Introducing a Pole Concept for Nodule Growth in the Thyroid Gland: Taller-than-Wide Shape, Frequency, Location and Risk of Malignancy of Thyroid Nodules in an Area with Iodine Deficiency
Abstract
:1. Introduction
- How frequent is TTW growth of thyroid nodules?
- Where are TTW nodules located within the thyroid gland?
- What is the risk for malignancy in TTW nodules?
- Finally, a pole concept for nodule growth is developed.
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patients
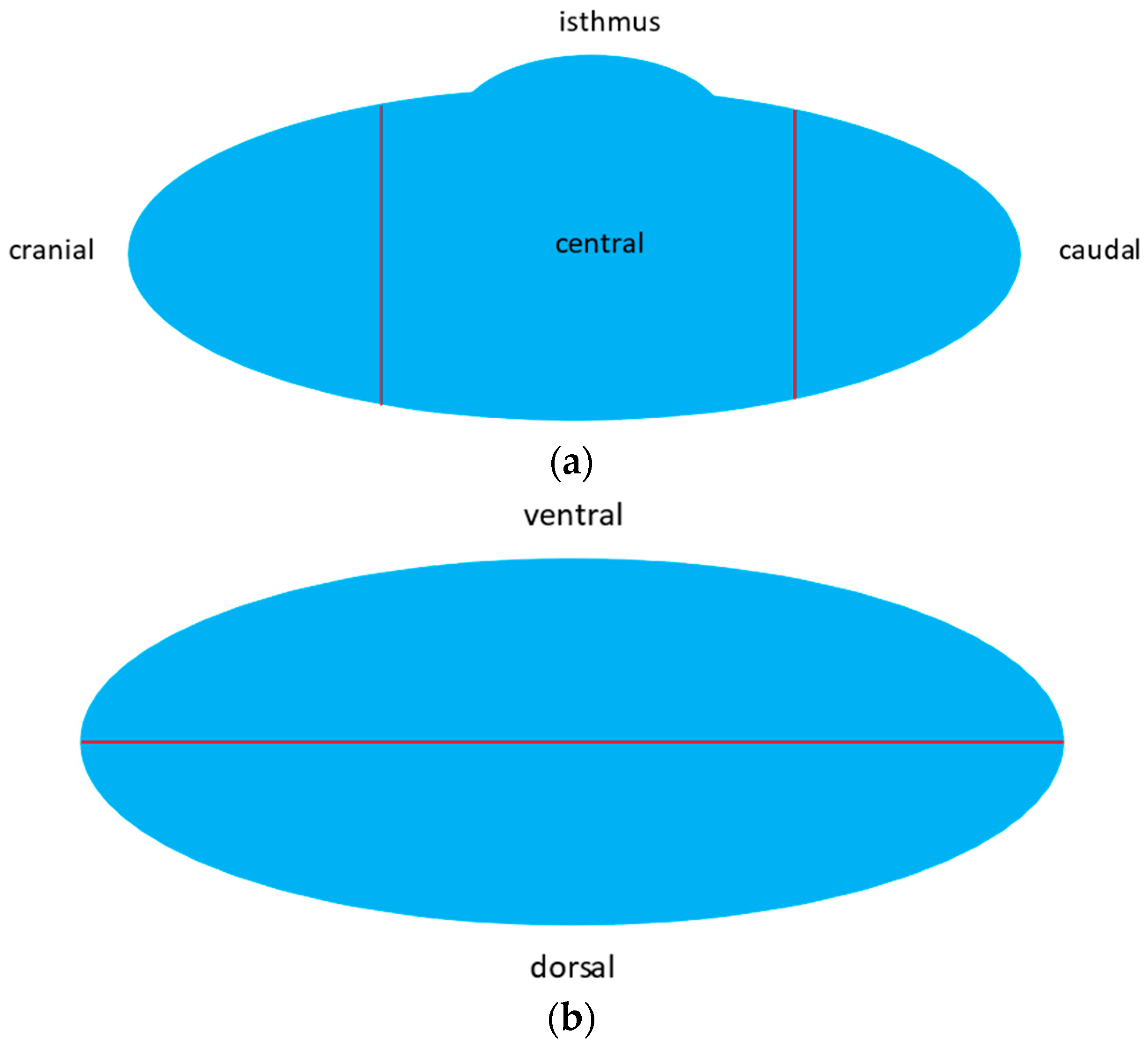
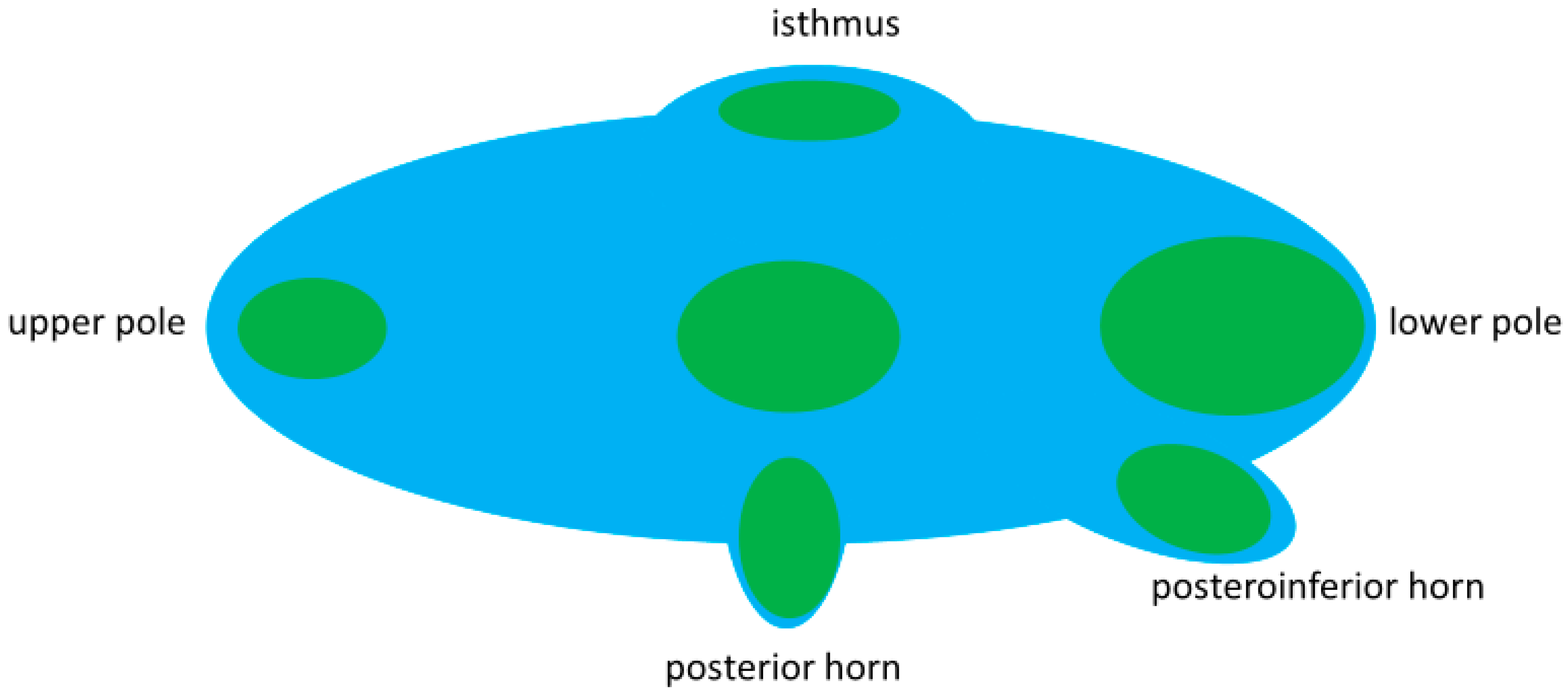
3.2. Anatomy
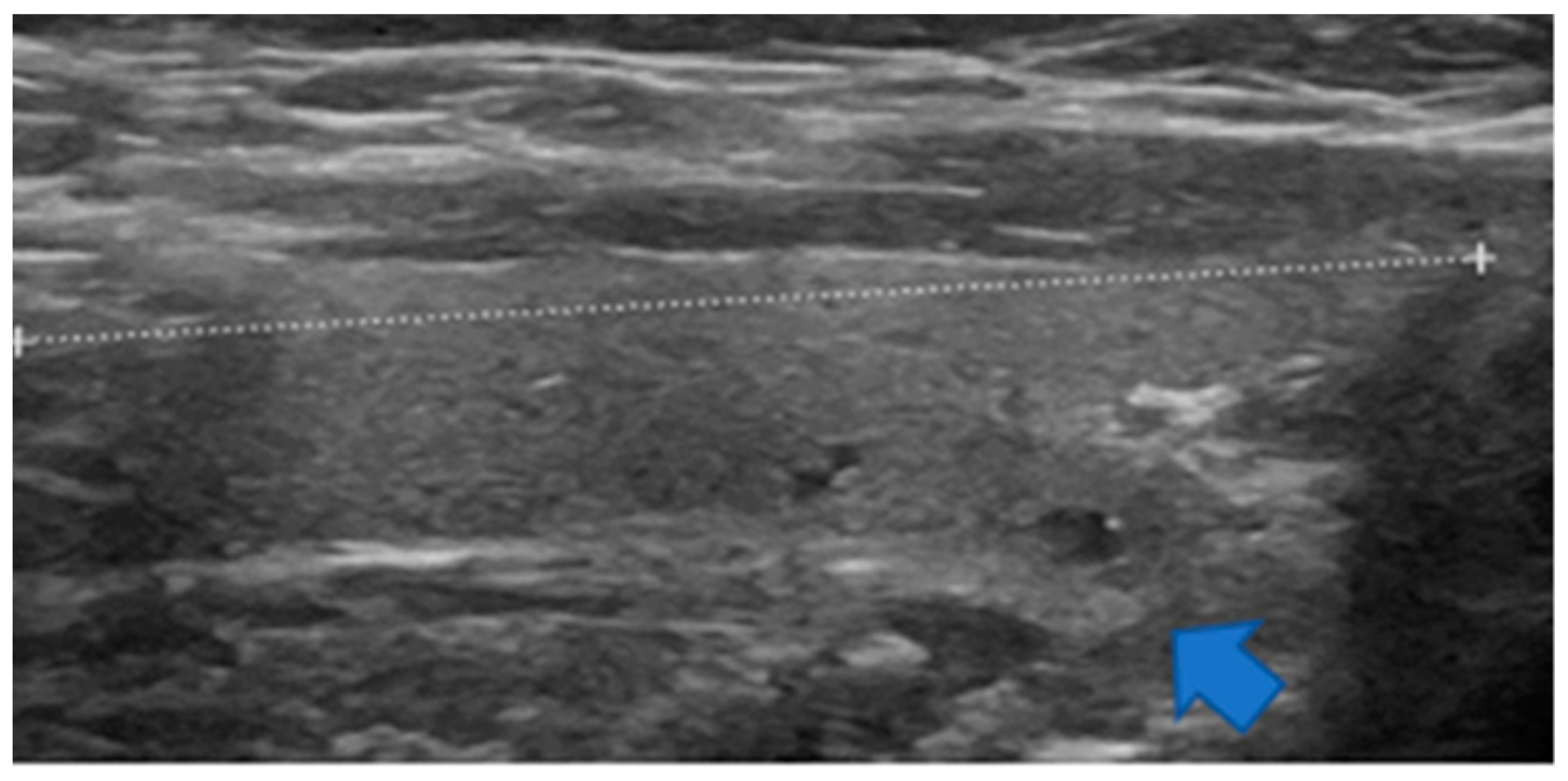
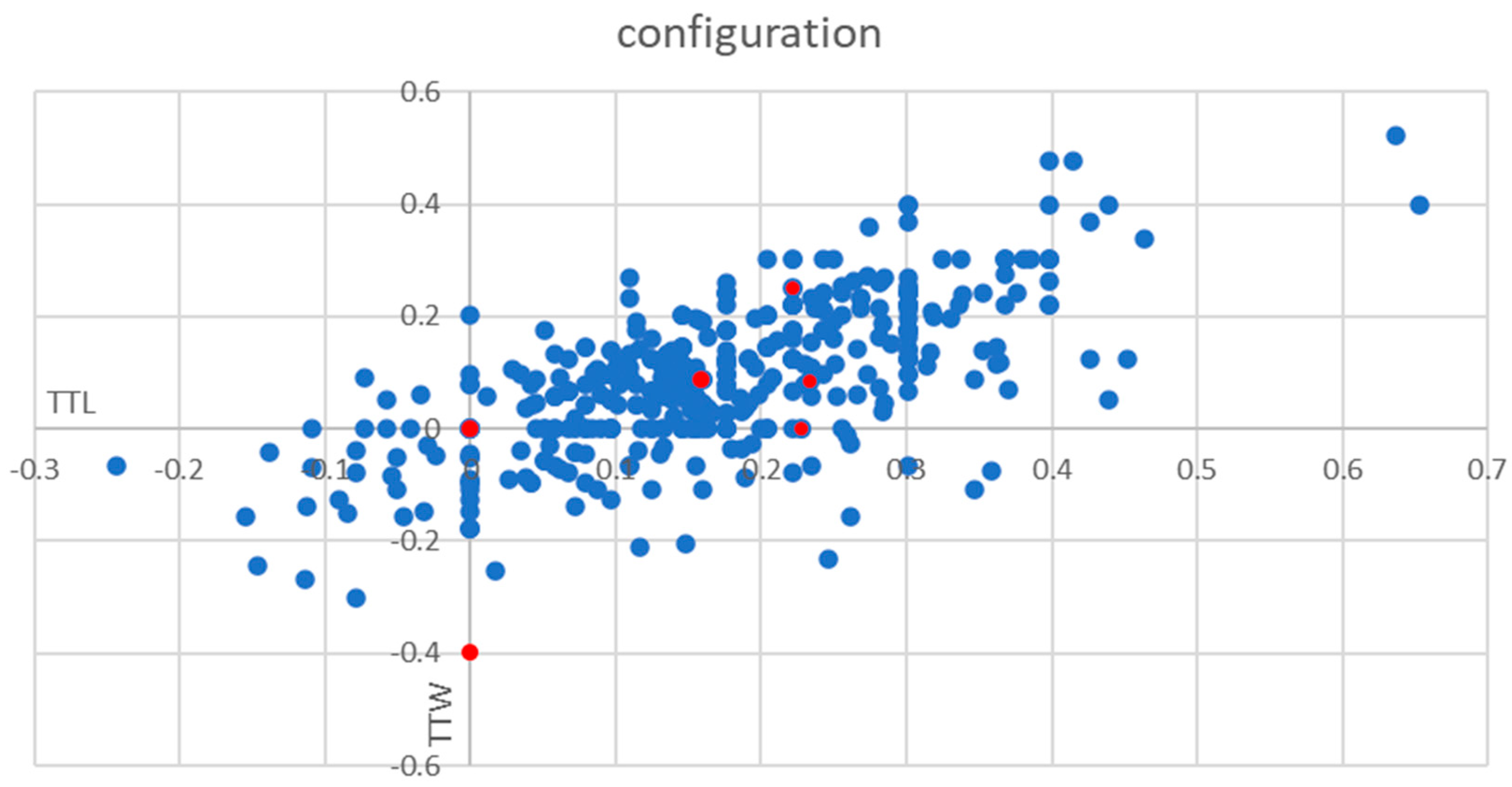
3.3. Frequency of TTW Nodules (Part 1—Prospective Part)
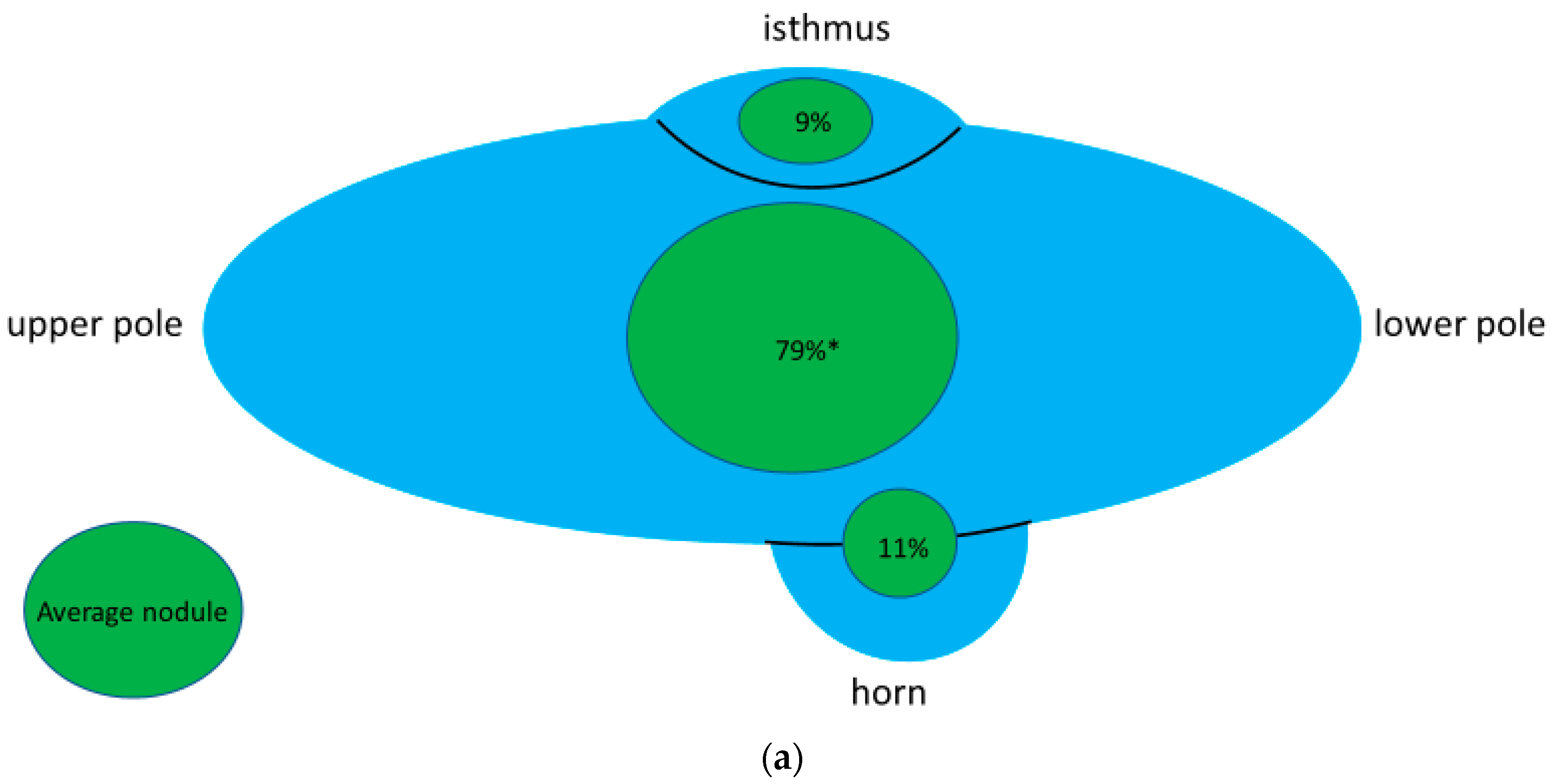
3.4. Location of Thyroid Nodules in Thyroid Gland
3.5. Location and Risk of Malignancy for TTW Nodules (Part 2—Retrospective Part)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carle, A.; Krejbjerg, A.; Laurberg, P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 465–479. [Google Scholar] [CrossRef]
- Schaffner, M.; Rochau, U.; Mühlberger, N.; Conrads-Frank, A.; Qerimi Rushaj, V.; Sroczynski, G.; Koukkou, E.; Thuesen, B.H.; Völzke, H.; Oberaigner, W.; et al. The economic impact of prevention, monitoring and treatment strategies for iodine deficiency disorders in Germany. Endocr. Connect. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seifert, P.; Schenke, S.; Zimny, M.; Stahl, A.; Grunert, M.; Klemenz, B.; Freesmeyer, M.; Kreissl, M.C.; Herrmann, K.; Görges, R. Diagnostic Performance of Kwak, EU, ACR, and Korean TIRADS as Well as ATA Guidelines for the Ultrasound Risk Stratification of Non-Autonomously Functioning Thyroid Nodules in a Region with Long History of Iodine Deficiency: A German Multicenter Trial. Cancers 2021, 13, 4467. [Google Scholar] [CrossRef] [PubMed]
- Paschke, R.; Schmid, K.W.; Gartner, R.; Mann, K.; Dralle, H.; Reiners, C. Epidemiology, pathophysiology, guideline-adjusted diagnostics, and treatment of thyroid nodules. Med. Klin. (Munich) 2010, 105, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, C.; Castellano, M.; Pirola, I.; Gandossi, E.; De Martino, E.; Cumetti, D.; Agosti, B.; Rosei, E.A. Thyroid nodule shape suggests malignancy. Eur. J. Endocrinol. 2006, 155, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef]
- Ha, E.J.; Moon, W.J.; Na, D.G.; Lee, Y.H.; Choi, N.; Kim, S.J.; Kim, J.K. A Multicenter Prospective Validation Study for the Korean Thyroid Imaging Reporting and Data System in Patients with Thyroid Nodules. Korean J. Radiol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Horvath, E.; Silva, C.F.; Majlis, S.; Rodriguez, I.; Skoknic, V.; Castro, A.; Rojas, H.; Niedmann, J.P.; Madrid, A.; Capdeville, F.; et al. Prospective validation of the ultrasound based TIRADS (Thyroid Imaging Reporting And Data System) classification: Results in surgically resected thyroid nodules. Eur. Radiol. 2017, 27, 2619–2628. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remonti, L.R.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C.; Gross, J.L. Thyroid ultrasound features and risk of carcinoma: A systematic review and meta-analysis of observational studies. Thyroid 2015, 25, 538–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, J.P.; Gionfriddo, M.R.; Al Nofal, A.; Boehmer, K.R.; Leppin, A.L.; Reading, C.; Callstrom, M.; Elraiyah, T.A.; Prokop, L.J.; Stan, M.N.; et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Campanella, P.; Ianni, F.; Rota, C.A.; Corsello, S.M.; Pontecorvi, A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: A systematic review and meta-analysis. Eur. J. Endocrinol. 2014, 170, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Na, D.G.; Baek, J.H.; Sung, J.Y.; Kim, J.H.; Kim, J.K.; Choi, Y.J.; Seo, H. Thyroid imaging reporting and data system risk stratification of thyroid nodules: Categorization based on solidity and echogenicity. Thyroid 2016, 26, 562–572. [Google Scholar] [CrossRef]
- Shin, H.S.; Na, D.G.; Paik, W.; Yoon, S.J.; Gwon, H.Y.; Noh, B.J.; Kim, W.J. Malignancy risk stratification of thyroid nodules with macrocalcification and rim calcification based on ultrasound patterns. Korean J. Radiol. 2021, 22, 663–671. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, H.S.; Kim, E.K.; Moon, H.J.; Kwak, J.Y. Malignancy risk stratification of thyroid nodules: Comparison between the thyroid imaging reporting and data system and the 2014 American Thyroid Association management guidelines. Radiology 2016, 278, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Moon, W.J.; Jung, S.L.; Lee, J.H.; Na, D.G.; Baek, J.H.; Lee, Y.H.; Kim, J.; Kim, H.S.; Byun, J.S.; Lee, D.H. Benign and malignant thyroid nodules: US differentiation—Multicenter retrospective study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef]
- Kim, S.Y.; Na, D.G.; Paik, W. Which ultrasound image plane is appropriate for evaluating the taller-than-wide sign in the risk stratification of thyroid nodules? Eur. Radiol. 2021, 31, 7605–7613. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef]
- Madelung, O.W. Anatomisches und Chirurgisches über die Glandula thyreoidea accessoria. Arch. Klin. Chir. 1879, 24, 71–107. [Google Scholar]
- Zuckerkandl, E. Die Epithelkörperchen von Didelphys azara nebst Bemerkungen über die Epithelkörperchen des Menschen. Über den hinteren Schilddrüsenfortsatz. Anat. Hefte 1902, 19, 62–82. [Google Scholar] [CrossRef]
- Schweizer, I.; Gemsenjäger, E. Struma mit Dysphagie: Altes und neues Wissen. Schweiz. Med. Forum 2004, 4, 934–936. [Google Scholar]
- Hassan, A.A. Zuckerkandl’s Tubercle. J. Am. Coll. Surg. 2003, 197, 880–882. [Google Scholar] [CrossRef]
- Mirilas, P.; Skandalakis, J.E. Zuckerkandl’s tubercle: Hannibal ad Portas. J. Am. Coll. Surg. 2003, 196, 796–801. [Google Scholar] [CrossRef]
- Baskin Sr, H.J.; Duick, D.S.; Levine, R.A. Thyroid Ultrasound and Ultrasound-Guided FNA, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 6; pp. 99–126. [Google Scholar]
- Schenke, S.; Seifert, P.; Zimny, M.; Winkens, T.; Binse, I.; Görges, R. Risk Stratification of Thyroid Nodules Using the Thyroid Imaging Reporting and Data System (TIRADS): The Omission of Thyroid Scintigraphy Increases the Rate of Falsely Suspected Lesions. J. Nucl. Med. 2019, 60, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, P.; Görges, R.; Zimny, M.; Kreissl, M.C.; Schenke, S. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine 2020, 67, 143–154. [Google Scholar] [CrossRef]
- Hisham, A.N.; Aina, E.N. Zuckerkandl’s tubercle of the thyroid gland in association with pressure symptoms: A coincidence or consequence? Aust. N. Z. J. Surg. 2000, 70, 251–253. [Google Scholar] [CrossRef]
- Gauger, P.G.; Delbridge, L.W.; Thompson, N.W.; Crummer, P.; Reeve, T.S. Incidence and importance of the tubercle of Zuckerkandl in thyroid surgery. Eur. J. Surg. 2001, 167, 249–254. [Google Scholar]
- Yun, J.S.; Lee, J.S.; Jung, J.J.; Nam, K.H.; Chung, W.Y.; Chang, H.S.; Park, C.S. The Zuckerkandl’s tubercle: A useful anatomical landmark for detecting both the recurrent laryngeal nerve and the superior parathyroid during thyroid surgery. Endocr. J. 2008, 55, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Sheahan, P.; Murphy, M.S. Thyroid tubercle of Zuckerkandl: Importance in thyroid surgery. Laryngoscope 2011, 121, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Won, H.J.; Won, H.S.; Kwak, D.S.; Jang, J.; Jung, S.L.; Kim, I.B. Zuckerkandl Tubercle of the Thyroid Gland: Correlations between Findings of Anatomic Dissections and CT Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1416–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, C.; Cuvelier, P.; Biet, A.; Boute, P.; Laude, M.; Strunski, V. Thyroid tubercle of Zuckerkandl: Anatomical and surgical experience from 79 thyroidectomies. J. Laryngol. Otol. 2009, 123, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Eszlinger, M.; Ullmann, M.; Ruschenburg, I.; Böhme, K.; Görke, F.; Franzius, C.; Adam, S.; Molwitz, T.; Landvogt, C.; Amro, B.; et al. Low Malignancy Rates in Fine-Needle Aspiration Cytologies in a Primary Care Setting in Germany. Thyroid 2017, 27, 1385–1392. [Google Scholar] [CrossRef]
- Zhang, F.; Oluwo, O.; Castillo, F.B.; Gangula, P.; Castillo, M.; Farag, F.; Zakaria, S.; Zahedi, T. Thyroid nodule location on ultrasonography as a predictor of malignancy. Endocr. Pract. 2019, 25, 131–137. [Google Scholar] [CrossRef]
- Ramundo, V.; Lamartina, L.; Falcone, R.; Ciotti, L.; Lomonaco, C.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Durante, C.; Grani, G. Is thyroid nodule location associated with malignancy risk? Ultrasonography 2019, 38, 231–235. [Google Scholar] [CrossRef]
- Duman, G.; Sariakcali, B. Thyroid Nodules Located in the Lower Pole Have a Higher Risk of Malignancy than Located in the Isthmus: A Single-Center Experience. Int. J. Endocrinol. 2021, 2021, 9940995. [Google Scholar] [CrossRef]
- Jasim, S.; Baranski, T.J.; Teefey, S.A.; Middleton, W.D. Investigating the Effect of Thyroid Nodule Location on the Risk of Thyroid Cancer. Thyroid 2020, 30, 401–407. [Google Scholar] [CrossRef]



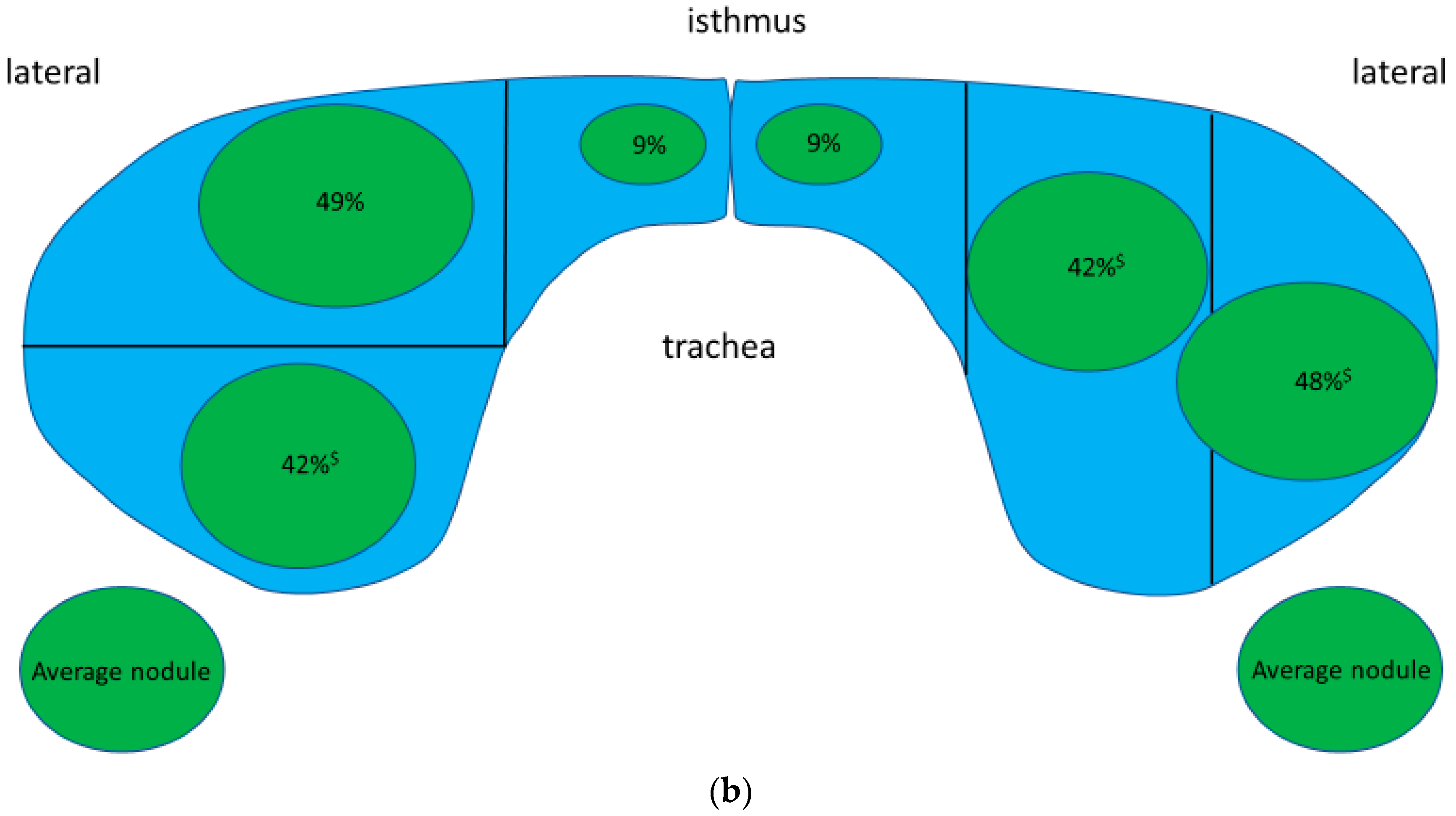
| Location | TTW Nodules (n = 74) | Non-TTW Nodules (n = 367) |
|---|---|---|
| dorsal position (n = 216) | 51 | 165 |
| non-dorsal position (n = 225) | 23 | 202 |
| Relationship with Horn | TTW Nodules (n = 74) | Non-TTW Nodules (n = 367) |
|---|---|---|
| association to horn (n = 51) | 21 | 30 |
| no association to horn (n = 390) | 53 | 337 |
| Category | Non-TTW Nodules (n = 367) % | TTW Nodules (n = 74) % | |
|---|---|---|---|
| composition | solid | 71 | 75 |
| mixed | 16 | 12 | |
| spongiform | 8 | 6 | |
| cystic | 6 | 7 | |
| echogenity | hypoechoic | 34 | 32 |
| isoechoic | 52 | 51 | |
| hyperechoic | 7 | 9 | |
| anechoic | 8 | 9 | |
| margin | smooth | 91 | 81 |
| lobulated/irregular/ill-defined | 9 | 19 | |
| calcification | none | 72 | 68 |
| microcalcification | 3 | 6 | |
| other | 25 | 26 |
| Location | Malignant TTW Nodules (n = 62) | Benign TTW Nodules (n = 101) |
|---|---|---|
| dorsal position (n = 78) | 22 | 56 |
| non-dorsal position (n = 85) | 40 | 45 |
| Relationship with Horn | Malignant TTW Nodules (n = 62) | Benign TTW Nodules (n = 101) |
|---|---|---|
| association to horn (n = 35) | 6 | 29 |
| no association to horn (n = 128) | 56 | 72 |
| Category | Malignant TTW Nodules (n = 62) % | Benign TTW Nodules (n = 101) % | |
|---|---|---|---|
| composition | solid | 96 | 85 |
| mixed | 4 | 14 | |
| cystic | 0 | 1 | |
| echogenicity | hypoechoic | 85 | 65 |
| isoechoic | 15 | 34 | |
| hyperechoic | 0 | 0 | |
| anechoic | 0 | 1 | |
| margin | smooth | 22 | 75 |
| lobulated/irregular/ill-defined | 78 | 25 | |
| calcification | none | 31 | 62 |
| microcalcification | 51 | 20 | |
| other | 18 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, M.; Schenke, S.A.; Zimny, M.; Görges, R.; Grunert, M.; Groener, D.; Seifert, P.; Stömmer, P.E.; Kreissl, M.C.; Stahl, A.R.; et al. Introducing a Pole Concept for Nodule Growth in the Thyroid Gland: Taller-than-Wide Shape, Frequency, Location and Risk of Malignancy of Thyroid Nodules in an Area with Iodine Deficiency. J. Clin. Med. 2022, 11, 2549. https://doi.org/10.3390/jcm11092549
Petersen M, Schenke SA, Zimny M, Görges R, Grunert M, Groener D, Seifert P, Stömmer PE, Kreissl MC, Stahl AR, et al. Introducing a Pole Concept for Nodule Growth in the Thyroid Gland: Taller-than-Wide Shape, Frequency, Location and Risk of Malignancy of Thyroid Nodules in an Area with Iodine Deficiency. Journal of Clinical Medicine. 2022; 11(9):2549. https://doi.org/10.3390/jcm11092549
Chicago/Turabian StylePetersen, Manuela, Simone A. Schenke, Michael Zimny, Rainer Görges, Michael Grunert, Daniel Groener, Philipp Seifert, Peter E. Stömmer, Michael C. Kreissl, Alexander R. Stahl, and et al. 2022. "Introducing a Pole Concept for Nodule Growth in the Thyroid Gland: Taller-than-Wide Shape, Frequency, Location and Risk of Malignancy of Thyroid Nodules in an Area with Iodine Deficiency" Journal of Clinical Medicine 11, no. 9: 2549. https://doi.org/10.3390/jcm11092549
APA StylePetersen, M., Schenke, S. A., Zimny, M., Görges, R., Grunert, M., Groener, D., Seifert, P., Stömmer, P. E., Kreissl, M. C., Stahl, A. R., & on behalf of the German TIRADS Study Group. (2022). Introducing a Pole Concept for Nodule Growth in the Thyroid Gland: Taller-than-Wide Shape, Frequency, Location and Risk of Malignancy of Thyroid Nodules in an Area with Iodine Deficiency. Journal of Clinical Medicine, 11(9), 2549. https://doi.org/10.3390/jcm11092549







