Neurogenic Bowel Dysfunction in Children and Adolescents
Abstract
1. Introduction
2. Methods
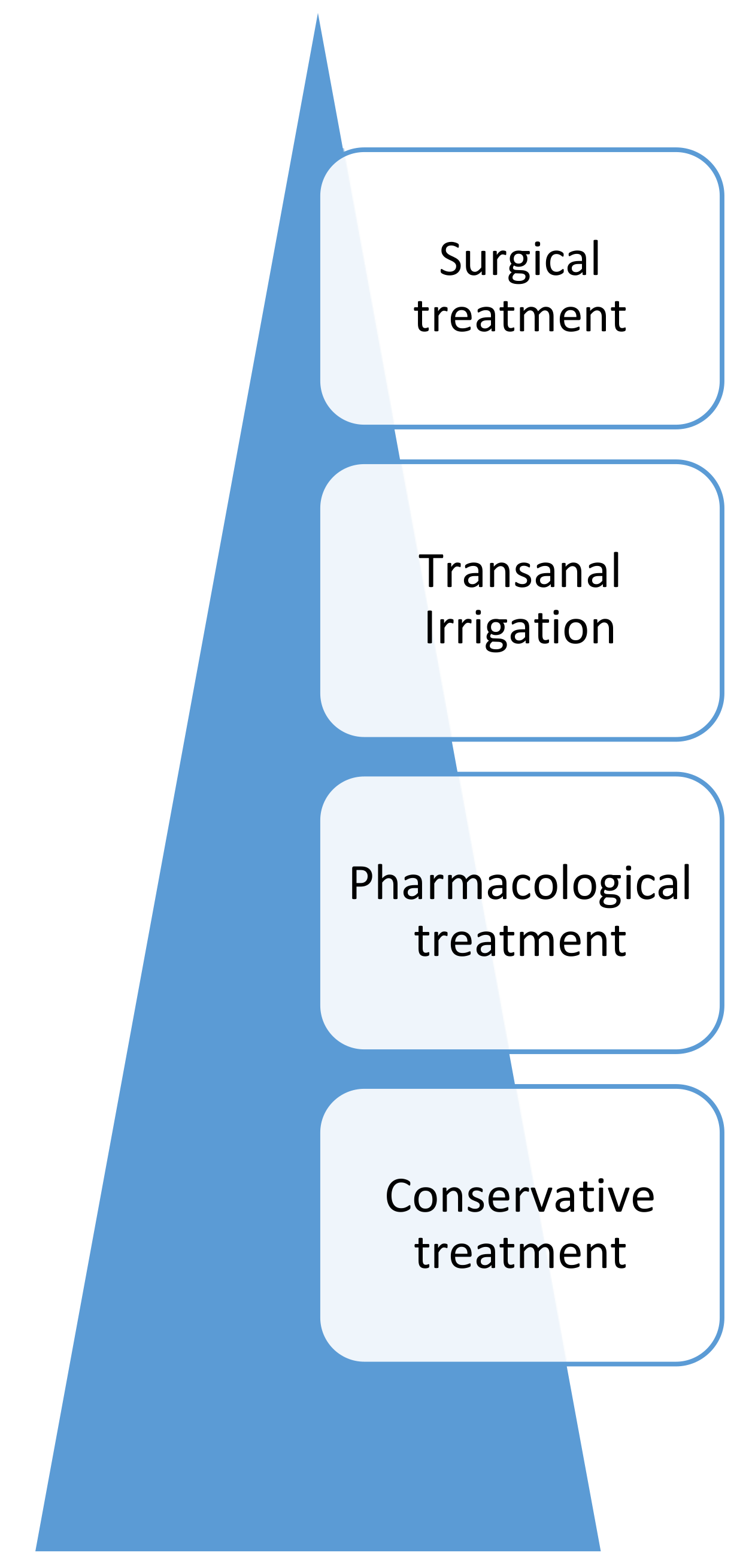
- The causes and pathophysiology of NBD in children and adolescents
- The conservative and medical management of NBD in children and adolescents
- The surgical management of NBD in children and adolescents.
3. Results
3.1. The Causes and Pathophysiology of NBD in Children and Adolescents
3.1.1. Causes
Myelodysplasia
Sacral Agenesis
Anorectal Malformation
Cerebral Palsy
Muscular Dystrophies and Mitochondrial Disorders
Acquired Brain Injury
Acquired Pelvic Injury
Acquired Spinal Cord Injury
Down’s Syndrome
Autism
Transverse Myelitis
Guillain–Barré Syndrome
Cauda Equina Syndrome
Multiple Sclerosis
Acute Disseminated Encephalomyelitis and Meningitis-Retention Syndrome
Spinal Canal Stenosis
Other Rare Pediatric Neurological Disease
Summary
3.1.2. Pathophysiology
Impact of Anatomical Location of Nerve Damage
Tools for Assessment of NBD
3.2. The Conservative and Pharmacological Management of NBD in Children and Adolescents
3.2.1. Starting a Bowel Management Program
3.2.2. The Pediatric Neurogenic Bowel Dysfunction Score
3.2.3. Conservative Treatments
Dietary Patterns, Particularly Fiber
Oral Fluid Intake
Physical Activity
Scheduled Defecation
Maximizing the Gastrocolic Reflex
Positioning
Abdominal Massage
Digital Anorectal Stimulation
Biofeedback and Physiotherapy
Non-invasive Electrical Stimulation
Transcutaneous Electrical Nerve Stimulation
Posterior Tibial Nerve Stimulation
Other Electrostimulation
3.2.4. Pharmacological Treatments
Probiotics
Oral Laxatives
- 2–5 years old: 0.4–1.2 g/day, in 1 or more doses;
- 6–11 years old: 1.2–2.4 g/day, in 1 or more doses;
- 12–18 years old: 2.4–4.8 g/day, in 1 or more doses.
- 2–10 years old: 5 mg once per day;
- 10–18 years old: 5–10 mg once per day.
- 2–6 years old: 2.5–5 mg/day in 1–2 doses;
- 6–12 years old: 7.5–10 mg/day in 1–2 doses;
- 12–18 years old: 15–20 mg/day in 1–2 doses.
Suppositories
Enemas
Transanal Irrigation
- Bulb syringe enemas are used for smaller volume enemas in older infants and young toddlers. The bulb is inserted through the anus and 60–90 mL of warm water can be instilled.
- Balloon enemas use a 24 Fr Foley catheter to administer a high-volume rectal enema. To have the enema administered, the patient must usually lie down, with the catheter’s balloon inflated inside the lower rectum to create a leak-proof seal above the anal canal. The individual must then transfer to the toilet to deflate the balloon and evacuate at the appropriate time, all of which can be quite challenging for a child who may have other disabilities.
- Cone enemas involve insertion of the tip of a graduated silicone cone until it occludes the anus (whether patulous or not) with a water-tight seal. This is simpler, less cumbersome, and somewhat less invasive than a balloon catheter and so may better suit younger children. The cone is connected to enema tubing in a similar manner to balloon enemas. Afterwards, the cone and tubing can easily be washed and re-used, making it relatively inexpensive compared to balloon enemas and specifically designed kits.
- Commercially available transanal irrigation (TAI) systems were designed to speed up the colonic washout process and increase independence in bowel management compared to the previous generic balloon catheter and cone techniques. All incorporate either a customized bag or chamber from where the irrigant solution drains along a tube ending in either a catheter or a cone that is passed through the anus to administer high-volume enemas over several minutes that have been shown on scintigraphy to clear far enough up the colon to render someone reliably clean for a few days [144].
Summary
3.3. The Surgical Management of NBD in Children and Adolescents
3.3.1. Sacral Nerve Modulation
3.3.2. Bowel Surgery
Malone Antegrade Continence Enema Procedure
Tube Cecostomy
Bowel Diversion
Bowel Resection
Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mugie, S.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of constipation in children and adults: A systematic review. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 3–18. [Google Scholar] [CrossRef]
- Rajindrajith, S.; Devanarayana, N.M.; Benninga, M.A. Review article: Faecal incontinence in children: Epidemiology, pathophysiology, clinical evaluation and management. Aliment. Pharmacol. Ther. 2013, 37, 37–48. [Google Scholar] [CrossRef]
- Mugie, S.M.; Di Lorenzo, C.; Benninga, M.A. Constipation in childhood. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M. Neurogenic continence. Part 3: Bowel management strategies. Br. J. Nurs 2008, 17, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Lie, H.R.; Bilenberg, N.; Laurberg, S. Bowel function in Danish children with myelomeningocele. APMIS Suppl. 2003, 109, 81–85. [Google Scholar]
- Midrio, P.; Mosiello, G.; Ausili, E.; Gamba, P.; Marte, A.; Lombardi, L.; Iacobelli, B.D.; Caponcelli, E.; Marrello, S.; Meroni, M.; et al. Peristeen ® transanal irrigation in paediatric patients with anorectal malformations and spinal cord lesions: A multicentre Italian study. Colorectal. Dis. 2016, 18, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Malone, P.S.; Wheeler, R.A.; Williams, J.E. Continence in patients with spina bifida: Long term results. Arch. Dis. Child. 1994, 70, 107–110. [Google Scholar] [CrossRef]
- Shandling, B.; Gilmour, R.F. The enema continence catheter in spina bifida: Successful bowel management. J. Pediatr. Surg. 1987, 22, 271–273. [Google Scholar] [CrossRef]
- Emmanuel, A.V.; Krogh, K.; Bazzocchi, G.; Leroi, A.M.; Bremers, A.; Leder, D.; van Kuppevelt, D.; Mosiello, G.; Vogel, M.; Perrouin-Verbe, B.; et al. Consensus review of best practice of transanal irrigation in adults. Spinal Cord 2013, 51, 732–738. [Google Scholar] [CrossRef]
- Bray, L.; Sanders, C. An evidence-based review of the use of transanal irrigation in children and young people with neurogenic bowel. Spinal Cord 2013, 51, 88–93. [Google Scholar] [CrossRef]
- Faaborg, P.M.; Christensen, P.; Kvitsau, B.; Buntzen, S.; Laurberg, S. Long-term outcome and safety of transanal colonic irrigation for neurogenic bowel dysfunction. Spinal Cord 2009, 47, 545–549. [Google Scholar] [CrossRef]
- Ausili, E.; Marte, A.; Brisighelli, G.; Midrio, P.; Mosiello, G.; La Pergola, E.; Lombardi, L.; Iacobelli, B.D.; Caponcelli, E.; Meroni, M.; et al. Short versus mid-long-term outcome of transanal irrigation in children with spina bifida and anorectal malformations. Childs Nerv. Syst. 2018, 34, 2471–2479. [Google Scholar] [CrossRef]
- Mosiello, G.; Marshall, D.; Rolle, U.; Cretolle, C.; Santacruz, B.G.; Frischer, J.; Benninga, M.A. Consensus review of best practice of transanal irrigation in children. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 343–352. [Google Scholar] [CrossRef]
- Fink, A.; Kosecoff, J.; Chassin, M.; Brook, R.H. Consensus methods: Characteristics and guidelines for use. Am. J. Public Health 1984, 74, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Christensen, P.; Laurberg, S. Colorectal symptoms in patients with neurological diseases. Acta Neurol. Scand. 2001, 103, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Volcik, K.A. Spina bifida and other neural tube defects. Curr. Probl. Pediatr. 2000, 30, 313–332. [Google Scholar] [CrossRef]
- Bauer, S.B. The management of the myelodysplastic child: A paradigm shift. BJU Int. 2003, 92, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Capitanucci, M.L.; Rivosecchi, M.; Silveri, M.; Lucchetti, M.C.; Mosiello, G.; De Gennaro, M. Neurovesical dysfunction due to spinal dysraphism in anorectal anomalies. Eur. J. Pediatr. Surg. 1996, 6, 159–162. [Google Scholar] [CrossRef]
- Rawashdeh, Y.F.; Austin, P.; Siggaard, C.; Bauer, S.B.; Franco, I.; de Jong, T.P.; Jorgensen, T.M. International Children’s Continence Society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol. Urodyn. 2012, 31, 615–620. [Google Scholar] [CrossRef]
- Guzman, L.; Bauer, S.B.; Hallett, M.; Khoshbin, S.; Colodny, A.H.; Retik, A.B. Evaluation and management of children with sacral agenesis. Urology 1983, 22, 506–510. [Google Scholar] [CrossRef]
- Borrelli, M.; Spinola, R.; Bruschini, H.; Walligora, M.; Nahas, W.C.; Freire, G.C.; Figueiredo, J.A.; De Goes, G.M.; Prado, M.J. Sacral agenesis: Why is it so frequently misdiagnosed? Urology 1985, 26, 351–355. [Google Scholar] [CrossRef]
- Mosiello, G.; Capitanucci, M.L.; Gatti, C.; Adorisio, O.; Lucchetti, M.C.; Silveri, M.; Schingo, P.S.M.; De Gennaro, M. How to investigate neurovesical dysfunction in children with anorectal malformations. J. Urol. 2003, 170, 1610–1613. [Google Scholar] [CrossRef]
- Peña, A. Posterior sagittal approach for the correction of anorectal malformations. Adv. Surg. 1986, 19, 69–100. [Google Scholar] [PubMed]
- Emir, H.; Soylet, Y. Neurovesical dysfunction in patients with anorectal malformations. Eur. J. Pediatr. Surg. 1998, 8, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K. Ultrasound examination of the neonatal spine. Australas J. Ultrasound. Med. 2011, 14, 39–41. [Google Scholar] [CrossRef]
- Richards, C.L.; Malouin, F. Cerebral palsy: Definition, assessment and rehabilitation. Handb. Clin. Neurol. 2013, 111, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Oktem, F.; Kisloglu, N.; Demirci, M.; Altuntas, I.; Kutluhan, S.; Dogan, M. Bladder and bowel control in children with cerebral palsy. Croat. Med. J. 2006, 47, 264–270. [Google Scholar]
- Von Wendt, L.; Similä, S.; Niskanen, P.; Järvelin, M.R. Development of bowel and bladder control in the mentally retarded. Dev. Med. Child Neurol. 1990, 32, 515–518. [Google Scholar] [CrossRef]
- Bohmer, C.J.; Taminiau, J.A.; Klinkenberg-Knol, E.C.; Meuwissen, S.G. The prevalence of constipation in institutionalized people with intellectual disability. J. Intellect. Disabil. Res. 2001, 45, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, C.M.; Goetze, O.; Latshang, T.D.; Bluemel, S.; Frauenfelder, T.; Bloch, K.E. Gastrointestinal Dysfunction in Patients with Duchenne Muscular Dystrophy. PLoS ONE 2016, 11, e0163779. [Google Scholar] [CrossRef]
- Crockett, C.D.; Bertrand, L.A.; Cooper, C.S.; Rahhal, R.M.; Liu, K.; Zimmerman, M.B.; Moore, S.A.; Mathews, K.D. Urologic and gastrointestinal symptoms in the dystroglycanopathies. Neurology 2015, 84, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Yadak, R.; Breur, M.; Bugiani, M. Gastrointestinal Dysmotility in MNGIE: From thymidine phosphorylase enzyme deficiency to altered interstitial cells of Cajal. Orphanet J. Rare Dis. 2019, 14, 33. [Google Scholar] [CrossRef]
- Barrett, T.G.; Scott-Brown, M.; Seller, A.; Bednarz, A.; Poulton, K.; Poulton, J. The mitochondrial genome in Wolfram syndrome. J. Med. Genet. 2000, 37, 463–466. [Google Scholar] [CrossRef]
- Chiminello, R.; Mosiello, G.; Castelli, E. Bladder and bowel dysfunctions in children with cerebral palsy and acquired brain injury: Are we able to define incidence and to predict them? Eur. Urol. Suppl. 2018, 17, e1179–e1180. [Google Scholar] [CrossRef]
- Berger, M.; Heinrich, M.; Lacher, M.; Hubertus, J.; Stehr, M.; von Schweinitz, D. Postoperative bladder and rectal function in children with sacro-coccygeal teratoma. Pediatr. Blood Cancer 2011, 56, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Mosiello, G.; Gatti, C.; De Gennaro, M.; Capitanucci, M.; Silveri, M.; Inserra, A.; Milano, G.; De Laurentis, C.; Boglino, C. Neurovesical dysfunction in children after treating pelvic neoplasms. BJU Int. 2003, 92, 289–292. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; O’Brien, C.; O’Connell, P.R.; O’Herlihy, C. Patterns of abnormal pudendal nerve function that are associated with postpartum fecal incontinence. Am. J. Obstet. Gynecol. 2003, 189, 730–735. [Google Scholar] [CrossRef]
- Ng, C.; Prott, G.; Rutkowski, S.; Li, Y.; Hansen, R.; Kellow, J.; Malcolm, A. Gastrointestinal symptoms in spinal cord injury: Relationships with level of injury and psychologic factors. Dis. Colon Rectum 2005, 48, 1562–1568. [Google Scholar] [CrossRef]
- Silveri, M.; Salsano, L.; Pierro, M.M.; Mosiello, G.; Capitanucci, M.L.; De Gennaro, M. Pediatric spinal cord injury: Approach for urological rehabilitation and treatment. J. Pediatr. Urol. 2006, 2, 10–15. [Google Scholar] [CrossRef]
- Valles, M.; Vidal, J.; Clave, P.; Mearin, F. Bowel dysfunction in patients with motor complete spinal cord injury: Clinical, neurological, and pathophysiological associations. Am. J. Gastroenterol. 2006, 101, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Middleton, J.W.; Malcolm, A. Bowel Dysfunction in Spinal Cord Injury. Curr. Gastroenterol. Rep. 2018, 20, 47. [Google Scholar] [CrossRef]
- Bhatt, N.R.; Murchison, L.; Yardy, G.; Kulkarni, M.; Mathur, A.B. Bladder bowel dysfunction in children with Down’s syndrome. Pediatr. Surg. Int. 2020, 36, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Handel, L.N.; Barqawi, A.; Checa, G.; Furness, P.D., 3rd; Koyle, M.A. Males with Down’s syndrome and nonneurogenic neurogenic bladder. J. Urol. 2003, 169, 646–649. [Google Scholar] [CrossRef]
- Gubbiotti, M.; Balboni, G.; Bini, V.; Elisei, S.; Bedetti, C.; Marchiafava, M.; Giannantoni, A. Bladder and Bowel Dysfunction, Adaptive Behaviour, and Psychiatric Profiles in Adults Affected by Autism Spectrum Disorders. Neurourol. Urodyn. 2019, 38, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Pidcock, F.S.; Krishnan, C.; Crawford, T.O.; Salorio, C.F.; Trovato, M.; Kerr, D.A. Acute transverse myelitis in childhood: Center-based analysis of 47 cases. Neurology 2007, 68, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Kalra, V.; Sharma, S.; Sahu, J.; Sankhyan, N.; Chaudhry, R.; Dhawan, B.; Mridula, B. Childhood acute transverse myelitis: Clinical profile, outcome, and association with antiganglioside antibodies. J. Child Neurol. 2009, 24, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, R.; Ikeuchi, Y.; Tomomasa, T.; Ushiku, H.; Ogawa, T.; Morikawa, A. Determinants of prognosis of acute transverse myelitis in children. Pediatr. Int. 2003, 45, 512–516. [Google Scholar] [CrossRef]
- Wolf, V.L.; Lupo, P.J.; Lotze, T.E. Pediatric transverse myelitis. J. Child Neurol. 2012, 27, 1426–1436. [Google Scholar] [CrossRef]
- Hughes, R.A.; Cornblath, D.R. Guillain-Barre syndrome. Lancet 2005, 366, 1653–1666. [Google Scholar] [CrossRef]
- Sawai, S.; Sakakibara, R.; Uchiyama, T.; Liu, Z.; Yamamoto, T.; Ito, T.; Kuwabara, S.; Kanai, K.; Asahina, M.; Yamanaka, T.; et al. Acute motor axonal neuropathy presenting with bowel, bladder, and erectile dysfunction. J. Neurol. 2007, 254, 250–252. [Google Scholar] [CrossRef]
- Zochodne, D.W. Autonomic involvement in Guillain-Barre syndrome: A review. Muscle Nerve 1994, 17, 1145–1155. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Aylott, C.E.; Grevitt, M.P.; Hegarty, J. Cauda equina syndrome: Factors affecting long-term functional and sphincteric outcome. Spine 2007, 32, 207–216. [Google Scholar] [CrossRef]
- Podnar, S. Bowel dysfunction in patients with cauda equina lesions. Eur. J. Neurol. 2006, 13, 1112–1117. [Google Scholar] [CrossRef]
- Podnar, S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve 2007, 35, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, E.; Marriott, J.J.; Jette, N.; Pringsheim, T.; Makhani, N.; Morrow, S.A.; Fisk, J.D.; Evans, C.; Béland, S.G.; Kulaga, S.; et al. Incidence and prevalence of multiple sclerosis in Europe: A systematic review. BMC Neurol. 2013, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Beland, S.G.; Kulaga, S.; Wolfson, C.; Kingwell, E.; Marriott, J.; Koch, M.; Makhani, N.; Morrow, S.; Fisk, J.; et al. Incidence and prevalence of multiple sclerosis in the Americas: A systematic review. Neuroepidemiology 2013, 40, 195–210. [Google Scholar] [CrossRef]
- Mah, J.K.; Thannauser, J.E. Management of multiple sclerosis in adolescents, current treatment options and related adherence issues. Adolesc. Health Med. Ther. 2010, 1, 31–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuntz, N.; Chabas, D.; Weinstock-Guttman, B.; Chitnis, T.; Yeh, E.A.; Krupp, L.; Ness, J.; Rodriguez, M.; Waubant, E. Treatment of multiple sclerosis in children and adolescents. Expert Opin. Pharmacother. 2010, 11, 505–520. [Google Scholar] [CrossRef]
- Nortvedt, M.W.; Riise, T.; Frugard, J.; Mohn, J.; Bakke, A.; Skar, A.B. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult. Scler. 2007, 13, 106–112. [Google Scholar] [CrossRef]
- Munteis, E.; Andreu, M.; Tellez, M.J.; Mon, D.; Ois, A.; Roquer, J. Anorectal dysfunction in multiple sclerosis. Mult. Scler. 2006, 12, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Tenembaum, S.; Chitnis, T.; Ness, J.; Hahn, J.S. International Pediatric MSSG. Acute disseminated encephalomyelitis. Neurology 2007, 68 (Suppl. S2), S23–S36. [Google Scholar] [CrossRef]
- Schwarz, S.; Mohr, A.; Knauth, M.; Wildemann, B.; Storch-Hagenlocher, B. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology 2001, 22, 1313–1318. [Google Scholar] [CrossRef]
- Sakakibara, R.; Uchiyama, T.; Liu, Z.; Yamamoto, T.; Ito, T.; Uzawa, A.; Suenaga, T.; Kanai, K.; Awa, Y.; Sugiyama, Y.; et al. Meningitis-retention syndrome. An unrecognized clinical condition. J. Neurol. 2005, 252, 1495–1499. [Google Scholar] [CrossRef]
- Wender, M. Acute disseminated encephalomyelitis (ADEM). J. Neuroimmunol. 2011, 231, 92–99. [Google Scholar] [CrossRef]
- Goh, K.J.; Khalifa, W.; Anslow, P.; Cadoux-Hudson, T.; Donaghy, M. The clinical syndrome associated with lumbar spinal stenosis. Eur. Neurol. 2004, 52, 242–249. [Google Scholar] [CrossRef]
- Schkrohowsky, J.G.; Hoernschemeyer, D.G.; Carson, B.S.; Ain, M.C. Early presentation of spinal stenosis in achondroplasia. J. Pediatr. Orthop. 2007, 27, 119–122. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Oakes, W.J.; Blount, J.P. Isolated atlantal stenosis in a patient with idiopathic growth hormone deficiency, and Klippel-Feil and Duane’s syndromes. Childs Nerv. Syst. 2005, 21, 421–424. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.J.; Shaw, P.J. Diagnosis and management of motor neurone disease. BMJ 2008, 336, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Szobor, A.; Máttyus, A.; Molnár, J. Myasthenia gravis in childhood and adolescence. Report on 209 patients and review of the literature. Acta Paediatr. Hung. 1988, 29, 299–312. [Google Scholar]
- Silveri, M.; De Gennaro, M.; Gatti, C.; Bizzarri, C.; Mosiello, G.; Cappa, M. Voiding dysfunction in x-linked adrenoleukodystrophy: Symptom score and urodynamic findings. J. Urol. 2004, 171, 2651–2653. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, J.H.; Cho, M.H.; Choi, Y.H.; Kim, S.H.; Im, Y.J.; Park, K.; Kang, H.G.; Chae, J.H.; Cheong, H.I. Urological Problems in Patients with Menkes Disease. J. Korean Med. Sci. 2019, 34, e4. [Google Scholar] [CrossRef]
- Chung, E.A.; Emmanuel, A.V. Gastrointestinal symptoms related to autonomic dysfunction following spinal cord injury. Prog. Brain Res. 2006, 152, 317–333. [Google Scholar] [CrossRef]
- Brookes, S.J.; Dinning, P.G.; Gladman, M.A. Neuroanatomy and physiology of colorectal function and defaecation: From basic science to human clinical studies. Neurogastroenterol. Motil. 2009, 21 (Suppl. S2), 9–19. [Google Scholar] [CrossRef]
- Palit, S.; Lunniss, P.J.; Scott, S.M. The physiology of human defecation. Dig. Dis. Sci. 2012, 57, 1445–1464. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Nielsen, J.; Djurhuus, J.C.; Mosdal, C.; Sabroe, S.; Laurberg, S. Colorectal function in patients with spinal cord lesions. Dis. Colon Rectum 1997, 40, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Leduc, B.E.; Giasson, M.; Favreau-Ethier, M.; Lepage, Y. Colonic transit time after spinal cord injury. J. Spinal Cord Med. 1997, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Mosdal, C.; Laurberg, S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord 2000, 38, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Benninga, M.A. Pathophysiology of pediatric fecal incontinence. Gastroenterology 2004, 126 (Suppl. S1), S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.V.; Chung, E.A.; Kamm, M.A.F.; Middleton, F. Relationship between gut-specific autonomic testing and bowel dysfunction in spinal cord injury patients. Spinal Cord 2009, 47, 623–627. [Google Scholar] [CrossRef][Green Version]
- Banwell, J.G.; Creasey, G.H.; Aggarwal, A.M.; Mortimer, J.T. Management of the neurogenic bowel in patients with spinal cord injury. Urol. Clin. N. Am. 1993, 20, 517–526. [Google Scholar] [CrossRef]
- Camilleri, M.; Bharucha, A.E. Gastrointestinal dysfunction in neurologic disease. Semin. Neurol. 1996, 16, 203–216. [Google Scholar] [CrossRef]
- Stiens, S.A.; Bergman, S.B.; Goetz, L.L. Neurogenic bowel dysfunction after spinal cord injury: Clinical evaluation and rehabilitative management. Arch. Phys. Med. Rehabil. 1997, 78 (Suppl. S3), S86–S102. [Google Scholar] [CrossRef]
- Doolin, E. Bowel management for patients with myelodysplasia. Surg. Clin. N. Am. 2006, 86, 505–514. [Google Scholar] [CrossRef]
- Garman, K.M.; Ficca, M. Managing encopresis in the elementary school setting: The school nurse’s role. J. Sch. Nurs. 2012, 28, 175–180. [Google Scholar] [CrossRef] [PubMed]
- McClurg, D.; Norton, C. What is the best way to manage neurogenic bowel dysfunction? BMJ 2016, 354, i3931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velde, S.V.; Biervliet, S.V.; Bruyne, R.D.; Winckel, M.V. A systematic review on bowel management and the success rate of the various treatment modalities in spina bifida patients. Spinal Cord 2013, 51, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Wide, P.; Glad Mattsson, G.; Drott, P.; Mattsson, S. Independence does not come with the method—Treatment of neurogenic bowel dysfunction in children with myelomeningocele. Acta Paediatr. 2014, 103, 1159–1164. [Google Scholar] [CrossRef]
- Benevento, B.T.; Sipski, M.L. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys. Ther. 2002, 82, 601–612. [Google Scholar] [CrossRef]
- Benninga, M.A.; Tabbers, M.M.; van Rijn, R.R. How to use a plain abdominal radiograph in children with functional defecation disorders. Arch. Dis. Child Educ. Pract. Ed. 2016, 101, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Rhee, P.L. How to interpret a functional or motility test—Colon transit study. J. Neurogastroenterol. Motil. 2012, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Noviello, C.; Cobellis, G.; Papparella, A.; Amici, G.; Martino, A. Role of anorectal manometry in children with severe constipation. Colorectal. Dis. 2009, 11, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Baharestani, M.M.; Ratliff, C.R. Pressure ulcers in neonates and children: An NPUAP white paper. Adv. Skin. Wound Care 2007, 20, 208–220. [Google Scholar] [CrossRef]
- Kewalramani, L.S. Autonomic dysreflexia in traumatic myelopathy. Am. J. Phys. Med. 1980, 59, 1–21. [Google Scholar]
- Krogh, K.; Christensen, P.; Sabroe, S.; Laurberg, S. Neurogenic bowel dysfunction score. Spinal Cord 2006, 44, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Hannan, M.; Cassidy, B.; Hidas, G.; Selby, B.; Khoury, A.E.; McLorie, G. Development, reliability and validation of a neurogenic bowel dysfunction score in pediatric patients with spina bifida. Neurourol. Urodynam. 2016, 35, 212–217. [Google Scholar] [CrossRef]
- Williams, C.L.; Dwyer, J.; Agostoni, C.; Hillemeier, C.; Kimm, S.Y.S.; Kwiterovich, P.O.; McClung, J.; Nicklas, T.A.; Saldanha, L. A Summary of Conference Recommendations on Dietary Fiber in Childhood. Pediatrics 1995, 96, 1023–1028. [Google Scholar]
- Suares, N.C.; Ford, A.C. Systematic review: The effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther. 2011, 33, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Markland, A.D.; Palsson, O.; Goode, P.S.; Burgio, K.L.; Busby-Whitehead, J.; Whitehead, W.E. Association of low dietary intake of fiber and liquids with constipation: Evidence from the National Health and Nutrition Examination Survey. Am. J. Gastroenterol. 2013, 108, 796–803. [Google Scholar] [CrossRef]
- Cameron, K.J.; Nyulasi, I.B.; Collier, G.R.; Brown, D.J. Assessment of the effect of increased dietary fibre intake on bowel function in patients with spinal cord injury. Spinal Cord 1996, 34, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Levitt, M.A.; Bauer, C.; Jackson, L.; Holder, M.; Peña, A. Treatment of fecal incontinence with a comprehensive bowel management program. J. Pediatr. Surg. 2009, 44, 1278–1283. [Google Scholar] [CrossRef]
- Choi, E.K.; Shin, S.H.; Im, Y.J.; Kim, M.J.; Han, S.W. The effects of transanal irrigation as a stepwise bowel management program on the quality of life of children with spina bifida and their caregivers. Spinal Cord 2013, 51, 384–388. [Google Scholar] [CrossRef][Green Version]
- Krassioukov, A.; Eng, J.J.; Claxton, G.; Sakakibara, B.M.; Shum, S. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord 2010, 48, 718–733. [Google Scholar] [CrossRef]
- Lemelle, J.L.; Guillemin, F.; Aubert, D.; Guys, J.M.; Lottmann, H.; Lortat-Jacob, S.; Moscovici, J.; Mouriquand, P.; Ruffion, A.; Schmitt, M. A multicentre study of the management of disorders of defecation in patients with spina bifida. Neurogastroenterol. Motil. 2006, 18, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; p. 638. [Google Scholar]
- Peters, H.P.; De Vries, W.R.; Vanberge-Henegouwen, G.P.; Akkermans, L.M. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Speelman, A.D.; van de Warrenburg, B.P.; van Nimwegen, M.; Petzinger, G.M.; Munneke, M.; Bloem, B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011, 7, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Meshkinpour, H.; Selod, S.; Movahedi, H.; Nami, N.; James, N.; Wilson, A. Effects of regular exercise in management of chronic idiopathic constipation. Dig. Dis. Sci. 1998, 43, 2379–2383. [Google Scholar] [CrossRef]
- Wald, A. Constipation in the primary care setting: Current concepts and misconceptions. Am. J. Med. 2006, 119, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.; Meshkinpour, H.; Vandenberg, K.; James, N.; Cohen, A.; Wilson, A. Effects of exercise on total and segmental colon transit. J. Clin. Gastroenterol. 1993, 16, 300–303. [Google Scholar] [CrossRef]
- Aaronson, M.J.; Freed, M.M.; Burakoff, R. Colonic myoelectric activity in persons with spinal cord injury. Dig. Dis. Sci. 1985, 30, 295–300. [Google Scholar] [CrossRef]
- Menardo, G.; Bausano, G.; Corazziari, E.; Fazio, A.; Marangi, A.; Genta, V.; Marenco, G. Large-bowel transit in paraplegic patients. Dis. Colon Rectum 1987, 30, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.A.; Morren, G.L.; Ryn, A.K.; Hallböök, O. Rectal pressure response to a meal in patients with high spinal cord injury. Arch. Phys. Med. Rehabil. 2003, 84, 108–111. [Google Scholar] [CrossRef]
- Sinclair, M. The use of abdominal massage to treat chronic constipation. J. Bodyw. Mov. Ther. 2011, 15, 436–445. [Google Scholar] [CrossRef]
- Han, T.R.; Kim, J.H.; Kwon, B.S. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord 1998, 36, 485–490. [Google Scholar] [CrossRef]
- Ayaş, S.; Leblebici, B.; Sözay, S.; Bayramoğlu, M.; Niron, E.A. The effect of abdominal massage on bowel function in patients with spinal cord injury. Am. J. Phys. Med. Rehabil. 2006, 85, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.J.; Chen, B.N.; Costa, M.; Humphreys, C.M. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J. Physiol. 1999, 516, 525–538. [Google Scholar] [CrossRef]
- Liu, Z.; Sakakibara, R.; Odaka, T.; Uchiyama, T.; Yamamoto, T.; Ito, T.; Hattori, T. Mechanism of abdominal massage for difficult defecation in a patient with myelopathy (HAM/TSP). J. Neurol. 2005, 252, 1280–1282. [Google Scholar] [CrossRef]
- Lämås, K.; Lindholm, L.; Stenlund, H.; Engström, B.; Jacobsson, C. Effects of abdominal massage in management of constipation—A randomized controlled trial. Int. J. Nurs. Stud. 2009, 46, 759–767. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.J.; Oh, J.; Lee, M.N.; Kim, S.; Kang, K.A. The effects of massage therapy on physical growth and gastrointestinal function in premature infants: A pilot study. J. Child Health Care 2016, 20, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Aşt, T.A. The Effect of Abdominal Massage on Constipation and Quality of Life. Gastroenterol. Nurs. 2016, 39, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A. Recto-colic reflex: Role in the defecation mechanism. Int. Surg. 1996, 81, 292–294. [Google Scholar]
- Whitehead, W.E.; Parker, L.H.; Masek, B.J.; Cataldo, M.F.; Freeman, J.M. Biofeedback treatment of fecal incontinence in patients with myelomeningocele. Dev. Med. Child Neurol. 1981, 23, 313–322. [Google Scholar] [CrossRef]
- Wald, A. Use of biofeedback in treatment of fecal incontinence in patients with meningomyelocele. Pediatrics 1981, 68, 45–49. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Parker, L.; Bosmajian, L.; Morrill-Corbin, E.D.; Middaugh, S.; Garwood, M.; Cataldo, M.F.; Freeman, J. Treatment of fecal incontinence in children with spina bifida: Comparison of biofeedback and behavior modification. Arch. Phys. Med. Rehabil. 1986, 67, 218–224. [Google Scholar]
- Loening-Baucke, V.; Desch, L.; Wolraich, M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Dev. Med. Child Neurol. 1988, 30, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Benninga, M.A.; Bharucha, A.E.; Chiarioni, G.; Di Lorenzo, C.; Whitehead, W.E. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol. Motil. 2015, 27, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.L.; Lordêlo, P.; Farias, T.; Barroso, U., Jr. Evaluation of constipation after parasacral transcutaneous electrical nerve stimulation in children with lower urinary tract dysfunction—A pilot study. J. Pediatr. Urol. 2013, 9, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Ismail, K.A.; Chase, J.; Gibb, S.; Clarke, M.; Catto-Smith, A.G.; Robertson, V.J.; Hutson, J.M.; Southwell, B.R. Daily transabdominal electrical stimulation at home increased defecation in children with slow-transit constipation: A pilot study. J. Pediatr. Surg. 2009, 44, 2388–2392. [Google Scholar] [CrossRef]
- Wright, A.J.; Haddad, M. Electroneurostimulation for the management of bladder bowel dysfunction in childhood. Eur. J. Paediatr. Neurol. 2017, 21, 67–74. [Google Scholar] [CrossRef]
- Mentes, B.B.; Yüksel, O.; Aydin, A.; Tezcaner, T.; Leventoğlu, A.; Aytaç, B. Posterior tibial nerve stimulation for faecal incontinence after partial spinal injury: Preliminary report. Tech. Coloproctol. 2007, 11, 115–119. [Google Scholar] [CrossRef]
- Leroi, A.M.; Siproudhis, L.; Etienney, I.; Damon, H.; Zerbib, F.; Amarenco, G.; Vitton, V.; Faucheron, J.L.; Thomas, C.; Mion, F.; et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: A randomized trial (CONSORT 1a). Am. J. Gastroenterol. 2012, 107, 1888–1896. [Google Scholar] [CrossRef]
- Palmer, L.S.; Richards, I.; Kaplan, W.E. Transrectal electrostimulation therapy for neuropathic bowel dysfunction in children with myelomeningocele. J. Urol. 1997, 157, 1449–1452. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, M.J.; Kim, J.H.; Hong, C.H.; Kim, J.W.; Noh, J.Y. Intravesical electrical stimulation improves neurogenic bowel dysfunction in children with spina bifida. J. Urol. 2004, 171, 2648–2650. [Google Scholar] [CrossRef]
- Coccorullo, P.; Strisciuglio, C.; Martinelli, M.; Miele, E.; Greco, L.; Staiano, A. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: A double-blind, randomized, placebo-controlled study. J. Pediatr. 2010, 157, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Rendeli, C.; Ausili, E.; Tabacco, F.; Focarelli, B.; Pantanella, A.; Di Rocco, C.; Genovese, O.; Fundarò, C. Polyethylene glycol 4000 vs. lactulose for the treatment of neurogenic constipation in myelomeningocele children: A randomized-controlled clinical trial. Aliment. Pharmacol. Ther. 2006, 23, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- House, J.G.; Stiens, S.A. Pharmacologically initiated defecation for persons with spinal cord injury: Effectiveness of three agents. Arch. Phys. Med. Rehabil. 1997, 78, 1062–1065. [Google Scholar] [CrossRef]
- Dunn, K.L.; Galka, M.L. A comparison of the effectiveness of Therevac SB and bisacodyl suppositories in SCI patients’ bowel programs. Rehabil. Nurs 1994, 19, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, J.H. Improved bowel care with a polyethylene glycol based bisacadyl suppository. J. Spinal. Cord. Med. 1997, 20, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M.; Emmanuel, A. Neurogenic bowel management. In Pelvic Organ Dysfunction in Neurological Disease—Clinical Management and Rehabilitation; Fowler, C., Panicker, J., Emmanuel, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 138–152. [Google Scholar]
- Bischoff, A.; Levitt, M.A.; Peña, A. Bowel management for the treatment of pediatric fecal incontinence. Pediatr. Surg. Int. 2009, 25, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; De La Torre, L.; Belkind-Gerson, J.; Lovell, M.; Ketzer, J.; Bealer, J.; Bischoff, A. Enema-Induced spastic left colon syndrome: An unintended consequence of chronic enema use. J. Pediatr. Surg. 2021, 56, 424–428. [Google Scholar] [CrossRef]
- Christensen, P.; Olsen, N.; Krogh, K.; Bacher, T.; Laurberg, S. Scintigraphic assessment of retrograde colonic washout in fecal incontinence and constipation. Dis. Colon Rectum 2003, 46, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ausili, E.; Focarelli, B.; Tabacco, F.; Murolo, D.; Sigismondi, M.; Gasbarrini, A.; Rendeli, C. Transanal irrigation in myelomeningocele children: An alternative, safe and valid approach for neurogenic constipation. Spinal Cord 2010, 48, 560–565. [Google Scholar] [CrossRef][Green Version]
- Kelly, M.S.; Dorgalli, C.; McLorie, G.; Khoury, A.E. Prospective evaluation of Peristeen® transanal irrigation system with the validated neurogenic bowel dysfunction score sheet in the pediatric population. Neurourol. Urodyn. 2017, 36, 632–635. [Google Scholar] [CrossRef]
- Gourcerol, G.; Vitton, V.; Leroi, A.M.; Michot, F.; Abysique, A.; Bouvier, M. How sacral nerve stimulation works in patients with faecal incontinence. Colorectal. Dis. 2011, 13, e203–e211. [Google Scholar] [CrossRef]
- Sheldon, R.; Kiff, E.S.; Clarke, A.; Harris, M.L.; Hamdy, S. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. Br. J. Surg. 2005, 92, 1423–1431. [Google Scholar] [CrossRef]
- Patton, V.; Stewart, P.; Lubowski, D.Z.; Cook, I.J.; Dinning, P.G. Sacral Nerve Stimulation Fails to Offer Long-term Benefit in Patients With Slow-Transit Constipation. Dis. Colon Rectum 2016, 59, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Van der Wilt, A.A.; van Wunnik, B.P.W.; Sturkenboom, R.; Han-Geurts, I.J.; Melenhorst, J.; Benninga, M.A.; Baeten, C.G.M.I.; Breukink, S.O. Sacral neuromodulation in children and adolescents with chronic constipation refractory to conservative treatment. Int. J. Colorectal. Dis. 2016, 31, 1459–1466. [Google Scholar] [CrossRef]
- Holzer, B.; Rosen, H.R.; Novi, G.; Ausch, C.; Hölbling, N.; Schiessel, R. Sacral nerve stimulation for neurogenic faecal incontinence. Br. J. Surg. 2007, 94, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, N.; Madersbacher, H.; Wyndaele, J.J.; Apostolidis, A.; Drake, M.J.; Gajewski, J.; Heesakkers, J.; Panicker, J.; Radziszewski, P.; Sakakibara, R.; et al. Neurogenic bowel dysfunction: Clinical management recommendations of the Neurologic Incontinence Committee of the Fifth International Consultation on Incontinence 2013. Neurourol. Urodyn. 2018, 37, 46–53. [Google Scholar] [CrossRef]
- Kelly, M.S.; Wiener, J.S.; Liu, T.; Patel, P.; Castillo, H.; Castillo, J.; Dicianno, B.E.; Jasien, J.; Peterson, P.; Routh, J.C.; et al. Neurogenic bowel treatments and continence in children and adults with myelomeningocele. J. Pediatr. Rehabil. Med. 2020, 13, 685–693. [Google Scholar] [CrossRef]
- Ambartsumyan, L.; Rodriguez, L. Bowel management in children with spina bifida. J. Pediatr. Rehabil. Med. 2018, 11, 293–301. [Google Scholar] [CrossRef]
- Gor, R.A.; Katorski, J.P.; Elliott, S.P. Medical and surgical management of neurogenic bowel. Curr. Opin. Urol. 2016, 26, 369–375. [Google Scholar] [CrossRef]
- Johnston, A.W.; Wiener, J.S.; Purves, J.T. Pediatric neurogenic bladder and bowel dysfunction: Will my child ever be out of diapers? Eur. Urol. Focus 2020, 6, 838–867. [Google Scholar] [CrossRef]
- Wong, D.W.; Jensen, L.L.; Bartolo, D.C.C.; Rothenberger, D.A. Artificial anal sphincter. Dis. Colon Rectum 1996, 39, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.M.; Malone, P.S. The Malone antegrade continence enema (MACE). J. Pediatr. Surg. 1995, 30, 68–71. [Google Scholar] [CrossRef]
- Ok, J.; Kurzrock, E.A. Objective measurement of quality of life changes after ACE Malone using FICQOL survey. J. Pediatr. Urol. 2011, 7, 389–393. [Google Scholar] [CrossRef][Green Version]
- Brinas, P.; Zalay, N.; Philis, A.; Castel-Lacanal, E.; Barrieu, M.; Portier, G. Use of Malone antegrade continence enemas in neurological bowel dysfunction. J. Visc. Surg. 2020, 157, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Bani-Hani, A.H.; Cain, M.P.; Kaefer, M.; Meldrum, K.K.; King, S.; Johnson, C.S.; Rink, R.C. The Malone Antegrade Continence Enema: Single Institutional Review. J. Urol. 2008, 180, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- VanderBrink, B.A.; Cain, M.P.; Kaefer, M.; Meldrum, K.K.; Misseri, R.; Rink, R.C. Outcomes following Malone antegrade continence enema and their surgical revisions. J. Pediatr. Surg. 2013, 48, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.; Beasley, S.; Robertson, R.; Morreau, P.N. Comparison of results of laparoscopic and open antegrade continence enema procedures. Pediatr. Surg. Int. 1999, 15, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Beasley, S.W.; Maoate, K. Appendicostomy Stomas and Antegrade Colonic Irrigation After Laparoscopic Antegrade Continence Enema. J. Laparoendosc. Adv. Surg. Tech. 2006, 16, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Malone, P.; Curry, J.; Osborne, A. The antegrade continence enema procedure why, when and how? World J. Urol. 1998, 16, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Bevill, M.D.; Bonnett, K.; Arlen, A.; Cooper, C.; Baxter, C.; Storm, D.W. Outcomes and satisfaction in pediatric patients with Chait cecostomy tubes. J. Pediatr. Urol. 2017, 13, 365–370. [Google Scholar] [CrossRef]
- Rawat, D.J.; Haddad, M.; Geoghegan, N.; Clarke, S.; Fell, J.M. Percutaneous endoscopic colostomy of the left colon: A new technique for management of intractable constipation in children. Gastrointest. Endosc. 2004, 60, 39–43. [Google Scholar] [CrossRef]
- Kelly, M.S. Malone antegrade continence enemas vs. cecostomy vs. transanal irrigation—What is new and how do we counsel patients? Curr. Urol. Rep. 2019, 20, 41. [Google Scholar] [CrossRef]
- Beckers, G.M.A.; de Meij, T.G.J. Peritonitis After Endoscopic Caecostomy With a Chait Trapdoor Catheter: What Lessons Can Be Learned? J. Pediatr. Gastroenterol. Nutr. 2012, 55, 217–218. [Google Scholar] [CrossRef]
- Hocevar, B.; Gray, M. Intestinal diversion (colostomy or ileostomy) in patients with severe bowel dysfunction following spinal cord injury. J. Wound Ostomy Cont. Nurs. 2008, 35, 159–166. [Google Scholar] [CrossRef]
- Coggrave, M.; Ingram, R.M.; Gardner, B.P.; Norton, C.S. The impact of stoma for bowel management after spinal cord injury. Spinal Cord 2012, 50, 848–852. [Google Scholar] [CrossRef]
- Branagan, G.; Tromans, A.; Finnis, D. Effect of stoma formation on bowel care and quality of life in patients with spinal cord injury. Spinal Cord 2003, 41, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.A.; Bonne Lee, B.; Muhlmann, M. Outcomes following stoma formation in patients with spinal cord injury. Colorectal Dis. 2019, 21, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.; Dukes, S.; Bryan, S.; Branagan, G. Early colostomy formation can improve independence following spinal cord injury and increase acceptability of bowel management. Top. Spinal Cord Inj. Rehabil. 2019, 25, 23–30. [Google Scholar] [CrossRef]
- Gasior, A.; Reck, C.; Vilanova-Sanchez, A.; Diefenbach, K.A.; Yacob, D.; Lu, P.; Vaz, K.; Di Lorenzo, C.; Levitt, M.A.; Wood, R.J. Surgical management of functional constipation: An intermediate report of a new approach using laparoscopic sigmoid resection combined with Malone appendicostomy. J. Pediatr. Surg. 2018, 53, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, J.A.; Zobell, S.; Leeflang, E.J.; Cao, D.; Mammen, L.; Rollins, M.D. Intermediate and long-term outcomes of a bowel management program for children with severe constipation or fecal incontinence. J. Pediatr. Surg. 2020, 55, 545–548. [Google Scholar] [CrossRef]
- Halleran, D.R.; Sloots, C.E.J.; Fuller, M.K.; Diefenbach, K. Adjuncts to bowel management for fecal incontinence and constipation, the role of surgery; appendicostomy, cecostomy, neoappendicostomy, and colonic resection. Sem. Pediatr. Surg. 2020, 29, 150998. [Google Scholar] [CrossRef] [PubMed]
- Siminas, S.; Losty, P.D. Current surgical management of pediatric idiopathic constipation: A systematic review of published studies. Ann. Surg. 2015, 262, 925–933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosiello, G.; Safder, S.; Marshall, D.; Rolle, U.; Benninga, M.A. Neurogenic Bowel Dysfunction in Children and Adolescents. J. Clin. Med. 2021, 10, 1669. https://doi.org/10.3390/jcm10081669
Mosiello G, Safder S, Marshall D, Rolle U, Benninga MA. Neurogenic Bowel Dysfunction in Children and Adolescents. Journal of Clinical Medicine. 2021; 10(8):1669. https://doi.org/10.3390/jcm10081669
Chicago/Turabian StyleMosiello, Giovanni, Shaista Safder, David Marshall, Udo Rolle, and Marc A. Benninga. 2021. "Neurogenic Bowel Dysfunction in Children and Adolescents" Journal of Clinical Medicine 10, no. 8: 1669. https://doi.org/10.3390/jcm10081669
APA StyleMosiello, G., Safder, S., Marshall, D., Rolle, U., & Benninga, M. A. (2021). Neurogenic Bowel Dysfunction in Children and Adolescents. Journal of Clinical Medicine, 10(8), 1669. https://doi.org/10.3390/jcm10081669





