Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort
Abstract
:1. Introduction
2. Materials and Methods
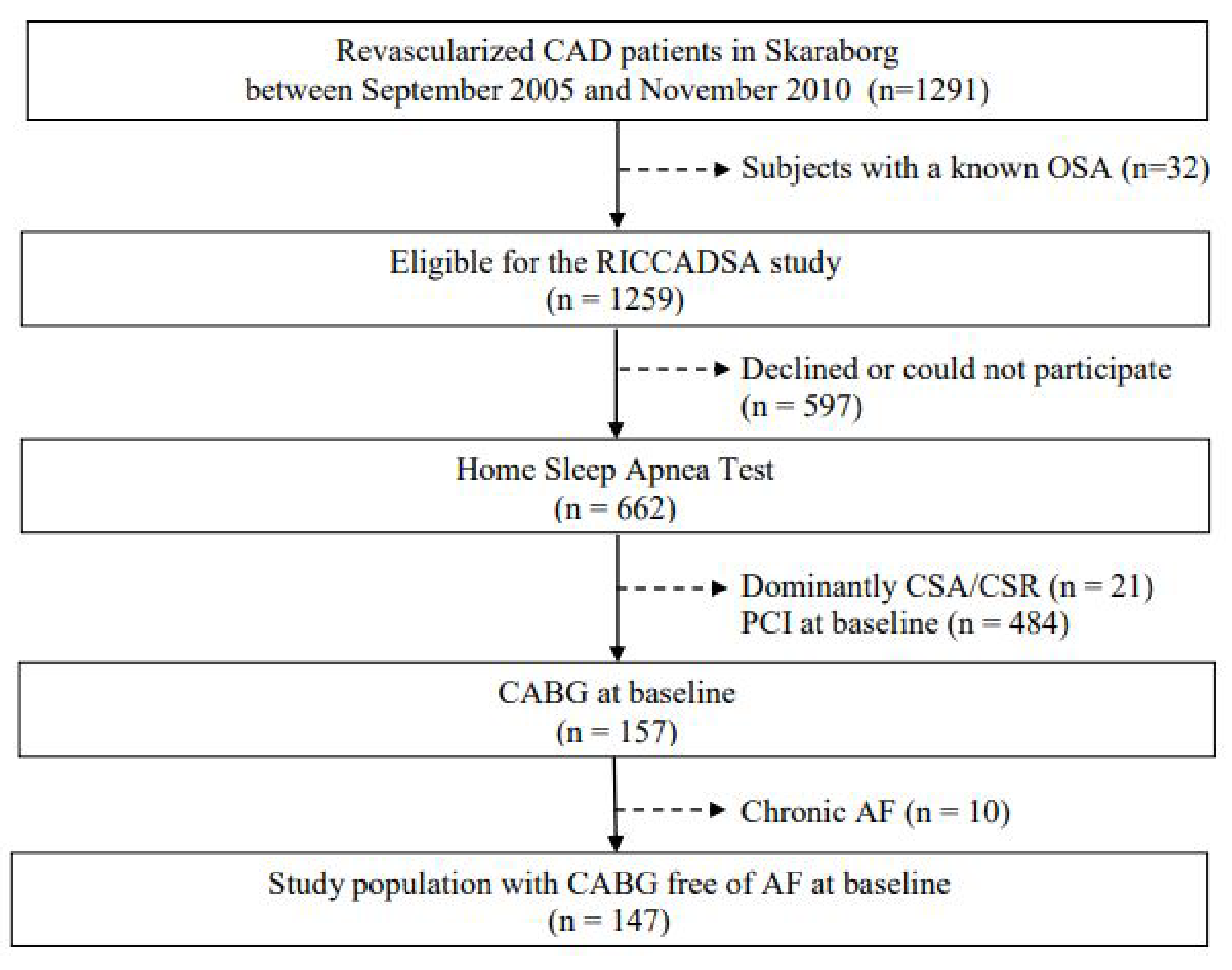
2.1. Study Population
2.2. Definition of Comorbidities
2.3. Sleep Studies, Group Allocation
2.4. Blood Sampling
2.5. Transthoracic Echocardiography
2.6. Statistical Analysis
2.7. Outcomes and Sample Size
3. Results
3.1. The Entire Study Population and Participants at Follow-Up
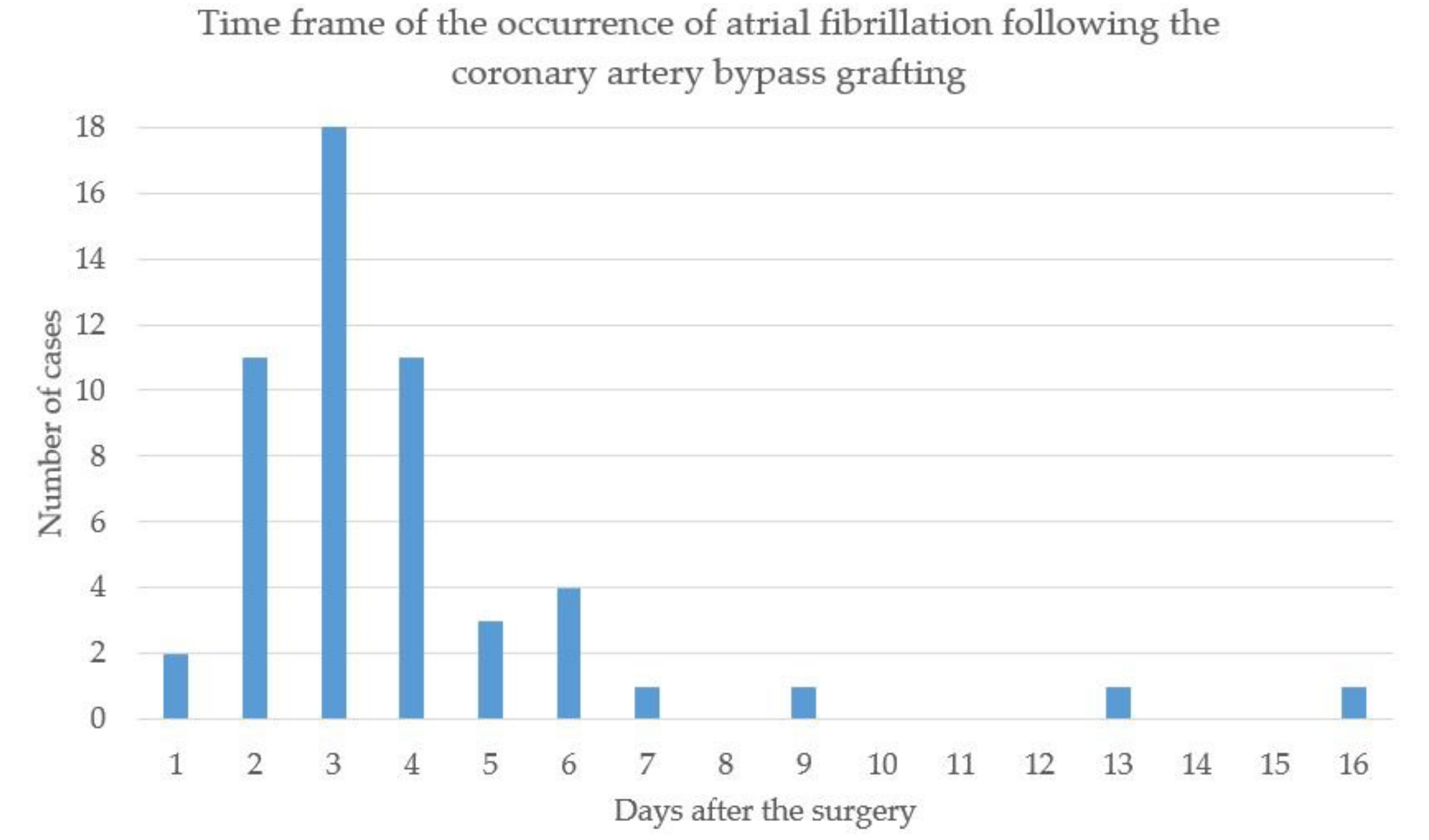
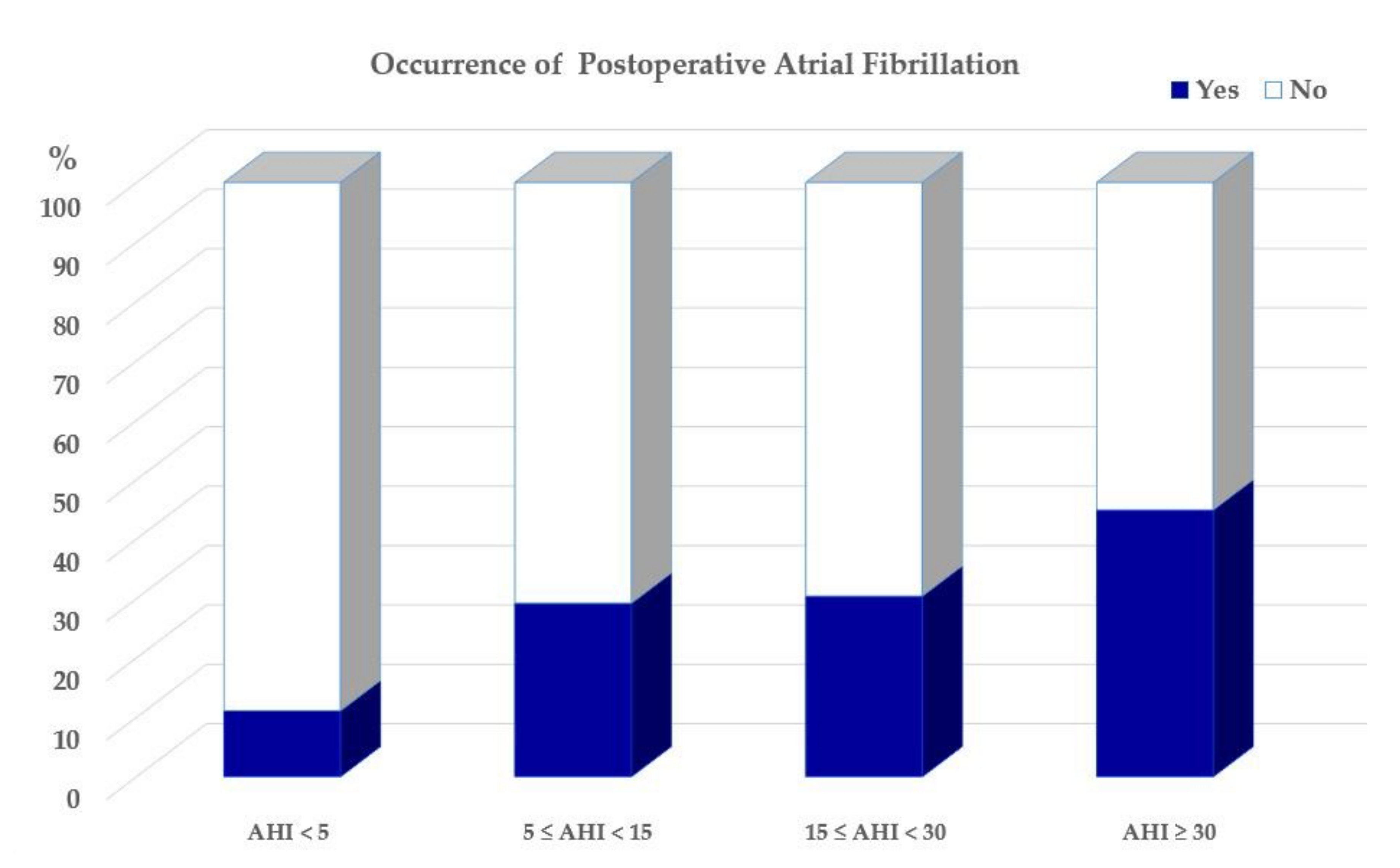
3.2. Occurrence of POAF and Its Association with OSA
3.3. Long-Term Outcomes
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilaveris, P.E.; Kennedy, H.L. Silent atrial fibrillation: Epidemiology, diagnosis, and clinical impact. Clin. Cardiol. 2017, 40, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Wolf, P.A. Awareness of the Role of Atrial Fibrillation as a Cause of Ischemic Stroke. Stroke 2014, 45, e19–e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wańkowicz, P.; Nowacki, P.; Gołąb-Janowska, M. Atrial fibrillation risk factors in patients with ischemic stroke. Arch. Med. Sci. 2021, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Echahidi, N.; Pibarot, P.; O’Hara, G.; Mathieu, P. Mechanisms, Prevention, and Treatment of Atrial Fibrillation after Cardiac Surgery. J. Am. Coll. Cardiol. 2008, 51, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Ailawadi, G.; Kirkwood, K.A.; Perrault, L.P.; Parides, M.K.; et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kang, D.R.; Uhm, J.-S.; Shim, J.; Sung, J.-H.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; Joung, B. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am. Heart J. 2014, 167, 593–600.e1. [Google Scholar] [CrossRef]
- Konstantino, Y.; Yovel, D.Z.; Friger, M.D.; Sahar, G.; Knyazer, B.; Amit, G. Postoperative Atrial Fibrillation Following Coronary Artery Bypass Graft Surgery Predicts Long-Term Atrial Fibrillation and Stroke. Isr. Med. Assoc. J. 2016, 18, 744–748. [Google Scholar]
- Lin, M.-H.; Kamel, H.; Singer, D.E.; Wu, Y.-L.; Lee, M.; Ovbiagele, B. Perioperative/Postoperative Atrial Fibrillation and Risk of Subsequent Stroke and/or Mortality. Stroke 2019, 50, 1364–1371. [Google Scholar] [CrossRef]
- AlTurki, A.; Marafi, M.; Proietti, R.; Cardinale, D.; Blackwell, R.; Dorian, P.; Bessissow, A.; Vieira, L.; Greiss, I.; Essebag, V.; et al. Major Adverse Cardiovascular Events Associated with Postoperative Atrial Fibrillation after Noncardiac Surgery: A Systematic Review and Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2020, 13, e007437. [Google Scholar] [CrossRef]
- Villareal, R.P.; Hariharan, R.; Liu, B.C.; Kar, B.; Lee, V.V.; Elayda, M.; Lopez, J.A.; Rasekh, A.; Wilson, J.M.; Massumi, A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J. Am. Coll. Cardiol. 2004, 43, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariscalco, G.; Klersy, C.; Zanobini, M.; Banach, M.; Ferrarese, S.; Borsani, P.; Cantore, C.; Biglioli, P.; Sala, A. Atrial Fibrillation After Isolated Coronary Surgery Affects Late Survival. Circulation 2008, 118, 1612–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; for the Investigators of the Ischemia Research and Education Foundation and the Multicenter Study of Perioperative Ischemia Research Group. A Multicenter Risk Index for Atrial Fibrillation after Cardiac Surgery. JAMA J. Am. Med. Assoc. 2004, 291, 1720–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Goyal, S.K.; Sharma, A. Atrial fibrillation in obstructive sleep apnea. World J. Cardiol. 2013, 5, 157–163. [Google Scholar] [CrossRef]
- Chan, M.T.V.; Wang, C.Y.; Seet, E.; Tam, S.; Lai, H.Y.; Chew, E.F.F.; Wu, W.K.K.; Cheng, B.C.P.; Lam, C.K.M.; Short, T.G.; et al. Association of Unrecognized Obstructive Sleep Apnea with Postoperative Cardiovascular Events in Patients Undergoing Major Noncardiac Surgery. JAMA J. Am. Med. Assoc. 2019, 321, 1788–1798. [Google Scholar] [CrossRef] [Green Version]
- Mooe, T.; Gullsby, S.; Rabben, T.; Eriksson, P. Sleep-disordered breathing: A novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron. Artery Dis. 1996, 7, 475–478. [Google Scholar] [CrossRef]
- Qaddoura, A.; Kabali, C.; Drew, D.; van Oosten, E.M.; Michael, K.A.; Redfearn, D.P.; Simpson, C.S.; Baranchuk, A. Obstructive Sleep Apnea as a Predictor of Atrial Fibrillation after Coronary Artery Bypass Grafting: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2014, 30, 1516–1522. [Google Scholar] [CrossRef]
- Feng, T.R.; White, R.S.; Ma, X.; Askin, G.; Pryor, K.O. The effect of obstructive sleep apnea on readmissions and atrial fibrillation after cardiac surgery. J. Clin. Anesth. 2019, 56, 17–23. [Google Scholar] [CrossRef]
- Van Oosten, E.M.; Hamilton, A.; Petsikas, D.; Payne, D.; Redfearn, D.P.; Zhang, S.; Hopman, W.M.; Baranchuk, A. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Boyce, S.W.; Hill, P.C.; Bafi, A.S.; Xue, Z.; Lindsay, J.; Corso, P.J. Association of Body Mass Index with New-Onset Atrial Fibrillation after Coronary Artery Bypass Grafting Operations. Ann. Thorac. Surg. 2011, 91, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Thunström, E.; Kallryd, A.; Herlitz, J.; Ejdebäck, J. Rationale and design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea—RICCADSA trial. Scand. Cardiovasc. J. 2009, 43, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; WHO Technical Report Series 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; Darien, I., Ed.; American Academy of Sleep Medicine: Darien, IO, USA, 2014. [Google Scholar]
- Peker, Y.; Thunström, E.; Glantz, H.; Wegscheider, K.; Zu Eulenburg, C. Outcomes in coronary artery disease patients with sleepy obstructive sleep apnoea on CPAP. Eur. Respir. J. 2017, 50, 1700749. [Google Scholar] [CrossRef] [Green Version]
- Glantz, H.; Thunström, E.; Johansson, M.C.; Guron, C.W.; Uzel, H.; Ejdebäck, J.; Nasic, S.; Peker, Y. Obstructive sleep apnea is independently associated with worse diastolic function in coronary artery disease. Sleep Med. 2015, 16, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Glantz, H.; Johansson, M.C.; Thunström, E.; Guron, C.W.; Uzel, H.; Saygin, M.; Herlitz, J.; Peker, Y. Effect of CPAP on diastolic function in coronary artery disease patients with nonsleepy obstructive sleep apnea: A randomized controlled trial. Int. J. Cardiol. 2017, 241, 12–18. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Kofidis, T.; Chan, S.-P.; Ong, T.-H.; Yeo, T.-C.; Tan, H.-C.; Lee, C.-H. Sleep apnoea and unscheduled re-admission in patients undergoing coronary artery bypass surgery. Atherosclerosis 2015, 242, 128–134. [Google Scholar] [CrossRef]
- Nagappa, M.; Ho, G.; Patra, J.; Wong, J.; Singh, M.; Kaw, R.; Cheng, D.; Chung, F. Postoperative Outcomes in Obstructive Sleep Apnea Patients Undergoing Cardiac Surgery: A Systematic Review and Meta-analysis of Comparative Studies. Anesth. Analg. 2017, 125, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Ahlsson, A.; Fengsrud, E.; Bodin, L.; Englund, A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur. J. Cardio Thorac. Surg. 2010, 37, 1353–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawitz, O.K.; Gulack, B.C.; Brennan, J.M.; Thibault, D.P.; Wang, A.; O’Brien, S.M.; Schroder, J.N.; Gaca, J.G.; Smith, P.K. Association of postoperative complications and outcomes following coronary artery bypass grafting. Am. Heart J. 2020, 222, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Aranki, S.F.; Shaw, D.P.; Adams, D.H.; Rizzo, R.J.; Couper, G.S.; VanderVliet, M.; Collins, J.J.; Cohn, L.H.; Burstin, H.R. Predictors of Atrial Fibrillation after Coronary Artery Surgery: Current trends and impact on hospital resources. Circulation 1996, 94, 390–397. [Google Scholar] [CrossRef]
- Daubert, M.A.; Whellan, D.J.; Woehrle, H.; Tasissa, G.; Anstrom, K.J.; Lindenfeld, J.; Benjafield, A.; Blase, A.; Punjabi, N.; Fiuzat, M.; et al. Treatment of sleep-disordered breathing in heart failure impacts cardiac remodeling: Insights from the CAT-HF Trial. Am. Heart J. 2018, 201, 40–48. [Google Scholar] [CrossRef]
- Freeman, J.V.; Simon, D.N.; Go, A.S.; Spertus, J.; Fonarow, G.C.; Gersh, B.J.; Hylek, E.M.; Kowey, P.R.; Mahaffey, K.W.; Thomas, L.E.; et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 393–402. [Google Scholar] [CrossRef] [Green Version]

| POAF n = 48 | No POAF n = 99 | |
|---|---|---|
| Age *, yrs | 66.5 ± 7.5 | 63.1 ± 8.7 |
| Male sex, % | 89.6 | 84.8 |
| BMI, kg/m2 | 28.0 ± 4.5 | 27.7 ± 4.1 |
| Obesity % | 22.9 | 24.2 |
| AHI categories *, % | ||
| <5.0 events/h (no OSA) | 4.2 | 16.2 |
| 5.0–14.9 events/h (mild) | 14.6 | 17.2 |
| 15.0–29.9 events/h (moderate) | 35.4 | 39.4 |
| ≥30.0 events/h (severe) | 45.8 | 27.3 |
| ESS ≥ 10, % | 37.5 | 32.3 |
| Current smoking, % | 4.2 | 14.1 |
| Hypertension, % | 64.6 | 61.2 |
| Diabetes, % | 33.3 | 21.2 |
| Stroke, % | 4.2 | 11.2 |
| Lung disease, % | 8.3 | 8.1 |
| Diuretic use, % | 34.3 | 30.9 |
| β blocker use, % | 89.2 | 89.7 |
| Aspirin use, % | 80.0 | 95.8 |
| Clopidogrel use, % | 4.6 | 1.5 |
| Warfarin use, % | 13.7 | 1.5 |
| CCB use, % | 18.2 | 17.0 |
| ACE inhibitor use, % | 34.3 | 37.2 |
| ARB use, % | 11.4 | 7.8 |
| Lipid-lowering agent use, % | 93.7 | 97.5 |
| Echocardiography† | n = 39 | n = 75 |
| LAD *, mm | 45.6 ± 5.9 | 43.4 ± 5.7 |
| LVEF % | 54.8 ± 8.4 | 56.9 ± 5.0 |
| p-NT-proBNP, ng/mL | 705.2 ± 1164.5 | 419.3 ± 416.5 |
| OR | Lower | Upper | p Value | |
|---|---|---|---|---|
| Age, years | 1.05 | 1.01 | 1.01 | 0.024 |
| Male sex | 1.54 | 0.52 | 4.51 | 0.435 |
| BMI, kg/m2 | 1.02 | 0.94 | 1.10 | 0.690 |
| Obesity | 0.93 | 0.41 | 2.10 | 0.860 |
| Current smoking | 0.26 | 0.06 | 1.21 | 0.087 |
| Hypertension | 1.16 | 0.56 | 2.37 | 0.694 |
| Diabetes | 1.86 | 0.86 | 4.01 | 0.115 |
| Lung disease | 1.03 | 0.30 | 3.62 | 0.958 |
| AHI, events/h | 1.03 | 1.01 | 1.05 | 0.003 |
| ODI, events/h | 1.04 | 1.01 | 1.06 | 0.007 |
| T90%, % | 1.01 | 0.99 | 1.02 | 0.545 |
| AHI categories | ||||
| <5.0 events/h | 1 | |||
| 5.0–14.9 events/h | 3.29 | 0.59 | 18.27 | 0.173 |
| 15.0–29.9 events/h | 3.49 | 0.72 | 16.87 | 0.105 |
| ≥ 30 events/h | 6.52 | 1.35 | 31.46 | 0.020 |
| LAD, mm | 1.07 | 0.99 | 1.14 | 0.068 |
| LVEF % | 1.05 | 0.98 | 1.11 | 0.157 |
| p-NT-proBNP, pg/mL | 1.00 | 1.00 | 1.00 | 0.090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peker, Y.; Holtstrand-Hjälm, H.; Celik, Y.; Glantz, H.; Thunström, E. Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort. J. Clin. Med. 2022, 11, 2459. https://doi.org/10.3390/jcm11092459
Peker Y, Holtstrand-Hjälm H, Celik Y, Glantz H, Thunström E. Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort. Journal of Clinical Medicine. 2022; 11(9):2459. https://doi.org/10.3390/jcm11092459
Chicago/Turabian StylePeker, Yüksel, Henrik Holtstrand-Hjälm, Yeliz Celik, Helena Glantz, and Erik Thunström. 2022. "Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort" Journal of Clinical Medicine 11, no. 9: 2459. https://doi.org/10.3390/jcm11092459
APA StylePeker, Y., Holtstrand-Hjälm, H., Celik, Y., Glantz, H., & Thunström, E. (2022). Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort. Journal of Clinical Medicine, 11(9), 2459. https://doi.org/10.3390/jcm11092459







