Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Plasma TCZ Concentration Measurement
2.3. Plasma IL-6 and sIL-6R Assessment
2.4. HLA Antibody Analysis
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients
3.2. Variability of Trough TCZ Concentrations
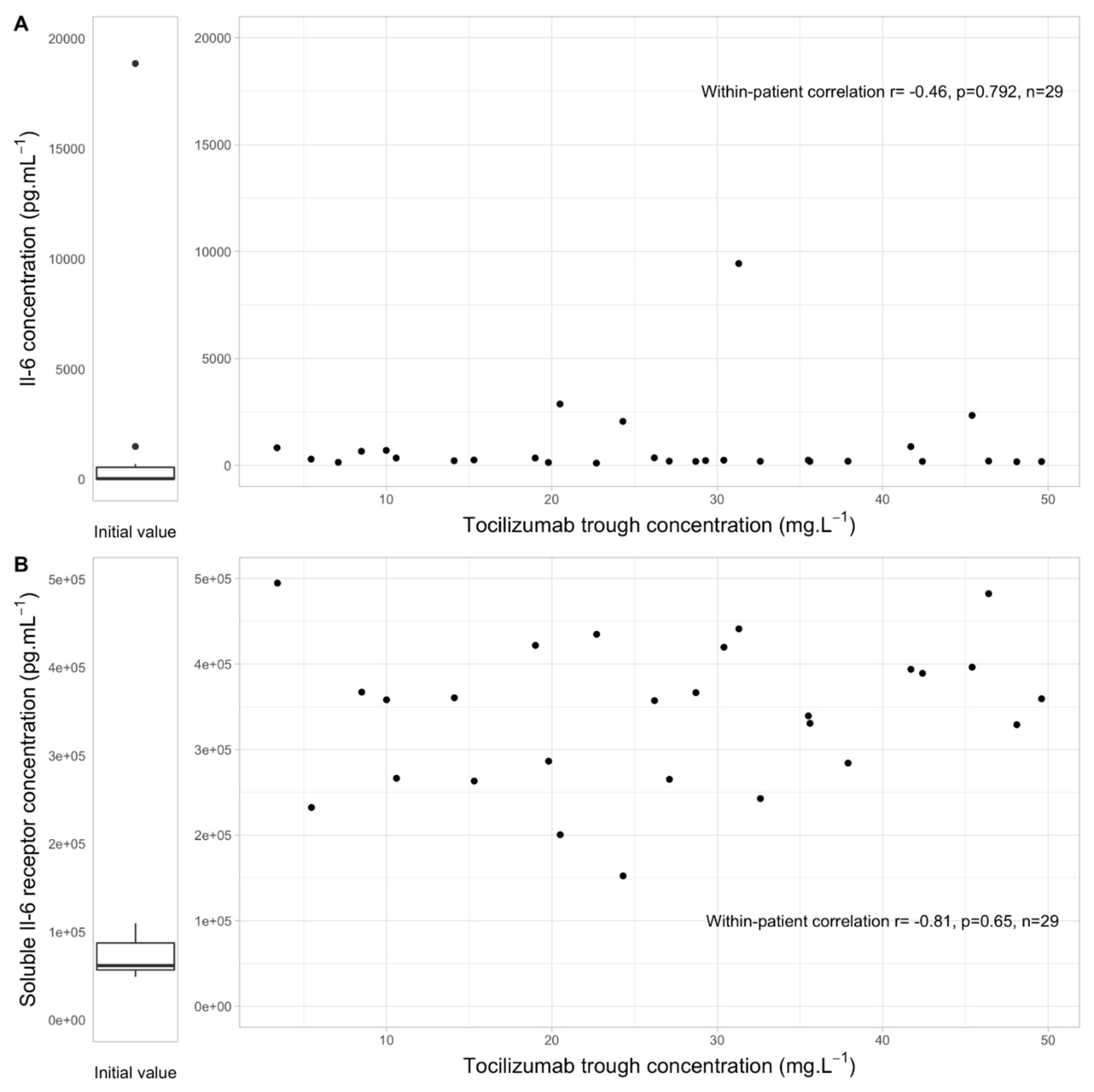
3.3. Link between Tocilizumab Trough Concentrations, IL6 and sIL6-R Levels
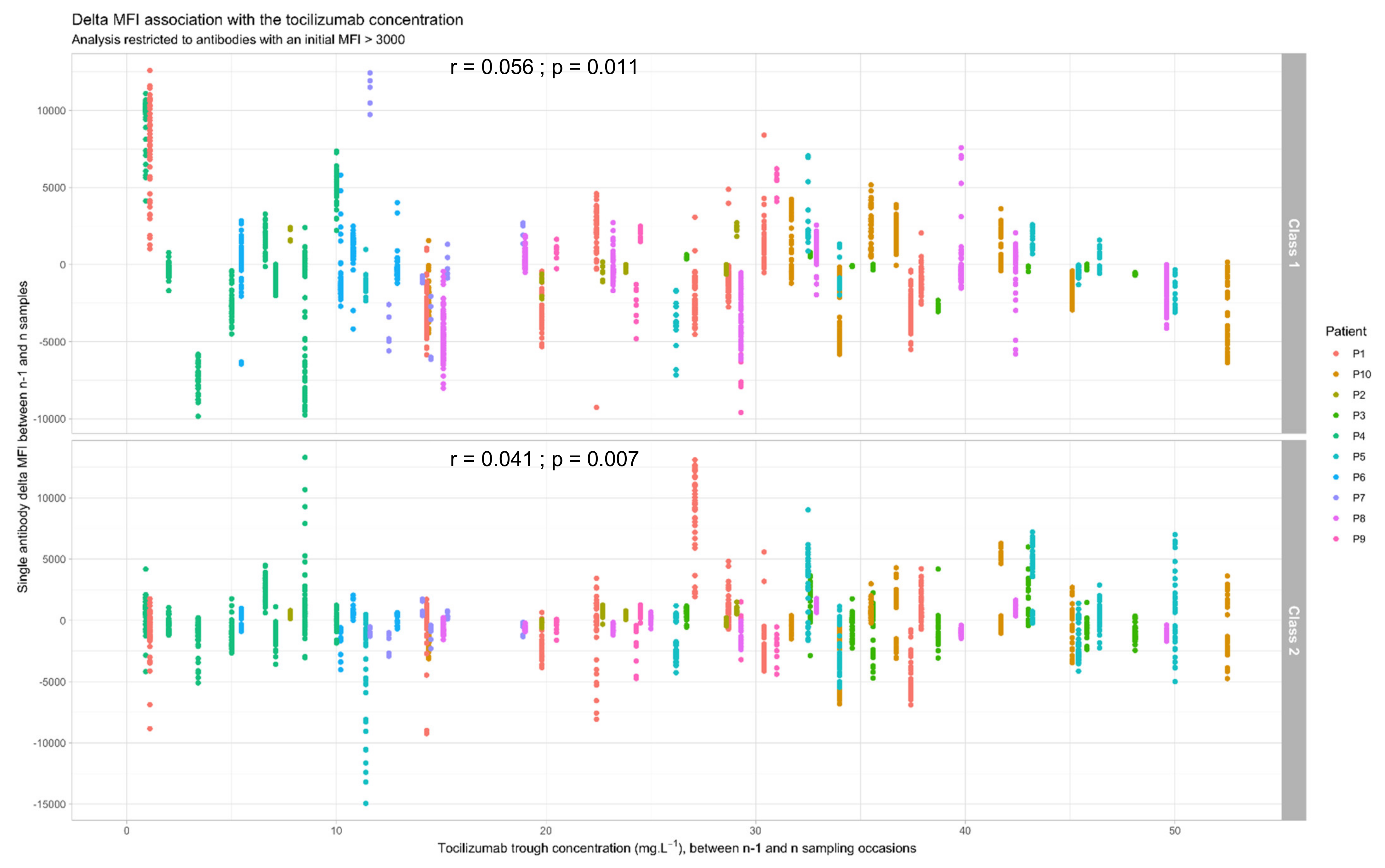
3.4. Link between Tocilizumab Trough Concentrations and Anti-HLA Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malvezzi, P.; Jouve, T.; Noble, J.; Rostaing, L. Desensitization in the Setting of HLA-Incompatible Kidney Transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2018, 16, 367–375. [Google Scholar]
- Noble, J.; Metzger, A.; Bennani, H.N.; Daligault, M.; Masson, D.; Terrec, F.; Imerzoukene, F.; Bardy, B.; Fiard, G.; Marlu, R.; et al. Apheresis Efficacy and Tolerance in the Setting of HLA-Incompatible Kidney Transplantation. J. Clin. Med. 2021, 10, 1316. [Google Scholar] [CrossRef]
- Orandi, B.J.; Luo, X.; Massie, A.B.; Garonzik-Wang, J.M.; Lonze, B.; Ahmed, R.; Van Arendonk, K.J.; Stegall, M.D.; Jordan, S.C.; Oberholzer, J.; et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N. Engl. J. Med. 2016, 374, 940–950. [Google Scholar] [CrossRef]
- Manook, M.; Koeser, L.; Ahmed, Z.; Robb, M.; Johnson, R.; Shaw, O.; Kessaris, N.; Dorling, A.; Mamode, N. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: A matched cohort analysis. Lancet 2017, 389, 727–734. [Google Scholar] [CrossRef]
- Vo, A.A.; Choi, J.; Kim, I.; Louie, S.; Cisneros, K.; Kahwaji, J.; Toyoda, M.; Ge, S.; Haas, M.; Puliyanda, D.; et al. A Phase I/II Trial of the Interleukin-6 Receptor–Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation 2015, 99, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Lukovsky, M.; Toyoda, M.; Wang, J.; Reinsmoen, N.L.; Lai, C.-H.; Peng, A.; Villicana, R.; Jordan, S.C. Rituximab and Intravenous Immune Globulin for Desensitization during Renal Transplantation. N. Engl. J. Med. 2008, 359, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habal, M.V.; Farr, M.; Restaino, S.; Chong, A. Desensitization in the Era of Precision Medicine: Moving from the Bench to Bedside. Transplantation 2019, 103, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Daligault, M.; Bardy, B.; Noble, J.; Bourdin, A.; Masson, D.; Bennani, H.N.; Bugnazet, M.; Malvezzi, P.; Rostaing, L.; Jouve, T. Marginal Impact of Tocilizumab Monotherapy on Anti-HLA Alloantibodies in Highly Sensitized Kidney Transplant Candidates. Transplant. Direct 2021, 7, e690. [Google Scholar] [CrossRef] [PubMed]
- Jouve, T.; Laheurte, C.; Noble, J.; Weinhard, J.; Daligault, M.; Renaudin, A.; Bennani, H.N.; Masson, D.; Gravelin, E.; Bugnazet, M.; et al. Immune responses following tocilizumab therapy to desensitize HLA-sensitized kidney-transplant candidates. Am. J. Transplant. 2021. [Google Scholar] [CrossRef]
- Truffot, A.; Gautier-Veyret, E.; Baillet, A.; Jourdil, J.; Stanke-Labesque, F.; Gottenberg, J. Variability of rituximab and tocilizumab trough concentrations in patients with rheumatoid arthritis. Fundam. Clin. Pharmacol. 2021, 35, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Bejan-Angoulvant, T.; Passot, C.; Mulleman, D.; Paintaud, G. Clinical Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies Approved to Treat Rheumatoid Arthritis. Clin. Pharmacokinet. 2015, 54, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Kneepkens, E.L.; van den Oever, I.; Plasencia, C.H.; Pascual-Salcedo, D.; De Vries, A.; Hart, M.; Nurmohamed, M.T.; Balsa, A.; Rispens, T.; Wolbink, G.J. Serum tocilizumab trough concentration can be used to monitor systemic IL-6 receptor blockade in patients with rheumatoid arthritis: A prospective observational cohort study. Scand. J. Rheumatol. 2016, 46, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; Hsu, J.C.; Lu, P.; Fettner, S.; Zhang, X.; Douglass, W.; Bao, M.; Rowell, L.; Burmester, G.R.; Kivitz, A. Pharmacokinetic and Pharmacodynamic Analysis of Subcutaneous Tocilizumab in Patients with Rheumatoid Arthritis From 2 Randomized, Controlled Trials: SUMMACTA and BREVACTA. J. Clin. Pharmacol. 2016, 57, 459–468. [Google Scholar] [CrossRef]
- Benucci, M.; Meacci, F.; Grossi, V.; Infantino, M.; Manfredi, M.; Bellio, E.; Bellio, V.; Gobbi, F.L.; Bazzichi, L.; Paolo, P.M.; et al. Correlations between immunogenicity, drug levels, and disease activity in an Italian cohort of rheumatoid arthritis patients treated with tocilizumab. Biol. Targets Ther. 2016, 10, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Willeman, T.; Jourdil, J.-F.; Gautier-Veyret, E.; Bonaz, B.; Stanke-Labesque, F. A multiplex liquid chromatography tandem mass spectrometry method for the quantification of seven therapeutic monoclonal antibodies: Application for adalimumab therapeutic drug monitoring in patients with Crohn’s disease. Anal. Chim. Acta 2019, 1067, 63–70. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Calculating correlation coefficients with repeated observations: Part 1-correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.; Grange, S.; Woodworth, T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J. Clin. Pharmacol. 2010, 50, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Sigaux, J.; Hamze, M.; Daien, C.; Morel, J.; Krzysiek, R.; Pallardy, M.; Maillere, B.; Mariette, X.; Miceli-Richard, C. Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Jt. Bone Spine 2017, 84, 39–45. [Google Scholar] [CrossRef]
- Arad, U.; Elkayam, O. Association of Serum Tocilizumab Trough Concentrations with Clinical Disease Activity Index Scores in Adult Patients with Rheumatoid Arthritis. J. Rheumatol. 2019, 46, 1577–1581. [Google Scholar] [CrossRef]
- Ruperto, N.; Brunner, H.I.; Ramanan, A.V.; Horneff, G.; Cuttica, R.; Henrickson, M.; Anton, J.; Boteanu, A.L.; Penades, I.C.; Minden, K.; et al. Subcutaneous dosing regimens of tocilizumab in children with systemic or polyarticular juvenile idiopathic arthritis. Rheumatology 2021, 60, 4568–4580. [Google Scholar] [CrossRef] [PubMed]
- Bastida, C.; Soy, D.; Ruiz-Esquide, V.; Sanmartí, R.; Huitema, A.D. Exposure-response modeling of tocilizumab in rheumatoid arthritis using continuous composite measures and their individual components. Br. J. Clin. Pharmacol. 2019, 85, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Torne, C.; Ortiz, M.D.A.; Moya, P.; Hernandez, M.V.; Reina, D.; Castellvi, I.; De Agustin, J.J.; de la Fuente, D.; Corominas, H.; Sanmarti, R.; et al. The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin. Arthritis Rheum. 2018, 47, 757–764. [Google Scholar] [CrossRef] [PubMed]

| Characteristics at Tocilizumab Initiation | Values |
|---|---|
| Female | 3 (30) |
| Age, years | 52 (34–61) |
| Body-mass index, kg/m2 | 24.0 (22.2–26.5) |
| Weight, kg | 70.5 (60.0–74.0) |
| Etiology of end-stage renal disease | |
| Glomerulonephritis | 5 (50) |
| Uropathy | 2 (20) |
| Others | 3 (30) |
| Number of subjects with previous transplant | 4 (40) |
| Number of tocilizumab infusions | 9 (7–10) |
| Creatinine, µmol·L−1 | 867 (795–1058) |
| IL-6, pg·mL−1 | 26.8 (6.2–544) |
| sIL-6R, pg·mL−1 | 61,786 (56,820–87,334) |
| Number of antibodies with an initial MFI > 3000 per patient, class 1 | 25.5 (6.5–47) |
| Number of antibodies with an initial MFI > 3000 per patient, class 2 | 26.5 (12.2–37.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truffot, A.; Jouve, T.; Noble, J.; Bardy, B.; Malvezzi, P.; Rostaing, L.; Stanke-Labesque, F.; Gautier-Veyret, E. Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization. J. Clin. Med. 2022, 11, 91. https://doi.org/10.3390/jcm11010091
Truffot A, Jouve T, Noble J, Bardy B, Malvezzi P, Rostaing L, Stanke-Labesque F, Gautier-Veyret E. Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization. Journal of Clinical Medicine. 2022; 11(1):91. https://doi.org/10.3390/jcm11010091
Chicago/Turabian StyleTruffot, Aurélie, Thomas Jouve, Johan Noble, Béatrice Bardy, Paolo Malvezzi, Lionel Rostaing, Françoise Stanke-Labesque, and Elodie Gautier-Veyret. 2022. "Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization" Journal of Clinical Medicine 11, no. 1: 91. https://doi.org/10.3390/jcm11010091
APA StyleTruffot, A., Jouve, T., Noble, J., Bardy, B., Malvezzi, P., Rostaing, L., Stanke-Labesque, F., & Gautier-Veyret, E. (2022). Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization. Journal of Clinical Medicine, 11(1), 91. https://doi.org/10.3390/jcm11010091








