Comparison of Plasma Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: II Analysis of Deposition Process
Abstract
1. Introduction
2. Results
2.1. Electron Ionization
2.2. GC-MS Measurements
2.3. Optical Emission Spectroscopy of Plasma
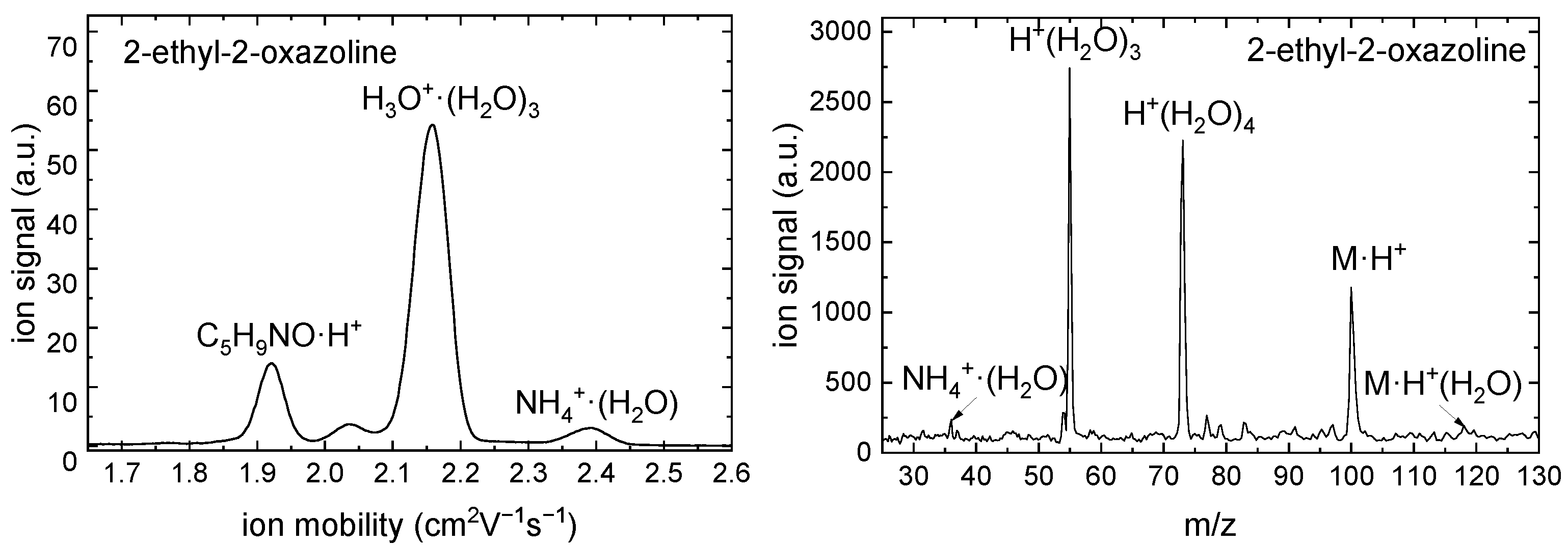
2.4. IMS-MS Measurement
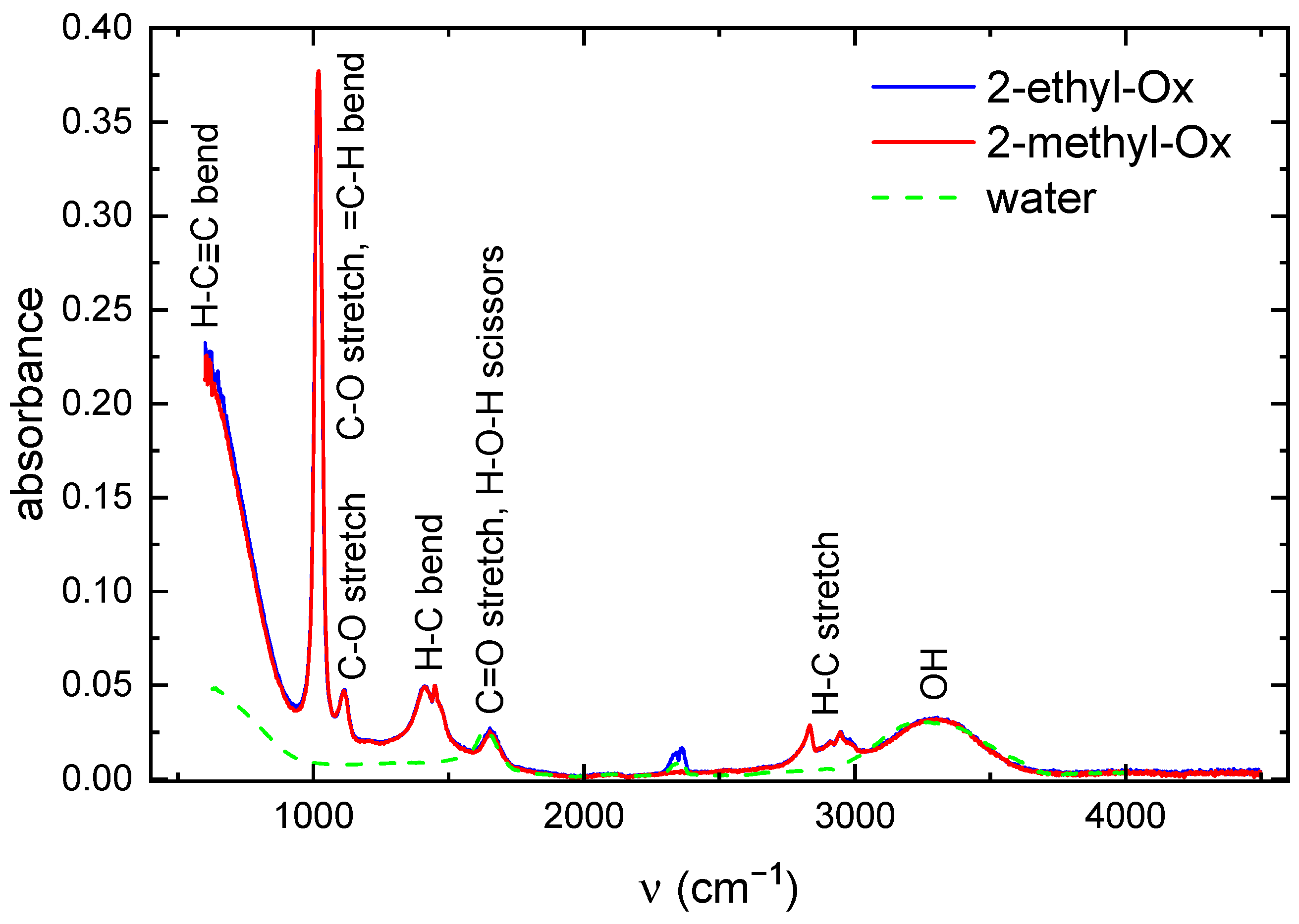
2.5. FTIR Analysis and Evaluation of Antibacterial Activity of Film Extract
3. Discussion and Conclusions
4. Materials and Methods
4.1. Materials
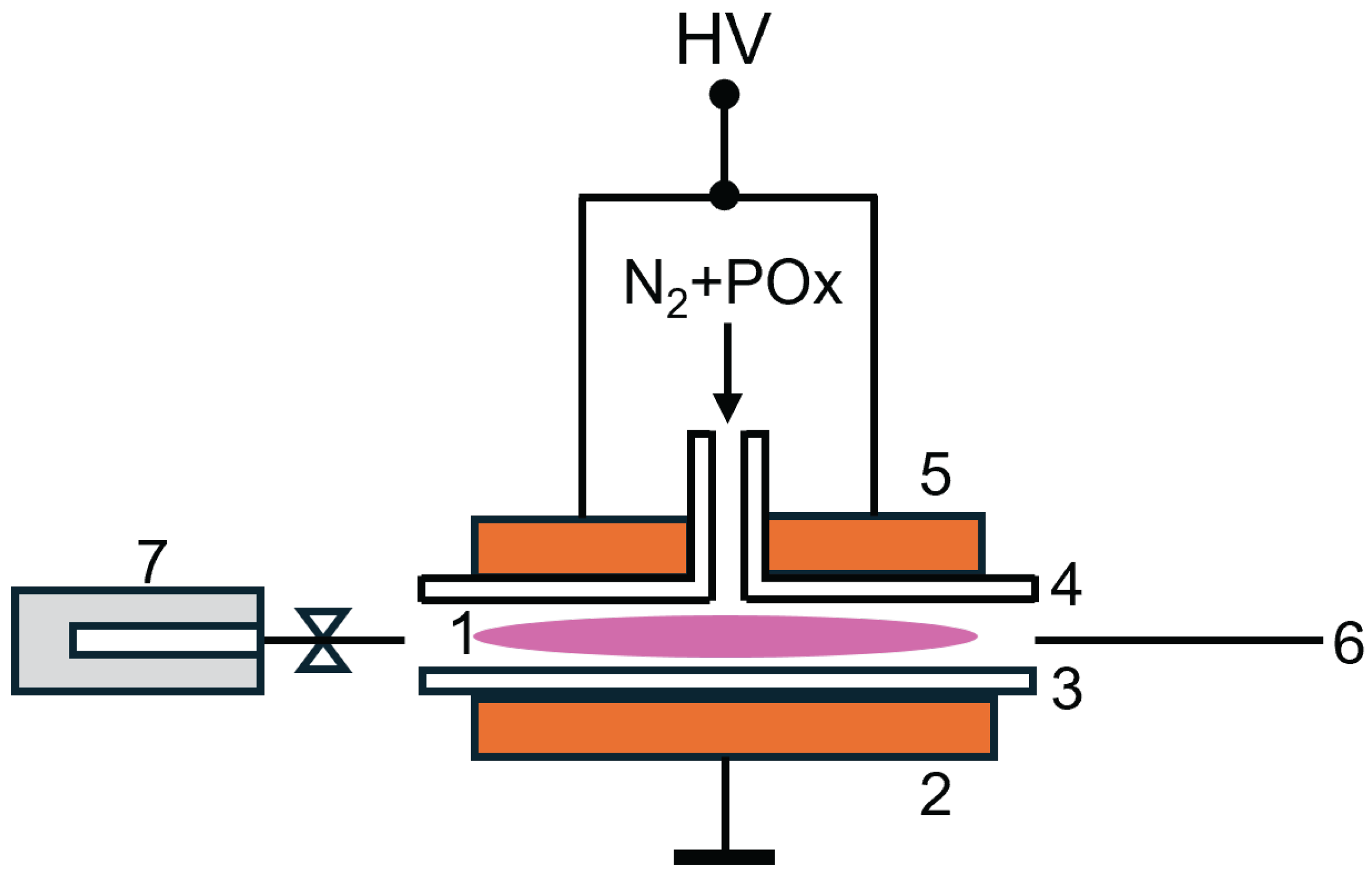
4.2. Plasma Reactor
4.3. Electron Ionization Measurement
4.3.1. Experiment
4.3.2. Theory
4.4. GC-MS Measurement
4.5. OES Measurement
4.6. IMS-MS Measurement
4.7. FTIR Analysis of Film Extract
4.8. Evaluation of Antibacterial Activity of Film Extract
Author Contributions
Funding
Conflicts of Interest
References
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Branch, D.W.; Wheeler, B.C.; Brewer, G.J.; Leckband, D.E. Long-term stability of grafted polyethylene glycol surfaces for use with microstamped substrates in neuronal cell culture. Biomaterials 2001, 22, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Pulpytel, J.; Mirshahi, M.; Arefi-Khonsari, F. Cell Resistant Peptidomimetic Poly (2-ethyl-2-oxazoline) Coatings Developed by Low Pressure Inductively Excited Pulsed Plasma Polymerization for Biomedical Purpose. Plasma Process. Polym. 2015, 12, 519–532. [Google Scholar] [CrossRef]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef]
- Cavallaro, A.A.; Macgregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling Properties of Plasma-Deposited Oxazoline-Based Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef]
- Zanini, S.; Zoia, L.; Dell’Orto, E.C.; Natalello, A.; Villa, A.M.; Della Pergola, R.; Riccardi, C. Plasma polymerized 2-ethyl-2-oxazoline: Chemical characterization and study of the reactivity towards different chemical groups. Mater. Des. 2016, 108, 791–800. [Google Scholar] [CrossRef]
- Stahel, P.; Mazankova, V.; Tomeckova, K.; Matouskova, P.; Brablec, A.; Prokes, L.; Jurmanova, J.; Bursikova, V.; Pribyl, R.; Lehocky, M.; et al. Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films-Antibacterial Properties and Cytocompatibility Performance. Polymers 2019, 11, 2069. [Google Scholar] [CrossRef]
- Mazankova, V.; Stahel, P.; Matouskova, P.; Brablec, A.; Cech, J.; Prokes, L.; Bursikova, V.; Stupavska, M.; Lehocky, M.; Ozaltin, K.; et al. Atmospheric Pressure Plasma Polymerized 2-Ethyl-2-oxazoline Based Thin Films for Biomedical Purposes. Polymers 2020, 12, 2679. [Google Scholar] [CrossRef]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Available online: https://www.iso.org/standard/54431.html (accessed on 13 December 2023).
- Snirer, M.; Kudrle, V.; Toman, J.; Jasek, O.; Jurmanova, J. Structure of microwave plasma-torch discharge during graphene synthesis from ethanol. Plasma Sources Sci. Technol. 2021, 30, 065020. [Google Scholar] [CrossRef]
- Borkhari, A.F.; Moravsky, L.; Matejcik, S. An atmospheric pressure field effect ionisation source for ion mobility spectrometry. Anal. Methods 2022, 14, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Volynets, A.V.; Lopaev, V.D.; Rakhimova, V.T.; Chukalovsky, A.A.; Mankelevich, Y.A.; Popov, N.A.; Zotovich, I.A.; Rakhimov, A.T. N2 dissociation and kinetics of N(4S) atoms in nitrogen DC glow discharge. J. Phys. D Appl. Phys. 2018, 51, 364002. [Google Scholar] [CrossRef]
- Guerra, V.; Sá, P.; Loureiro, J. Role played by the N2(A ) metastable in stationary N2 and N2-O2 discharges. J. Phys. D Appl. Phys. 2001, 34, 1745–1755. [Google Scholar] [CrossRef]
- Pintassilgo, C.D.; Jaoul, C.; Loureiro, J.; Belmonte, T.; Czerwiec, T. Kinetic modelling of a N2 flowing microwave discharge with CH4 addition in the post-discharge for nitrocarburizing treatments. J. Phys. D Appl. Phys. 2007, 40, 3620. [Google Scholar] [CrossRef]
- Torokova, L.; Watson, J.; Krcma, F.; Mazankova, V.; Mason, N.J.; Horvath, G.; Matejcik, S. Gas Chromatography Analysis of Discharge Products in N2-CH4 Gas Mixture at Atmospheric Pressure: Study of Mimic Titan’s Atmosphere. Contrib. Plasma Phys. 2015, 55, 470–480. [Google Scholar] [CrossRef]
- Jasek, O.; Toman, J.; Snirer, M.; Jurmanova, J.; Kudrle, V.; Michalicka, J.; Vsiansky, D.; Pavlinak, D. Microwave plasma-based high temperature dehydrogenation of hydrocarbons and alcohols as a single route to highly efficient gas phase synthesis of freestanding graphene. Nanotechnology 2021, 32, 505608. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Dias, A.; Henriques, J.; Rego, A.; Ferraria, A.; Abrashev, M.V.; Dias, F.M.; Luhrs, C.C.; et al. On the plasma-based growth of ‘flowing’ graphene sheets at atmospheric pressure conditions. Plasma Sources Sci. Technol. 2015, 25, 015013. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Liu, S.; Wang, S.; Mi, J. Preparation of medical hydrophilic and antibacterial silicone rubber via surface modification. RSC Adv. 2021, 11, 39950–39957. [Google Scholar] [CrossRef]
- Stahel, P.; Mazankova, V.; Prokes, L.; Bursikova, V.; Stupavska, M.; Lehocky, M.; Pistekova, H.; Ozaltin, K.; Trunec, D. Comparison of Plasma-Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: I Film Properties. Int. J. Mol. Sci. 2023, 24, 17455. [Google Scholar] [CrossRef]
- Stepankova, K.; Muellerova, M.; Zidek, S.; Pistekova, H.; Urbanek, P.; Stahel, P.; Trunec, D.; Popelka, A.; Kallingal, N.; Mozetic, M.; et al. Plasma Polymerization of Pentane and Hexane for Antibacterial and Biocompatible Thin Films. Plasma Process. Polym. 2025, 22, 2400266. [Google Scholar] [CrossRef]
- Massines, F.; Gherardi, N.; Naude, N.; Segur, P. Glow and Townsend dielectric barrier discharge in various atmosphere. Plasma Phys. Control. Fusion 2005, 47, B577. [Google Scholar] [CrossRef]
- Trunec, D.; Navratil, Z.; Tomekova, J.; Mazankova, V.; Durcanyova, S.; Zahoranova, A. Chemical composition of gaseous products generated by coplanar barrier discharge in air and N2/O2 mixtures. Plasma Sources Sci. Technol. 2022, 31, 115011. [Google Scholar] [CrossRef]
- Lerouge, S.; Major, A.; Girault-Lauriault, P.L.; Raymond, M.A.; Laplante, P.; Soulez, G.; Mwale, F.; Wertheimer, M.R.; Hebert, M.J. Nitrogen-rich coatings for promoting healing around stent-grafts after endovascular aneurysm repair. Biomaterials 2007, 28, 1209–1217. [Google Scholar] [CrossRef]
- Stano, M.; Matejcik, S.; Skalny, J.; Mark, T. Electron impact ionization of CH4: Ionization energies and temperature effects. J. Phys. B At. Mol. Opt. Phys. 2003, 36, 261–271. [Google Scholar] [CrossRef]
- Papp, P.; Urban, J.; Matejčík, Š.; Stano, M.; Ingolfsson, O. Dissociative electron attachment to gas phase valine: A combined experimental and theoretical study. J. Chem. Phys. 2006, 125, 204301. [Google Scholar] [CrossRef]
- Milosavljević, A.R.; Kočišek, J.; Papp, P.; Kubala, D.; Marinković, B.P.; MacH, P.; Urban, J.; Matejčík, Š. Electron impact ionization of furanose alcohols. J. Chem. Phys. 2010, 132, 1–11. [Google Scholar] [CrossRef]
- Papp, P.; Lacko, M.; Mészáros, D.; Stano, M.; Mach, P.; Matejčík, Š. An experimental and theoretical study of electron impact ionization and dissociative electron attachment to trimethyl borate. Int. J. Mass Spectrom. 2014, 365–366, 157–162. [Google Scholar] [CrossRef]
- Lacko, M.; Papp, P.; Wnorowski, K.; Matejčík, Š. Electron-induced ionization and dissociative ionization of iron pentacarbonyl molecules. Eur. Phys. J. D 2015, 69, 84. [Google Scholar] [CrossRef]
- Papp, P.; Danko, M.; Matejčík, Š. Electron ionization and photoionization of cyclopropylamine. Int. J. Mass Spectrom. 2020, 455, 116390. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. SRD 69; Argon; National Institute of Standards and Technology: Gaithersburg, MD, USA; U.S. Department of Commerce: Gaithersburg, MD, USA, 1 October 2023. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440371&Units=SI&Mask=20#Ion-Energetics (accessed on 6 July 2025).
- Wannier, G.H. The threshold law for single ionization of atoms or ions by electrons. Phys. Rev. 1953, 90, 817–825. [Google Scholar] [CrossRef]
- Read, F.H. Extensions of the Wannier theory for near-threshold excitation and ionisation of atoms by electron impact. J. Phys. B At. Mol. Phys. 1984, 17, 3965–3986. [Google Scholar] [CrossRef]
- Gstir, B.; Denifl, S.; Hanel, G.; Rümmele, M.; Fiegele, T.; Cicman, P.; Stano, M.; Matejcik, S.; Scheier, P.; Becker, K.; et al. Electron impact multiple ionization of neon, argon and xenon atoms close to threshold: Appearance energies and Wannier exponents. J. Phys. B At. Mol. Opt. Phys. 2002, 35, 2993–3007. [Google Scholar] [CrossRef]
- Gstir, B.; Denifl, S.; Hanel, G.; Rümmele, M.; Fiegele, T.; Stano, M.; Feketeova, L.; Matejcik, S.; Becker, K.; Scheier, P.; et al. High resolution multiple electron impact ionisation of He, Ne, Ar, Kr and Xe atoms close to threshold: Appearance energies and Wannier exponents. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 205, 413–416. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Roothaan, C.C.J. New Developments in Molecular Orbital Theory. Rev. Mod. Phys. 1951, 23, 69–89. [Google Scholar] [CrossRef]
- Berthier, G. Extension de la methode du champ moleculaire self-consistent a l’etude des couches incompletes. Comptes Rendus Hebd. Des SéAnces L’AcadéMie Des Sci. 1954, 238, 91–93. [Google Scholar]
- Pople, J.A.; Nesbet, R.K. Self-Consistent Orbitals for Radicals. J. Chem. Phys. 1954, 22, 571–572. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. Semi-direct algorithms for the MP2 energy and gradient. Chem. Phys. Lett. 1990, 166, 281–289. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Sæbø, S.; Almlöf, J. Avoiding the integral storage bottleneck in LCAO calculations of electron correlation. Chem. Phys. Lett. 1989, 154, 83–89. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Head-Gordon, T. Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 1994, 220, 122–128. [Google Scholar] [CrossRef]
- Raghavachari, K.; Pople, J.A. Approximate fourth-order perturbation theory of the electron correlation energy. Int. J. Quantum Chem. 1978, 14, 91–100. [Google Scholar] [CrossRef]
- Raghavachari, K.; Frisch, M.J.; Pople, J.A. Contribution of triple substitutions to the electron correlation energy in fourth order perturbation theory. J. Chem. Phys. 1980, 72, 4244–4245. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Simmie, J.M.; Somers, K.P. Benchmarking Compound Methods (CBS-QB3, CBS-APNO, G3, G4, W1BD) against the Active Thermochemical Tables: A Litmus Test for Cost-Effective Molecular Formation Enthalpies. J. Phys. Chem. A 2015, 119, 7235–7246. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Q.D.; Sun, M.M.; Yin, G.; Liang, J. Benchmark calculations for bond dissociation energies and enthalpy of formation of chlorinated and brominated polycyclic aromatic hydrocarbons. RSC Adv. 2021, 11, 29690–29701. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Crosmer, W.; Thomas, N.; Tsang, P.; Duckett, R. Cold Trap Fractionation as an Organic Analysis Technique. Rev. Sci. Instrum. 1973, 44, 837–843. [Google Scholar] [CrossRef]
- Michalczuk, B.; Moravsky, L.; Papp, P.; Mach, P.; Sabo, M.; Matejcik, S. Isomer and conformer selective atmospheric pressure chemical ionisation of dimethyl phthalate. Phys. Chem. Chem. Phys. 2019, 21, 13679–13685. [Google Scholar] [CrossRef]
- Moravsky, L.; Borkhari, A.F.; Adamov, A.Y.; Sysoev, A.A.; Papp, P.; Matejcik, S. Negative Atmospheric Pressure Chemical Ionization of Chlorinated Hydrocarbons Studied by Ion Mobility Spectrometry (IMS) and IMS-MS Techniques. J. Am. Soc. Mass Spectrom. 2022, 33, 1569–1576. [Google Scholar] [CrossRef]
- Moravsky, L.; Michalczuk, B.; Hrda, J.; Hamaguchi, S.; Matejcik, S. Monitoring of nonthermal plasma degradation of phthalates by ion mobility spectrometry. Plasma Process. Polym. 2021, 18, e2100032. [Google Scholar] [CrossRef]

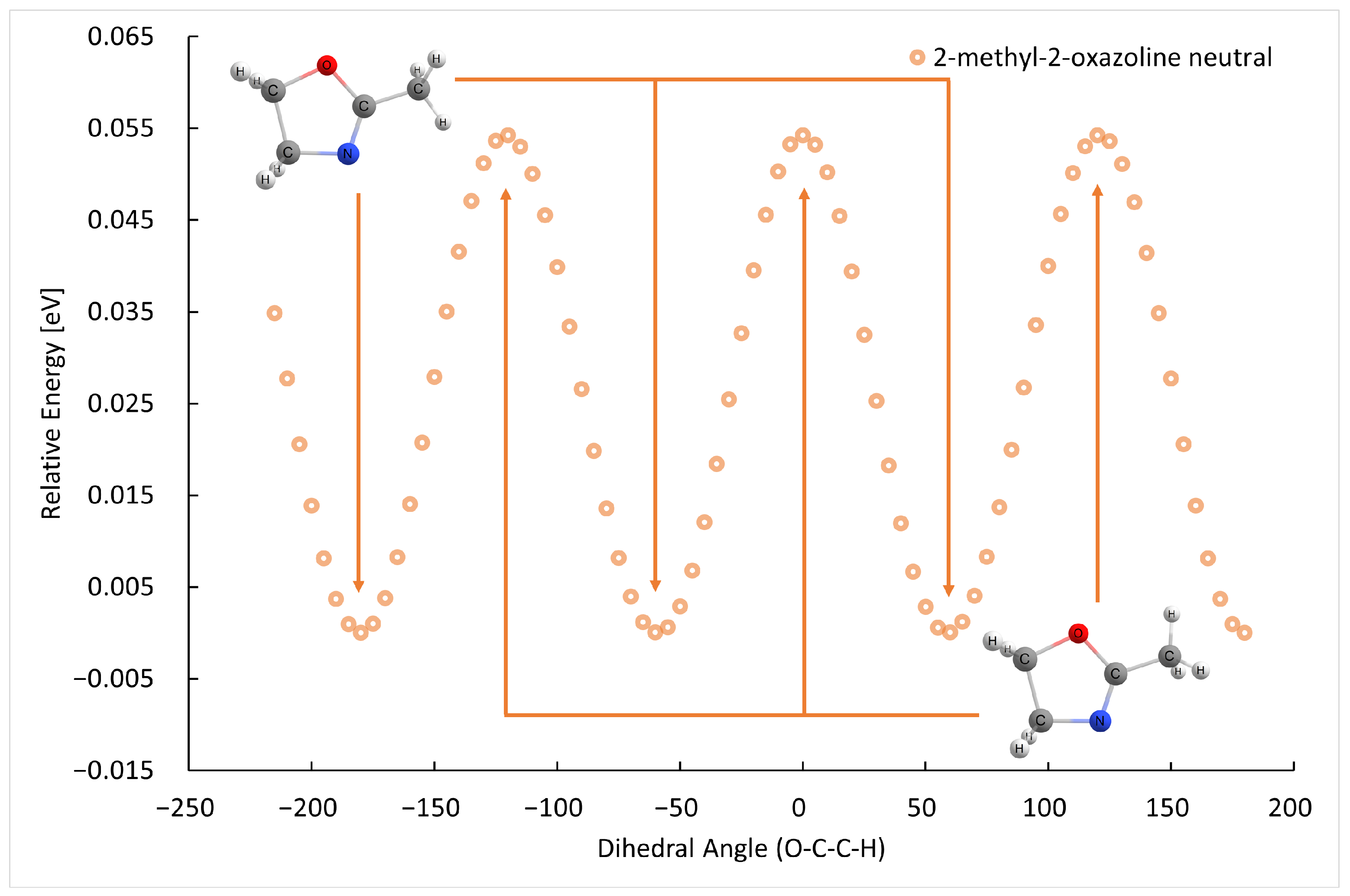
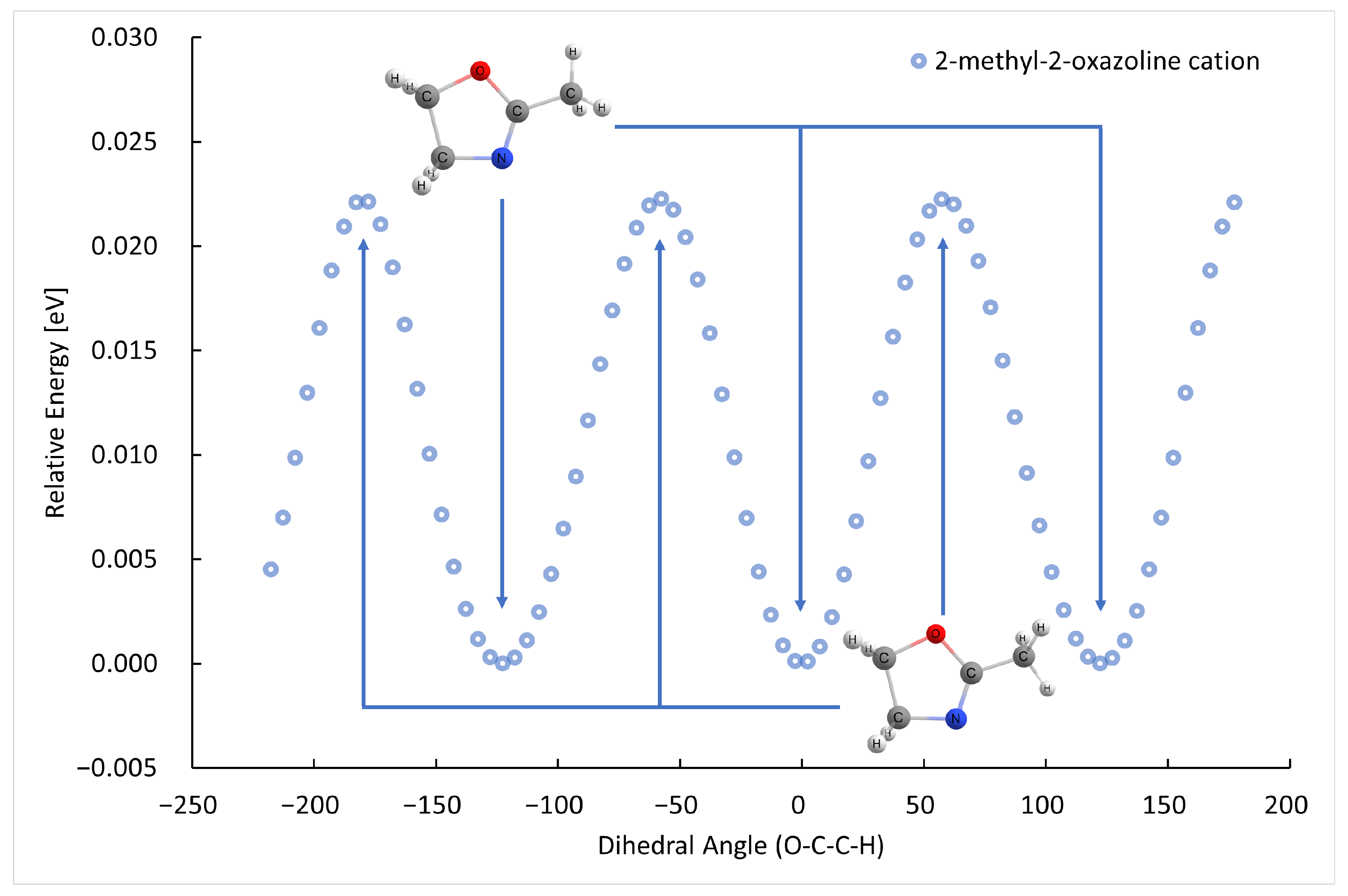
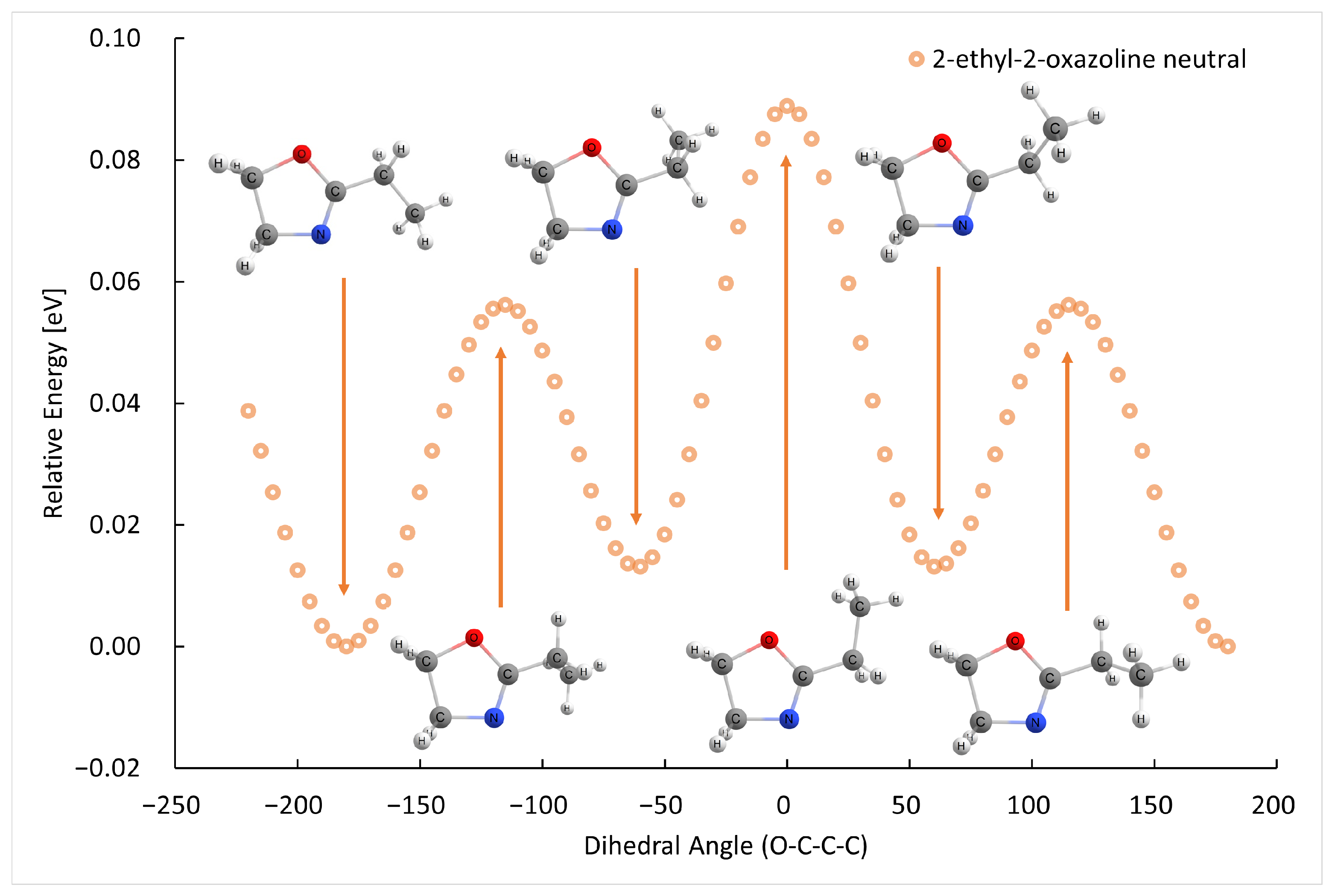
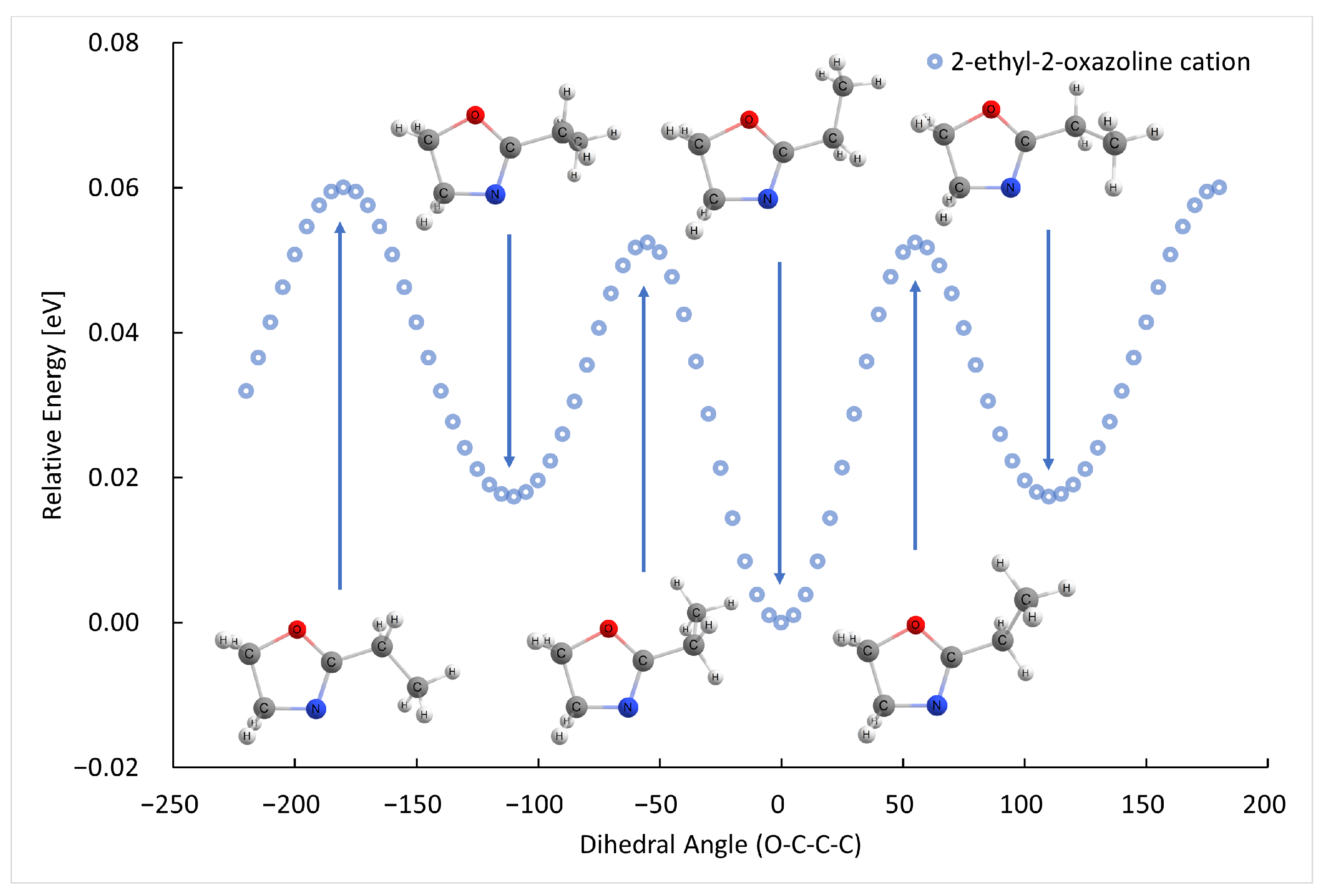
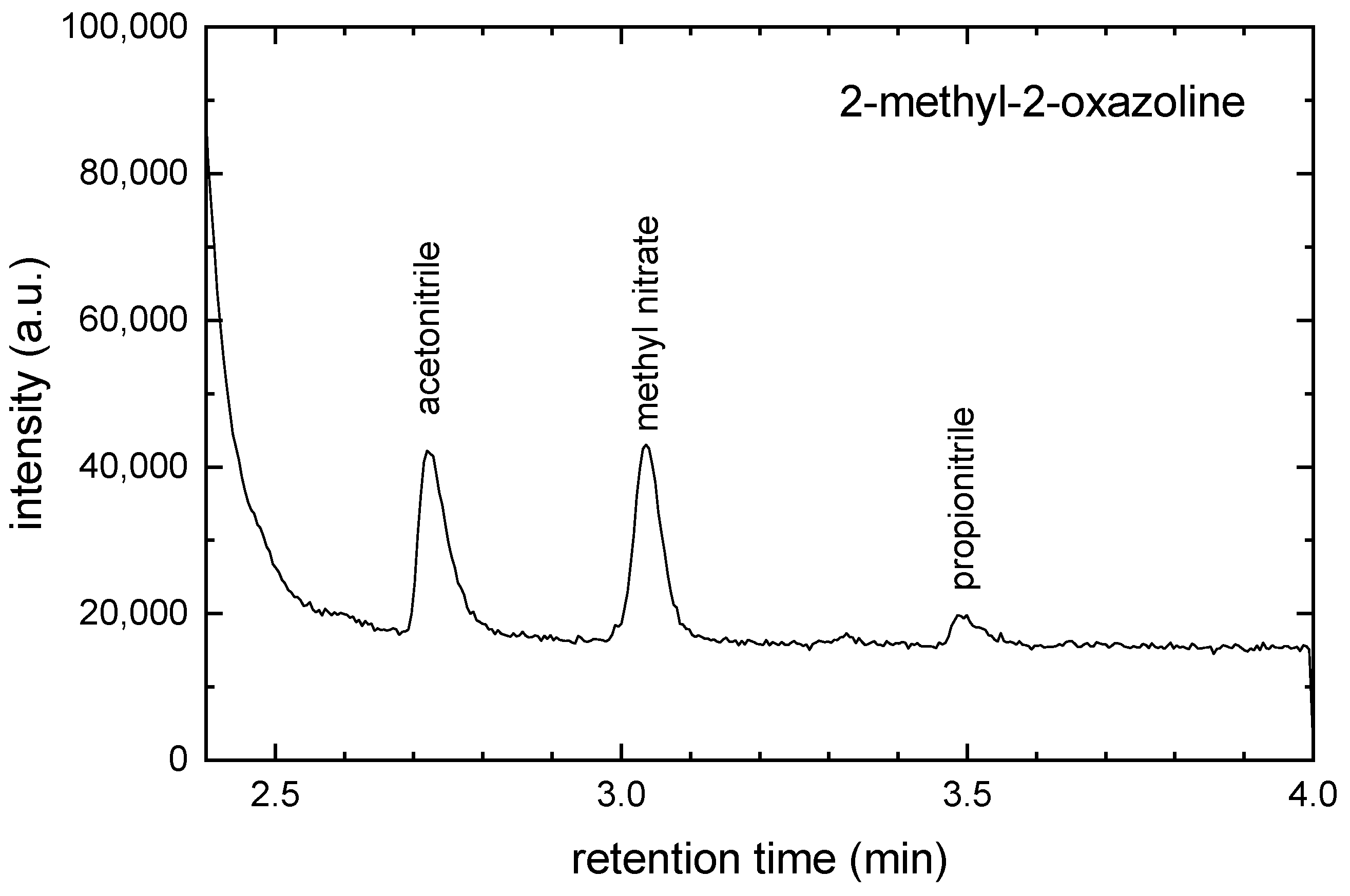
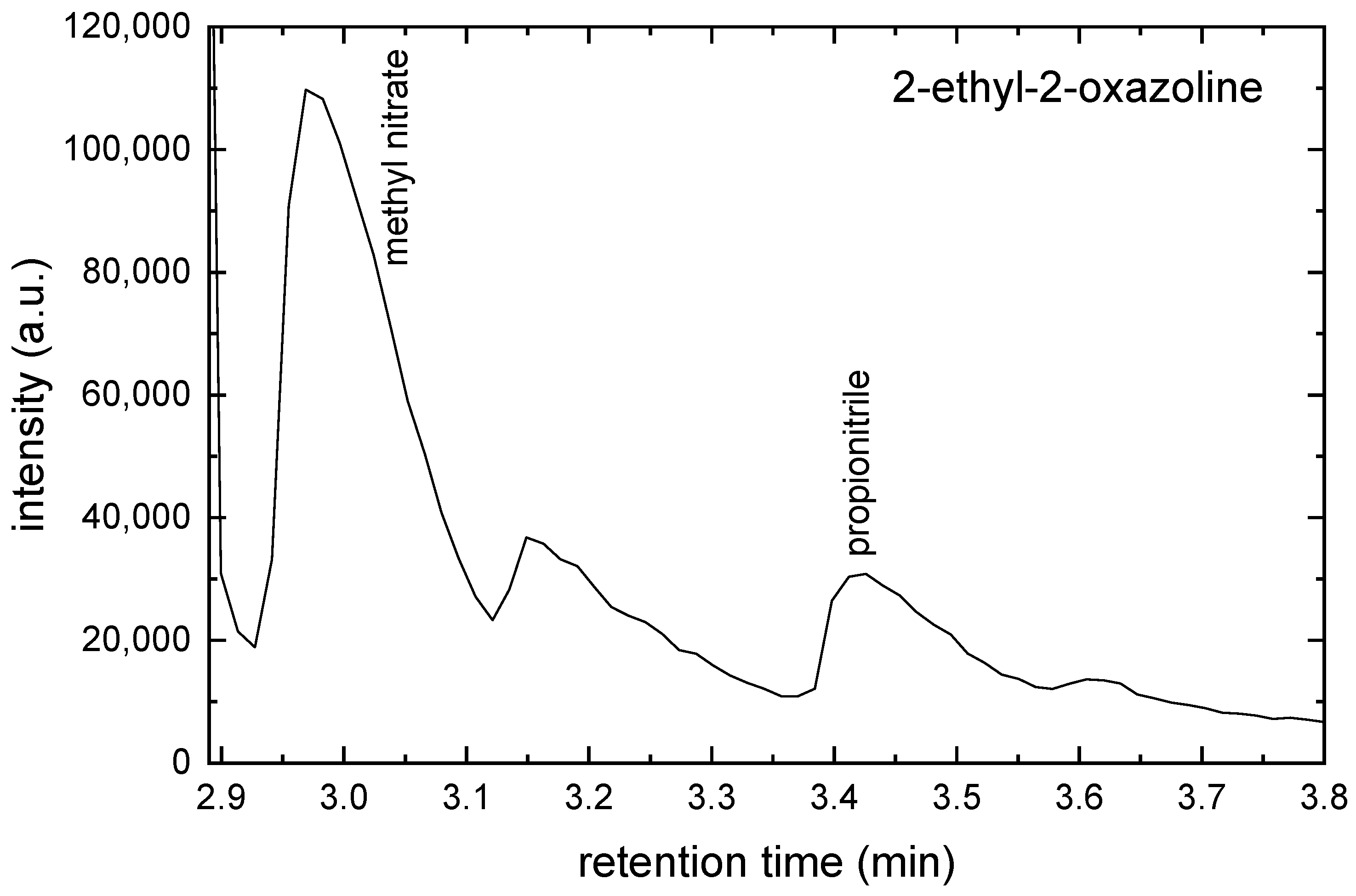
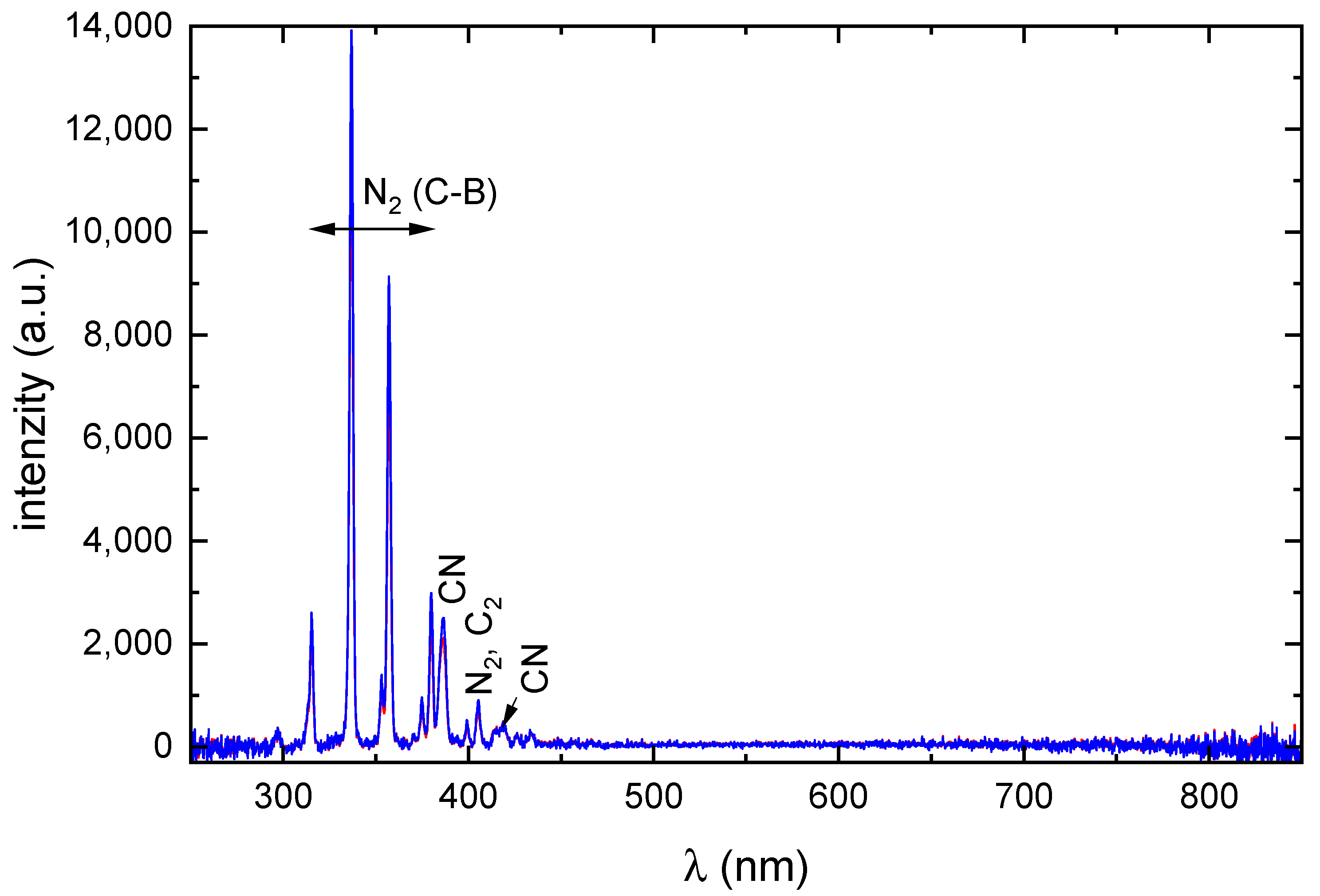
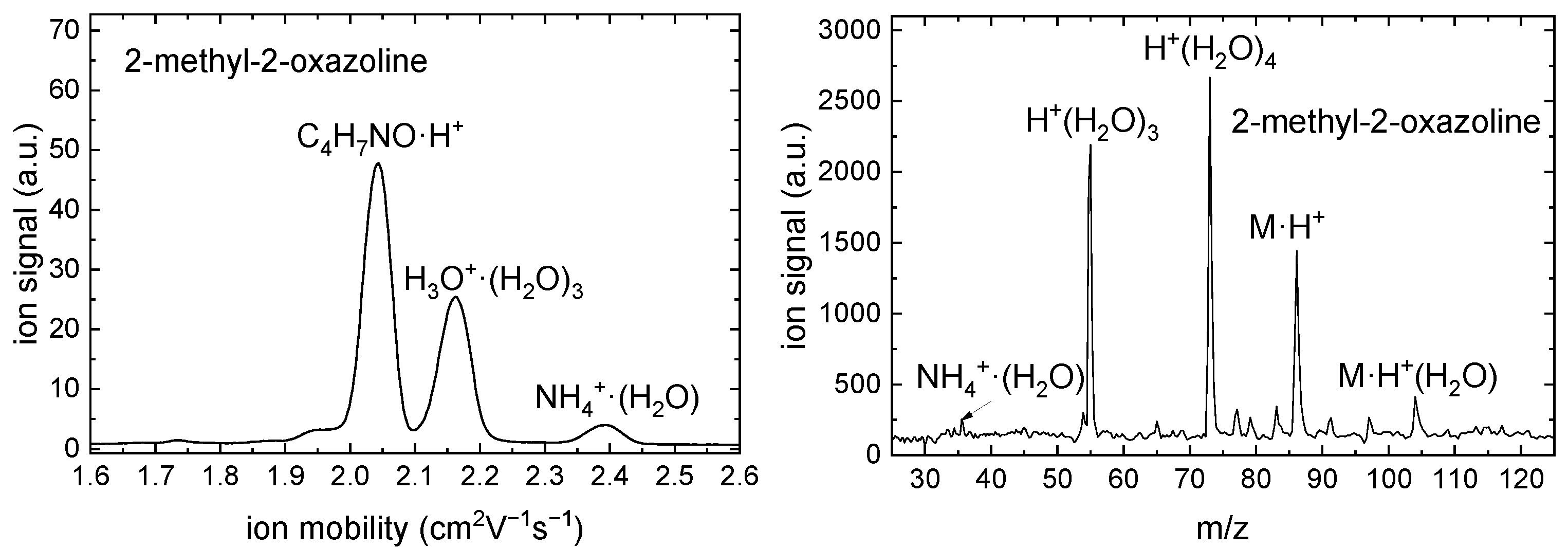

| m (a.u.) | Cation | Neutral | AE G4 (eV) | AE Exp. (eV) |
|---|---|---|---|---|
| 85 | NO-C | – | 9.14 | 9.09 |
| 84 | OCCHNC-C | H | 10.44 | 9.90 |
| 56 | C-CH=N-C | CH=O | 10.19 | 10.11 |
| 55 | C-C=N-C | C=O | 10.14 | 10.10 |
| 45 | C-CH-O | C-C≡N | 10.18 | 10.59 |
| 44 | C-CH- | -C≡N | 10.57 | 10.50 |
| 43 | C-C= | =C=N + | 10.81 | 10.40 |
| m (a.u.) | Cation | Neutral | AE G4 (eV) | AE Exp. (eV) |
|---|---|---|---|---|
| 99 | – | 9.03 | 9.15 | |
| 98 | OCCHNC- | H | 10.37 | 10.40 |
| 70 | C-N=C- | CH=O | 9.54 | 10.00 |
| 69 | C=N-C- | C=O | 10.04 | 10.40 |
| 54 | =O + | 12.46 | 12.23 | |
| 45 | -C≡N | 10.46 | 10.60 | |
| 44 | -C≡N | 10.60 | 10.50 | |
| 41 | -CH=O | 12.59 | 12.00 |
| Radicals | DE G4 (eV) |
|---|---|
| cyc(NC) + | 4.77 |
| cyc() + | 1.5 |
| + | 4.1 |
| Radicals | DE G4 (eV) |
|---|---|
| cyc(NC) + | 3.61 |
| cyc(NC) + | 4.66 |
| cyc() + | 1.53 |
| + | 4.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, P.; Mazánková, V.; Moravský, L.; Blaško, J.; Sťahel, P.; Prokeš, L.; Horňák, R.; Lehocký, M.; Pištěková, H.; Trunec, D. Comparison of Plasma Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: II Analysis of Deposition Process. Int. J. Mol. Sci. 2025, 26, 8641. https://doi.org/10.3390/ijms26178641
Papp P, Mazánková V, Moravský L, Blaško J, Sťahel P, Prokeš L, Horňák R, Lehocký M, Pištěková H, Trunec D. Comparison of Plasma Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: II Analysis of Deposition Process. International Journal of Molecular Sciences. 2025; 26(17):8641. https://doi.org/10.3390/ijms26178641
Chicago/Turabian StylePapp, Peter, Věra Mazánková, Ladislav Moravský, Ján Blaško, Pavel Sťahel, Lubomír Prokeš, Radek Horňák, Marián Lehocký, Hana Pištěková, and David Trunec. 2025. "Comparison of Plasma Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: II Analysis of Deposition Process" International Journal of Molecular Sciences 26, no. 17: 8641. https://doi.org/10.3390/ijms26178641
APA StylePapp, P., Mazánková, V., Moravský, L., Blaško, J., Sťahel, P., Prokeš, L., Horňák, R., Lehocký, M., Pištěková, H., & Trunec, D. (2025). Comparison of Plasma Polymerized Thin Films Deposited from 2-Methyl-2-oxazoline and 2-Ethyl-2-oxazoline: II Analysis of Deposition Process. International Journal of Molecular Sciences, 26(17), 8641. https://doi.org/10.3390/ijms26178641








