Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Procedure
2.2. Process Followed for the Selection of Articles
2.3. Data Extraction
2.4. Methodological Quality
3. Results
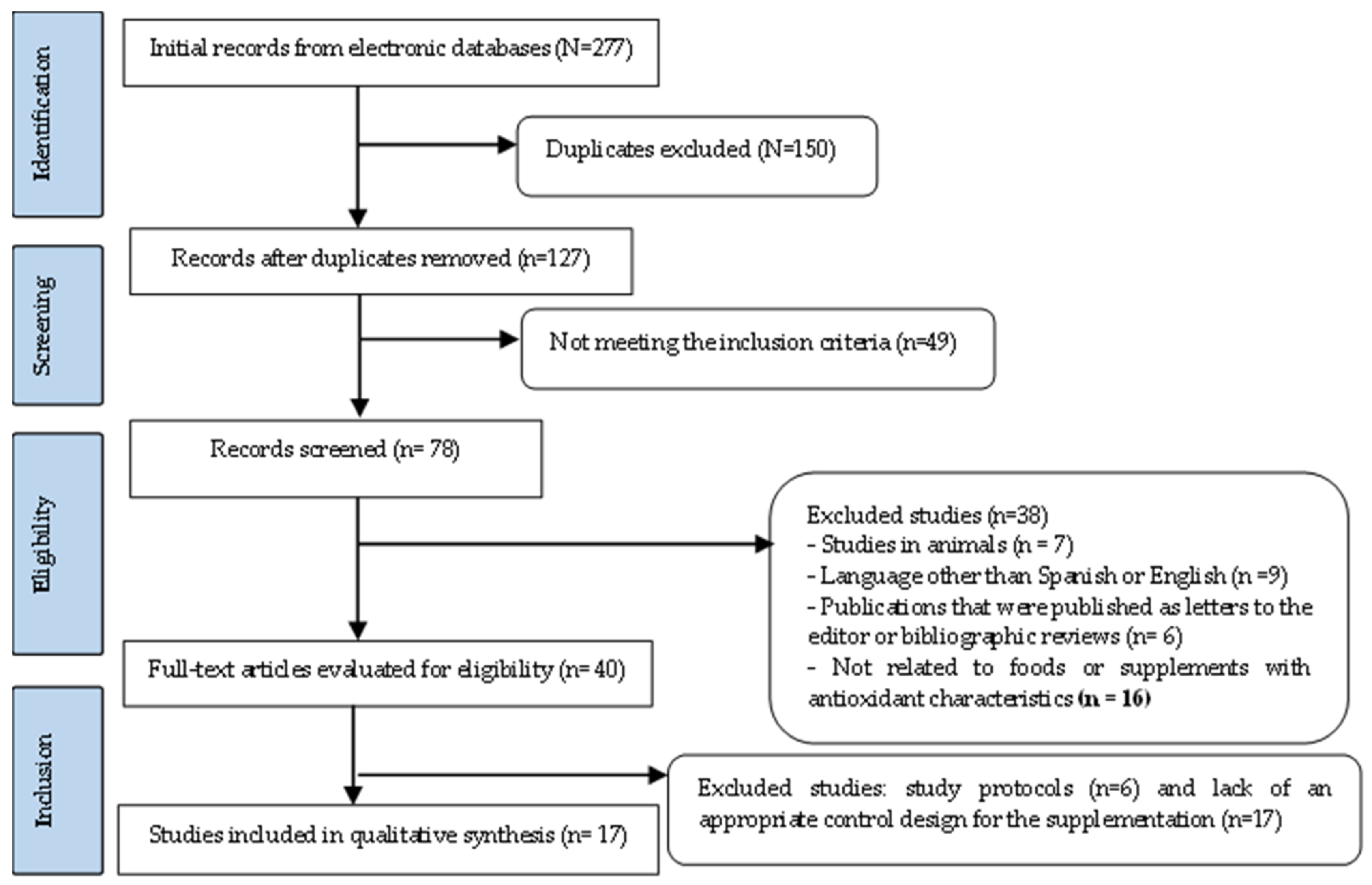
3.1. Selection of Studies
3.2. Results of the Quality Assessment
3.3. Descriptive Information of the Selected Articles Included in the Systematic Review
4. Discussion
4.1. Study Limitation
4.2. Recommendations for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wessely, S.; Hotopf, M. Is fibromyalgia a distinct clinical entity? Historical and epidemiological evidence. Baillieres Best. Pract. Res. Clin. Rheum. 1999, 13, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M. Clinical manifestations and diagnosis of fibromyalgia. Rheum. Dis. Clin. N. Am. 2009, 35, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 20, 426130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Smith, H.S.; Harris, R.; Clauw, D. Fibromyalgia: An afferent processing disorder leading to a complex pain generalized syndrome. Pain Phys. 2011, 14, 217–245. [Google Scholar] [CrossRef]
- Maffei, M.E. Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef]
- Wolfe, F.; Egloff, N.; Häuser, W. Widespread Pain and Low Widespread Pain Index Scores among Fibromyalgia-positive Cases Assessed with the 2010/2011 Fibromyalgia Criteria. J. Rheum. 2016, 43, 1743–1748. [Google Scholar] [CrossRef]
- Cuyul-Vásquez, I.; Araya-Quintanilla, F.; Gutiérrez-Espinoza, H. Comment on Siracusa, et al. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. Int. J. Mol. Sci. 2021, 22, 9075. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Brito, R.G.; Santos, P.L.; Almeida, M.; Pina, L.T.S.; Antoniolli, A.R.; Almeida, J.R.G.d.S.; Picot, L.; Gokhan, G.; Quintans, J.S.S.; Júnior, L.J. Natural Products as Promising Pharmacological Tools for the Management of Fibromyalgia Symptoms—A Review. In Discussions of Unusual Topics in Fibromyalgia; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, R.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Trovato, A.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; di Paola, R.; et al. Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®. Biomedicines 2021, 13, 1683. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Salvemini, D. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Siddharth, K.D.; Abbas, A.M. Some oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndrome. Int. J. Rheum. Dis. 2017, 20, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, M.D.; de Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuroendocrinol. Lett. 2010, 31, 2–4. [Google Scholar]
- Akkuş, S.; Nazıroğlu, M.; Eriş, S.; Yalman, K.; Yılmaz, N.; Yener, M. Levels of lipid peroxidation, nitric oxide, and antioxidant vitamins in plasma of patients with fibromyalgia. Cell Biochem. Funct. 2009, 27, 181–185. [Google Scholar] [CrossRef]
- Sakarya, S.T.; Akyol, Y.; Bedir, A.; Canturk, F. The relationship between serum antioxidant vitamins, magnesium levels, and clinical parameters in patients with primary fibromyalgia syndrome. Clin. Rheum. 2011, 30, 1039–1043. [Google Scholar] [CrossRef] [Green Version]
- Sendur, O.F.; Tastaban, E.; Turan, Y.; Ulman, C. The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheum. Int. 2008, 28, 1117–1121. [Google Scholar] [CrossRef]
- Gómez-Centeno, A.; Ramentol, M.; Alegre, C. Nutritional Supplementation with Coenzyme Q10, Tryptophan and Magnesium in Fibromyalgia Treatment: A Letter to Editor. Reumatol. Clín. 2020, 26, 62–63. [Google Scholar]
- Rus, A.; Molina, F.; Martínez-Ramírez, M.J.; Aguilar-Ferrándiz, M.E.; Carmona, R.; Del Moral, M.L. Effects of olive oil consumption on cardiovascular risk factors in patients with fibromyalgia. Nutrients 2020, 12, 918. [Google Scholar] [CrossRef] [Green Version]
- Gilron, I.; Robb, S.; Tu, D.; Holden, R.; Towheed, T.; Ziegler, D.; Wang, L.; Milev, R.; Gray, C. Double-blind, randomized, placebo-controlled crossover trial of alpha-lipoic acid for the treatment of fibromyalgia pain: The IMPALA trial. Pain 2021, 162, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional Interventions in the Management of Fibromyalgia Syndrome. Nutrients 2020, 20, 2525. [Google Scholar] [CrossRef] [PubMed]
- Andretta, A.; Batista, E.D.; Schieferdecker, M.E.M.; Petterle, R.R.; Boguszewski, C.L.; Paiva, E.D.S. Relation between magnesium and calcium and parameters of pain, quality of life and depression in women with fibromyalgia. Adv. Rheum. 2019, 59, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortancil, O.; Sanli, A.; Eryuksel, R.; Basaran, A.; Ankarali, H. Association between serum ferritin level and fibromyalgia syndrome. Eur. J. Clin. Nutr. 2010, 64, 308–312. [Google Scholar] [CrossRef]
- Wu, Z.; Malihi, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. The association between vitamin D concentration and pain: A systematic review and meta-analysis. Public Health Nutr. 2018, 21, 2022–2037. [Google Scholar] [CrossRef]
- Makrani, A.H.; Afshari, M.; Ghajar, M.; Forooghi, Z.; Moosazadeh, M. Vitamin D and fibromyalgia: A meta-analysis. Korean J. Pain 2017, 30, 250–257. [Google Scholar] [CrossRef]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef]
- Saaiq, M.; Ashraf, B. Modifying “Pico” Question into “Picos” Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J. Plast. Surg. 2017, 6, 390–392. [Google Scholar]
- Sullivan, J.L. The Pain Catastrophizing Scale: User Manual; McGill University: Montreal, QC, Canada, 2009; pp. 1–36. [Google Scholar]
- Crichton, N. Visual analogue scale (VAS). J. Clin. Nurs. 2001, 10, 697–706. [Google Scholar]
- Von, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 2015, 50, 133–149. [Google Scholar]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Sarmer, S.; Ergin, S.; Yavuzer, G. The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheum. Int. 2000, 20, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Jensen, M.P.; Thornby, J.I.; Shanti, B.F. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain 2004, 5, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. McMaster Critical Review Form-Quantitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998. [Google Scholar]
- Barmarki, M.; Maindet-Dominici, C.; Nizard, J.; Baron, D.; Russ, I.; Fardellone, P.; Ginies, P.; Marc, J.F.; Conrozier, T.; Bertin, P. Multicenter, Prospective, Controlled Double-Blind Study Comparing Fib-19-01, A Phytotherapy Treatment, To A Dietary Supplement and to Conventional Care in Patients Suffering from Fibromyalgia. Altern. Ther. Health Med. 2019, 25, 46–53. [Google Scholar]
- San Mauro, I.; López, S.; Collado, L.; Sanz, S.; Garican, E. Anti-inflammatory and antioxidant feeding and supplementation may serve as adjuvants in women with fibromyalgia. J. Nutr. Intermed. Metab. 2019, 15, 3–9. [Google Scholar] [CrossRef]
- Sawaddiruk, P.; Apaijai, N.; Paiboonworachat, S.; Kaewchur, T.; Kasitanon, N.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, N.; Chattipakorn, S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic. Res. 2019, 53, 901–909. [Google Scholar] [CrossRef]
- Boomershine, C.S.; Koch, T.A.; Morris, D. A blinded, randomized, placebo-controlled study to investigate the efficacy and safety of ferric carboxymaltose in iron-deficient patients with fibromyalgia. Rheum. Ther. 2018, 5, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Rus, A.; Molina, F.; Ramos, M.M.; Martínez-Ramírez, M.J.; del Moral, M.L. Extra Virgin Olive Oil Improves Oxidative Stress, Functional Capacity, and Health-Related Psychological Status in Patients with Fibromyalgia: A Preliminary Study. Biol. Res. Nurs. 2017, 19, 106–115. [Google Scholar] [CrossRef]
- Umeda, M.; Kempka, L.; Weatherby, A.; Greenlee, B.; Mansion, K. Effects of caffeinated chewing gum on muscle pain during submaximal isometric exercise in individuals with fibromyalgia. Physiol. Behav. 2016, 1, 139–145. [Google Scholar] [CrossRef]
- Di Pierro, F.; Rossi, A.; Consensi, A.; Giacomelli, C.; Bazzichi, L. Role for a water-soluble form of CoQ10 in female subjects affected by fibromyalgia. A preliminary study. Clin. Exp. Rheum. 2017, 35 (Suppl. 105), 20–27. [Google Scholar]
- Iqbal, R.; Mughal, M.; Asghar, M.; Shaheen, N.; Ahmad, N.; Farman, S.; Saeed, M.; Khan, I.; Arshad, M. Effect of Vitamins C, E and Nigella sativa Seeds on Antioxidant Activity in Fibromyalgia Patients. Pak. J. Zool. 2015, 47, 7–13. [Google Scholar]
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheum. Int. 2013, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.G.; Calich, A.L.; Tritto, A.C.C.; de Sa Pinto, A.L.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine supplementation in fibromyalgia: A randomized, double-blind, placebo-controlled trial. Arthritis Care Res. 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, M.D.; Cano-García, F.J.; Alcocer-Gómez, E.; De Miguel, M.; Sánchez-Alcázar, J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q₁₀ effect on clinical improvement. PLoS ONE 2012, 7, e35677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahner-Roedler, D.L.; Thompson, J.M.; Luedtke, C.A.; King, S.M.; Cha, S.S.; Elkin, P.L.; Bruce, B.K.; Townsend, C.O.; Bergeson, J.R.; Eickhoff, A.L.; et al. Dietary soy supplement on fibromyalgia symptoms: A randomized, double-blind, placebo-controlled, early phase trial. Evid. Based Complement. Altern. Med. 2011, 2011, 350697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliot, D.L.; Kuehl, K.S.; Jones, K.D.; Dulacki, K. Using an eccentric exercise-testing protocol to assess the beneficial effects of tart cherry juice in fibromyalgia patients. Integr. Med. 2010, 9, 24–29. [Google Scholar]
- Rossini, M.; di Munno, O.; Valentini, G.; Bianchi, G.; Biasi, G.; Cacace, E.; Malesci, D.; la Montagna, G.; Viapiana, O.; Adami, S. Double-blind, multicenter trial comparing acetyl l-carnitine with placebo in the treatment of fibromyalgia patients. Clin. Exp. Rheum. 2007, 25, 182–188. [Google Scholar]
- Merchant, R.E.; Andre, C.A.; Wise, C.M. Nutritional supplementation with Chlorella pyrenoidosa for fibromyalgia syndrome: A double-blind, placebo-controlled, crossover study. J. Musculoskelet. Pain 2001, 9, 37–54. [Google Scholar] [CrossRef]
- Russell, I.J.; Michalek, J.E.; Flechas, J.D.; Abraham, G.E. Treatment of fibromyalgia syndrome with super malic: A randomized, double blind, placebo controlled, crossover pilot study. J. Rheum. 1995, 22, 953–958. [Google Scholar]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 23, 1166. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; di Lascio, S.; Fornasari, D. Acetyl-l-carnitine in chronic pain: A narrative review. Pharm. Res. 2021, 6, 105874. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Rizzo, M.; Krogager, C.; Kennedy, C.; Georges, C.M.G.; Knežević, T.; Liberopoulos, E.; Vallée, A.; Pérez-Martínez, P.; Wenstedt, E.F.E.; et al. Safety Evaluation of α-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants 2020, 19, 1011. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic acid as a biological antioxidant. Free. Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can coenzyme Q10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Signal. 2013, 20, 1356–1361. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef]
- Kaartinen, K.; Lammi, K.; Hypen, M.; Nenonen, M.; Hanninen, O.; Rauma, A.L. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheum. 2000, 29, 308–313. [Google Scholar] [CrossRef]
- Rousset, F.; Grange, L.; Minh Vu Chuong, N.; Pinosa-Zezza, C.; Gaudin, P.; Conrozier, T.; Morel, F.; Lardy, B. Impact of the Addition of Ginger Extract and Copper Sulphate to Glucosamine Sulphate on Il-1Beta-Stimulated Chondrocytes. J. Rheum. Dis. Treat. 2016, 2, 038. [Google Scholar] [CrossRef]
- Naziroǧlu, M.; Akkuş, S.; Soyupek, F.; Yalman, K.; Çelik, O.; Eriş, S.; Uslusoy, G.A. Vitamins C and e treatment combined with exercise modulates oxidative stress markers in blood of patients with fibromyalgia: A controlled clinical pilot study. Stress 2010, 13, 498–505. [Google Scholar] [CrossRef]
- Navarini, L.; Afeltra, A.; Gallo Afflitto, G.; Margiotta, D.P.E. Polyunsaturated fatty acids: Any role in rheumatoid arthritis? Lipids Health Dis. 2017, 16, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [Green Version]
| Author/s | Items according to Critical Review By McMaster | T1 | % | MQ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Gilron I, et al., 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Barmaki M, et al., 2019 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 12 | 75 | G |
| San Mauro I, et al., 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 81.25 | VG |
| Sawaddiruk P, et al., 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Boomershine, et al., 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 15 | 93.75 | E |
| Rus A, et al., 2017 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 81.25 | VG |
| Umeda M, et al., 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Di Pierro F, et al., 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 13 | 81.25 | VG |
| Iqbaq R, et al., 2015 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| Bagis, et al., 2013 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 14 | 87.5 | VG |
| Alves, et al., 2013 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 81.25 | VG |
| Cordero, et al., 2012 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 13 | 81.25 | VG |
| Wahner-Roedler, et al., 2011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Elliot D, et al., 2010 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.75 | E |
| Rossini, et al., 2007 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 81.25 | VG |
| Merchant, et al., 2001 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 12 | 75 | G |
| Russel, et al., 1995 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 12 | 75 | G |
| T2 + A1:S14 | 19 | 19 | 19 | 19 | 11 | 15 | 9 | 11 | 19 | 19 | 19 | 11 | 19 | 17 | 19 | 10 | |||
| Author/s, Year of Study | Country | Participants, Sex | Age (±) | Intervention | Placebo | Study Duration |
|---|---|---|---|---|---|---|
| Gilron I, et al., 2021 [22] | Canada | n = 22 women, 5 men | 47 ± 6.72 | 27 | - | 10 weeks *G |
| Barmaki M, et al., 2019 [37] | France | n = 100 all women | 49 ± 7.12 | I1 = 36; I2 =33 | 31 | 24 weeks |
| San Mauro I, et al., 2019 [38] | Spain | n = 13 all women | 51.46 ± 8.04 | 6 | 7 | 4 weeks |
| Sawaddiruk P, et al., 2019 [39] | Thailand | n = 2 men, 9 women | 46 ± 11 | 5 | 6 | 10 weeks |
| Boomershine, et al., 2018 [40] | US | n = 80 women, 1 man | 42,5 ± 10.9 | 41 | 40 | 6 weeks |
| Rus A, et al., 2017 [41] | Spain | n = 23 all women | 50.88 ± 6.5 | 11 | 12 | 3 weeks |
| Umeda M, et al., 2016 [42] | US | n = 23 all women | 43.57 ± 18.49 | 12 | 11 | 3 sessions |
| Di Pierro F, et al., 2016 [43] | Italy | n = 22 all women | 53 ± 9.1 | 12 | 10 | 24 weeks |
| Iqbaq R, et al., 2015 [44] | Pakistan | n = 50 all woman | 37.87 ± 1.68 | 50 | 16 | 8 weeks |
| Bagis, et al., 2013 [45] | Turkey | n = 80 all women | 41.4 ± 10.5 | 60 | 20 ** | 8 weeks |
| Alves, et al., 2013 [46] | Brazil | n = 28 all women | 48.85 ± 9.25 | 15 | 13 | 16 weeks |
| Cordero, et al., 2012 [47] | Spain | n = 35 all women | 45.75 ± 4.5 | 20 | 15 ** | 12 weeks |
| Wahner-Roedler, et al., 2011 [48] | US | n = 50 all woman | 47.7 ± 4.25 | 25 | 25 | 6 weeks |
| Elliot D, et al., 2010 [49] | US | n = 14 all women | 51 ± 2.0 | 14 | - | 4 weeks *E |
| Rossini, et al., 2007 [50] | Italy | n = 89 all women | 46.8 ± 5.05 | 47 | 42 | 10 weeks |
| Merchant, et al., 2001 [51] | US | n = 43 all women | 47.1 ± 9 | 22 | 21 | 12 weeks |
| Russel, et al., 1995 [52] | US | n = 21 women, 3 men | 49 | 12 | 12 | 4 weeks |
| Study | Intervention Group (IG) | Control | Pain Scale | Results | Effect for Pain |
|---|---|---|---|---|---|
| Gilron I, et al., 2021 [22] | IG = ALA * 1663 mg/day for 5 weeks and placebo during the second 5 weeks. IGP = the first 5 weeks were treated with placebo and ALA for the next 5 weeks. | Placebo | FIQ, BPI, VAS | For women, the perception of pain for all scales with respect to the placebo group was for ALA of (p = 0.13) and for men (p = 0.01). | Neutral effect for women and beneficial for men. |
| Barmaki M, et al., 2019 [37] | G1 = Fibromyalgine® (Fib) (vitamin C, acerola ginger root, freeze-dried royal jelly), 2 capsules/day; G2 = food supplement (FS), 2 capsules/day; G3 = control arm not receiving any supplementation. | NoST | FIQ, VAS | The supplementation with Fibromyalgine® showed an improvement in pain intensity on the FIQ scale (p < 0.001). | Positive benefit. |
| San Mauro I, et al., 2019 [38] | Turmeric supplement 500 mg/day, gluten-free diet and low in histamine. | NoST | CPGS, PCS | PCS (p = 0.190), GPGS (p = 0.671). | Neutral benefit. |
| Sawaddiruk P, et al., 2019 [39] | G1 = CoQ10 supplementation 300 mg/day+ pregabalin (150 mg/day); G2 = placebo + pregabalin (150 mg/day) for 40 days. At day 40, patients who received CoQ10 therapy were switched to placebo, and vice versa. | Placebo | VAS, PPT | Decrease in VAS and increase in PPT significantly increased in pregabalin-treated FM patients with CoQ10, compared to those treated with pregabalin and placebo alone. | Positive benefit. |
| Boomershine, et al., 2018 [40] | Ferric carboxymaltose 15 mg/kg (up to 750 mg). | Placebo | FIQ, BPI | Greater improvements from baseline to day 42 were observed for ferric carboxymaltose vs. placebo in FIQ total score and BPI total score. | Positive benefit. |
| Rus A, et al., 2017 [41] | Extra virgin olive oil (EVOO) 50 mL/day. Control group = Refined olive oil (ROO) 50 mL/day. | Control | FIQ, VAS | In the EVOO group, a decrease in FIQ (p < 0.011) was observed, but not in pain (p < 0.279) compared to the ROO consumption group. | Neutral benefit. |
| Umeda M, et al., 2016 [42] | Gum with 100 mg of caffeine. | Placebo | SF-MPQ, PPI, VAS | Pain results improved in the experimental group measured with SF-MPQ (p = 0.006). Pain measured with VAS (p = 0.396)/PPI (p = 0. 87). | Neutral benefit. |
| Di Pierro F, et al., 2016 [43] | 200 mg × 2/day CoQ10 formula. | Control | VAS, FIQ | Statistical significance is only evidenced p <0.005, for the pain scale (VAS), in the rest the results were not significant. | Positive benefit. |
| Iqbaq, et al., 2015 [44] | Vitamin C (200 mg daily), E (200 mg daily) and Nigella sativa seeds (13 mg 4–5 times daily). | Control | VAS | VAS (p < 0.05). | Positive benefit. |
| Bagis, et al., 2013 [45] | IG1 (n = 20) Mg citrate 300 mg/day; IG2 (n = 20) amitriptyline 10 mg/day; GI3 (n = 20) Mg citrate 300 mg/day + amitriptyline 10 mg/day. | NA | VAS, FIQ | Positive effects on all pain parameters with the combination of amitriptyline + magnesium citrate proved to be effective on all pain parameters (p < 0.001). | Positive effects combination of amitriptyline + magnesium citrate. |
| Alves, et al., 2013 [46] | 20 gm of creatine monohydrate for 5 days divided into 4 equal doses, followed by 5 gm/day as a single dosage throughout the trial. | Placebo | MPQ, FIQ | FIQ and MPQ (p > 0.005). | Neutral benefit. |
| Cordero, et al., 2012 [47] | 300 mg/day of CoQ10. | Control | FIQ, VAS | There was a decrease in the FIQ score (p < 0.001) and VAS (p < 0.001) in the experimental group compared with the control group. | Positive benefit. |
| Wahner-Roedler, et al., 2011 [48] | Soy protein (20 g), soy isoflavone (160 mg) (1 serving daily). | Placebo | FIQ | No significant differences between groups. | Neutral benefit. |
| Elliot D, et al., 2010 [49] | GE = received tart cherry juice, 2 bottles/day, morning and evening. | Placebo | VAS | There were no significant differences for either group in terms of pain (p > 0.005). | Neutral benefit. |
| Rossini, et al., 2007 [50] | 1000 mg acetyl L-carnitine (LAC) or placebo. | Placebo | VAS | VAS (p < 0.02). | Positive benefit. |
| Merchant, et al., 2001 [51] | Sun Chlorella™ green algae and Wakasa Gold Chlorella™ (500 g and 100 mL/day, respectively) | Placebo | VAS | VAS (p < 0.05). | Positive benefit. |
| Russel, et al., 1995 [52] | 200 mg malic acid + 50 mg magnesium, from 3 capsules up to 6 per day. | Placebo | VAS | Pain with this supplement was not significantly different from placebo (P5 0.7). | Neutral benefit. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Araque, A.; Verde, Z.; Torres-Ortega, C.; Sainz-Gil, M.; Velasco-Gonzalez, V.; González-Bernal, J.J.; Mielgo-Ayuso, J. Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia—A Systematic Review. J. Clin. Med. 2022, 11, 2462. https://doi.org/10.3390/jcm11092462
Fernández-Araque A, Verde Z, Torres-Ortega C, Sainz-Gil M, Velasco-Gonzalez V, González-Bernal JJ, Mielgo-Ayuso J. Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia—A Systematic Review. Journal of Clinical Medicine. 2022; 11(9):2462. https://doi.org/10.3390/jcm11092462
Chicago/Turabian StyleFernández-Araque, Ana, Zoraida Verde, Clara Torres-Ortega, Maria Sainz-Gil, Veronica Velasco-Gonzalez, Jerónimo Javier González-Bernal, and Juan Mielgo-Ayuso. 2022. "Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia—A Systematic Review" Journal of Clinical Medicine 11, no. 9: 2462. https://doi.org/10.3390/jcm11092462
APA StyleFernández-Araque, A., Verde, Z., Torres-Ortega, C., Sainz-Gil, M., Velasco-Gonzalez, V., González-Bernal, J. J., & Mielgo-Ayuso, J. (2022). Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia—A Systematic Review. Journal of Clinical Medicine, 11(9), 2462. https://doi.org/10.3390/jcm11092462








