Area-under-the-Curve-Based Mycophenolate Mofetil Dosage May Contribute to Decrease the Incidence of Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. HSCT Procedure
2.3. GvHD Prophylaxis
2.4. MPA AUC0–12 Monitoring and Dose Tailoring
2.5. Statistical Analysis
3. Results
3.1. Study Population and Transplant Characteristics
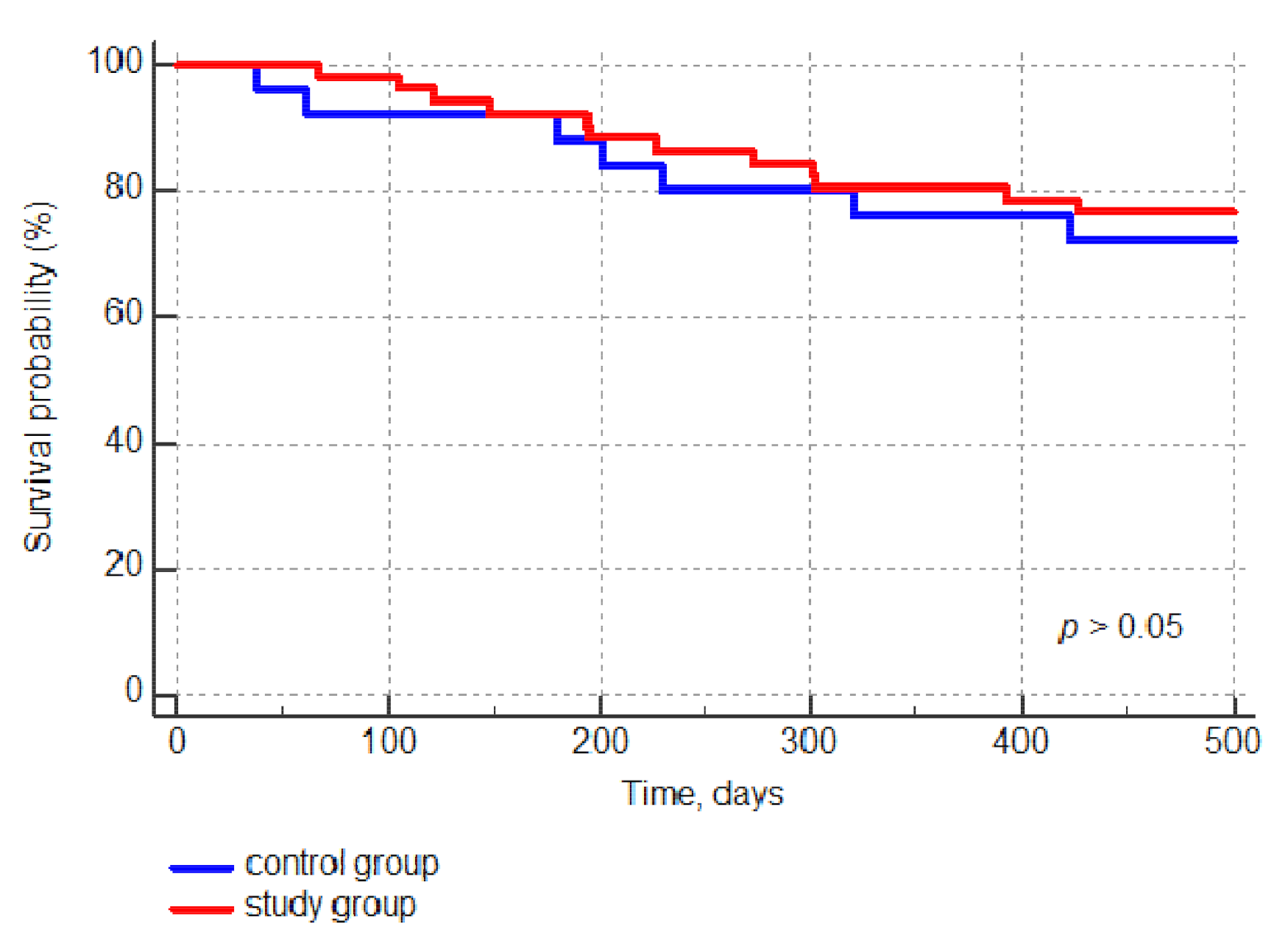
3.2. Primary Outcome: 12-Month Acute and Chronic GvHD Incidence
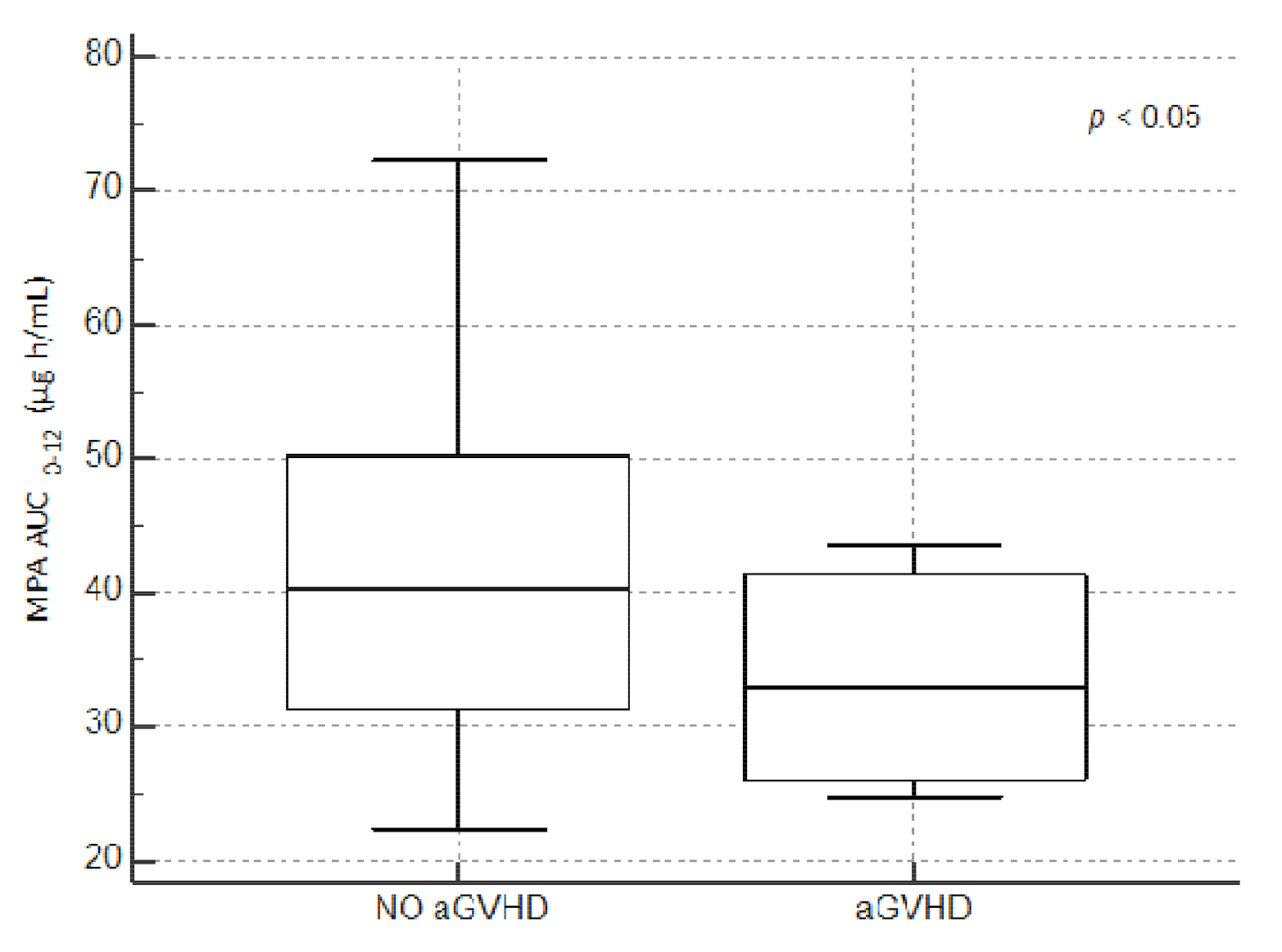
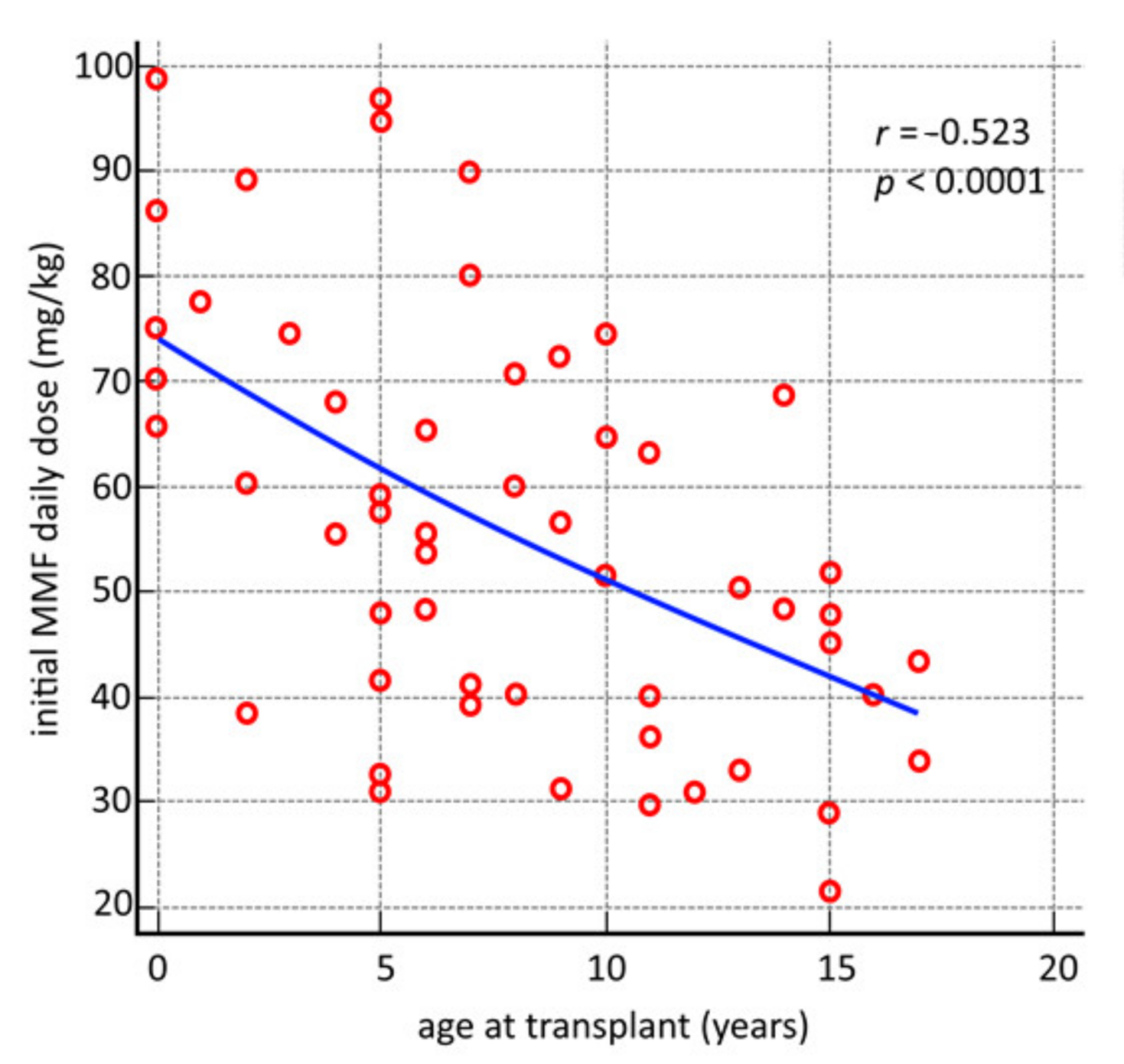
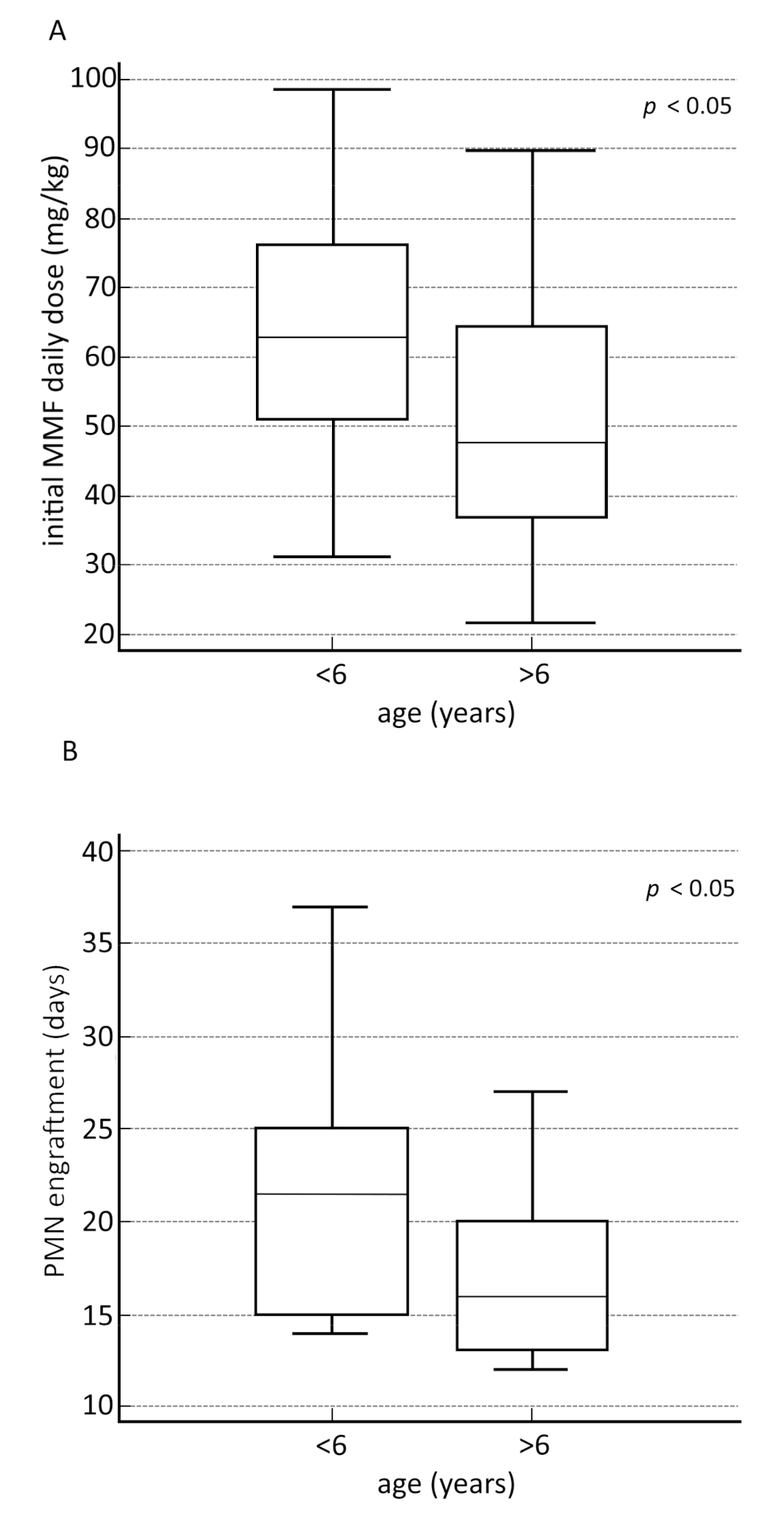
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharfan-Dabaja, M.; Mhaskar, R.; Reljic, T.; Pidala, J.; Perkins, J.B.; Djulbegovic, B.; Kumar, A. Mycophenolate mofetil versus methotrexate for prevention of graft-versus-host disease in people receiving allogeneic hematopoietic stem cell transplantation. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Okamura, A.; Yamamori, M.; Shimoyama, M.; Kawano, Y.; Kawano, H.; Kawamori, Y.; Nishikawa, S.; Minagawa, K.; Yakushijin, K.; Katayama, Y.; et al. Pharmacokinetics-based optimal dose-exploration of mycophenolate mofetil in allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2008, 88, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jacobsohn, D.A.; Vogelsang, G.B. Acute graft versus host disease. Orphanet. J. Rare Dis. 2007, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, R.; Yeshurun, M.; Vidal, L.; Shpilberg, O.; Gafter-Gvili, A. Mycophenolate mofetil vs. methotrexate for the prevention of graft-versus-host-disease--systematic review and meta-analysis. Leuk. Res. 2014, 38, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Yamamori, M.; Katayama, Y.; Matsui, T. Mycophenolate mofetil: Fully utilizing its benefits for GvHD prophylaxis. Int. J. Hematol. 2012, 96, 10–25. [Google Scholar] [CrossRef]
- Storb, R.; Deeg, H.J.; Whitehead, J.; Appelbaum, F.; Beatty, P.; Bensinger, W.; Buckner, C.D.; Clift, R.; Doney, K.; Farewell, V.; et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 1986, 314, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chow, S.L.D. Clinical Pharmacokinetics of Mycophenolic Acid in Hematopoietic Stem Cell Transplantation Recipients. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 183–189. [Google Scholar] [CrossRef]
- Von Vietinghoff, S.; Ouyang, H.; Ley, K. Mycophenolic acid suppresses granulopoiesis by inhibition of interleukin-17 production. Kidney Int. 2010, 78, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Arndt, C.; Beck, J.F.; Gruhn, B. A pediatric prognostic score for patients undergoing allogeneic hematopoietic stem cell transplantation. Eur. J. Haematol. 2014, 93, 509–515. [Google Scholar] [CrossRef]
- Maximova, N.; Schillani, G.; Simeone, R.; Maestro, A.; Zanon, D. Comparison of Efficacy and Safety of Caspofungin Versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis. Adv. Ther. 2017, 34, 1184–1199. [Google Scholar] [CrossRef]
- Haentzschel, I.; Freiberg-Richter, J.; Platzbecker, U.; Kiani, A.; Schetelig, J.; Illmer, T.; Ehninger, G.; Schleyer, E.; Bornhäuser, M. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008, 42, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.T.; Hoecker, B.; Armstrong, V.W.; Oellerich, M.; Tönshoff, B. Validation of an abbreviated pharmacokinetic profile for the estimation of mycophenolic acid exposure in pediatric renal transplant recipients. Ther. Drug Monit. 2006, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Militano, O.; Jin, Z.; Figurski, M.; Shaw, L.; Moore, V.; Morris, E.; Tallamy, B.; van deVen, C.; Ayello, J.; et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol. Blood Marrow Transplant. 2010, 16, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiehl, M.G.; Shipkova, M.; Basara, N.; Blau, I.W.; Schutz, E.; Armstrong, V.W.; Oellerich, M.; Fauser, A.A. Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin. Biochem. 2000, 33, 203–208. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Shaw, B.E.; Tredger, J.M.; Brown, N.W.; Kulkarni, S.; Saso, R.; Evans, S.; Treleaven, J.; Davies, F.E.; Ethell, M.E.; et al. Mycophenolic acid trough level monitoring: Relevance in acute and chronic graft versus host disease and its relation with albumin. Clin. Transplant. 2011, 25, 222–227. [Google Scholar] [CrossRef]
- Licata, S.; Maximova, N.; Baraldo, M. Estimated AUC of Mycophenolate mofetil in pediatric patients with hematopoietic cell transplantation using a reduced sampling model validated for kidney transplant. Oral Presentation. In Proceedings of the 37th National Congress of the Italian Society of Pharmacology (SIP), Naples, Italy, 27–30 October 2015. [Google Scholar]
- Windreich, R.M.; Goyal, R.K.; Joshi, R.; Kenkre, T.S.; Howrie, D.; Venkataramanan, R. A Pilot Study of Continuous Infusion of Mycophenolate Mofetil for Prophylaxis of Graft-versus-Host-Disease in Pediatric Patients. Biol. Blood Marrow Transplant. 2016, 22, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.M.; Holt, D.W.; Oellerich, M.; Meiser, B.; van Gelder, T. Current issues in therapeutic drug monitoring of mycophenolic acid: Report of a roundtable discussion. Ther. Drug Monit. 2001, 23, 305–315. [Google Scholar] [CrossRef]
- Hesselink, D.A.; van Hest, R.M.; Mathot, R.A.; Bonthuis, F.; Weimar, W.; de Bruin, R.W.; van Gelder, T. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am. J. Transplant. 2005, 5, 987–994. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin. Pharmacokinet. 2007, 46, 13–58. [Google Scholar] [CrossRef]
- Jenke, A.; Renner, U.; Richte, M.; Freiberg-Richter, J.; Platzbecker, U.; Helwig, A.; Thiede, H.M.; Schäfer-Eckart, K.; Ehninger, G.; Bornhäuser, M. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin. Transplant. 2001, 15, 176–184. [Google Scholar] [CrossRef]
- Shaw, L.M.; Korecka, M.; Aradhye, S.; Grossman, R.; Bayer, L.; Innes, C.; Cucciara, A.; Barker, C.; Naji, A.; Nicholls, A.; et al. Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J. Clin. Pharmacol. 2000, 40, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Long-Boyle, J.; Rydholm, N.; Orchard, P.J.; Tolar, J.; Smith, A.R.; Jacobson, P.; Brundage, R. Population pharmacokinetics of unbound mycophenolic acid in pediatric and young adult patients undergoing allogeneic hematopoietic cell transplantation. J. Clin. Pharmacol. 2012, 52, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.L.; Flournoy, N.; Thomas, E.D.; Prentice, R.; Fefer, A.; Buckner, C.D.; Storb, R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 1979, 10, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.M.; Aksentijevich, I.; Arber, D.A.; Barrett, J.; Brentjens, R.J.; Brufsky, J.; Cortes, J.; De Lima, M.; Forman, S.J.; Fuchs, E.J.; et al. The Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of acute leukemia. J. Immunother. Cancer 2020, 8, e000810. [Google Scholar] [CrossRef]



| Pre-Transplant Baseline Characteristics. | Study Group | Historical Group | p-Value |
|---|---|---|---|
| Number of patients (%) | 51 (100) | 25 (100) | - |
| Sex, number (%): | |||
| Male | 29 (56.9) | 16 (57.9) | NS |
| Female | 22 (43.1) | 9 (41.1) | |
| Age at transplant, years, median (IQR) | 7 (5–11) | 7 (4–12) | NS |
| Underlying disease, number (%): | |||
| Acute lymphoblastic leukemia | 23 (45.1) | 11 (44.0) | NS |
| Acute myeloid leukemia | 5 (9.8) | 3(12.0) | NS |
| Myelodysplastic syndrome | 11 (21.6) | 4 (16.0) | NS |
| Nonmalignant disorders | 9 (17.6) | 5 (20.0) | NS |
| Solid tumor | 3 (5.5) | 2(8.0) | NS |
| Disease risk, number (%): | |||
| Standard | 27 (49.1) | 14 (56.0) | NS |
| High | 24 (47.1) | 11 (44.0) | NS |
| Myeloablative conditioning, number (%): | |||
| MCHT-based | 34 (66.7) | 16 (64.0) | NS |
| TBI-based | 19 (34.5) | 9 (36.0) | NS |
| Donor type, number (%): | |||
| Matched unrelated donor | 47 (92.2) | 20 (80.0) | NS |
| Haploidentical donor | 4 (7.8) | 5 (20.0) | NS |
| Female donor to male recipient, number (%) | 10 (18.2) | 7 (28.0) | NS |
| Graft source, number (%): | |||
| Bone marrow | 35 (68.6) | 20 (80.0) | NS |
| Peripheral blood stem cells | 18 (35.3) | 5 (20.0) | NS |
| Graft cellular composition, median (IQR): | |||
| CD 34+ cells × 106/kg | 6.32 (4.7–9.31) | 5.27 (3.87–7.04) | NS |
| TNC × 108/kg | 5.22 (4.43–8.01) | 4.51 (3.95–5.46) | NS |
| GvHD prophylaxis, number (%): | |||
| Tacrolimus + MMF | 47 (92.2) | - | - |
| Cyclosporin + MTX + MMF | - | 23 (92.0) | - |
| Sirolimus + MMF | 4 (7.8) | 2 (8.0) | NS |
| VARIABLES | WHOLE COHORT | AGE 0–6 YEARS | AGE 7–17 YEARS | p-Value |
|---|---|---|---|---|
| Patients, number (%) | 55 | 24 (43.6) | 31 (56.4) | - |
| MMF initial daily dose, mg/kg, median (IQR) | 55.5 (40–70.4) | 62.9 (49.7–76.8) | 47.6 (36.4–64.7) | 0.00852 |
| First MPA AUC0–12, μg h/mL, median (IQR): | 30.4 (26.5–41.2) | 28 (24.1–35.6) | 32.8 (28.7–43.9) | 0.00988 |
| <30, number (%) | 26 (47.3) | 16 (61.5) | 10 (38.5) | 0.0152 |
| 30–60, number (%) | 24 (43.6) | 7 (29.2) | 17 (70.8) | 0.0991 |
| >60, number (%) | 5 (9.1) | 1 (2) | 4 (80) | 0.3728 |
| MMF final daily dose, mg/kg, median (IQR) | 59.2 (48.1–85.4) | 82.1 (56.4–94.1) | 52.1 (40.4–68) | 0.00048 |
| Last MPA AUC0–12, μg h/mL, median (IQR): | 40.2 (30.3–47.7) | 40 (29.1–44) | 40.3 (30.3–49.5) | 0.64135 |
| <30, number (%) | 13 (23.6) | 6 (46.2) | 7 (53.8) | 1 |
| 30–60, number (%) | 36 (65.5) | 14 (38.9) | 22 (61.1) | 0.3973 |
| >60, number (%) | 6 (10.9) | 4 (66.6) | 2 (33.4) | 0.3866 |
| MMF prophylaxis duration, days, median (IQR) | 26 (20–31) | 23.5 (19–29.7) | 28 (23–31) | 0.06637 |
| Number of AUC0–12 performed, median (IQR) | 2 (2–3) | 2 (2–2.7) | 2 (2–3) | 0.41593 |
| MMF dosage change, number, median (IQR) | 1 (1–1) | 1 (0–1) | 1 (1–1) | 0.31518 |
| Author | Type of Study | Number of Patients | Median Age at Transplant (Years) | GvHD Prophylaxis Regimen with MMF (Dose and Route of Administration) | Acute GvHD Incidence | Moderate or Severe Chronic GvHD | Median Time to Neutrophil Engraftment (Days) |
|---|---|---|---|---|---|---|---|
| Bolwell, 2004 | Randomized controlled | 40 | 49 (19–60) | 500 mg 3 times daily either IV or orally | grade I–II: 52% grade II–IV: 48% | 63% | 11 (range 8–24) |
| Kiehl, 2002 | Randomized controlled, multicenter | 45 | not available | (1) 1 g twice daily (n = 14) (2) 1.5 g twice daily (n = 18) IV | (1) grade II–IV: 43% (2) grade II–IV: 66% | not available | not available |
| Perkins, 2010 | Randomized controlled, single center | 92 | 49.9 (23–66.2) | 30 mg/kg/ day IV in two divided doses | grade II–IV: 78% grade III–IV: 19% | 38% | 15 |
| Hamilton, 2014 | Retrospective study | 241 | 47 (19–68) | First given at a dose of 500 IV three times a day >increased to 1000 mg twice daily | grade II–IV: 37% grade II–IV: 17% | 28% | 11 |
| Windreich, 2016 | Prospective | 19 | 6 months to 21 years | 15 mg/kg every 8 h from day 0 and administered as a constant 2-h infusion > a target total MPA AUC0–24 of 40 to 80 µg/mL/h. While on IV CI of MMF, total MPA levels were measured 3 times weekly and MMF dose adjusted to maintain a total MPA Css of 1.7 to 3.3 mg/mL | Six of 18 assessable patients (33%) developed grades II to IV acute GVHD (mean MPA levels were 0.5 mg/mL in those with acute GVHD versus 0.6 mg/ mL in those without acute GVHD (p 0.40). | Two of 15 developed moderate severity chronic GVHD | 18 of 19 patients (95%): median of 13 days (range, 8 to 41) - BMT: engrafted at 11 days (range, 8 to 43); - CB: 17 days (range, 15 to 41) (p < 0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlone, G.; Simeone, R.; Baraldo, M.; Maestro, A.; Zanon, D.; Barbi, E.; Maximova, N. Area-under-the-Curve-Based Mycophenolate Mofetil Dosage May Contribute to Decrease the Incidence of Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients. J. Clin. Med. 2021, 10, 406. https://doi.org/10.3390/jcm10030406
Carlone G, Simeone R, Baraldo M, Maestro A, Zanon D, Barbi E, Maximova N. Area-under-the-Curve-Based Mycophenolate Mofetil Dosage May Contribute to Decrease the Incidence of Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients. Journal of Clinical Medicine. 2021; 10(3):406. https://doi.org/10.3390/jcm10030406
Chicago/Turabian StyleCarlone, Giorgia, Roberto Simeone, Massimo Baraldo, Alessandra Maestro, Davide Zanon, Egidio Barbi, and Natalia Maximova. 2021. "Area-under-the-Curve-Based Mycophenolate Mofetil Dosage May Contribute to Decrease the Incidence of Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients" Journal of Clinical Medicine 10, no. 3: 406. https://doi.org/10.3390/jcm10030406





