Combined Photodynamic Therapy and Chemotherapy Using Local Intra-Arterial Intratumoral Administration of Chlorin e6 and Cisplatin: First Clinical Observations
Abstract
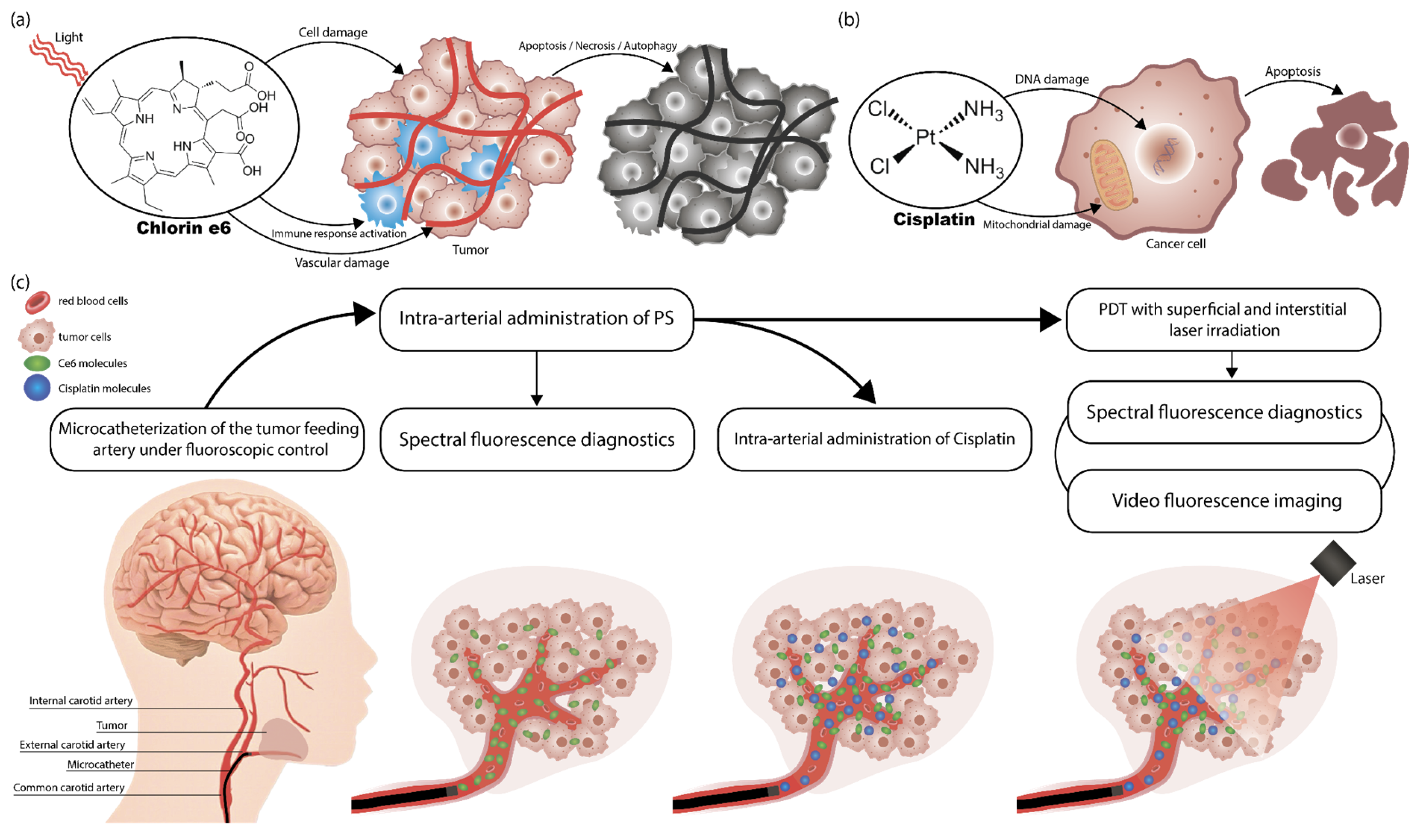
1. Introduction
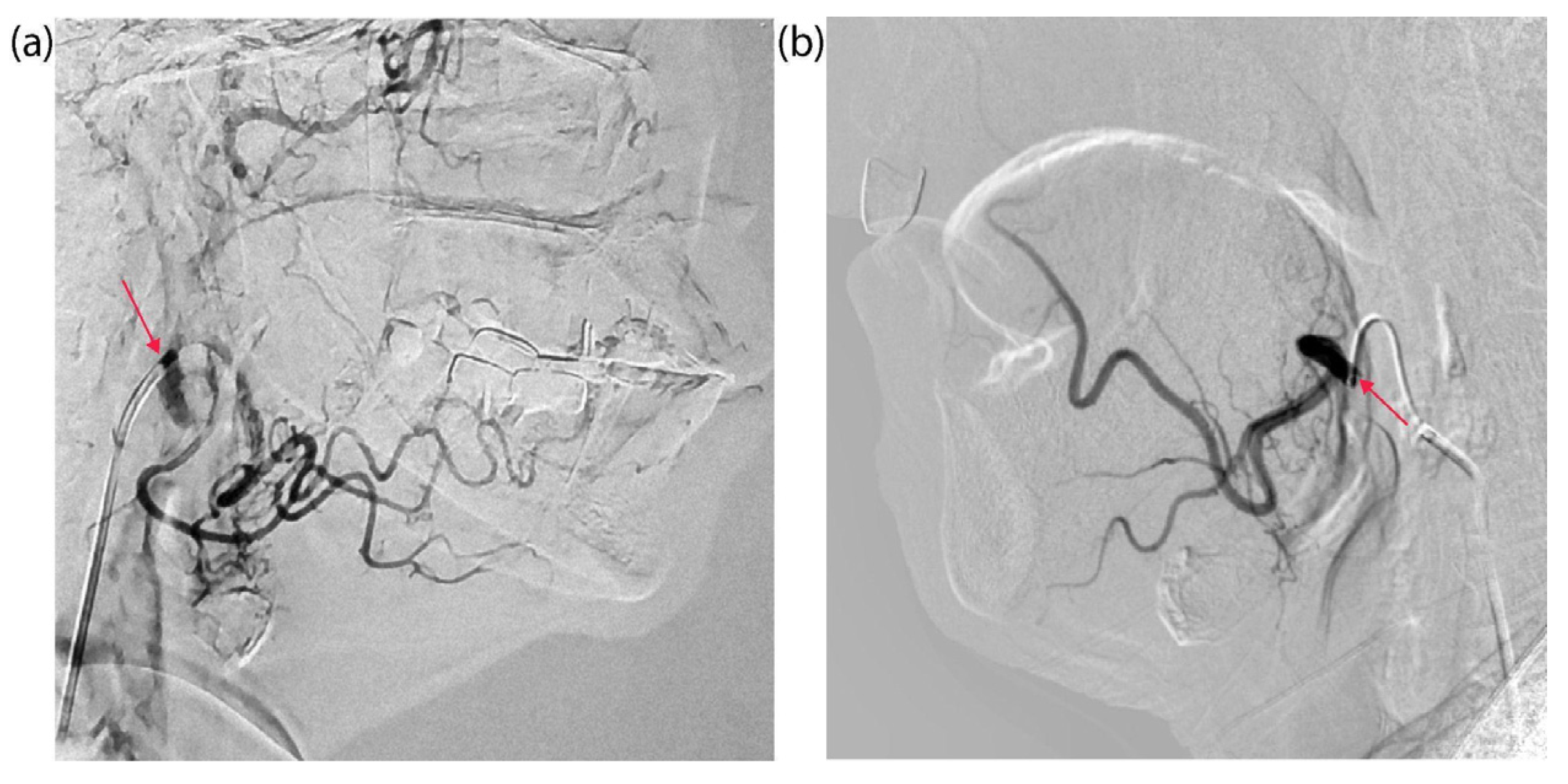
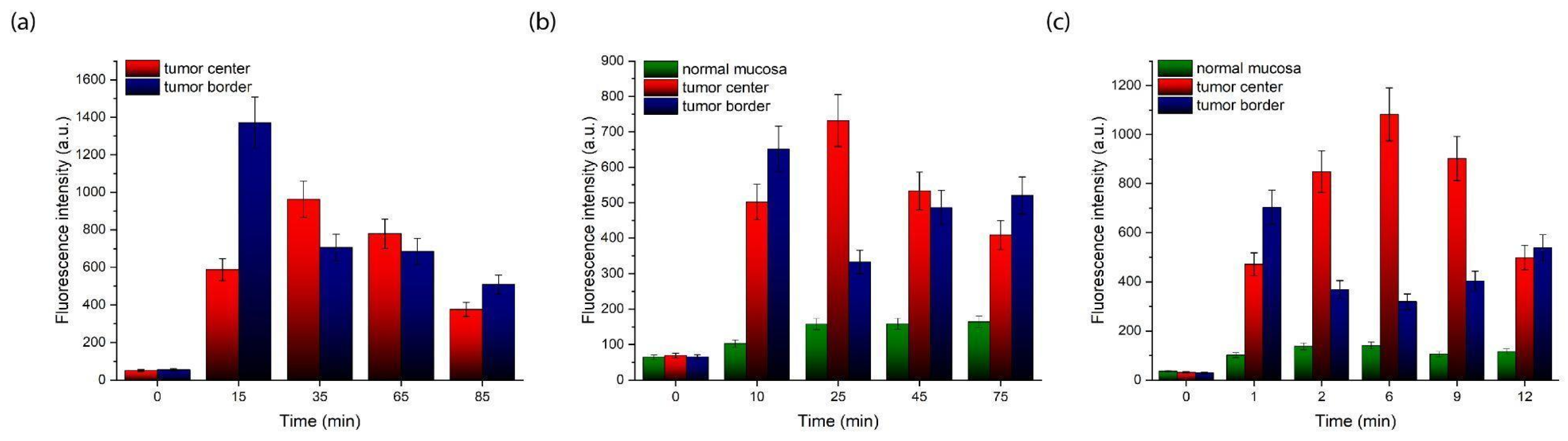
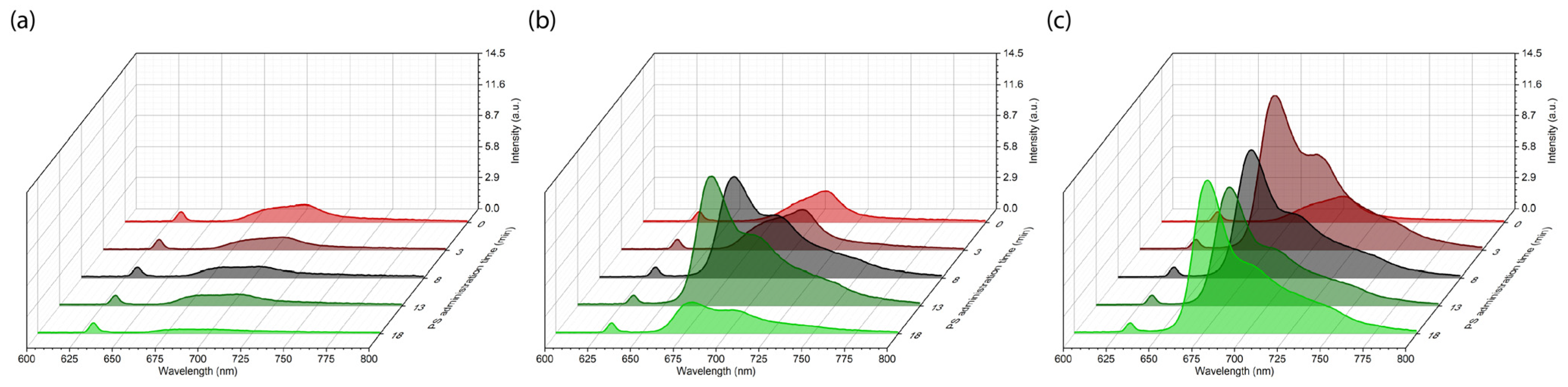
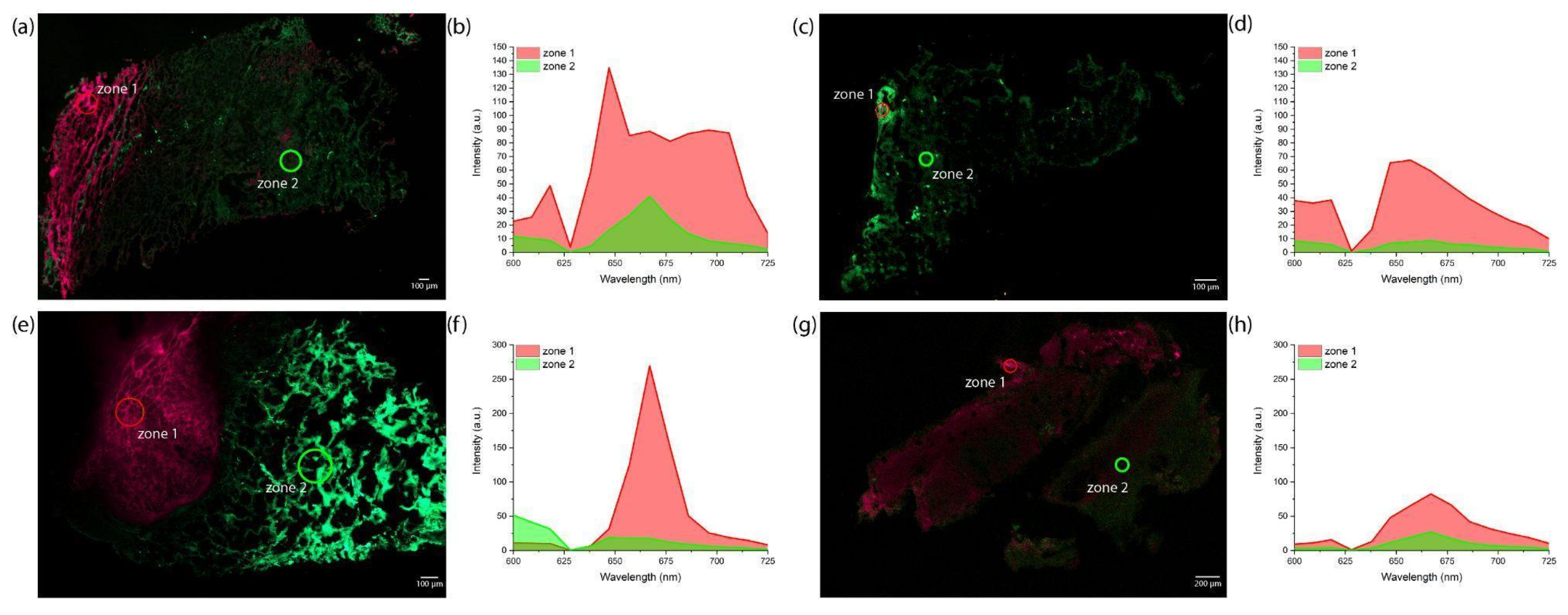
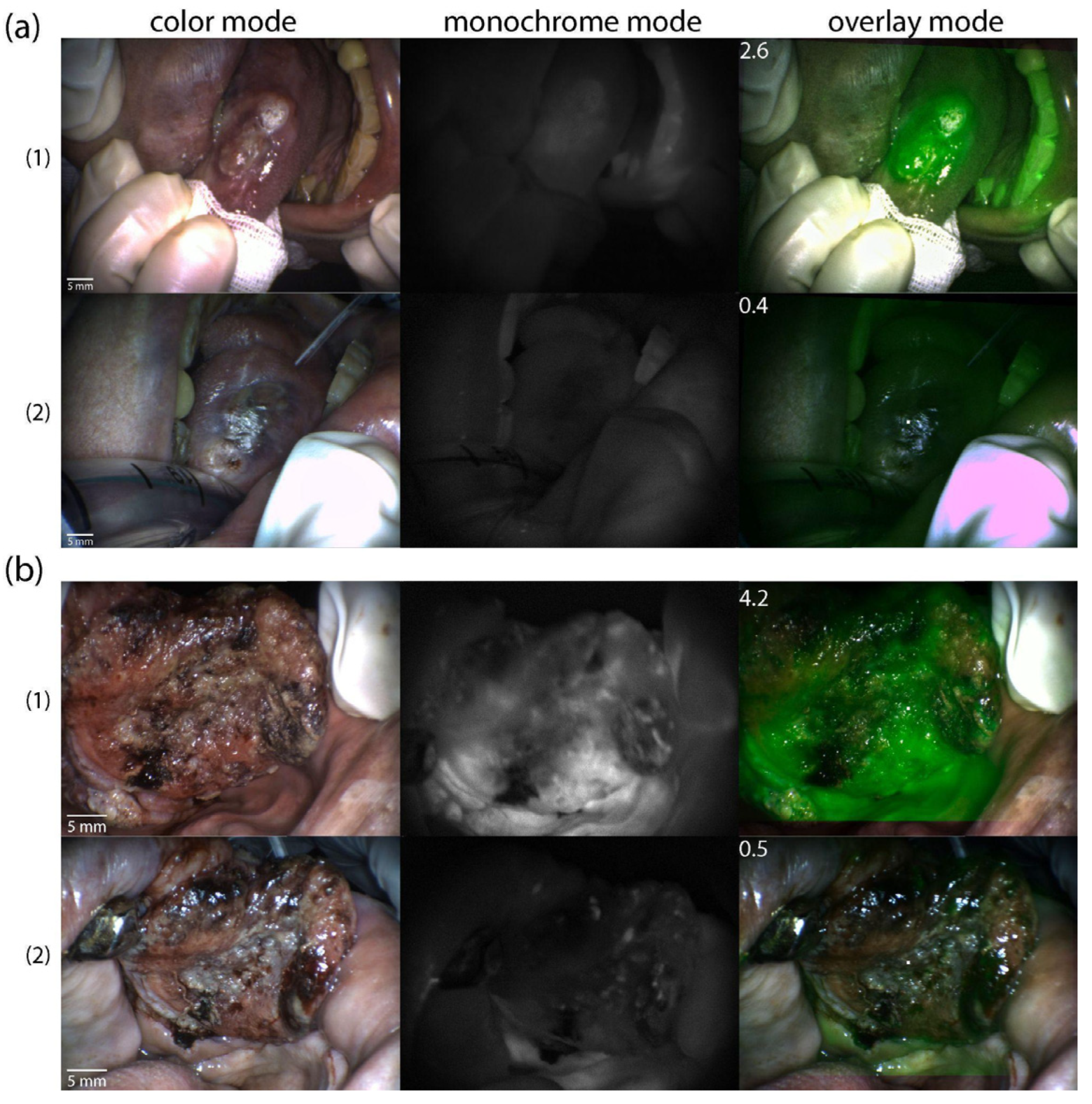
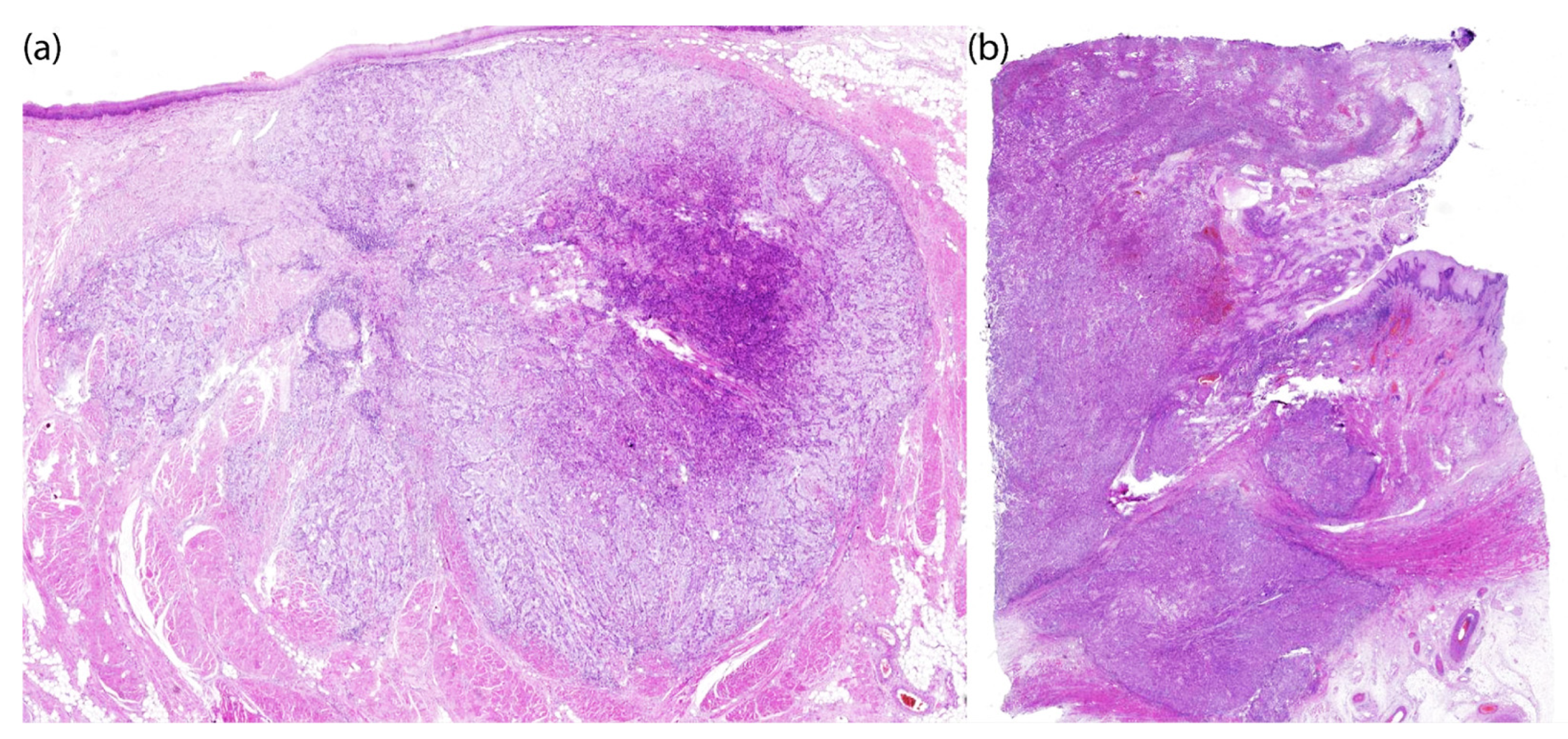
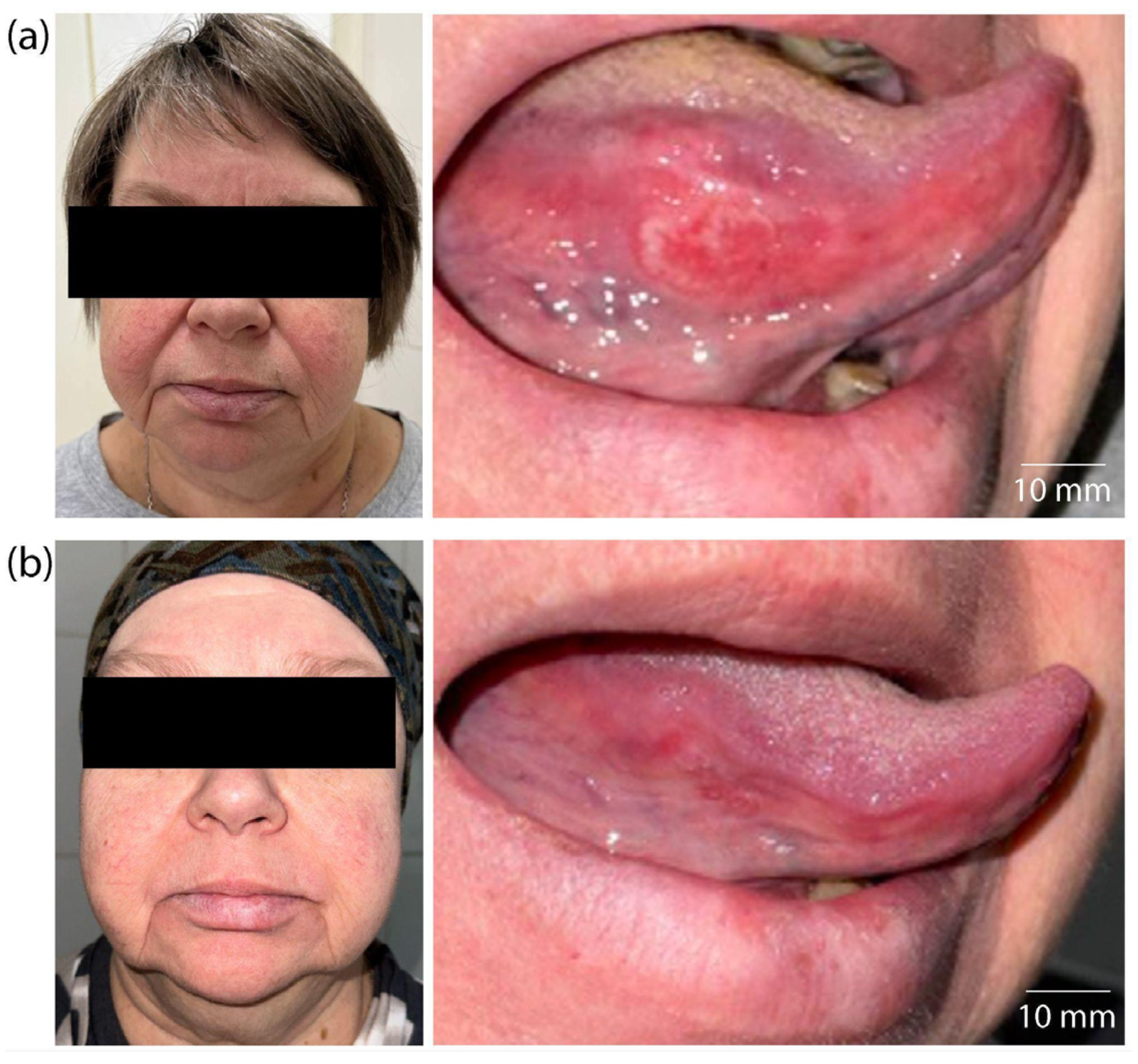
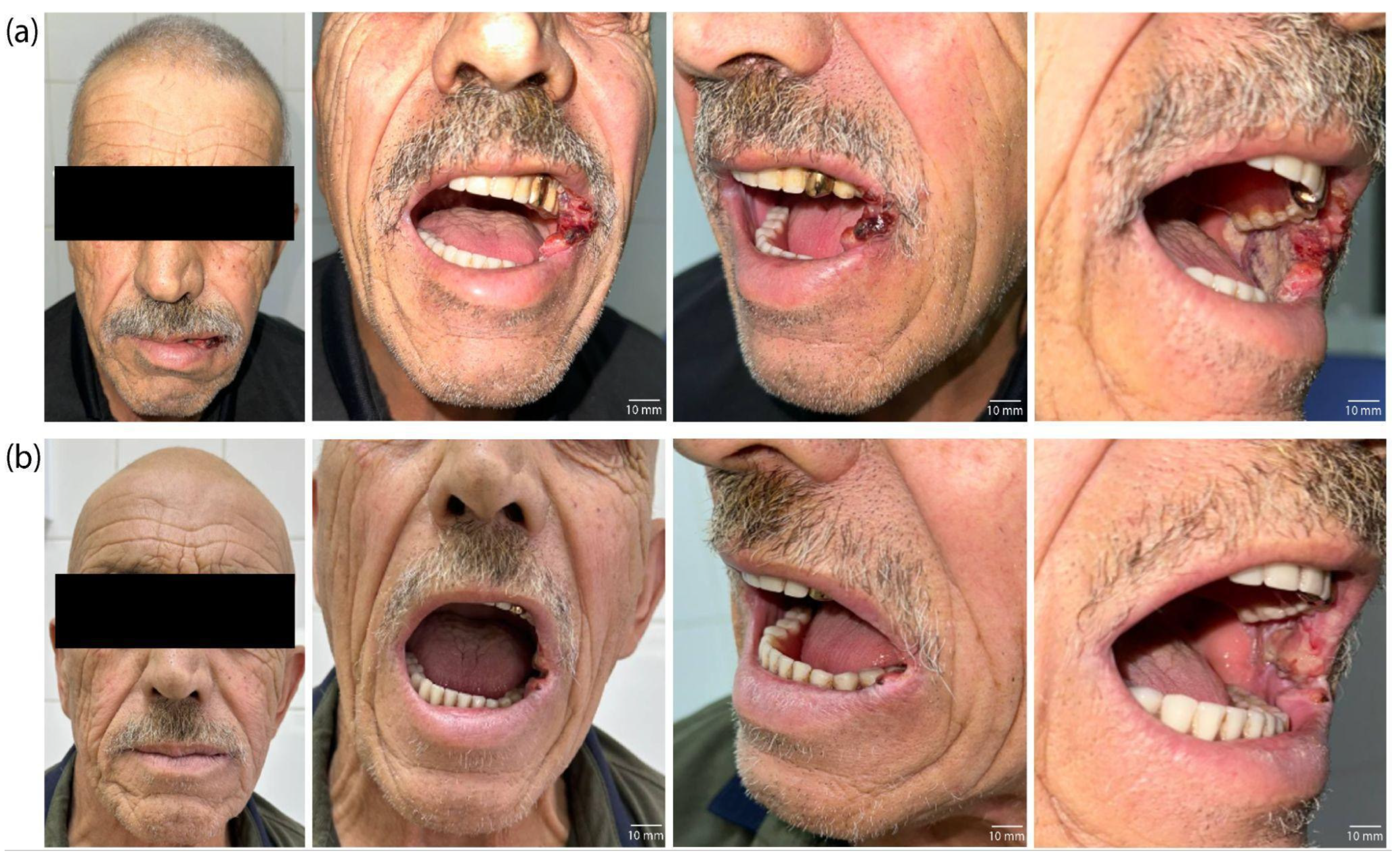
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemotherapy
4.2. Tumor Catheterization
4.3. Photosensitizer
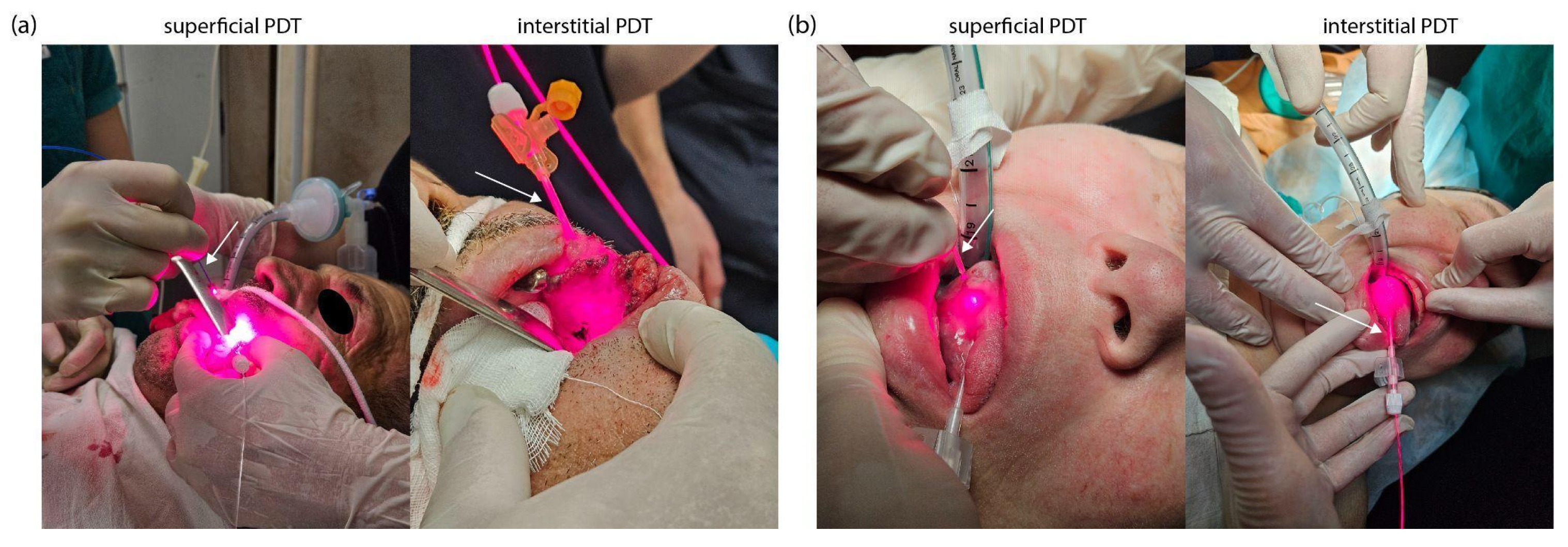
4.4. Photodynamic Therapy
4.5. Spectral-Fluorescence Diagnostics
4.6. Video Fluorescence Imaging
4.7. Confocal Microscopy
4.8. Histology
4.9. Statistical Analysis
5. Conclusions
6. Patients
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HNSCC | Head and neck squamous cell carcinoma |
| PDT | Photodynamic therapy |
| Ce6 | Chlorin e6 |
| PS | Photosensitizer |
| WHO | World Health Organization |
| ECM | Extracellular matrix |
| TME | Tumor microenvironment |
| ROS | Reactive oxygen species |
| Pt(II) | Platinum(II) |
| CDDP | Cis-diamminedichloridoplatinum(II) |
| CBDCA | cis-diammine-1,1-cyclobutanedicarboxylatoplatinum(II) |
| 1-OHP | trans-R,R-cyclohexane-1,2-diamineoxalateplatinum(II) |
| DNA | Deoxyribonucleic acid |
| NCC | Nonresectable cholangiocarcinoma |
| EHCC | Extrahepatic cholangiocarcinoma |
| HCC | Hilar cholangiocarcinoma |
| 9-HPbD | 9-hydroxypheophorbide α |
| mTHPC | Meta-tetra(hydroxyphenyl)chlorin |
| ZnPc | Zinc phthalocyanine |
| DOX | Doxorubicin |
| CT | Computed tomography |
| ECA | External carotid artery |
| PpIX | Protoporphyrin IX |
| EPR | Enhanced permeability and retention |
| OSCCs | Oral squamous cell carcinomas |
| DDR1 | Discoidin domain receptor 1 |
| PR | Partial response |
| CR | Complete response |
References
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services; News; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 1 February 2024).
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- World Cancer Research Fund. Mouth and Oral Cancer Statistics; World Cancer Research Fund International: London, UK, 2023; Available online: https://www.wcrf.org/preventing-cancer/cancer-statistics/mouth-and-oral-cancer-statistics/ (accessed on 27 November 2024).
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral cancer prevalence, mortality, and costs in medicaid and commercial insurance claims data. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017, 171, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Ros, M.; Saltel, F. A complex and evolutive character: Two face aspects of ECM in tumor progression. Front. Oncol. 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Bressmann, T. Speech disorders related to head and neck cancer: Laryngectomy, glossectomy, and velopharyngeal and maxillofacial defects. In The Handbook of Language and Speech Disorders; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 495–527. [Google Scholar]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent advances in the treatment of bone metastases and primary bone tumors: An up-to-date review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—How we can rise to the challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Zagury-Orly, I.; Khaouam, N.; Noujaim, J.; Desrosiers, M.Y.; Maniakas, A. The effect of radiation and chemoradiation therapy on the head and neck mucosal microbiome: A review. Front. Oncol. 2021, 11, 784457. [Google Scholar] [CrossRef]
- Albano, D.; Benenati, M.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Cozzi, D.; De Robertis, R.; Gentili, F.; Grazzini, I.; et al. Imaging side effects and complications of chemotherapy and radiation therapy: A pictorial review from head to toe. Insights Into Imaging 2021, 12, 76. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Vogelius, I.R.; Hodgson, D.; Howell, R.; Jackson, A.; Hua, C.H.; Olch, A.J.; Ronckers, C.; Kremer, L.; Milano, M.; et al. Radiation dose-volume-response relationships for adverse events in childhood cancer survivors: Introduction to the scientific issues in PENTEC. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 338–353. [Google Scholar] [CrossRef]
- Brook, I. Early side effects of radiation treatment for head and neck cancer. Cancer/Radiothérapie 2021, 25, 507–513. [Google Scholar] [CrossRef]
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P. Combinations of photodynamic therapy with other minimally invasive therapeutic technologies against cancer and microbial infections. Int. J. Mol. Sci. 2023, 24, 10875. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Chang, J.E. Enhancing cancer treatment through combined approaches: Photodynamic therapy in concert with other modalities. Pharmaceutics 2024, 16, 1420. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.S.; Bhattarai, H.K. Singlet oxygen, photodynamic therapy, and mechanisms of cancer cell death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Hackbarth, S.; Islam, R.; Šubr, V.; Etrych, T.; Fang, J. Singlet oxygen in vivo: It is all about intensity. J. Pers. Med. 2022, 12, 891. [Google Scholar] [CrossRef]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Makarov, V.I.; Pominova, D.V.; Ryabova, A.V.; Romanishkin, I.D.; Voitova, A.V.; Steiner, R.W.; Loschenov, V.B. Theranostic properties of crystalline aluminum phthalocyanine nanoparticles as a photosensitizer. Pharmaceutics 2022, 14, 2122. [Google Scholar] [CrossRef]
- Lou, P.J.; Jones, L.; Hopper, C. Clinical outcomes of photodynamic therapy for head-and-neck cancer. Technol. Cancer Res. Treat. 2003, 2, 311–317. [Google Scholar] [CrossRef]
- Lange, C.; Bednarski, P.J. Evaluation for synergistic effects by combinations of photodynamic therapy (PDT) with temoporfin (mTHPC) and Pt (II) complexes carboplatin, cisplatin or oxaliplatin in a set of five human cancer cell lines. Int. J. Mol. Sci. 2018, 19, 3183. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S. Platinum-based cancer chemotherapeutics: Recent trends and future perspectives. Curr. Chin. Sci. 2022, 2, 275–293. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Viana Cabral, F.; Quilez Alburquerque, J.; Roberts, H.J.; Hasan, T. Shedding light on chemoresistance: The perspective of photodynamic therapy in cancer management. Int. J. Mol. Sci. 2024, 25, 3811. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Rajabi Kouchi, F.; Eixenberger, J.; Shirini, D.; Estrada, D.; Shirini, F. Synergic antitumor effect of photodynamic therapy and chemotherapy mediated by nano drug delivery systems. Pharmaceutics 2022, 14, 322. [Google Scholar] [CrossRef]
- Compagnin, C.; Mognato, M.; Celotti, L.; Canti, G.; Palumbo, G.; Reddi, E. Cell proliferation and cell cycle alterations in oesophageal p53-mutated cancer cells treated with cisplatin in combination with photodynamic therapy. Cell Prolif. 2010, 43, 262–274. [Google Scholar] [CrossRef]
- Nonaka, M.; Ikeda, H.; Inokuchi, T. Effect of combined photodynamic and chemotherapeutic treatment on lymphoma cells in vitro. Cancer Lett. 2002, 184, 171–178. [Google Scholar] [CrossRef]
- Javani Jouni, F.; Abdollahi, V.; Zadehmodarres, S.; Abbasinia, H.; Asnaashari, M.; Zafari, J. Combination of cisplatin treatment and photodynamic therapy attenuates cisplatin-induced cell toxicity in A2780 and A2780-CP cervical cancer cell lines. Lasers Med. Sci. 2022, 37, 1175–1180. [Google Scholar] [CrossRef]
- Mao, W.; Sun, Y.; Zhang, H.; Cao, L.; Wang, J.; He, P. A combined modality of carboplatin and photodynamic therapy suppresses epithelial-mesenchymal transition and matrix metalloproteinase-2 (MMP-2)/MMP-9 expression in HEp-2 human laryngeal cancer cells via ROS-mediated inhibition of MEK/ERK signalling pathway. Lasers Med. Sci. 2016, 31, 1697–1705. [Google Scholar] [CrossRef]
- He, P.; Ahn, J.C.; Shin, J.I.; Hwang, H.J.; Kang, J.W.; Lee, S.J.; Chung, P.S. Enhanced apoptotic effect of combined modality of 9-hydroxypheophorbide α-mediated photodynamic therapy and carboplatin on AMC-HN-3 human head and neck cancer cells. Oncol. Rep. 2009, 21, 329–334. [Google Scholar] [PubMed]
- Hwang, H.; Biswas, R.; Chung, P.S.; Ahn, J.C. Modulation of EGFR and ROS induced cytochrome c release by combination of photodynamic therapy and carboplatin in human cultured head and neck cancer cells and tumor xenograft in nude mice. J. Photochem. Photobiol. B Biol. 2013, 128, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chang, J.E.; Jheon, S.; Han, S.J.; Kim, J.K. Enhanced production of reactive oxygen species in HeLa cells under concurrent low-dose carboplatin and Photofrin® photodynamic therapy. Oncol. Rep. 2018, 40, 339–345. [Google Scholar]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M.; et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef]
- Tabero, A.; Planas, O.; Gallavardin, T.; Nieves, I.; Nonell, S.; Villanueva, A. Smart dual-functionalized gold nanoclusters for spatio-temporally controlled delivery of combined chemo-and photodynamic therapy. Nanomaterials 2020, 10, 2474. [Google Scholar] [CrossRef]
- Wentrup, R.; Winkelmann, N.; Mitroshkin, A.; Prager, M.; Voderholzer, W.; Schachschal, G.; Jürgensen, C.; Büning, C. Photodynamic therapy plus chemotherapy compared with photodynamic therapy alone in hilar nonresectable cholangiocarcinoma. Gut Liver 2016, 10, 470. [Google Scholar] [CrossRef]
- Chhatre, S.; Vachani, A.; Allison, R.R.; Jayadevappa, R. Survival outcomes with photodynamic therapy, chemotherapy and radiation in patients with stage III or stage IV non-small cell lung cancer. Cancers 2021, 13, 803. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A promising photosensitizer in photo-based cancer nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Gilyadova, A.; Ishchenko, A.; Shiryaev, A.; Alekseeva, P.; Efendiev, K.; Karpova, R.; Loshchenov, M.; Loschenov, V.; Reshetov, I. Phototheranostics of cervical neoplasms with chlorin e6 photosensitizer. Cancers 2022, 14, 211. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Musaev, G.K.; Loshenov, M.V.; Borodkin, A.V.; Levkin, V.V.; Okhotnikova, N.L.; Volkov, V.V.; Makarov, V.I.; Loshenov, V.B. Fluorescence diagnosis and photodynamic therapy in combined treatment of cholangiocarcinoma. Biomed. Photonics 2017, 5, 15–24. [Google Scholar] [CrossRef][Green Version]
- Maltsev, D.S.; Kulikov, A.N.; Vasiliev, A.S.; Chhablani, J. Safety and Efficacy of Photodynamic Therapy with Chlorin E6 in Chronic Central Serous Chorioretinopathy. Retina 2024, 44, 1387–1393. [Google Scholar] [CrossRef]
- Gilyadova, A.V.; Ishchenko, A.A.; Samoilova, S.V.; Shiryaev, A.A.; Novruzaliyeva, M.F.; Efendiev, K.T.; Alekseeva, P.M.; Loschenov, V.B.; Reshetov, I.V. Comparative study of treatment efficacy in severe intraepithelial squamous cell lesions and preinvasive cervical cancer by conization and chlorin e6-mediated fluorescence-assisted systemic photodynamic therapy. Photodiagnosis Photodyn. Ther. 2024, 46, 104060. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Smirnova, N.L.; Privalov, O.A.; Moryganova, T.M.; Strelnikov, A.I.; Morshnev, P.K.; Koifman, O.I.; Lyubimtsev, A.V.; Kustova, T.V.; Berezin, D.B. Transurethral resection of non-muscle invasive bladder tumors combined with fluorescence diagnosis and photodynamic therapy with chlorin e6-type photosensitizers. J. Clin. Med. 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Onodera, M.; Kitahara, S.; Sato, Y.; Kawamata, T.; Muragaki, Y.; Masamune, K. Prognostic Factors and Talaporfin Sodium Concentration in Photodynamic Therapy for Recurrent Grade 4 Glioma. Pharmaceuticals 2025, 18, 583. [Google Scholar] [CrossRef]
- Wiehe, A.; Senge, M.O. The Photosensitizer Temoporfin (m THPC)–chemical, Pre-clinical and Clinical Developments in the Last Decade. Photochem. Photobiol. 2023, 99, 356–419. [Google Scholar] [CrossRef]
- Kübler, A.C.; De Carpentier, J.; Hopper, C.; Leonard, A.G.; Putnam, G. Treatment of squamous cell carcinoma of the lip using Foscan-mediated photodynamic therapy. Int. J. Oral Maxillofac. Surg. 2001, 30, 504–509. [Google Scholar] [CrossRef]
- Fontana, L.C.; Pinto, J.G.; Magalhães, J.A.; Tada, D.B.; de Almeida, R.M.S.; Pacheco-Soares, C.; Ferreira-Strixino, J. Comparison of the photodynamic effect of two chlorins, photodithazine and fotoenticine, in gliosarcoma cells. Photochem 2022, 2, 165–180. [Google Scholar] [CrossRef]
- Čunderlíková, B.; Kongshaug, M.; Gangeskar, L.; Moan, J. Increased binding of chlorin e6 to lipoproteins at low pH values. Int. J. Biochem. Cell Biol. 2000, 32, 759–768. [Google Scholar] [CrossRef]
- Sun, H.; Ong, Y.; Kim, M.M.; Dimofte, A.; Singhal, S.; Cengel, K.A.; Yodh, A.G.; Zhu, T.C. A Comprehensive Study of Reactive Oxygen Species Explicit Dosimetry for Pleural Photodynamic Therapy. Antioxidants 2024, 13, 1436. [Google Scholar] [CrossRef]
- Gonzalez-Carmona, M.A.; Bolch, M.; Jansen, C.; Vogt, A.; Sampels, M.; Mohr, R.U.; van Beekum, K.; Mahn, R.; Praktiknjo, M.; Nattermann, J.; et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment. Pharmacol. Ther. 2019, 49, 437–447. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Zhu, Y.; Pan, W.; Ma, W.Q.; Shao, A.L. Photodynamic therapy combined with local chemotherapy for the treatment of advanced esophagocardiac carcinoma. Photodiagnosis Photodyn. Ther. 2007, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Cheon, Y.K.; Lee, E.J.; Lee, T.Y.; Shim, C.S. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2013, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, K.; Alekseeva, P.; Linkov, K.; Shiryaev, A.; Pisareva, T.; Gilyadova, A.; Reshetov, I.; Voitova, A.; Loschenov, V. Tumor fluorescence and oxygenation monitoring during photodynamic therapy with chlorin e6 photosensitizer. Photodiagnosis Photodyn. Ther. 2024, 45, 103969. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef]
- Zavedeeva, V.E.; Efendiev, K.T.; Kustov, D.M.; Loschenova, L.Y.; Loschenov, V.B. Dual-Wavelength Fluorescence Study of In Vivo Accumulation and Formation of 5-ALA-Induced Porphyrins. Biomed. Photonics 2025, 14, 36–46. [Google Scholar]
- Kiening, M.; Lange, N. A recap of heme metabolism towards understanding protoporphyrin IX selectivity in cancer cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef]
- Efendiev, K.T.; Alekseeva, P.M.; Shiryaev, A.A.; Skobeltsin, A.S.; Solonina, I.L.; Fatyanova, A.S.; Reshetov, I.V.; Loschenov, V.B. Preliminary low-dose photodynamic exposure to skin cancer with chlorin e6 photosensitizer. Photodiagnosis Photodyn. Ther. 2022, 38, 102894. [Google Scholar] [CrossRef]
- Artsemyeva, T.P.; Tzerkovsky, D.A. Efficacy of photodynamic therapy with chlorine-based photosensitizer in the treatment of basal cell carcinomas. J. Anal. Oncol. 2023, 12, 15–23. [Google Scholar] [CrossRef]
- Strojan, P.; Vermorken, J.B.; Beitler, J.J.; Saba, N.F.; Haigentz Jr, M.; Bossi, P.; Worden, F.P.; Langendijk, J.A.; Eisbruch, A.; Mendenhall, W.M.; et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2016, 38, E2151–E2158. [Google Scholar] [CrossRef]
- Huang, R.; Boltze, J.; Li, S. Strategies for improved intra-arterial treatments targeting brain tumors: A systematic review. Front. Oncol. 2020, 10, 1443. [Google Scholar] [CrossRef]
- Kumagai, T.; Takeda, N.; Fukase, S.; Koshu, H.; Inoue, A.; Ibuchi, Y.; Yoneoka, Y. Intra-arterial chemotherapy for malignant tumors of head and neck region using three types of modified injection method. Interv. Neuroradiol. 2003, 9, 113–123. [Google Scholar] [CrossRef]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Su, H.; Mu, G.; Sun, J.H.; Tan, C.P.; Liang, X.; Ji, L.; Mao, Z.W. Co-delivery of cisplatin prodrug and chlorin e6 by mesoporous silica nanoparticles for chemo-photodynamic combination therapy to combat drug resistance. ACS Appl. Mater. Interfaces 2016, 8, 13332–13340. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Qiu, H.; Huang, K.; Xu, Q.; Zhou, B.; Zhang, L.; Zhou, M.; Yi, X. A co-delivery system based on chlorin e6-loaded ROS-sensitive polymeric prodrug with self-amplified drug release to enhance the efficacy of combination therapy for breast tumor cells. Front. Bioeng. Biotechnol. 2023, 11, 1168192. [Google Scholar] [CrossRef] [PubMed]
- El Herch, I.; Tornaas, S.; Dongre, H.N.; Costea, D.E. Heterogeneity of cancer-associated fibroblasts and tumor-promoting roles in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2024, 11, 1340024. [Google Scholar] [CrossRef] [PubMed]
- Griso, A.B.; Acero-Riaguas, L.; Castelo, B.; Cebrián-Carretero, J.L.; Sastre-Perona, A. Mechanisms of cisplatin resistance in HPV negative head and neck squamous cell carcinomas. Cells 2022, 11, 561. [Google Scholar] [CrossRef]
- Lai, S.L.; Tan, M.L.; Hollows, R.J.; Robinson, M.; Ibrahim, M.; Margielewska, S.; Parkinson, E.K.; Ramanathan, A.; Zain, R.B.; Mehanna, H.; et al. Collagen induces a more proliferative, migratory and chemoresistant phenotype in head and neck cancer via DDR1. Cancers 2019, 11, 1766. [Google Scholar] [CrossRef]
- Yu, T.T.; Hu, J.; Li, Q.R.; Peng, X.C.; Xu, H.Z.; Han, N.; Li, L.; Yang, X.; Xu, X.; Yang, Z.; et al. Chlorin e6-induced photodynamic effect facilitates immunogenic cell death of lung cancer as a result of oxidative endoplasmic reticulum stress and DNA damage. Int. Immunopharmacol. 2023, 115, 109661. [Google Scholar] [CrossRef]
- Lintern, N.; Smith, A.M.; Jayne, D.G.; Khaled, Y.S. Photodynamic stromal depletion in pancreatic ductal adenocarcinoma. Cancers 2023, 15, 4135. [Google Scholar] [CrossRef]
- Gaber, S.A.A.; Müller, P.; Zimmermann, W.; Hüttenberger, D.; Wittig, R.; Kader, M.H.A.; Stepp, H. ABCG2-mediated suppression of chlorin e6 accumulation and photodynamic therapy efficiency in glioblastoma cell lines can be reversed by KO143. J. Photochem. Photobiol. B Biol. 2018, 178, 182–191. [Google Scholar] [CrossRef]
- Robey, R.W.; Steadman, K.; Polgar, O.; Bates, S.E. ABCG2-mediated transport of photosensitizers: Potential impact on photodynamic therapy. Cancer Biol. Ther. 2005, 4, 195–202. [Google Scholar] [CrossRef]
- Yin, W.; Xiang, D.; Wang, T.; Zhang, Y.; Pham, C.V.; Zhou, S.; Jiang, G.; Hou, Y.; Zhu, Y.; Han, Y.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Huang, Z.; Chen, X.; Yan, X.; Zhai, J.; Ma, Y. Evaluation of curcumin-mediated photodynamic therapy on the reverse of multidrug resistance in tumor cells. RSC Adv. 2020, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Shiryaev, A.; Alekseeva, P.; Efendiev, K.; Yakovlev, D.; Moskalev, A.; Kozlikina, E.; Kharnas, P.; Reshetov, I.; Levkin, V.; Loshchenov, V. Investigated spectral-fluorescent properties of endogenous porphyrins of the wild boar hepatobiliary system optimize the diagnostics and treatment of cholangiocarcinoma with FD and PDT. Opt. Eng. 2020, 59, 061615. [Google Scholar] [CrossRef]
- Chitose, S.I.; Chijiwa, H.; Maeda, A.; Umeno, H.; Nakashima, T.; Kiyokawa, K.; Hayabuchi, N.; Fujita, H. Evaluation of overall tumor cellularity after neoadjuvant chemotherapy in patient with locally advanced hypopharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 2391–2399. [Google Scholar] [CrossRef]
- Bond, C.; Lancaster, G.A.; Campbell, M.; Chan, C.; Eddy, S.; Hopewell, S.; Mellor, K.; Thabane, L.; Eldridge, S. Pilot and feasibility studies: Extending the conceptual framework. Pilot Feasibility Stud. 2023, 9, 24. [Google Scholar] [CrossRef]
- Teresi, J.A.; Yu, X.; Stewart, A.L.; Hays, R.D. Guidelines for designing and evaluating feasibility pilot studies. Med. Care 2022, 60, 95–103. [Google Scholar] [CrossRef]


| Study | PS | Chemotherapeutic Agent | Administration Method | Cancer Type | Key Findings |
|---|---|---|---|---|---|
| Wentrup R. et al., 2016 [40] | Photofrin II® | Cisplatin/Oxaliplatin/Gemcitabine | Intravenous (PS) + Systemic (Chemo) | Hilar NCC | Combined therapy improved survival vs. PDT alone. |
| Gonzalez-Carmona M. A. et al., 2019 [54] | Photosan®/Photofrin®/Foscan® (Temoporfin, mTHPC) | Gemcitabine/Cisplatin | Intravenous (PS) + Systemic (Chemo) | Advanced EHCC | Combination PDT and chemotherapy was well tolerated and resulted in significantly longer survival than chemotherapy alone. |
| Zhang N. Z. et al., 2007 [55] | Photocarcinorin (PSD-007) | 5-fluorouracil | Intravenous (PS) + Local Intra-Arterial (Chemo) | Advanced Esophagocardiac Carcinoma | Therapeutic effect of the PDT can be further improved when combined with local chemotherapy. |
| Hong M. J. et al., 2013 [56] | Photofrin® | Gemcitabine/Cisplatin | Intravenous (PS) + Systemic (Chemo) | Advanced HCC | PDT with chemotherapy results in longer survival than PDT alone. |
| Current study | Ce6 (Photoran E6®) | Cisplatin | Local Intra-Arterial (PS) + Local Intra-Arterial (Chemo) | Advanced HNSCC | Targeted delivery of both agents (PS and Chemo), reduced doses, and showed high efficacy and safety. |
| Parameter | Patient PCM | Patient KIA |
|---|---|---|
| Gender | male | female |
| Age | 64 years | 70 years |
| Weight | 63 kg | 103 kg |
| Body Mass Index | 23.7 | 39.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efendiev, K.; Shiryaev, A.; Rakhmanova, A.; Pisareva, T.; Mamedova, A.; Samoylova, S.; Reshetov, I.; Skobeltsin, A.; Krivetskaya, A.; Ryabova, A.; et al. Combined Photodynamic Therapy and Chemotherapy Using Local Intra-Arterial Intratumoral Administration of Chlorin e6 and Cisplatin: First Clinical Observations. Int. J. Mol. Sci. 2025, 26, 8640. https://doi.org/10.3390/ijms26178640
Efendiev K, Shiryaev A, Rakhmanova A, Pisareva T, Mamedova A, Samoylova S, Reshetov I, Skobeltsin A, Krivetskaya A, Ryabova A, et al. Combined Photodynamic Therapy and Chemotherapy Using Local Intra-Arterial Intratumoral Administration of Chlorin e6 and Cisplatin: First Clinical Observations. International Journal of Molecular Sciences. 2025; 26(17):8640. https://doi.org/10.3390/ijms26178640
Chicago/Turabian StyleEfendiev, Kanamat, Artem Shiryaev, Aidai Rakhmanova, Tatiana Pisareva, Alena Mamedova, Svetlana Samoylova, Igor Reshetov, Alexey Skobeltsin, Anna Krivetskaya, Anastasia Ryabova, and et al. 2025. "Combined Photodynamic Therapy and Chemotherapy Using Local Intra-Arterial Intratumoral Administration of Chlorin e6 and Cisplatin: First Clinical Observations" International Journal of Molecular Sciences 26, no. 17: 8640. https://doi.org/10.3390/ijms26178640
APA StyleEfendiev, K., Shiryaev, A., Rakhmanova, A., Pisareva, T., Mamedova, A., Samoylova, S., Reshetov, I., Skobeltsin, A., Krivetskaya, A., Ryabova, A., Makarov, V., & Loschenov, V. (2025). Combined Photodynamic Therapy and Chemotherapy Using Local Intra-Arterial Intratumoral Administration of Chlorin e6 and Cisplatin: First Clinical Observations. International Journal of Molecular Sciences, 26(17), 8640. https://doi.org/10.3390/ijms26178640







