on DM and regular exercise, with their baseline characteristics presented in Table 1. More than half of the total patients had DM; 36.1% did not exercise regularly, and 18.1% exercised regularly. Approximately 46% of the patients had no DM, of which 29.0% did not exercise regularly, and 16.8% exercise regularly. The mean age of patients was 67.3 ± 11.7 years, and 80.8% were men. A total of 1207 patients experienced symptoms of clinical manifestation, such as intermittent claudication (59.6%), critical limb ischemia (32.2%), and abnormal skin color (4.5%). The numbers of patients diagnosed with hypertension, DM, and dyslipidemia were 864 (68.4%), 685 (54.2%), and 570 (45.1%), respectively.

Table 1.
Baseline characteristics between groups (n = 1263).
There was a significant group difference in multilevel disease (p = 0.002). The aortoiliac lesions were more frequent in Group A, while infrapopliteal lesions were more frequent in Group D. The femoropopliteal lesions did not differ between the groups (p = 0.341). Balloon angiography was performed in approximately 97%, while stent insertion was performed in 56.6%.
3.2. Major Adverse Events
The total number of patients with MALE and MACE during the study period is summarized in Table 2. The total number of patients with MALE was 465 (36.8%), and those with MACE numbered 158 (12.5%). MALE occurred most frequently in Group D (DM/non-regular exercise, 42.8%), with significant group differences (χ2 = 29.47, p < 0.001). MACE also occurred most frequently in Group D (50.0%), with significant group differences (χ2 = 27.42, p < 0.001). Major amputation, endovascular revascularization, death, and myocardial infarction occurred most frequently in Group D, with significant differences between groups.

Table 2.
Major adverse events between groups (n = 1263).
The Kaplan–Meier survival curves for MALE- and MACE-free survival showed significant group differences (log-rank test, p < 0.001). Group D showed the poorest prognosis in the MALE- and MACE-free survival (Figure 2).
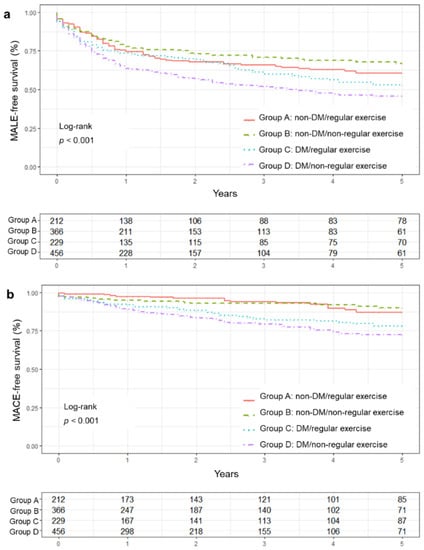
Figure 2.
Major adverse events-free survival curve between groups. (a) MALE-free survival curve, (b) MACE-free survival curve. DM, diabetes mellitus; MACE, major adverse cardiovascular events; MALE, major adverse limb events.
3.3. Hazard Ratios of MALE and MACE
Before analyzing the Cox regression model, we tested the proportional hazards assumption using the Schoenfeld residuals test. The results show no violation of the proportional hazards assumption (MALE, p = 0.216; MACE, p = 0.089); therefore, we estimated the HR with 95% CI using the Cox regression model. We performed a univariate analysis for factors that affected the major adverse events (Table 3). For MALE, patients with critical limb ischemia had a 2.16 times higher incidence (95% CI 1.79–2.61) than those without. The MACE incidence was 2.07 times higher in Group C (DM/regular exercise; 95% CI 1.18–3.64) and 2.73 times higher in Group D (DM/non-regular exercise; 95% CI 1.63–4.56) compared to Group A (non-DM/regular exercise). Characteristics of groups, higher age, sex (female), critical limb ischemia, chronic kidney disease, congestive heart failure, and longer DM duration were the factors associated with an increased risk of MALE and MACE. In addition, hypertension (HR = 1.83, 95% CI 1.25–2.69) and coronary artery disease (HR = 1.63, 95% CI 1.19–2.23) also increased the risk of MACE.

Table 3.
Univariate analysis of factors associated with hazard ratios of major adverse events.
The Cox regression analysis for multivariate analysis showed that patients with critical limb ischemia, chronic kidney disease, and congestive heart failure had 1.85-, 1.40-, and 1.36-times higher risks of MALE, respectively (Table 4). The incidence of MACE was 2.77 times higher in patients with chronic kidney disease. The major adverse events incidence was not statistically significant between the four groups.

Table 4.
Multivariate analysis of factors associated with hazard ratios of major adverse events.
4. Discussion
This study investigated the associated factors of MALE and MACE after endovascular revascularization in PAD patients based on the grouping by DM and regular exercise. In the present study, group differences were not statistically significant in MALE and MACE incidence after endovascular revascularization. Target lesion status (location, length of lesions, and degree of calcification) or clinical ischemia before the endovascular revascularization affects the vessel patency [2] associated with exercise maintenance and clinical prognosis. Thus, the CLI and CKD associated with the vascular condition before the procedure may be at high risk for the incidence of MALE and MACE. The risk factors for major adverse events found in this study were critical limb ischemia, chronic kidney disease, and congestive heart failure for MALE, and higher age, critical limb ischemia, and chronic kidney disease for MACE. These results are similar to those of previous studies identifying the risk factors for adverse clinical outcomes of PAD [2,6,23,24]. In particular, chronic kidney disease, a common independent predictor of major adverse events, is consistent with poorer outcomes in patients with PAD [25,26].
The multivariate analysis showed that the DM and non-regular exercise group had a higher risk of MALE and MACE than the non-DM and regular exercise group, but it was not statistically significant. There were significant group differences in MALE- and MACE-free survival curves, and the DM and non-regular exercise group had a shorter major adverse event-free survival than others. In the univariate analysis, the DM and non-regular exercise group had a higher risk of major adverse events with statistical significance. Direct comparisons with previous studies are difficult because there is no study on the association between DM, regular exercise, and major adverse events in patients with PAD; however, a cohort study that examined PAD patients after 30 days post-revascularization showed the increased likelihood of a major adverse event for an increased frailty score [27] affected by physical activity and exercise [28]. Although our study did not show statistical significance, regular exercise may be associated with decreased major adverse events. It is necessary to investigate the relationship between major adverse events and exercise habits in future studies.
In this study, 34 patients (5.0%) in the DM groups and 11 patients (1.9%) in the non-DM groups underwent major amputation. In addition, non-DM groups also had a lower incidence of MALE and MACE than DM groups (p < 0.001). In the previous studies, regular exercise was shown to decrease overall mobility loss and lower limb amputation and maintain the functional status and walking performance of patients [2,17,19]. Therefore, healthcare providers need to ensure early detection and adequate management of people at risk to lower the incidence of major adverse events by encouraging regular exercise.
Our study showed that 36.8% of the patients experienced MALE, and 12.5% experienced MACE during the follow-up. In a previous study using healthcare claims data, patients with PAD experienced MALE (22.9%) and MACE (11.3%) during a mean of a 1.8-year follow-up period [29]. A five-year follow-up study with PAD patients using EMR data reported that 38.2% experienced MALE, and 17.0% experienced MACE [6]. The incidence of MALE was almost three times that of MACE in this study, which was similar to other studies, despite slight differences in the composition of diseases in MALE and MACE and the study samples.
Our study had several limitations. First, we extracted regular exercise data from self-reported data recorded in the EMRs. Self-reported regular exercise data can be used as an indicator of patients’ exercise habits, but more objective indicators of frequency, intensity, and duration of exercise were not reflected. However, EMRs contain a vast number of real-world data used in many studies investigating the clinical course of diseases and predicting adverse clinical outcomes [6,30]. Therefore, this study is meaningful because it used EMR data to investigate long-term clinical outcomes in PAD patients. Second, as the study data were collected from a tertiary hospital’s EMRs, the generalizability may be limited; however, this tertiary hospital adopted a full EMR system in which rich clinical documents are stored electronically [31]. Finally, as this was a retrospective longitudinal study, we could not control other potential confounding variables during the follow-up period. In addition, in this study, due to the limitations of the relevant information stored in EMRs, clinical manifestations, not Rutherford or Fontaine classifications, which classify symptoms in PAD patients, were reported.
5. Conclusions
As far as we know, this is the first study to investigate the associated factors of MALE and MACE after endovascular revascularization in patients with PAD focusing on DM and regular exercise. Critical limb ischemia and chronic kidney disease increase the risks of MALE and MACE in patients with PAD. Further study is needed to explore this finding and determine the long-term effects of the trajectory change in exercise habits and DM management. Additionally, in clinical practice, target management of PAD patients with DM, critical limb ischemia, and chronic kidney disease is essential to reduce the risk of major adverse events after endovascular revascularization.
Author Contributions
Conceptualization, M.K., Y.S.Y., Y.-G.K. and M.C.; Data curation, M.K.; Formal analysis, M.K. and Y.S.Y.; Funding acquisition, M.C.; Investigation, M.K. and Y.S.Y.; Methodology, M.K., Y.S.Y., Y.-G.K. and M.C.; Project administration, M.C.; Resources, M.C.; Supervision, M.C.; Validation, Y.-G.K. and M.C.; Visualization, M.K.; Writing—original draft, M.K. and Y.S.Y.; Writing—review and editing, M.K., Y.-G.K. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C1007185) and by the Brain Korea 21 FOUR Project funded by the National Research Foundation (NRF) of Korea, Yonsei University College of Nursing.
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of the Yonsei University Health System (IRB number: 4-2019-0720).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
The authors thank the department of medical records of Severance Hospital in South Korea for helping to extract the EMR data and providing advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360, j5842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Writing Committee Members; Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary. J. Am. Coll. Cardiol. 2017, 69, 1465–1508. [Google Scholar] [CrossRef] [PubMed]
- Treat-Jacobson, D.; McDermott, M.M.; Bronas, U.G.; Campia, U.; Collins, T.C.; Criqui, M.H.; Gardner, A.W.; Hiatt, W.R.; Regensteiner, J.G.; Rich, K.; et al. Optimal Exercise Programs for Patients with Peripheral Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e10–e33. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, S.H.; Joh, J.H. Risk factors for asymptomatic peripheral arterial disease in Korean population: Lessons from a community-based screening. Ann. Surg. Treat. Res. 2019, 97, 210–216. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Lee, Y.J.; Ko, Y.G.; Kang, T.S.; Lim, S.H.; Hong, S.J.; Ahn, C.-M.; Kim, J.-S.; Kim, B.-K.; Choi, D.; et al. Optimal strategy for antiplatelet therapy after endovascular revas-cularization for lower extremity peripheral artery disease. JACC Cardiovasc. Interv. 2019, 12, 2359–2370. [Google Scholar] [CrossRef]
- Fowkes, F.; Aboyans, V.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Ryu, G.W.; Choi, M. Functional Status and Health-Related Quality of Life in Patients with Peripheral Artery Disease: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 10941. [Google Scholar] [CrossRef]
- McDermott, M.M.; Kibbe, M.R. Improving lower extremity functioning in peripheral artery disease: Exercise, endovascular revascularization, or both? JAMA 2017, 317, 689–690. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Stabler, T.; Kenjale, A.; Ham, K.; Jelesoff, N.; Allen, J. Potential mechanisms for reduced delivery of nitric oxide to peripheral tissues in diabetes mellitus. Ann. N. Y. Acad. Sci. 2010, 1203, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kreutzburg, T.; Peters, F.; Riess, H.C.; Hischke, S.; Marschall, U.; Kriston, L.; L’Hoest, H.; Sedrakyan, A.; Debus, E.S.; Behrendt, C.-A.; et al. Editor’s Choice—Comorbidity patterns among Patients with peripheral arterial occlusive disease in Germany: A trend analysis of health insurance claims data. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, E.; Sartori, M.; Pacelli, A.; Conti, E.; Cosmi, B. Coronary artery disease and restenosis after peripheral endovascular intervention are predictors of poor outcome in peripheral arterial disease. Acta Cardiol. 2020, 75, 649–656. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018. World Health Organization. 2018. Available online: https://www.who.int/publications/i/item/ncd-country-profiles-2018 (accessed on 17 March 2022).
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025. 2019. Available online: https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (accessed on 17 March 2022).
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M. Step-Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients with Peripheral Artery Disease: A Randomized Controlled Trial. J. Am. Heart Assoc. 2014, 3, e001107. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Abbott, K. Association of diabetic peripheral arterial disease and objectively-measured physical activity: NHANES 2003–2004. J. Diabetes Metab. Disord. 2014, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Scott, K.J.; Blevins, S.M. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: A randomized controlled trial. Circulation 2011, 123, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, E.J.; Chen, D.C.; Westin, G.G.; Singh, S.; McCoach, C.E.; Bang, H.; Yeo, K.; Anderson, D.; Amsterdam, E.A.; Laird, J.R. Adherence to Guideline-Recommended Therapy Is Associated with Decreased Major Adverse Cardiovascular Events and Major Adverse Limb Events Among Patients with Peripheral Arterial Disease. J. Am. Heart Assoc. 2014, 3, e000697. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.S.; Geraghty, P.J.; Bradbury, A.W.; Hevelone, N.; Lipsitz, S.R.; Moneta, G.L.; Nehler, M.R.; Powell, R.J.; Sidawy, A.N. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J. Vasc. Surg. 2009, 50, 1462–1473. [Google Scholar] [CrossRef] [Green Version]
- Toney-Butler, T.J.; Unison-Pace, W.J.; Nursing Admission Assessment and Examination. StatPearls Publishing LLC. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493211/ (accessed on 14 September 2021).
- Patel, V.I.; Mukhopadhyay, S.; Guest, J.M.; Conrad, M.F.; Watkins, M.T.; Kwolek, C.J.; LaMuraglia, G.M.; Cambria, R.P. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J. Vasc. Surg. 2014, 59, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Vanassche, T.; Verhamme, P.; Anand, S.S.; Shestakovska, O.; Fox, K.; Bhatt, D.L.; Avezum, A.; Alings, M.; Aboyans, V.; Maggioni, A.P.; et al. Risk factors and clinical outcomes in chronic coronary and peripheral artery disease: An analysis of the randomized, double-blind COMPASS trial. Eur. J. Prev. Cardiol. 2020, 27, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.; Coresh, J.; Arima, H.; Ärnlöv, J.; Cirillo, M.; Ebert, N.; Hiramoto, J.S.; Kimm, H.; Shlipak, M.G.; et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017, 5, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, P.; Aboyans, V.; Desormais, I.; Kowalsky, T.; Cambou, J.P.; Constans, J.; Rivière, A.B. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J. Vasc. Surg. 2013, 58, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Najafi, B.; Veranyan, N.; Zulbaran-Rojas, A.; Park, C.; Nguyen, H.; Nakahara, Q.K.; Elizondo-Adamchik, H.; Chung, J.; Mills, J.L.; Montero-Baker, M.; et al. Association Between Wearable Device–Based Measures of Physical Frailty and Major Adverse Events Following Lower Extremity Revascularization. JAMA Netw. Open 2020, 3, e2020161. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Simpson, A.; Bhagnani, T.; Leeper, N.J.; Murphy, B.; Nordstrom, B.; Ting, W.; Zhao, Q.; Berger, J.S. Incidence and Cost of Major Adverse Cardiovascular Events and Major Adverse Limb Events in Patients with Chronic Coronary Artery Disease or Peripheral Artery Disease. Am. J. Cardiol. 2019, 123, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Jeong, G.Y.; Jeong, O.S.; Chang, D.K.; Cha, W.C. Machine Learning and Initial Nursing Assessment-Based Triage System for Emergency Department. Health Inform. Res. 2020, 26, 13–19. [Google Scholar] [CrossRef]
- Park, Y.-T.; Kim, D.; Park, R.W.; Atalag, K.; Kwon, I.H.; Yoon, D.; Choi, M. Association between Full Electronic Medical Record System Adoption and Drug Use: Antibiotics and Polypharmacy. Health Inform. Res. 2020, 26, 68–77. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).