Lead Dependent Tricuspid Valve Dysfunction-Risk Factors, Improvement after Transvenous Lead Extraction and Long-Term Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Baseline Parameters
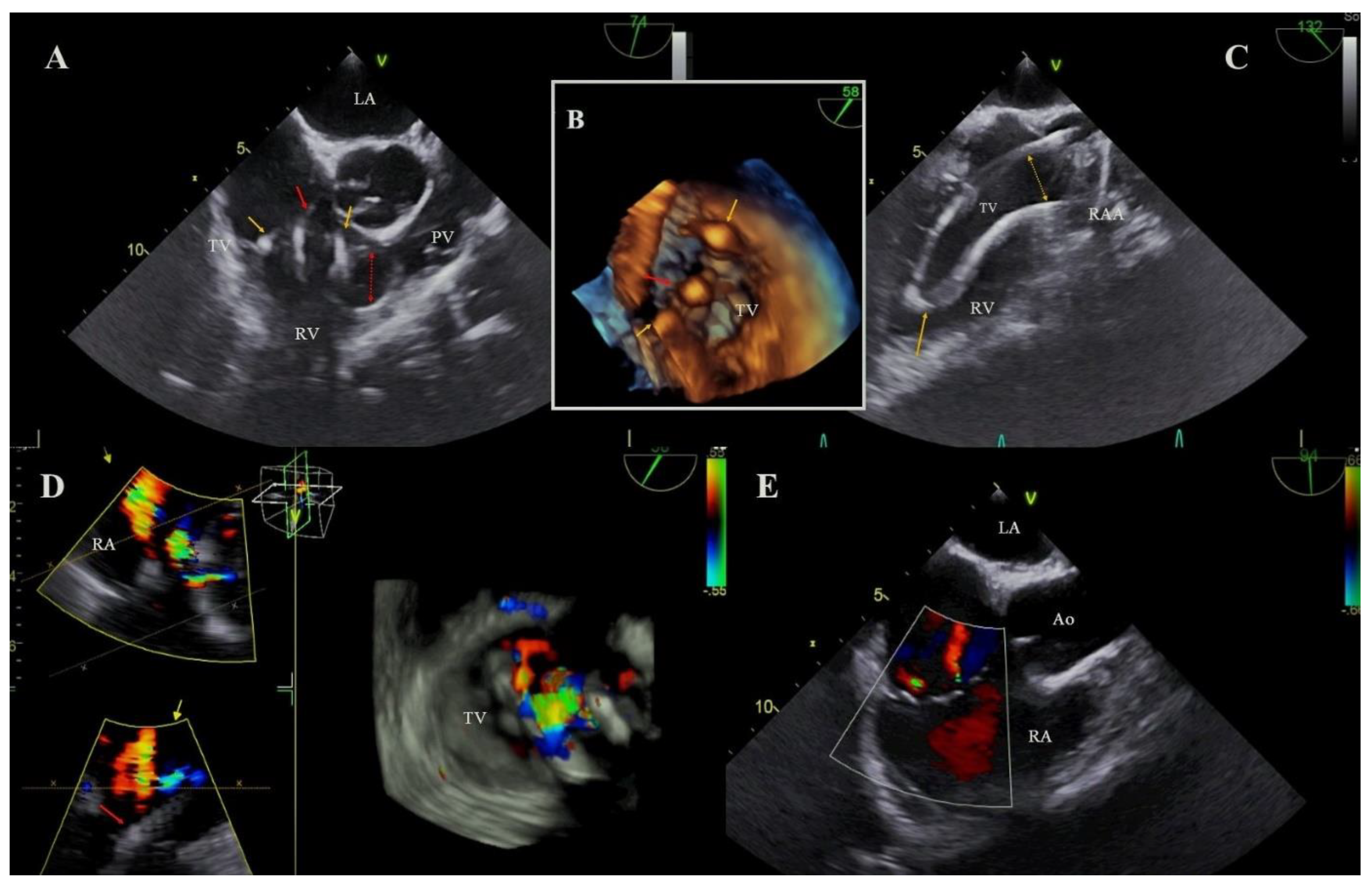
2.3. Echocardiography
2.4. Definitions
2.5. Transvenous Lead Extraction Procedure
2.6. Indications for Transvenous Lead Extraction in Whole Examined Population of Patients
2.7. Statistical Analysis
2.8. Approval of the Bioethics Committee
3. Results
3.1. Baseline Characteristics
3.2. Pacing System and TLE-Related Factors
3.3. Echocardiographic Findings
3.4. Univariate and Multivariate Logistic Regression of Risk Factors for LDTVD
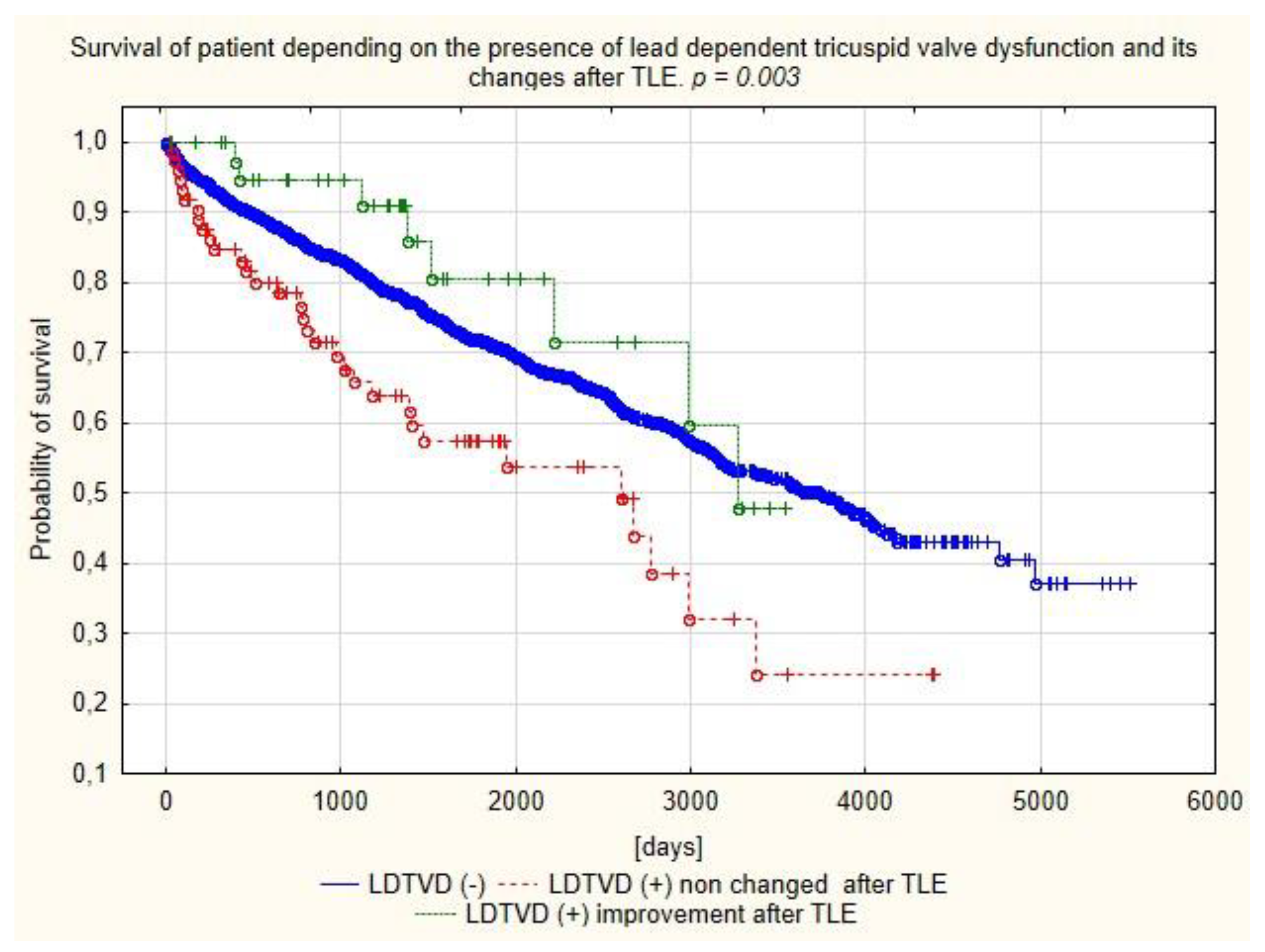
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nachnani, G.H.; Gooch, A.S.; Hsu, I. Systolic Murmurs Induced by Pacemaker Catheters. Arch. Intern. Med. 1969, 124, 202–205. [Google Scholar] [CrossRef]
- Gould, L.; Reddy, C.V.; Yacob, U.; Teich, M.; DeMartino, A.; DePalma, D.; Gomprecht, R.F. Perforation of the tricuspid valve by a transvenous pacemaker. JAMA 1974, 230, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Aldrich, H.R.; Lieberman, E.H.; Lamas, G.A.; Agatston, A.S. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am. J. Cardiol. 1998, 82, 1130–1132. [Google Scholar] [CrossRef]
- De Cock, C.C.; Vinkers, M.; Van Campe, L.C.; Verhorst, P.M.; Visser, C.A. Long-term outcome of patients with multiple (≥3) noninfected transvenous leads: A clinical and echocardiographic study. Pacing Clin. Electrophysiol. 2000, 23, 423–426. [Google Scholar] [CrossRef]
- Klutstein, M.; Balkin, J.; Butnaru, A.; Ilan, M.; Lahad, A.; Rosenmann, D. Tricuspid incompetence following permanent pacemaker implantation. Pacing Clin. Electrophysiol. 2009, 32 (Suppl. S1), S135–S137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Spevack, D.M.; Tunick, P.A.; Bullinga, J.R.; Kronzon, I.; Chinitz, L.A.; Reynolds, H.R. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: An observational study. J. Am. Soc. Echocardiogr. 2008, 21, 284–287. [Google Scholar] [CrossRef]
- Al-Bawardy, R.; Krishnaswamy, A.; Rajeswaran, J.; Bhargava, M.; Wazni, O.; Wilkoff, B.; Tuzcu, E.M.; Martin, D.; Thomas, J.; Blackstone, E.; et al. Tricuspid regurgitation and implantable devices. Pacing Clin. Electrophysiol. 2015, 38, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Friedman, S.E.; Kono, A.T.; Greenberg, M.L.; Palac, R.T. Tricuspid Regurgitation Following Implantation of Endocardial Leads: Incidence and Predictors. Pacing Clin. Electrophysiol. 2015, 38, 1267–1274. [Google Scholar] [CrossRef]
- Saito, M.; Iannaccone, A.; Kaye, G.; Negishi, K.; Kosmala, W.; Marwick, T.H.; PROTECT-PACE Investigator. Effect of Right Ventricular Pacing on Right Ventricular Mechanics and Tricuspid Regurgitation in Patients With High-Grade Atrioventricular Block and Sinus Rhythm (from the Protection of Left Ventricular Function During Right Ventricular Pacing Study). Am. J. Cardiol. 2015, 116, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Fanari, Z.; Hammami, S.; Hammami, M.B.; Shuraih, M. The effects of right ventricular apical pacing with transvenous pacemaker and implantable cardioverter defibrillator on mitral and tricuspid regurgitation. J. Electrocardiol. 2015, 48, 791–797. [Google Scholar] [CrossRef]
- Arabi, P.; Özer, N.; Ateş, A.H.; Yorgun, H.; Oto, A.; Aytemir, K. Effects of pacemaker and implantable cardioverter defibrillator electrodes on tricuspid regurgitation and right sided heart functions. Cardiol. J. 2015, 22, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Leibowitz, D.W.; Rosenheck, S.; Pollak, A.; Geist, M.; Gilon, D. Transvenous pacemaker leads do not worsen tricuspid regurgitation: A prospective echocardiographic study. Cardiology 2000, 93, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Kucukarslan, N.; Kirilmaz, A.; Ulusoy, E.; Yokusoglu, M.; Gramatnikovski, N.; Ozal, E.; Tatar, H. Tricuspid insufficiency does not increase early after permanent implantation of pacemaker leads. J. Card. Surg. 2006, 21, 391–394. [Google Scholar] [CrossRef]
- Webster, G.; Margossian, R.; Alexander, M.E.; Cecchin, F.; Triedman, J.K.; Walsh, E.P.; Berul, C.I. Impact of transvenous ventricular pacing leads on tricuspid regurgitation in pediatric and congenital heart disease patients. J. Interv. Card. Electrophysiol. 2008, 21, 65–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.; Kim, D.Y.; Cho, I.; Hong, G.R.; Ha, J.W.; Shim, C.Y. Prevalence, predictors, and prognosis of tricuspid regurgitation following permanent pacemaker implantation. PLoS ONE 2020, 15, e0235230. [Google Scholar] [CrossRef]
- Riesenhuber, M.; Spannbauer, A.; Gwechenberger, M.; Pezawas, T.; Schukro, C.; Stix, G.; Schneider, M.; Goliasch, G.; Anvari, A.; Wrba, T.; et al. Pacemaker lead-associated tricuspid regurgitation in patients with or without pre-existing right ventricular dilatation. Clin. Res. Cardiol. 2021, 110, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Fang, H.Y.; Chen, H.C.; Chen, Y.L.; Tsai, T.H.; Pan, K.L.; Lin, Y.S.; Liu, W.H.; Chen, M.C. Progressive tricuspid regurgitation and elevated pressure gradient after transvenous permanent pacemaker implantation. Clin. Cardiol. 2021, 44, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017, 14, e503–e551. [Google Scholar] [CrossRef] [Green Version]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohaissen, M.A.; Chan, K.L. Tricuspid Regurgitation Following Implantation of a Pacemaker/Cardioverter-Defibrillator. Curr. Cardiol. Rep. 2013, 15, 357. [Google Scholar] [CrossRef] [PubMed]
- Anvardeen, K.; Rao, R.; Hazra, S.; Hay, K.; Dai, H.; Stoyanov, N.; Birnie, D.; Dwivedi, G.; Chan, K.L. Lead-Specific Features Predisposing to the Development of Tricuspid Regurgitation After Endocardial Lead Implantation. CJC Open 2019, 31, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Polewczyk, A.; Kutarski, A.; Tomaszewski, A.; Brzozowski, W.; Czajkowski, M.; Polewczyk, M.; Janion, M. Lead dependent tricuspid dysfunction: Analysis of the mechanism and management in patients referred for transvenous lead extraction. Cardiol. J. 2013, 20, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Najib, M.Q.; Vittala, S.S.; Challa, S.; Raizada, A.; Tondato, F.J.; Lee, H.R.; Chaliki, H.P. Predictors of severe tricuspid regurgitation in patients with permanent pacemaker or automatic implantable cardioverter-defibrillator leads. Tex. Heart Inst. J. 2013, 40, 529–533. [Google Scholar]
- Mediratta, A.; Addetia, K.; Yamat, M.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Weinert, L.; Maffessanti, F.; Jeevanandam, V.; Mor-Avi, V.; et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc. Imaging 2014, 7, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Addetia, K.; Maffessanti, F.; Mediratta, A.; Yamat, M.; Weinert, L.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Patel, A.R.; Kruse, E.; et al. Impact of implantable transvenous device lead location on severity of tricuspid regurgitation. J. Am. Soc. Echocardiogr. 2014, 27, 1164–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.X.; Qian, J.; Hou, F.Q.; Liu, Y.N.; Mao, J.H. Comparison of right ventricular apex and right ventricular outflow tract septum pacing in the elderly with normal left ventricular ejection fraction: Long-term follow-up. Kardiol. Pol. 2012, 70, 1130–1139. [Google Scholar]
- Schleifer, J.W.; Pislaru, S.V.; Lin, G.; Powell, B.D.; Espinosa, R.; Koestler, C.; Thome, T.; Polk, L.; Li, Z.; Asirvatham, S.J.; et al. Effect of ventricular pacing lead position on tricuspid regurgitation: A randomized prospective trial. Heart Rhythm. 2018, 15, 1009–1016. [Google Scholar] [CrossRef]
- Nazmul, M.N.; Cha, Y.M.; Lin, G.; Asirvatham, S.J.; Powell, B.D. Percutaneous pacemaker or implantable cardioverter-defibrillator lead removal in an attempt to improve symptomatic tricuspid regurgitation. Europace 2013, 15, 409–413. [Google Scholar] [CrossRef]
- Migliore, F.; Zorzi, A.; Bertaglia, E.; Leoni, L.; Siciliano, M.; De Lazzari, M.; Ignatiuk, B.; Veronese, M.; Verlato, R.; Tarantini, G.; et al. Incidence, management, and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin. Electrophysiol. 2014, 37, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Höke, U.; Auger, D.; Thijssen, J.; Wolterbeek, R.; van der Velde, E.T.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart 2014, 100, 960–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delling, F.N.; Hassan, Z.K.; Piatkowski, G.; Tsao, C.W.; Rajabali, A.; Markson, L.J.; Zimetbaum, P.J.; Manning, W.J.; Chang, J.D.; Mukamal, K.J. Tricuspid regurgitation and mortality in patients with transvenous permanent pacemaker leads. Am. J. Cardiol. 2016, 117, 988–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Prelininary and verifired diagnosis of LDTVD | Effect of TLE on severity of TR in pts with LDTVD impression of echocardioigraphist | ||||
| Preliminary diagnosis of LDTVD | 125 | 4.90% | Insignificant | 73 | 61.34% |
| Confirmed diagnosis of LDTVD | 119 | 4.44% | Perceptible | 33 | 27.73% |
| All patients | 2678 | 100.00% | Significant | 13 | 10.92% |
| Main/predominant Indications for lead extraction in patient with LDTVD | All LDTVD patients | 119 | 100.00% | ||
| Symptomatic lead dependent TV dysfunction | 45 | 37.82% | |||
| Lead damage/dysfunction (lead replacement) | 39 | 32.78% | Changes of degree of TR after TLE (degrees) | ||
| Systemic infection or local or mixed infection | 21 | 17.65% | No change (the same) | 77 | 64.71% |
| Upgrading, downgrading, prevention of lead abandonment | 11 | 9.24% | Reduction of TR for 1 degree | 38 | 31.93% |
| Recapture venous access (symptomatic occlusion, lead replacement/upgrading) | 3 | 2.52% | Reduction of TR for 2 degrees | 4 | 3.36% |
| All LDTVD patients | 119 | 100.00% | All LDTVD patients | 119 | 100.00% |
| Mechanism of TV dysfunction (partial immobilisation of the leaf or irritation causing degeneration) | Average right ventricular lead dwell time | 104.57 | SD 69.8 | ||
| Propping upward the leaflet by the lead | 45 | 37.82% | LDTVD and cardiac surgery after TLE | ||
| Drawing down f the leaflet by the lead (immobilisation) | 57 | 47.90% | Indication reached—observation | 22 | 18.49% |
| Impingement of the leaflet by the lead presence (irritation) | 3 | 2.52% | No indication—observation only | 74 | 62.19% |
| Perforation of the leaflet with the lead | 3 | 2.52% | Referred for TV plastic | 18 | 15.13% |
| Connection of lead with the lead with scar | 11 | 9.24% | Not considered (contraindication, lack of agreement) | 5 | 4.20% |
| All LDTVD patients | 119 | 100.00% | ALL patients with LDTVD | 119 | 100.00% |
| Patient-Related Potential Risk Factors of LDTVD | LDTVD (All Patients with LDTVD) | NO LDTVD (Control Group) | |||
|---|---|---|---|---|---|
| Number of patients/number of the group | 119 | 1 | 2559 | 2 | p |
| Presented values | Count/average | %/Sd | Count/average | %/Sd | 1 vs. 2 |
| Patient’s age during TLE | 68.09 | 15.05 | 66.90 | 14.52 | 0.087 |
| Patient’s age during first system implantation | 58.43 | 18.96 | 58.32 | 16.18 | 0.485 |
| Sex (% of female patients) | 67 | 56.30% | 995 | 38.88% | 0.001 |
| Baseline heart diseases: IHD, MI | 69 | 57.98% | 1494 | 58.38% | 0.995 |
| Baseline heart diseases: primary cardiomyopathy | 13 | 10.92% | 343 | 13.40% | 0.562 |
| Baseline heart diseases: valvular heart disease | 10 | 8.40% | 59 | 2.31% | 0.001 |
| Baseline heart diseases: post-inflammatory, congenital, channelopathies, neurocardiogenic, unknown | 27 | 22.69% | 662 | 25.87% | 0.566 |
| NYHA class I–IV | 2.20 | 0.80 | 1.84 | 0.67 | <0.001 |
| AF permanent | 50 | 42.02% | 565 | 22.08% | 0.001 |
| Hypertension | 66 | 55.46% | 1487 | 58.11% | 0.777 |
| Diabetes (any) | 21 | 17.65% | 534 | 20.87% | 0.515 |
| Renal failure (any) | 34 | 28.57% | 538 | 21.02% | 0.052 |
| Valvular implant presence | 83 | 18.49% | 156 | 6.10% | 0.001 |
| Mechanical valve presence | 15 | 12.61% | 15 | 3.59% | 0.001 |
| Previous sternotomy | 24 | 20.17% | 359 | 14.03% | 0.07 |
| Long-term anticoagulation | 73 | 61.35% | 1000 | 39.08% | 0.001 |
| Charlson’s index (points) | 4.99 | 3.56 | 4.83 | 3.69 | 0.352 |
| LDTVD (All Patients with LDTVD) | NO LDTVD (Control Group) | ||||
|---|---|---|---|---|---|
| Number of Patients Number of the Group | 119 | 1 | 2559 | 2 | p |
| Presented Values | Count/Average | %/ Sd | Count/Average | %/Sd | 1 vs. 2 |
| TLE Indications | |||||
| LRIE certain with or without pocket infection | 13 | 10.92% | 458 | 17.90% | 0.078 |
| LRIE probable with or without pocket infection | 4 | 3.36% | 159 | 6.21% | 0.279 |
| Local/isolated pocket infection | 4 | 3.36% | 206 | 8.05% | 0.099 |
| All infections | 21 | 17.65% | 823 | 32.16% | 0.002 |
| Non-infectious prophylactic indications | 5 | 4.20% | 86 | 3.36% | 0.788 |
| Non-infectious therapeutic indications | 93 | 78.15% | 1650 | 64.48% | 0.002 |
| All non-infectious indications | 98 | 82.35% | 1736 | 67.84% | 0.001 |
| System and history of pacing | |||||
| Device type—PM (AAI, VVI, DDD, CRT-P) | 100 | 84.03% | 1786 | 69.79% | 0.001 |
| Device type—ICD (VVI, DDD) | 9 | 7.56% | 577 | 22.55% | 0.001 |
| Device type—CRT-D | 10 | 8.40% | 196 | 7.66% | 0.865 |
| Number of leads in the system before TLE | 1.84 | 0.70 | 1.82 | 0.62 | 0.971 |
| Presence of abandoned lead before TLE | 16 | 13.45% | 270 | 10.55% | 0.363 |
| Number of abandoned leads before TLE | 0.21 | 0.60 | 0.14 | 0.43 | 0.636 |
| Number of leads in the heart before TLE | 2.03 | 0.82 | 1.95 | 0.73 | 0.459 |
| 4 and >4 leads before TLE | 7 | 5.88% | 73 | 2.85% | 0.096 |
| One ICD lead before TLE | 18 | 15.13% | 762 | 29.78% | 0.001 |
| 2 or more ICD leads before TLE | 2 | 1.68% | 18 | 7.03% | 0.493 |
| Apical RV lead location (lead analysis) | 101 | 41.91% | 2058 | 41.39% | 0.166 |
| Out of apical (septal, outfow tract, anterior wall) RV lead location (lead analysis) | 30 | 12.45% | 483 | 9.71% | 0.092 |
| Previous TLE in history | 8 | 6.72% | 135 | 5.28% | 0.603 |
| Upgrading or downgrading with lead abandonment | 11 | 9.24% | 158 | 6.17% | 0.229 |
| Excessive long lead loop in the atrium (fluoroscopy) | 18 | 15.13% | 311 | 12.15% | 0.374 |
| Excessive lead loop crossing TV or in the ventricle (fluoroscopy)—A | 24 | 20.17% | 116 | 4.53% | 0.001 |
| Fluoroscopic impression of lead collision with TV (without loop) to tense or to long—B | 25 | 21.01% | 15 | 0.59% | 0.001 |
| Fluoroscopic impression of lead loop collision with TV—C | 25 | 21.01% | 115 | 4.49% | 0.001 |
| All lead’s collision with TV (A + B + C) | 74 | 62.19% | 246 | 9.61% | 0.001 |
| Dwell time of oldest one lead in the patient before TLE | 116.82 | 81.90 | 103.73 | 75.64 | 0.106 |
| Mean lead dwell time (in the patient) before TLE (in months) | 107.73 | 68.90 | 95.83 | 66.98 | 0.058 |
| TLE Procedure Complicity, Efficacy, Complications, Outcomes and Long-Term Mortality after TLE | LDTVD (All Patients with LDTVD) | NO LDTVD (Control Group) | |||
|---|---|---|---|---|---|
| Number of Patients Number of the Group | 119 | 1 | 2559 | 2 | p |
| Presented Values | Count/Average | %/Sd | Count/Average | %/Sd | 1 vs. 2 |
| TLE procedure complexity | |||||
| Procedure duration (sheath to sheath) | 29.66 | 35.90 | 14.44 | 21.60 | 0.242 |
| Average time of single lead extraction (sheath-to sheath/number of extracted leads) | 11.05 | 17.24 | 8.64 | 12.03 | 0.512 |
| Technical problem during TLE (any) | 29 | 24.37% | 531 | 20.75% | 0.356 |
| Number of big technical problems | 1.71 | 1.15 | 1.34 | 0.65 | 0.153 |
| One technical problem only | 16 | 13.45% | 322 | 12.58% | 0.842 |
| Two or more technical Problems | 12 | 10.08% | 113 | 4.42% | 0.007 |
| Utility of additional tools | |||||
| Evolution (old and new) or TighRail | 6 | 5.04% | 38 | 1.49% | 0.008 |
| Lasso catheter/snare | 9 | 7.56% | 94 | 3.67% | 0.050 |
| Basket catheter | 1 | 0.84% | 19 | 0.74% | 0.683 |
| TLE efficacy and complications | |||||
| Major complications (any) | 1 | 0.84% | 50 | 1.95% | 0.613 |
| Hemopericardium | 1 | 0.84% | 28 | 1.09% | 0.834 |
| Haemothorax | 0 | 0.00% | 3 | 0.31% | 0.298 |
| Tricuspid valve damage (significant) during TLE | 0 | 0.00% | 17 | 0.66% | 0.771 |
| Rescue cardiac surgery | 0 | 0.00% | 23 | 0.90% | 0.604 |
| Minor complications (any) | 14 | 11.76% | 204 | 7.98% | 0.172 |
| Death procedure related (intra, post-procedural) | 0 | 0.00% | 0 | 0.00% | N |
| Death indication-related (intra, post-procedural) | 0 | 0.00% | 1 | 0.04% | 0.026 |
| Partial radiological success (remained tip or < 4 cm lead fragment) | 10 | 8.40% | 93 | 3.63% | 0.014 |
| Full clinical success | 118 | 99.16% | 2504 | 97.85% | 0.17 |
| Full procedural success | 108 | 90.76% | 2446 | 95.58% | 0.191 |
| Long-term mortality after TLE | |||||
| Alive during 1658 ± 1203 (1–5519) days of follow up | 78 | 65.55% | 1796 | 70.18% | 0.329 |
| Death during all 1658 ± 1203 (1–5519) days of follow up | 41 | 34.45% | 763 | 29.82% | 0.329 |
| Echocardiographic Findings/Abnormalities Recorded in Patients with or without LDTVD | LDTVD (All Patients with LDTVD) | NO LDTVD (Control Group) | |||
|---|---|---|---|---|---|
| Number of Patients Number of the Group | 119 | 1 | 2559 | 2 | p |
| Presented Values | Count/Average | %/Sd | Count/Average | %/Sd | 1 vs. 2 |
| Echocardiography before and after TLE | |||||
| Average LVEF | 48.58 | 13.27 | 49.53 | 15.46 | 0.153 |
| Mitral regurgitation (significant) | 24/112 | 21.43% | 352/2528 | 13.92% | 0.037 |
| PASP (mmHg) | 40.73 | 13.41 | 30.49 | 13.16 | 0.001 |
| RV diameter (mm) | 35.51 | 7.65 | 31.24 | 6.00 | 0.001 |
| Tricuspid Regurgitation before TLE | |||||
| Non-significant (0, 1, 2 grade ) | 14/119 | 11.77% | 2123/2553 | 83.16% | 0.001 |
| Significant (3 grade) | 48/119 | 40.34% | 360/2553 | 14.10% | 0.001 |
| Severe (4 grade) | 57/119 | 47.90% | 70/2553 | 2.74% | 0.001 |
| Any shadows on the leads before TLE | |||||
| Any shadows on leads before TLE | 53/119 | 52.94% | 1264/2554 | 49.39% | 0.336 |
| Connecting tissue surrounding the lead | 7/119 | 5.88% | 262/2557 | 10.25% | 0.164 |
| Blood cloth on the lead | 11/119 | 9.24% | 164/2557 | 6.41% | 0.336 |
| Vegetation-like mass | 1/119 | 0.84% | 107/2557 | 4.18% | 0.116 |
| Thicker lead | 26/119 | 21.85% | 470/2557 | 18.38% | 0.406 |
| Vegetation | 16/119 | 13.45% | 450/2556 | 17.61% | 0.296 |
| Strong connective tissue scar connection of the lead with heart structures (any) | 28/115 | 24.35% | 315/2498 | 12.61% | 0.001 |
| Strong connective tissue scar connection of the lead with tricuspid apparatus | 17/119 | 14.29% | 124/2557 | 4.85% | 0.001 |
| Strong connective tissue scar connection of the lead with RA wall | 11/119 | 9.24% | 99/2557 | 3.87% | 0.008 |
| Strong connective tissue scar connection of the lead with SVC | 5/119 | 4.20% | 104/2557 | 4.07% | 0.869 |
| Strong connective tissue scar connection of the lead with RV wall | 14/119 | 11.77% | 157/2557 | 6.14% | 0.073 |
| Loops of the leads | |||||
| Excessive loops of the leads in the heart (any)/ECHO | 43/119 | 36.13% | 450/2558 | 17.59% | 0.001 |
| Excessive loop in the RA | 27/119 | 22.69% | 331/2558 | 12.94% | 0.001 |
| Excessive loop in the TV | 27/119 | 22.69% | 94/2558 | 2.68% | 0.001 |
| Excessive loop in the RV | 20/119 | 16.81% | 124/2558 | 4.85% | 0.001 |
| 19 LDTVD vs. Other (2559) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Patient’s age during TLE (by year) | 1.008 | 0.995–0.021 | 0.239 | |||
| Female gender | 1.927 | 1.327–1.797 | <0.001 | 2.441 | 1.514–3.937 | <0.001 |
| Baseline heart diseases: valvular heart disease | 3.248 | 1.860–1.669 | <0.001 | 2.755 | 1.153–6.580 | 0.022 |
| NYHA class (by 1) | 2.092 | 1.609–2.720 | <0.001 | 2.060 | 1.443–2.940 | <0.001 |
| PASP (by 1 mm Hg) | 1.047 | 1.035–1.060 | <0.001 | 1.030 | 1.014–1.046 | <0.001 |
| RV diameter (by 1 mm) | 1.099 | 1.073–1.126 | <0.001 | 1.079 | 1.043–1.116 | <0.001 |
| Permanent AF | 2.555 | 1.750–3.730 | <0.001 | 1.776 | 0.985–3.201 | 0.056 |
| Mitral valve insufficiency | 1.897 | 1.214–2.963 | 0.004 | 1.038 | 0.586–1.840 | 0.879 |
| Renal failure (any) | 1.447 | 0.956–2.191 | 0.081 | 1.058 | 0.631–1.773 | 0.831 |
| Valvular implant presence | 2.801 | 1.633–4.804 | <0.001 | 1.045 | 0.431–2.534 | 0.922 |
| Previous sternotomy | 1.377 | 0.855–2.216 | 0.187 | |||
| Long term anticoagulation | 2.254 | 1.547–3.284 | <0.000 | 1.152 | 0.634–2.092 | 0.643 |
| Presence of pacing leads only (AAI VVI VDD DDD CRTP) | 2.116 | 1.321–3.390 | 0.002 | 1.812 | 1.001–3.279 | 0.050 |
| Presence of HV lead(s) (without CRTD) | 0.406 | 0.238–0.693 | <0.001 | |||
| ≥4 leads before TLE | 1.848 | 0.788–4.334 | 0.158 | |||
| Mean lead dwell time before TLE (by year) | 1.030 | 0.999–1.063 | 0.062 | 0.969 | 0.925–1.015 | 0.184 |
| * Lead(s) collision with TV (including excessive loop (s) of lead (s)) | 12.765 | 8.506–19.16 | <0.001 | 15.283 | 9.101–25.663 | <0.001 |
| * Excessive loops of the leads in the heart (any)/ECHO | 2.790 | 1.878–4.143 | <0.001 | |||
| Excessive loop in the RA/ECHO | 2.031 | 1.290–3.197 | 0.002 | |||
| Excessive loop in the TV/ECHO | 7.630 | 4.701–12.385 | <0.001 | |||
| Excessive loop in the RV/ECHO | 4.034 | 2.382–6.830 | <0.001 | |||
| Strong connective tissue scar connection of the lead with heart structures (any) | 2.489 | 1.605–3.861 | <0.001 | |||
| Strong connective tissue scar connection of the lead with tricuspid apparatus | 3.397 | 1.938–5.954 | <0.001 | 2.004 | 0.958–4.196 | 0.065 |
| Strong connective tissue scar connection of the lead with RA wall | 2.934 | 1.520–5.663 | <0.001 | 3.601 | 1.639–7.912 | <0.001 |
| Strong connective tissue scar connection of the lead with RV wall | 2.313 | 1.289–4.151 | 0.005 | 2.151 | 0.992–4.663 | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomaszewski, A.; Brzozowski, W.; Szczęśniak-Stańczyk, D.; Duda, K.; Kutarski, A. Lead Dependent Tricuspid Valve Dysfunction-Risk Factors, Improvement after Transvenous Lead Extraction and Long-Term Prognosis. J. Clin. Med. 2022, 11, 89. https://doi.org/10.3390/jcm11010089
Polewczyk A, Jacheć W, Nowosielecka D, Tomaszewski A, Brzozowski W, Szczęśniak-Stańczyk D, Duda K, Kutarski A. Lead Dependent Tricuspid Valve Dysfunction-Risk Factors, Improvement after Transvenous Lead Extraction and Long-Term Prognosis. Journal of Clinical Medicine. 2022; 11(1):89. https://doi.org/10.3390/jcm11010089
Chicago/Turabian StylePolewczyk, Anna, Wojciech Jacheć, Dorota Nowosielecka, Andrzej Tomaszewski, Wojciech Brzozowski, Dorota Szczęśniak-Stańczyk, Krzysztof Duda, and Andrzej Kutarski. 2022. "Lead Dependent Tricuspid Valve Dysfunction-Risk Factors, Improvement after Transvenous Lead Extraction and Long-Term Prognosis" Journal of Clinical Medicine 11, no. 1: 89. https://doi.org/10.3390/jcm11010089
APA StylePolewczyk, A., Jacheć, W., Nowosielecka, D., Tomaszewski, A., Brzozowski, W., Szczęśniak-Stańczyk, D., Duda, K., & Kutarski, A. (2022). Lead Dependent Tricuspid Valve Dysfunction-Risk Factors, Improvement after Transvenous Lead Extraction and Long-Term Prognosis. Journal of Clinical Medicine, 11(1), 89. https://doi.org/10.3390/jcm11010089






