Are Hippocampal Hypoperfusion and ATP Depletion Prime Movers in the Genesis of Alzheimer’s Disease? A Review of Recent Pertinent Observations from Molecular Biology
Abstract
1. Introduction
2. Brain Ageing
3. A Role for ATP Depletion in the Genesis of AD
3.1. Matching ATP Production to Requirement in the Brain
3.2. Biosensors of ATP Status
3.2.1. AMPK
3.2.2. Sirtuins
3.2.3. Phosphofructokinase (PFK)
3.2.4. ATP Regulation by Mitochondrial Nucleotide Transporters
3.3. Hypoxia-Inducible Factor 1 (HIF-1) Mediates the Response to Hypoxia
Hypoxia Up-Regulated Mitochondrial Movement Regulator (HUMMR)
3.4. Mitochondrial-Derived Peptides (MDPs) and Nuclear-Encoded Microproteins
3.5. Spectrum of Molecules Involved in ATP Turnover
4. Brain Processes with Very High ATP Consumption/Turnover
4.1. The Malate-Aspartate Shuttle
4.2. The Glutamate/GABA/Glutamine Cycle
4.2.1. The Energy Cost of the Glutamate/GABA/Glutamine Cycle
4.2.2. Disturbances of the Glutamate/GABA/Glutamine Cycle in AD
4.2.3. Effects of Hypoxia/Ischaemia on the Glutamate/GABA/Glutamine Cycle
4.2.4. Promoting Anaplerosis in Astrocytes to Support Glutamine Synthesis
4.3. Axonal Transport Has a High Energy Requirement
4.3.1. Axonal Transport of Mitochondria
4.3.2. Role of Tau Protein in Axon Transport
4.3.3. Disordered Axonal Transport in AD
5. Effects of ATP Depletion on Lipid Metabolism
5.1. Glycerophospholipids
5.1.1. Synthesis
5.1.2. Physiological Functions
5.1.3. Pathophysiology
Membrane Peroxidation of PUFAs
5.1.4. Potential Role of Disordered Membrane Phospholipids in Promoting Aβ Production from Amyloid Precursor Protein (APP)
5.1.5. Disturbances of Membrane Lipids in AD
6. Hypoperfusion of the Hippocampus
6.1. Blood Supply to the Brain Cortex and Hippocampus
6.2. Features of the Hippocampal Vasculature Increase the Risk for Hypoperfusion
6.3. Neurovascular Coupling and the Effects of Hypoxia
6.4. Effects of Hypertension on Cerebral Blood Flow
7. Genomic, Proteomic, Metabolomic, and Imaging Investigations to Identify Causative Genes and Pathways in AD
7.1. Human Studies
7.2. Animal Studies
8. Discussion
8.1. How Could the Hypoperfusion/ATP Depletion Model Influence Clinical Practice?
8.1.1. Therapy
8.1.2. Potential Markers
8.2. Suggestions for Further Study
9. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK5′ | AMP-activated protein kinase |
| APH1 (Anterior Pharynx defective 1) | a component of the gamma-secretase complex |
| APP | Amyloid Beta Precursor Protein |
| BACE | Beta-Secretase APP Beta-Secretase |
| BBB | blood–brain barrier |
| CBF | cerebral blood flow |
| rCBF | regional cerebral blood flow |
| FDG-PET | fluorodeoxyglucose (FDG)-positive emission tomography (PET) |
| GSEA | gene set enrichment analysis |
| HDAC4 | Histone Deacetylase |
| HIF1α, | Hypoxia-inducible factor 1-alpha |
| HUMMR | hypoxia up-regulated mitochondrial movement regulator |
| LOAD | late-onset Alzheimer’s disease |
| MCI | Mild Cognitive Impairment |
| Miro1 and Miro2 | Mitochondrial Rho GTPase proteins |
| Mitochondrial-derived peptides: | |
| GAU | gene antisense ubiquitous |
| MOTS-c | Mitochondrial ORF of the 12S rRNA Type-C |
| MtALTND4 | protein encoded from an alternative open reading frame of the gene for the NADH-ubiquinone oxidoreductase chain 4 (ND4) protein |
| SHLP1 -SHLP6 | six small humanin-like peptides with 20–35 amino acids |
| SHMOOSE | Small Human Mitochondrial ORF Over SErine tRNA |
| MRI | Magnetic Resonance Imaging |
| NFTs | neurofibrillary tangles, |
| PEN2, | Gamma-secretase subunit |
| PET | positive emission tomography |
| PS1/PS2 | Presenilin 1/2 |
| PUFAs | polyunsaturated fatty acids |
| SAH | subarachnoid haemorrhage |
| SREBP-2 | Sterol regulatory element binding protein-2 |
| Transgenic mouse models: | |
| Tg25476 AD mice | overexpress a mutated form of APP (the ‘Swedish mutation’). Develop amyloid plaques and cognitive deficits |
| APPswe/PSEN1dE9 (PSAPP) | carry both the Swedish mutation and a presenilin mutation |
| 5xFAD mice | express 5 mutations in two genes (APP and Presenilin-1); have increased Aβpeptide, amyloid plaques, and cognitive deficits |
| 3xTG mice | express the Swedish mutation, and mutations in PSEN1and human Tau |
| Tg4-42 mouse model | express N-truncated 4-42 Aβ |
References
- Gómez-Isla, T.; Price, J.L.; McKeel, D.W., Jr.; Morris, J.C.; Growdon, J.H.; Hyman, B.T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 1996, 16, 4491–4500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, G.R.; Carlyle, B.C. Interrogation of the human cortical peptidome uncovers cell-type specific signatures of cognitive resilience against Alzheimer’s disease. Sci. Rep. 2024, 14, 7161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thangavel, R.; Kempuraj, D.; Stolmeier, D.; Anantharam, P.; Khan, M.; Zaheer, A. Glia maturation factor expression in entorhinal cortex of Alzheimer’s disease brain. Neurochem. Res. 2013, 38, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kapadia, A.; Billimoria, K.; Desai, P.; Grist, J.T.; Heyn, C.; Maralani, P.; Symons, S.; Zaccagna, F. Hypoperfusion Precedes Tau Deposition in the Entorhinal Cortex: A Retrospective Evaluation of ADNI-2 Data. J. Clin. Neurol. 2023, 19, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kahn, I.; Andrews-Hanna, J.R.; Vincent, J.L.; Snyder, A.Z.; Buckner, R.L. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008, 100, 129–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torrico, T.J.; Abdijadid, S. Neuroanatomy, Limbic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538491/ (accessed on 25 July 2025).
- Patel, A.; Biso, G.M.N.R.; Fowler, J.B. Neuroanatomy, Temporal Lobe. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519512/ (accessed on 15 June 2025).
- Baloyannis, S.J. Mitochondrial alterations in Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman spectroscopy and machine learning for biomedical applications: Alzheimer’s disease diagnosis based on the analysis of cerebrospinal fluid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119188. [Google Scholar] [CrossRef] [PubMed]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Li, L.; Cai, S.; Song, K.; Hu, S. Identification of novel risk genes for Alzheimer’s disease by integrating genetics from hippocampus. Sci. Rep. 2024, 14, 27484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.Y.; Lee, Y.S.; Liu, Y.L.; Hsu, W.C.; Ho, W.M.; Huang, Y.H.; Tsai, S.J.; Kuo, P.H.; Chen, Y.C. Association study of alcohol dehydrogenase and aldehyde dehydrogenase polymorphism with Alzheimer Disease in the Taiwanese Population. Front. Neurosci. 2021, 15, 625885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, C.Y.; Wu, S.M.; Kuan, Y.C.; Lin, Y.T.; Hsu, C.R.; Hsu, W.H.; Liu, Y.S.; Majumdar, A.; Stettler, M.; Yang, C.M.; et al. Associations between risk of Alzheimer’s disease and obstructive sleep apnea, intermittent hypoxia, and arousal responses: A pilot study. Front. Neurol. 2022, 13, 1038735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adlimoghaddam, A.; Sabbir, M.G.; Albensi, B.C. Ammonia as a Potential Neurotoxic Factor in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seiler, N. Ammonia and Alzheimer’s disease. Neurochem. Int. 2002, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.; Wolf, A.B.; Chavira, B.; Shonebarger, D.; Meckel, J.P.; Leung, L.; Ballina, L.; Ly, S.; Saini, A.; Jones, T.B.; et al. Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E ɛ4 Carriers. J. Alzheimers Dis. 2016, 53, 95–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yen, K.; Miller, B.; Kumagai, H.; Silverstein, A.; Cohen, P. Mitochondrial-derived microproteins: From discovery to function. Trends Genet. 2024, 41, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Jia, K.; Wang, T.; Guo, L.; Xuan, Z.; Michaelis, E.K.; Swerdlow, R.H.; Alzheimer’s Disease Neuroimaging Initiative; Du, H. Hippocampal transcriptome-wide association study and pathway analysis of mitochondrial solute carriers in Alzheimer’s disease. Transl. Psychiatry 2024, 14, 250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nordestgaard, L.T.; Tybjaerg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimers Dement. 2015, 11, 1430–1438. [Google Scholar] [CrossRef]
- Aikawa, T.; Holm, M.L.; Kanekiyo, T. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Roeck, A.; Van Broeckhoven, C.; Sleegers, K. The role of ABCA7 in Alzheimer’s disease: Evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019, 138, 201–220. [Google Scholar] [CrossRef]
- Picard, C.; Julien, C.; Frappier, J.; Miron, J.; Théroux, L.; Dea, D.; United Kingdom Brain Expression Consortium and for the Alzheimer’s Disease Neuroimaging Initiative; Breitner, J.C.S.; Poirier, J. Alterations in cholesterol metabolism-related genes in sporadic Alzheimer’s disease. Neurobiol. Aging 2018, 66, 180.e1–180.e9. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Zakeri, N.S.; Pashazadeh, S.; MotieGhader, H. Gene biomarker discovery at different stages of Alzheimer using gene co-expression network approach. Sci. Rep. 2020, 10, 12210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef]
- Lanoiselée, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.Y.; Bingham, E.J.; Twine, S.M.; Geiger, J.D.; Ghribi, O. Metabolomic Identification in cerebrospinal fluid of the effects of high dietary cholesterol in a rabbit model of Alzheimer’s Disease. Metabolomics 2012, 2, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aksman, L.M.; Oxtoby, N.P.; Scelsi, M.A.; Wijeratne, P.A.; Young, A.L.; Alves, I.L.; Collij, L.E.; Vogel, J.W.; Barkhof, F.; Alexander, D.C.; et al. ADNI. A data-driven study of Alzheimer’s disease related amyloid and tau pathology progression. Brain 2023, 146, 4935–4948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef] [PubMed]
- Lamari, F.; Rossignol, F.; Mitchell, G.A. Glycerophospholipids: Roles in Cell Trafficking and Associated Inborn Errors. J. Inherit. Metab. Dis. 2025, 48, e70019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halliwell, B.; Gutteridge, J.M. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet 1984, 1, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.; Pickard, J.D. Prostaglandins, thromboxane, leukotrienes and the cerebral circulation in health and disease. Adv. Tech. Stand. Neurosurg. 1985, 12, 3–90. [Google Scholar] [CrossRef] [PubMed]
- Feringa, F.M.; van der Kant, R. Cholesterol and Alzheimer’s Disease; from risk genes to pathological effects. Front. Aging Neurosci. 2021, 13, 690372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vetrivel, K.S.; Thinakaran, G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim. Biophys. Acta 2010, 1801, 860–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554–567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, A.C. Hippocampal vascular supply and its role in vascular cognitive impairment. Stroke 2023, 54, 673–685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petralia, R.S.; Mattson, M.P.; Yao, P.J. Communication breakdown: The impact of ageing on synapse structure. Ageing Res. Rev. 2014, 14, 31–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ledig, C.; Schuh, A.; Guerrero, R.; Heckemann, R.A.; Rueckert, D. Structural brain imaging in Alzheimer’s disease and mild cognitive impairment: Biomarker analysis and shared morphometry database. Sci. Rep. 2018, 8, 11258. [Google Scholar] [CrossRef]
- Ingram, T.; Chakrabarti, L. Proteomic profiling of mitochondria: What does it tell us about the ageing brain? Aging 2016, 8, 3161–3179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boveris, A.; Navarro, A. Brain mitochondrial dysfunction in aging. IUBMB Life 2008, 60, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, M.L.; Martínez, M.; De Juan, E.; Díez, A.; Bustos, G.; Miquel, J. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res. 1994, 644, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Rottenberg, H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem. Biophys. Res. Commun. 2000, 273, 603–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stauch, K.L.; Purnell, P.R.; Fox, H.S. Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function. Aging 2014, 6, 320–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stauch, K.L.; Purnell, P.R.; Villeneuve, L.M.; Fox, H.S. Proteomic analysis and functional characterization of mouse brain mitochondria during aging reveal alterations in energy metabolism. Proteomics 2015, 15, 1574–1586. [Google Scholar] [CrossRef]
- Groebe, K.; Krause, F.; Kunstmann, B.; Unterluggauer, H.; Reifschneider, N.H.; Scheckhuber, C.Q.; Sastri, C.; Stegmann, W.; Wozny, W.; Schwall, G.P.; et al. Differential proteomic profiling of mitochondria from Podospora anserina, rat and human reveals distinct patterns of age-related oxidative changes. Exp. Gerontol. 2007, 42, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, J.; Xin, Y.; Mi, X.; Wang, J.; Gao, Q.; Luo, H. Gene expression analysis reveals novel gene signatures between young and old adults in human prefrontal cortex. Front. Aging Neurosci. 2018, 10, 259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez-Cabrera, M.C.; Ferrando, B.; Brioche, T.; Sanchis-Gomar, F.; Viña, J. Exercise and antioxidant supplements in the elderly. J. Sport Health Sci. 2013, 2, 94–100. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Kenworthy, A.K. Lipid peroxidation drives liquid-liquid phase separation and disrupts raft protein partitioning in biological membranes. J. Am. Chem. Soc. 2024, 146, 1374–1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garin, C.M.; Nadkarni, N.A.; Pépin, J.; Flament, J.; Dhenain, M. Whole brain mapping of glutamate distribution in adult and old primates at 11.7T. Neuroimage 2022, 251, 118984. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Ferreira, T.; Rodriguez, S. Mitochondrial DNA: Inherent Complexities Relevant to Genetic Analyses. Genes 2024, 15, 617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.L.; Corey, C.; White, P.; Watson, A.; Gladwin, M.T.; Simon, M.A.; Shiva, S. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. JCI Insight 2018, 2, e91415. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Hardie, D.G. Keeping the home fires burning: AMP-activated protein kinase. J. R. Soc. Interface 2018, 15, 20170774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapa, R.; Moglad, E.; Afzal, M.; Gupta, G.; Bhat, A.A.; Hassan Almalki, W.; Kazmi, I.; Alzarea, S.I.; Pant, K.; Singh, T.G.; et al. The role of sirtuin 1 in ageing and neurodegenerative disease: A molecular perspective. Ageing Res. Rev. 2024, 102, 102545. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e27, Erratum in Cell 2024, 187, 1312–1313. https://doi.org/10.1016/j.cell.2024.01.049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Razick, D.I.; Akhtar, M.; Wen, J.; Alam, M.; Dean, N.; Karabala, M.; Ansari, U.; Ansari, Z.; Tabaie, E.; Siddiqui, S. The Role of Sirtuin 1 (SIRT1) in Neurodegeneration. Cureus 2023, 15, e40463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burtscher, J.; Denti, V.; Gostner, J.M.; Weiss, A.K.; Strasser, B.; Hüfner, K.; Burtscher, M.; Paglia, G.; Kopp, M.; Dünnwald, T. The interplay of NAD and hypoxic stress and its relevance for ageing. Ageing Res. Rev. 2025, 104, 102646. [Google Scholar] [CrossRef] [PubMed]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: From chemistry to the clinic. Clin. Epigenet. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Wu, Z.; Zhao, P. The protective effects of activating Sirt1/NF-κB pathway for neurological disorders. Rev. Neurosci. 2021, 33, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cimen, H.; Han, M.J.; Yang, Y.; Tong, Q.; Koc, H.; Koc, E.C. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 2010, 49, 304–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Liu, X.; Su, C.; Wu, F.; Sun, J.; Zhang, J.; Yang, X.; Zhang, C.; Zhou, Z.; Zhang, X.; et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bround, M.J.; Bers, D.M.; Molkentin, J.D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 2020, 144, A3–A13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gavaldà-Navarro, A.; Mampel, T.; Viñas, O. Changes in the expression of the human adenine nucleotide translocase isoforms condition cellular metabolic/proliferative status. Open Biol. 2016, 6, 150108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atlante, A.; Valenti, D. A walk in the memory, from the first functional approach up to its regulatory role of mitochondrial bioenergetic flow in health and disease: Focus on the adenine nucleotide translocator. Int. J. Mol. Sci. 2021, 22, 4164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, L.; Titus, A.S.; Banerjee, K.K.; George, S.; Lin, W.; Deota, S.; Saha, A.K.; Nakamura, K.; Gut, P.; Verdin, E.; et al. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via, AMPK. Aging 2013, 5, 835–849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Cheng, X.; Sun, H.; Li, Y.; Mei, W.; Zeng, C. Atractyloside Protect Mice Against Liver Steatosis by Activation of Autophagy via ANT-AMPK-mTORC1 Signaling Pathway. Front. Pharmacol. 2021, 12, 736655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Austin, J.; Aprille, J.R. Carboxyatractyloside-insensitive influx and efflux of adenine nucleotides in rat liver mitochondria. J. Biol. Chem. 1984, 259, 154–160. [Google Scholar] [CrossRef] [PubMed]
- del Arco, A.; Satrústegui, J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004, 279, 24701–24713. [Google Scholar] [CrossRef]
- Fiermonte, G.; De Leonardis, F.; Todisco, S.; Palmieri, L.; Lasorsa, F.M.; Palmieri, F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2004, 279, 30722–30730. [Google Scholar] [CrossRef]
- Aprille, J.R. Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. J. Bioenerg. Biomembr. 1993, 25, 473–481. [Google Scholar] [CrossRef]
- Anunciado-Koza, R.P.; Zhang, J.; Ukropec, J.; Bajpeyi, S.; Koza, R.A.; Rogers, R.C.; Cefalu, W.T.; Mynatt, R.L.; Kozak, L.P. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J. Biol. Chem. 2011, 286, 11659–11671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofherr, A.; Seger, C.; Fitzpatrick, F.; Busch, T.; Michel, E.; Luan, J.; Osterried, L.; Linden, F.; Kramer-Zucker, A.; Wakimoto, B.; et al. The mitochondrial transporter SLC25A25 links ciliary TRPP2 signaling and cellular metabolism. PLoS Biol. 2018, 16, e2005651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofherr, A.; Seger, C.; Fitzpatrick, F.; Busch, T.; Michel, E.; Luan, J.; Osterried, L.; Linden, F.; Kramer-Zucker, A.; Wakimoto, B.; et al. The mitochondrial transporter SLC25A25 links ciliary TRPP2 signaling and cellular metabolism, Supplement S2 Data. PLoS Biol. 2018, 16, e2005651. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, e2005651. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Jiang, X.; Wang, Y.; Li, C.; Lin, Z.; Wei, Y.; Ni, Q. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell. Mol. Neurobiol. 2024, 44, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, T.K.; Huang, C.R.; Lin, K.J.; Hsieh, Y.H.; Chen, S.D.; Lin, Y.C.; Chao, A.C.; Yang, D.I. Potential Roles of Hypoxia-Inducible Factor-1 in Alzheimer’s Disease: Beneficial or Detrimental? Antioxidants 2024, 13, 1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arany, Z.; Huang, L.E.; Eckner, R.; Bhattacharya, S.; Jiang, C.; Goldberg, M.A.; Bunn, H.F.; Livingston, D.M. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 12969–12973. [Google Scholar] [CrossRef]
- Ema, M.; Hirota, K.; Mimura, J.; Abe, H.; Yodoi, J.; Sogawa, K.; Poellinger, L.; Fujii-Kuriyama, Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999, 18, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.; Mottet, D.; Michel, G.; Roland, I.; Raes, M.; Remacle, J.; Michiels, C. Hypoxia-induced activation of HIF-1: Role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999, 460, 251–256. [Google Scholar] [CrossRef]
- Carrero, P.; Okamoto, K.; Coumailleau, P.; O’Brien, S.; Tanaka, H.; Poellinger, L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 2000, 20, 402–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragoussis, J.; Ratcliffe, P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009, 284, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ponka, P. Identification of a hypoxia response element in the transferrin receptor gene. J. Biol. Chem. 1999, 274, 24147–24152. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Feng, J.; Wu, G.; Wei, Z.; Wang, J.Z.; Zhang, B.; Liu, R.; Liu, F.; Wang, X.; Li, H.L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Lim, S.; Hoffman, D.; Aspenstrom, P.; Federoff, H.J.; Rempe, D.A. HUMMR, a hypoxia- and HIF-1alpha-inducible protein, alters mitochondrial distribution and transport. J. Cell Biol. 2009, 185, 1065–1081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.; Kim, S.J.; Kumagai, H.; Mehta, H.H.; Xiang, W.; Liu, J.; Yen, K.; Cohen, P. Peptides derived from small mitochondrial open reading frames: Genomic, biological, and therapeutic implications. Exp. Cell Res. 2020, 393, 112056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.; Kim, S.J.; Kumagai, H.; Yen, K.; Cohen, P. Mitochondria-derived peptides in aging and healthspan. J. Clin. Investig. 2022, 132, e158449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 2016, 8, 796–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kienzle, L.; Bettinazzi, S.; Choquette, T.; Brunet, M.; Khorami, H.H.; Jacques, J.F.; Moreau, M.; Roucou, X.; Landry, C.R.; Angers, A.; et al. A small protein coded within the mitochondrial canonical gene nd4 regulates mitochondrial bioenergetics. BMC Biol. 2023, 21, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yen, K.; Mehta, H.H.; Kim, S.J.; Lue, Y.; Hoang, J.; Guerrero, N.; Port, J.; Bi, Q.; Navarrete, G.; Brandhorst, S.; et al. The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan. Aging 2020, 12, 11185–11199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rathore, A.; Chu, Q.; Tan, D.; Martinez, T.F.; Donaldson, C.J.; Diedrich, J.K.; Yates, J.R., 3rd; Saghatelian, A. MIEF1 Microprotein Regulates Mitochondrial Translation. Biochemistry 2018, 57, 5564–5575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, C.S.; Jadiya, P.; Zhang, X.; McLendon, J.M.; Abouassaly, G.M.; Witmer, N.H.; Anderson, E.J.; Elrod, J.W.; Boudreau, R.L. Mitoregulin: A lncRNA-Encoded Microprotein that Supports Mitochondrial Supercomplexes and Respiratory Efficiency. Cell Rep. 2018, 23, 3710–3720.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makarewich, C.A.; Baskin, K.K.; Munir, A.Z.; Bezprozvannaya, S.; Sharma, G.; Khemtong, C.; Shah, A.M.; McAnally, J.R.; Malloy, C.R.; Szweda, L.I.; et al. MOXI is a mitochondrial micropeptide that enhances fatty acid beta-oxidation. Cell Rep. 2018, 23, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Xiao, M.H.; Chen, H.X.; Meng, Y.; Zhao, N.; Yang, L.; Tang, H.; Wang, J.L.; Liu, X.; Zhu, Y.; et al. A novel mitochondrial micropeptide MPM enhances mitochondrial respiratory activity and promotes myogenic differentiation. Cell Death Dis. 2019, 10, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yen, K.; Wan, J.; Mehta, H.H.; Miller, B.; Christensen, A.; Levine, M.E.; Salomon, M.P.; Brandhorst, S.; Xiao, J.; Kim, S.J.; et al. Humanin Prevents Age-Related Cognitive Decline in Mice and is Associated with Improved Cognitive Age in Humans. Sci. Rep. 2018, 8, 14212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.; Arpawong, T.E.; Jiao, H.; Kim, S.J.; Yen, K.; Mehta, H.H.; Wan, J.; Carpten, J.C.; Cohen, P. Comparing the Utility of Mitochondrial and Nuclear DNA to Adjust for Genetic Ancestry in Association Studies. Cells 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godoy, P.A.; Ramírez-Molina, O.; Fuentealba, J. Exploring the Role of P2X Receptors in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bennett, N.K.; Nguyen, M.K.; Darch, M.A.; Nakaoka, H.J.; Cousineau, D.; Ten Hoeve, J.; Graeber, T.G.; Schuelke, M.; Maltepe, E.; Kampmann, M.; et al. Defining the ATPome reveals cross-optimization of metabolic pathways. Nat. Commun. 2020, 11, 4319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinklarova, L.; Schmidt, M.; Benek, O.; Kuca, K.; Gunn-Moore, F.; Musilek, K. Friend or enemy? Review of 17β-HSD10 and its role in human health or disease. J. Neurochem. 2020, 155, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yip, Y.M.; Li, L. In silico construction of HK2-VDAC1 complex and investigating the HK2 binding-induced molecular gating mechanism of VDAC1. Mitochondrion 2016, 30, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the, CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Broeks, M.H.; Shamseldin, H.E.; Alhashem, A.; Hashem, M.; Abdulwahab, F.; Alshedi, T.; Alobaid, I.; Zwartkruis, F.; Westland, D.; Fuchs, S.; et al. MDH1 deficiency is a metabolic disorder of the malate-aspartate shuttle associated with early onset severe encephalopathy. Hum. Genet. 2019, 138, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Herrada-Soler, E.; Satrústegui, J.; Contreras, L.; Del Arco, A. AGC1 Deficiency: Pathology and Molecular and Cellular Mechanisms of the Disease. Int. J. Mol. Sci. 2022, 23, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lacabanne, D.; Sowton, A.P.; Jose, B.; Kunji, E.R.S.; Tavoulari, S. Current Understanding of Pathogenic Mechanisms and Disease Models of Citrin Deficiency. J. Inherit. Metab. Dis. 2025, 48, e70021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrústegui, J.; et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Arco, A.; González-Moreno, L.; Pérez-Liébana, I.; Juaristi, I.; González-Sánchez, P.; Contreras, L.; Pardo, B.; Satrústegui, J. Regulation of neuronal energy metabolism by calcium: Role of MCU and Aralar/malate-aspartate shuttle. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119468. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.G. Purine and Pyrimidine Metabolism. In Textbook of Biochemistry with Clinical Correlations, 5th ed.; Devlin, T.M., Ed.; Wiley-Liss: New York, NY, USA, 2002; pp. 825–860. [Google Scholar]
- Amaral, A.; Hadera, M.G.; Kotter, M.; Sonnewald, U. Oligodendrocytes Do Not Export NAA-Derived Aspartate In Vitro. Neurochem. Res. 2017, 42, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Rosko, L.; Smith, V.N.; Yamazaki, R.; Huang, J.K. Oligodendrocyte Bioenergetics in Health and Disease. Neuroscientist 2019, 25, 334–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Broeks, M.H.; van Karnebeek, C.D.M.; Wanders, R.J.A.; Jans, J.J.M.; Verhoeven-Duif, N.M. Inborn disorders of the malate aspartate shuttle. J. Inherit. Metab. Dis. 2021, 44, 792–808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, N.I.; Van Der Knaap, M.S. AGC1 deficiency and cerebral hypomyelination. N. Engl. J. Med. 2009, 361, 1997–1998. [Google Scholar] [CrossRef]
- Koch, J.; Broeks, M.H.; Gautschi, M.; Jans, J.; Laemmle, A. Inborn errors of the malate aspartate shuttle—Update on patients and cellular models. Mol. Genet. Metab. 2024, 142, 108520. [Google Scholar] [CrossRef] [PubMed]
- Ait-El-Mkadem, S.; Dayem-Quere, M.; Gusic, M.; Chaussenot, A.; Bannwarth, S.; Francois, B.; Genin, E.C.; Fragaki, K.; Volker-Touw, C.L.M.; Vasnier, C.; et al. Mutations in MDH2, encoding a Krebs cycle enzyme, cause early-onset severe encephalopathy. Am. J. Hum. Genet. 2017, 100, 151–159. [Google Scholar] [CrossRef] [PubMed]
- van Karnebeek, C.D.M.; Ramos, R.J.; Wen, X.Y.; Tarailo-Graovac, M.; Gleeson, J.G.; Skrypnyk, C.; Brand-Arzamendi, K.; Karbassi, F.; Issa, M.Y.; van der Lee, R.; et al. Bi-allelic GOT2 Mutations Cause a Treatable Malate-Aspartate Shuttle-Related Encephalopathy. Am. J. Hum. Genet. 2019, 105, 534–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jalil, M.A.; Begum, L.; Contreras, L.; Pardo, B.; Iijima, M.; Li, M.X.; Ramos, M.; Marmol, P.; Horiuchi, M.; Shimotsu, K.; et al. Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J. Biol. Chem. 2005, 280, 31333–31339. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Ramoz, N.; Barreto, M.; Gazdoiu, M.; Takahashi, N.; Gertner, M.; Dorr, N.; Gama Sosa, M.A.; De Gasperi, R.; Perez, G.; et al. Slc25a12 disruption alters myelination and neurofilaments: A model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol. Psychiatry 2010, 67, 887–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llorente-Folch, I.; Sahún, I.; Contreras, L.; Casarejos, M.J.; Grau, J.M.; Saheki, T.; Mena, M.A.; Satrústegui, J.; Dierssen, M.; Pardo, B. AGC1-malate aspartate shuttle activity is critical for dopamine handling in the nigrostriatal pathway. J. Neurochem. 2013, 124, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A. Glial Glutamine Homeostasis in Health and Disease. Neurochem. Res. 2023, 48, 1100–1128. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A.; Bak, L.K.; Waagepetersen, H.S. Astrocytic Control of Biosynthesis and Turnover of the Neurotransmitters Glutamate and, GABA. Front. Endocrinol. 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidoryk-Węgrzynowicz, M.; Adamiak, K.; Strużyńska, L. Astrocyte-Neuron Interaction via the Glutamate-Glutamine Cycle and Its Dysfunction in Tau-Dependent Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 3050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derouiche, A.; Frotscher, M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: Evidence for transmitter degradation. Brain Res. 1991, 552, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.C.; Billups, D.; Billups, B. Maintaining the presynaptic glutamate supply for excitatory neurotransmission. J. Neurosci. Res. 2015, 93, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.L.; Behar, K.L.; Hyder, F.; Shulman, R.G. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: Implications for brain function. Annu. Rev. Physiol. 2003, 65, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U. Glutamate synthesis has to be matched by its degradation—Where do all the carbons go? J. Neurochem. 2014, 131, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Drejer, J.; Hertz, L.; Schousboe, A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J. Neurochem. 1983, 41, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A.; Waagepetersen, H.S.; Sonnewald, U. Astrocytic pyruvate carboxylation: Status after 35 years. J. Neurosci. Res. 2019, 97, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zerangue, N.; Kavanaugh, M.P. Flux coupling in a neuronal glutamate transporter. Nature 1996, 383, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Bergles, D.E.; Jahr, C.E. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 1997, 19, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Mennerick, S.; Zorumski, C.F. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature 1994, 368, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.B.; Lee, M.L.; DaSilva, S. Glutamate Transporters and Mitochondria: Signaling, Co-compartmentalization, Functional Coupling, and Future Directions. Neurochem. Res. 2020, 45, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.M.; Koo, J.C.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate transporter coupling to Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Genda, E.N.; Jackson, J.G.; Sheldon, A.L.; Locke, S.F.; Greco, T.M.; O’Donnell, J.C.; Spruce, L.A.; Xiao, R.; Guo, W.; Putt, M.; et al. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J. Neurosci. 2011, 31, 18275–18288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, C.D.; Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Stadtman, E.R.; Floyd, R.A.; Markesbery, W.R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10540–10543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F.; St Clair, D.; Keller, J.N.; Pierce, W.M.; Klein, J.B.; Markesbery, W.R. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol. Dis. 2006, 22, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xian, X.; Li, L.; Yao, X.; Hu, Y.; Zhang, M.; Li, W. Ceftriaxone Improves Cognitive Function and Upregulates GLT-1-Related Glutamate-Glutamine Cycle in APP/PS1 Mice. J. Alzheimers Dis. 2018, 66, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, L.; Xian, X.; Liu, L.; Gao, J.; Li, W. Ceftriaxone regulates glutamate production and vesicular assembly in presynaptic terminals through GLT-1 in APP/PS1 mice. Neurobiol. Learn. Mem. 2021, 183, 107480. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Aksenova, M.V.; Carney, J.M.; Butterfield, D.A. Oxidative modification of glutamine synthetase by amyloid beta peptide. Free Radic. Res. 1997, 27, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Carney, J.M.; Mattson, M.P.; Aksenova, M.; Harris, M.; Wu, J.F.; Floyd, R.A.; Butterfield, D.A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 3270–3274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harris, M.E.; Hensley, K.; Butterfield, D.A.; Leedle, R.A.; Carney, J.M. Direct evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1–40) in cultured hippocampal neurons. Exp. Neurol. 1995, 131, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Herrup, K.; Chen, J.; Herrup, K. Glutamine acts as a neuroprotectant against DNA damage, beta-amyloid and H2O2-induced stress. PLoS ONE 2012, 7, e33177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buntup, D.; Skare, O.; Solbu, T.T.; Chaudhry, F.A.; Storm-Mathisen, J.; Thangnipon, W. Beta-amyloid 25-35 peptide reduces the expression of glutamine transporter SAT1 in cultured cortical neurons. Neurochem. Res. 2008, 33, 248–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulijewicz-Nawrot, M.; Syková, E.; Chvátal, A.; Verkhratsky, A.; Rodríguez, J.J. Astrocytes and glutamate homoeostasis in Alzheimer’s disease: A decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro 2013, 5, 273–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, J.V.; Skotte, N.H.; Christensen, S.K.; Polli, F.S.; Shabani, M.; Markussen, K.H.; Haukedal, H.; Westi, E.W.; Diaz-delCastillo, M.; Sun, R.C.; et al. Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death Dis. 2021, 12, 954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, J.V.; Christensen, S.K.; Westi, E.W.; Diaz-delCastillo, M.; Tanila, H.; Schousboe, A.; Aldana, B.I.; Waagepetersen, H.S. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105198. [Google Scholar] [CrossRef] [PubMed]
- 164 Masliah, E.; Alford, M.; DeTeresa, R.; Mallory, M.; Hansen, L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Abdul, H.M.; Sama, M.A.; Furman, J.L.; Mathis, D.M.; Beckett, T.L.; Weidner, A.M.; Patel, E.S.; Baig, I.; Murphy, M.P.; LeVine, H., 3rd; et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009, 29, 12957–12969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacob, C.P.; Koutsilieri, E.; Bartl, J.; Neuen-Jacob, E.; Arzberger, T.; Zander, N.; Ravid, R.; Roggendorf, W.; Riederer, P.; Grünblatt, E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J. Alzheimers Dis. 2007, 11, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Is ammonia a pathogenetic factor in Alzheimer’s disease? Neurochem. Res. 1993, 18, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, E.H.; Popek, M.; Frontczak-Baniewicz, M.; Utheim, T.P.; Albrecht, J.; Zielińska, M.; Chaudhry, F.A. Perturbation of astroglial Slc38 glutamine transporters by NH4+ contributes to neurophysiologic manifestations in acute liver failure. FASEB J. 2021, 35, e21588. [Google Scholar] [CrossRef] [PubMed]
- Gropman, A. Brain imaging in urea cycle disorders. Mol. Genet. Metab. 2010, 100 (Suppl. S1), S20–S30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maestri, N.E.; Lord, C.; Glynn, M.; Bale, A.; Brusilow, S.W. The phenotype of ostensibly healthy women who are carriers for ornithine transcarbamylase deficiency. Medicine 1998, 77, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Gyato, K.; Wray, J.; Huang, Z.J.; Yudkoff, M.; Batshaw, M.L. Metabolic and neuropsychological phenotype in women heterozygous for ornithine transcarbamylase deficiency. Ann. Neurol. 2004, 55, 80–86. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.C.; Jackson, J.G.; Robinson, M.B. Transient Oxygen/Glucose Deprivation Causes a Delayed Loss of Mitochondria and Increases Spontaneous Calcium Signaling in Astrocytic Processes. J. Neurosci. 2016, 36, 7109–7127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, A.R.; Willnow, T.E. Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System. Int. J. Mol. Sci. 2019, 20, 5671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mochel, F.; Duteil, S.; Marelli, C.; Jauffret, C.; Barles, A.; Holm, J.; Sweetman, L.; Benoist, J.F.; Rabier, D.; Carlier, P.G.; et al. Dietary anaplerotic therapy improves peripheral tissue energy metabolism in patients with Huntington’s disease. Eur. J. Hum. Genet. 2010, 18, 1057–1060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marin-Valencia, I.; Good, L.B.; Ma, Q.; Malloy, C.R.; Pascual, J.M. Heptanoate as a neural fuel: Energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J. Cereb. Blood Flow. Metab. 2013, 33, 175–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pascual, J.M.; Liu, P.; Mao, D.; Kelly, D.I.; Hernandez, A.; Sheng, M.; Good, L.B.; Ma, Q.; Marin-Valencia, I.; Zhang, X.; et al. Triheptanoin for glucose transporter type I deficiency (G1D): Modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement. JAMA Neurol. 2014, 71, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, X.; Wang, L.; Tandon, N.; Sun, H.; Tian, J.; Du, H.; Pascual, J.M.; Guo, L. Triheptanoin Mitigates Brain ATP Depletion and Mitochondrial Dysfunction in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 425–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aso, E.; Semakova, J.; Joda, L.; Semak, V.; Halbaut, L.; Calpena, A.; Escolano, C.; Perales, J.C.; Ferrer, I. Triheptanoin supplementation to ketogenic diet curbs cognitive impairment in APP/PS1 mice used as a model of familial Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Sheng, Z.H. Mitochondrial transport and docking in axons. Exp. Neurol. 2009, 218, 257–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berth, S.H.; Lloyd, T.E. Disruption of axonal transport in neurodegeneration. J. Clin. Investig. 2023, 133, e168554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cason, S.E.; Holzbaur, E.L.F. Selective motor activation in organelle transport along axons. Nat. Rev. Mol. Cell Biol. 2022, 23, 699–714. [Google Scholar] [CrossRef]
- Prezel, E.; Elie, A.; Delaroche, J.; Stoppin-Mellet, V.; Bosc, C.; Serre, L.; Fourest-Lieuvin, A.; Andrieux, A.; Vantard, M.; Arnal, I. Tau can switch microtubule network organizations: From random networks to dynamic and stable bundles. Mol. Biol. Cell 2018, 29, 154–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Z.; Schaedel, L.; Portran, D.; Aguilar, A.; Gaillard, J.; Marinkovich, M.P.; Théry, M.; Nachury, M.V. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 2017, 356, 328–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portran, D.; Schaedel, L.; Xu, Z.; Théry, M.; Nachury, M.V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [Google Scholar] [CrossRef] [PubMed]
- Saxton, W.M.; Hollenbeck, P.J. The axonal transport of mitochondria. J. Cell Sci. 2012, 125 Pt 9, 2095–2104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruppa, A.J.; Buss, F. Motor proteins at the mitochondria-cytoskeleton interface. J. Cell Sci. 2021, 134, jcs226084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D.; Reese, T.S.; Sheetz, M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985, 42, 39–50. [Google Scholar] [CrossRef]
- Du, J.; Wei, Y.; Liu, L.; Wang, Y.; Khairova, R.; Blumenthal, R.; Tragon, T.; Hunsberger, J.G.; Machado-Vieira, R.; Drevets, W.; et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc. Natl. Acad. Sci. USA 2010, 107, 11573–11578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banerjee, R.; Chakraborty, P.; Yu, M.C.; Gunawardena, S. A stop or go switch: Glycogen synthase kinase 3β phosphorylation of the kinesin 1 motor domain at Ser314 halts motility without detaching from microtubules. Development 2021, 148, dev199866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padzik, A.; Deshpande, P.; Hollos, P.; Franker, M.; Rannikko, E.H.; Cai, D.; Prus, P.; Mågård, M.; Westerlund, N.; Verhey, K.J.; et al. KIF5C S176 Phosphorylation Regulates Microtubule Binding and Transport Efficiency in Mammalian Neurons. Front. Cell. Neurosci. 2016, 10, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brady, S.T.; Morfini, G.A. Regulation of motor proteins, axonal transport deficits and adult-onset neurodegenerative diseases. Neurobiol. Dis. 2017, 105, 273–282. [Google Scholar] [CrossRef]
- Schwarz, T.L. Mitochondrial trafficking in neurons. Cold Spring Harb. Perspect. Biol. 2013, 5, a011304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fransson, S.; Ruusala, A.; Aspenström, P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006, 344, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Glater, E.E.; Megeath, L.J.; Stowers, R.S.; Schwarz, T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006, 173, 545–557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klosowiak, J.L.; Focia, P.J.; Chakravarthy, S.; Landahl, E.C.; Freymann, D.M.; Rice, S.E. Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO Rep. 2013, 14, 968–974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macaskill, A.F.; Rinholm, J.E.; Twelvetrees, A.E.; Arancibia-Carcamo, I.L.; Muir, J.; Fransson, A.; Aspenstrom, P.; Attwell, D.; Kittler, J.T. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 2009, 61, 541–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, Q.; Zakaria, H.M.; Simone, A.; Sheng, Z.H. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr. Biol. 2012, 22, 545–552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, K.E.; Sheetz, M.P. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004, 117, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Pilling, A.D.; Horiuchi, D.; Lively, C.M.; Saxton, W.M. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 2006, 17, 2057–2068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef]
- Elie, A.; Prezel, E.; Guérin, C.; Denarier, E.; Ramirez-Rios, S.; Serre, L.; Andrieux, A.; Fourest-Lieuvin, A.; Blanchoin, L.; Arnal, I. Tau co-organizes dynamic microtubule and actin networks. Sci. Rep. 2015, 5, 9964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadavath, H.; Jaremko, M.; Jaremko, Ł.; Biernat, J.; Mandelkow, E.; Zweckstetter, M. Folding of the tau protein on microtubules. Angew. Chem. Int. Ed. Engl. 2015, 54, 10347–10351. [Google Scholar] [CrossRef]
- Watamura, N.; Foiani, M.S.; Bez, S.; Bourdenx, M.; Santambrogio, A.; Frodsham, C.; Camporesi, E.; Brinkmalm, G.; Zetterberg, H.; Patel, S.; et al. In vivo hyperphosphorylation of tau is associated with synaptic loss and behavioral abnormalities in the absence of tau seeds. Nat. Neurosci. 2025, 28, 293–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roveta, F.; Bonino, L.; Piella, E.M.; Rainero, I.; Rubino, E. Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 11941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanfranchi, M.; Yandiev, S.; Meyer-Dilhet, G.; Ellouze, S.; Kerkhofs, M.; Dos Reis, R.; Garcia, A.; Blondet, C.; Amar, A.; Kneppers, A.; et al. The AMPK-related kinase NUAK1 controls cortical axons branching by locally modulating mitochondrial metabolic functions. Nat. Commun. 2024, 15, 2487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Xiong, G.-J.; Huang, N.; Sheng, Z.-H. The cross-talk of energy sensing and mitochondrial anchoring sustains synaptic efficacy by maintaining presynaptic metabolism. Nat. Metab. 2020, 2, 1077–1095. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rico, T.; Gilles, M.; Chauderlier, A.; Comptdaer, T.; Magnez, R.; Chwastyniak, M.; Drobecq, H.; Pinet, F.; Thuru, X.; Buée, L.; et al. Tau Stabilizes Chromatin Compaction. Front. Cell Dev. Biol. 2021, 9, 740550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magrin, C.; Bellafante, M.; Sola, M.; Piovesana, E.; Bolis, M.; Cascione, L.; Napoli, S.; Rinaldi, A.; Papin, S.; Paganetti, P. Tau protein modulates an epigenetic mechanism of cellular senescence in human SH-SY5Y neuroblastoma cells. Front. Cell Dev. Biol. 2023, 11, 1232963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stokin, G.B.; Lillo, C.; Falzone, T.L.; Brusch, R.G.; Rockenstein, E.; Mount, S.L.; Raman, R.; Davies, P.; Masliah, E.; Williams, D.S.; et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 2005, 307, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.W.; Chang, L.C.; Ma, T.; Oh, H.; French, B.; Puralewski, R.; Mathews, F.; Fang, Y.; Lewis, D.A.; Kennedy, J.L.; et al. Older molecular brain age in severe mental illness. Mol. Psychiatry 2021, 26, 3646–3656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vance, J.E.; Hayashi, H.; Karten, B. Cholesterol homeostasis in neurons and glial cells. Semin. Cell Dev. Biol. 2005, 16, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Sych, T.; Gurdap, C.O.; Wedemann, L.; Sezgin, E. How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes 2021, 11, 323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.A.; London, E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998, 164, 103–114. [Google Scholar] [CrossRef] [PubMed]
- London, E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.R.; Ghosh, S.; Veatch, S.L. Critical Phenomena in Plasma Membrane Organization and Function. Annu. Rev. Phys. Chem. 2021, 72, 51–72. [Google Scholar] [CrossRef]
- Levi, M.; Gratton, E. Visualizing the regulation of SLC34 proteins at the apical membrane. Pflugers Arch. 2019, 471, 533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sebastiao, A.M.; Colino-Oliveira, M.; Assaife-Lopes, N.; Dias, R.B.; Ribeiro, J.A. Lipid rafts, synaptic transmission and plasticity: Impact in age-related neurodegenerative diseases. Neuropharmacology 2013, 64, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Helms, J.B.; Zurzolo, C. Lipids as targeting signals: Lipid rafts and intracellular trafficking. Traffic 2004, 5, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bosiacki, M.; Pilarczyk, M.; Gąssowska-Dobrowolska, M.; Jarmużek, P.; Szućko-Kociuba, I.; Kulik-Sajewicz, J.; Chlubek, D.; Baranowska-Bosiacka, I. Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases. Cancers 2024, 16, 2115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.K. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr. Opin. Lipidol. 2012, 23, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Tashima, Y.; Houjou, T.; Fujita, M.; Yoko-o, T.; Jigami, Y.; Taguchi, R.; Kinoshita, T. Fatty acid remodeling of GPI-anchored proteins is required for their raft association. Mol. Biol. Cell 2007, 18, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol. 2020, 10, 190290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807, Erratum in J. Lipid Res. 2014, 55, 2444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, M.J.; Crameri, J.J.; Thorburn, D.R.; Frazier, A.E.; Stojanovski, D. Mitochondrial biology and dysfunction in secondary mitochondrial disease. Open Biol. 2022, 12, 220274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikon, N.; Ryan, R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilkerson, R.W.; Selker, J.M.; Capaldi, R.A. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 2003, 546, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005, 280, 29403–29408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schägger, H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braughler, J.M.; Duncan, L.A.; Chase, R. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.F. Overview of methods used for detecting lipid peroxidation. Methods Enzymol. 1984, 105, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Kono, N.; Ariyama, H.; Shindou, H.; Shimizu, T.; Arai, H. Lysophosphatidylcholine acyltransferase 1 protects against cytotoxicity induced by polyunsaturated fatty acids. FASEB J. 2016, 30, 2027–2039. [Google Scholar] [CrossRef]
- Rybnikova, E.; Damdimopoulos, A.E.; Gustafsson, J.A.; Spyrou, G.; Pelto-Huikko, M. Expression of novel antioxidant thioredoxin-2 in the rat brain. Eur. J. Neurosci. 2000, 12, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Phadnis, V.V.; Snider, J.; Varadharajan, V.; Ramachandiran, I.; Deik, A.A.; Lai, Z.W.; Kunchok, T.; Eaton, E.N.; Sebastiany, C.; Lyakisheva, A.; et al. MMD collaborates with ACSL4 and MBOAT7 to promote polyunsaturated phosphatidylinositol remodeling and susceptibility to ferroptosis. Cell Rep. 2023, 42, 113023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Lazarov, O.; Lee, M.; Peterson, D.A.; Sisodia, S.S. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 2002, 22, 9785–9793. [Google Scholar] [CrossRef]
- Koo, E.H.; Sisodia, S.S.; Archer, D.R.; Martin, L.J.; Weidemann, A.; Beyreuther, K.; Fischer, P.; Masters, C.L.; Price, D.L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl. Acad. Sci. USA 1990, 87, 1561–1565. [Google Scholar] [CrossRef]
- Capone, R.; Tiwari, A.; Hadziselimovic, A.; Peskova, Y.; Hutchison, J.M.; Sanders, C.R.; Kenworthy, A.K. The C99 domain of the amyloid precursor protein resides in the disordered membrane phase. J. Biol. Chem. 2021, 296, 100652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Allinson, T.M.; Parkin, E.T.; Turner, A.J.; Hooper, N.M. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003, 74, 342–352. [Google Scholar] [CrossRef]
- Iwatsubo, T. The g-secretase complex: Machinery for intramembrane proteolysis. Curr. Opin. Neurobiol. 2004, 14, 379–383. [Google Scholar] [CrossRef]
- Abraham, C.B.; Xu, L.; Pantelopulos, G.A.; Straub, J.E. Characterizing the transmembrane domains of ADAM10 and BACE1 and the impact of membrane composition. Biophys. J. 2023, 122, 3999–4010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Runz, H.; Rietdorf, J.; Tomic, I.; de Bernard, M.; Beyreuther, K.; Pepperkok, R.; Hartmann, T. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J. Neurosci. 2002, 22, 1679–1689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Yao, J.; Kim, T.W.; Tall, A.R. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J. Biol. Chem. 2003, 278, 27688–27694. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.L.; Elias, J.E.; Gygi, S.P.; Selkoe, D.J. Identification of beta-secretase (BACE1) substrates using quantitative proteomics. PLoS ONE 2009, 4, e8477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, N.; Martín, V.; Marín, R.; Moreno, D.; Ferrer, I.; Díaz, M. Altered lipid composition in cortical lipid rafts occurs at early stages of sporadic Alzheimer’s disease and facilitates APP/BACE1 interactions. Neurobiol. Aging 2014, 35, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; Lemere, C.A.; Selkoe, D.J.; Clemens, J.A. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol. Dis. 1996, 3, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rubinski, A.; Tosun, D.; Franzmeier, N.; Neitzel, J.; Frontzkowski, L.; Weiner, M.; Ewers, M. Lower Cerebral Perfusion Is Associated with Tau-PET in the Entorhinal Cortex across the Alzheimer’s Continuum. Neurobiol. Aging 2021, 102, 111–118. [Google Scholar] [CrossRef]
- Chao, L.L.; Buckley, S.T.; Kornak, J.; Schuff, N.; Madison, C.; Yaffe, K.; Miller, B.L.; Kramer, J.H.; Weiner, M.W. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 19–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Love, S.; Miners, J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattsson, N.; Tosun, D.; Insel, P.S.; Simonson, A.; Jack, C.R., Jr.; Beckett, L.A.; Donohue, M.; Jagust, W.; Schuff, N.; Weiner, M.W. Association of brain amyloid-β with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain A J. Neurol. 2014, 137 Pt 5, 1550–1561. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Montagne, A.; Sagare, A.P.; Nation, D.A.; Schneider, L.S.; Chui, H.C.; Harrington, M.G.; Pa, J.; Law, M.; Wang, D.J.J.; et al. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019, 15, 158–167, Erratum in Alzheimers Dement. 2022, 18, 522. https://doi.org/10.1002/alz.12483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de la Torre, J.C. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Small vessel disease and Alzheimer’s dementia: Pathological considerations. Cerebrovasc. Dis. 2002, 13, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Gorelick, P.B. Converging pathologic mechanisms in vascular and neurodegenerative dementia. Stroke 2003, 34, 335–337. [Google Scholar] [CrossRef]
- Landau, S.M.; Harvey, D.; Madison, C.M.; Koeppe, R.A.; Reiman, E.M.; Foster, N.L.; Weiner, M.W.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging Initiative. Associations between cognitive, functional, and FDG-PET measures of decline in AD and, MCI. Neurobiol. Aging 2011, 32, 1207–1218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landau, S.M.; Harvey, D.; Madison, C.M.; Reiman, E.M.; Foster, N.L.; Aisen, P.S.; Petersen, R.C.; Shaw, L.M.; Trojanowski, J.Q.; Jack, C.R., Jr.; et al. Alzheimer’s Disease Neuroimaging Initiative. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010, 75, 230–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ossenkoppele, R.; van der Flier, W.M.; Zwan, M.D.; Adriaanse, S.F.; Boellaard, R.; Windhorst, A.D.; Barkhof, F.; Lammertsma, A.A.; Scheltens, P.; van Berckel, B.N. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 2013, 80, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Protas, H.D.; Chen, K.; Langbaum, J.B.; Fleisher, A.S.; Alexander, G.E.; Lee, W.; Bandy, D.; de Leon, M.J.; Mosconi, L.; Buckley, S.; et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013, 70, 320–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spallazzi, M.; Dobisch, L.; Becke, A.; Berron, D.; Stucht, D.; Oeltze-Jafra, S.; Caffarra, P.; Speck, O.; Düzel, E. Hippocampal vascularization patterns: A high-resolution 7 Tesla time-of-flight magnetic resonance angiography study. Neuroimage Clin. 2019, 21, 101609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190, Erratum in Nat. Commun. 2021, 12, 4497. https://doi.org/10.1038/s41467-021-24833-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ando, S.; Tsukamoto, H.; Stacey, B.S.; Washio, T.; Owens, T.S.; Calverley, T.A.; Fall, L.; Marley, C.J.; Iannetelli, A.; Hashimoto, T.; et al. Acute hypoxia impairs posterior cerebral bioenergetics and memory in man. Exp. Physiol. 2023, 108, 1516–1530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geraghty, J.R.; Lara-Angulo, M.N.; Spegar, M.; Reeh, J.; Testai, F.D. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: Predictors and relationship to functional outcome. J. Stroke Cerebrovasc. Dis. 2020, 29, 105027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pickard, J.D.; Walker, V.; Perry, S.; Smythe, P.J.; Eastwood, S.; Hunt, R. Arterial eicosanoid production following chronic exposure to a periarterial haematoma. J. Neurol. Neurosurg. Psychiatry 1984, 47, 661–667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erdem, A.; Yaşargil, G.; Roth, P. Microsurgical anatomy of the hippocampal arteries. J. Neurosurg. 1993, 79, 256–265. [Google Scholar] [CrossRef] [PubMed]
- den Abeelen, A.S.; Lagro, J.; van Beek, A.H.; Claassen, J.A. Impaired cerebral autoregulation and vasomotor reactivity in sporadic Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Papadopoulos, P.; Hamel, E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: Impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br. J. Pharmacol. 2013, 170, 661–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roher, A.E.; Esh, C.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.C.; Seward, J.D.; Sue, L.I.; Beach, T.G. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2055–2062. [Google Scholar] [CrossRef]
- Roher, A.E.; Esh, C.; Rahman, A.; Kokjohn, T.A.; Beach, T.G. Atherosclerosis of cerebr arteries in Alzheimer disease. Stroke 2004, 35, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.; Düzel, S.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövden, M.; Lindenberger, U.; Bäckman, L.; Braun-Dullaeus, R.; et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 2015, 20, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Maksimovich, I.V. Differences in cerebral angioarchitectonics in Alzheimer’s Disease in comparison with other neurodegenerative and ischemic lesions. World J. Neurosci. 2018, 8, 454–469. [Google Scholar] [CrossRef]
- Lawley, J.S.; Macdonald, J.H.; Oliver, S.J.; Mullins, P.G. Unexpected reductions in regional cerebral perfusion during prolonged hypoxia. J. Physiol. 2017, 595, 935–947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mottahedin, A.; Prag, H.A.; Dannhorn, A.; Mair, R.; Schmidt, C.; Yang, M.; Sorby-Adams, A.; Lee, J.J.; Burger, N.; Kulaveerasingam, D.; et al. Targeting succinate metabolism to decrease brain injury upon mechanical thrombectomy treatment of ischemic stroke. Redox Biol. 2023, 59, 102600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beason-Held, L.L.; Moghekar, A.; Zonderman, A.B.; Kraut, M.A.; Resnick, S.M. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 2007, 38, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Kiwak, K.J.; Hekimian, K.; Levine, L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science 1984, 224, 886–889. [Google Scholar] [CrossRef]
- Hota, S.K.; Barhwal, K.; Singh, S.B.; Ilavazhagan, G. Differential temporal response of hippocampus, cortex and cerebellum to hypobaric hypoxia: A biochemical approach. Neurochem. Int. 2007, 51, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Kadar, T.; Marlowe, B.E.; Stillman, M.J.; Galli, R.L.; Levy, A.; Devine, J.A.; Lieberman, H.R. Morphological alterations in the hippocampus following hypobaric hypoxia. Hum. Exp. Toxicol. 1996, 15, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.S.; Almezgagi, M.; Zhang, Y.; Zhonglei, D.; Qinfang, Z.; Ali, M.; Shouket, Z.; Zhang, W. Experimental brain injury induced by acute hypobaric hypoxia stimulates changes in mRNA expression and stress marker status. Int. J. Res. Rev. 2021, 8, 242–247. [Google Scholar] [CrossRef]
- Ji, X.; Ferreira, T.; Friedman, B.; Liu, R.; Liechty, H.; Bas, E.; Chandrashekar, J.; Kleinfeld, D. Brain microvasculature has a common topology with local differences in geometry that match metabolic load. Neuron 2021, 109, 1168–1187.e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perosa, V.; Priester, A.; Ziegler, G.; Cardenas-Blanco, A.; Dobisch, L.; Spallazzi, M.; Assmann, A.; Maass, A.; Speck, O.; Oltmer, J.; et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain 2020, 143, 622–634. [Google Scholar] [CrossRef]
- Purnell, C.; Gao, S.; Callahan, C.M.; Hendrie, H.C. Cardiovascular risk factors and incident Alzheimer disease: A systematic review of the literature. Alzheimer Dis. Assoc. Disord. 2009, 23, 1–10. [Google Scholar] [CrossRef]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement. 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Death by a thousand cuts in Alzheimer’s disease: Hypoxia–the prodrome. Neurotox. Res. 2013, 24, 216–243. [Google Scholar] [CrossRef]
- Siachpazidou, D.I.; Stavrou, V.T.; Astara, K.; Pastaka, C.; Gogou, E.; Hatzoglou, C.; Economou, N.T.; Gourgoulianis, K.I. Alzheimer’s Disease in Patients with Obstructive Sleep Apnea Syndrome. Tanaffos 2020, 19, 176–185. [Google Scholar] [PubMed] [PubMed Central]
- Correia, S.C.; Moreira, P.I. Oxygen sensing and signaling in Alzheimer’s disease: A breathtaking story! Cell. Mol. Neurobiol. 2022, 42, 3–21. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Mohammed, R.; Ojha, U. What are the links between hypoxia and Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2019, 15, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological impacts of chronic hypoxia on Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 902–909. [Google Scholar] [CrossRef]
- Iadecola, C. Dangerous leaks: Blood-brain barrier woes in the aging hippocampus. Neuron 2015, 85, 231–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamori, Y.; Horie, R. Developmental course of hypertension and regional cerebral blood flow in stroke-prone spontaneously hypertensive rats. Stroke 1977, 8, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Guldbrandsen, A.; Vethe, H.; Farag, Y.; Oveland, E.; Garberg, H.; Berle, M.; Myhr, K.M.; Opsahl, J.A.; Barsnes, H.; Berven, F.S. In-depth characterization of the cerebrospinal fluid (CSF) proteome displayed through the CSF proteome resource (CSF-PR). Mol. Cell. Proteom. 2014, 13, 3152–3163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paglia, G.; Stocchero, M.; Cacciatore, S.; Lai, S.; Angel, P.; Alam, M.T.; Keller, M.; Ralser, M.; Astarita, G. Unbiased Metabolomic Investigation of Alzheimer’s Disease Brain Points to Dysregulation of Mitochondrial Aspartate Metabolism. J. Proteome Res. 2016, 15, 608–618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, J.W.; Fang, Z.Y.; Huang, Y.; Liuyang, Z.Y.; Zhang, X.L.; Wang, J.L.; Wei, H.; Wang, J.Z.; Wang, X.C.; Zeng, J.; et al. Application of Weighted Gene Co-Expression Network Analysis to Explore the Key Genes in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 65, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, B.; Wang, X.; Li, Y.; Chen, P.C.; Yu, K.; Dey, K.K.; Yarbro, J.M.; Han, X.; Lutz, B.M.; Rao, S.; et al. Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer’s Disease Progression. Neuron 2020, 105, 975–991.e7. [Google Scholar] [CrossRef]
- Liu, N.; Xu, J.; Liu, H.; Zhang, S.; Li, M.; Zhou, Y.; Qin, W.; Li, M.J.; Yu, C.; Alzheimer’s Disease Neuroimaging Initiative. Hippocampal transcriptome-wide association study and neurobiological pathway analysis for Alzheimer’s disease. PLoS Genet. 2021, 17, e1009363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horgusluoglu, E.; Neff, R.; Song, W.M.; Wang, M.; Wang, Q.; Arnold, M.; Krumsiek, J.; Galindo-Prieto, B.; Ming, C.; Nho, K.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI); Alzheimer Disease Metabolomics Consortium. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimers Dement. 2022, 18, 1260–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullins, R.; Kapogiannis, D. Alzheimer’s Disease-Related Genes Identified by Linking Spatial Patterns of Pathology and Gene Expression. Front. Neurosci. 2022, 16, 908650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, D.; Li, J.; Liu, H.; Wang, J.; Xia, Y.; Qiu, W.; Wang, N.; Wang, X.; Wang, X.; Ma, C.; et al. Integrative Transcriptomic Analyses of Hippocampal-Entorhinal System Subfields Identify Key Regulators in Alzheimer’s Disease. Adv. Sci. 2023, 10, e2300876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tandon, R.; Levey, A.I.; Lah, J.J.; Seyfried, N.T.; Mitchell, C.S. Machine Learning Selection of Most Predictive Brain Proteins Suggests Role of Sugar Metabolism in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 92, 411–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czech, C.; Berndt, P.; Busch, K.; Schmitz, O.; Wiemer, J.; Most, V.; Hampel, H.; Kastler, J.; Senn, H. Metabolite profiling of Alzheimer’s disease cerebrospinal fluid. PLoS ONE 2012, 7, e31501. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Liu, C.; Jones, D.P.; Uppal, K. Untargeted metabolomics reveal dysregulations in sugar, methionine, and tyrosine pathways in the prodromal state of AD. Alzheimers Dement. 2020, 12, e12064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Geus, M.B.; Leslie, S.N.; Lam, T.; Wang, W.; Roux-Dalvai, F.; Droit, A.; Kivisakk, P.; Nairn, A.C.; Arnold, S.E.; Carlyle, B.C. Mass spectrometry in cerebrospinal fluid uncovers association of glycolysis biomarkers with Alzheimer’s disease in a large clinical sample. Sci. Rep. 2023, 13, 22406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panyard, D.J.; McKetney, J.; Deming, Y.K.; Morrow, A.R.; Ennis, G.E.; Jonaitis, E.M.; Van Hulle, C.A.; Yang, C.; Sung, Y.J.; Ali, M.; et al. Large-scale proteome and metabolome analysis of CSF implicates altered glucose and carbon metabolism and succinylcarnitine in Alzheimer’s disease. Alzheimers Dement. 2023, 19, 5447–5470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, J.V.; Christensen, S.K.; Aldana, B.I.; Nissen, J.D.; Tanila, H.; Waagepetersen, H.S. Alterations in cerebral cortical glucose and glutamine metabolism precedes amyloid plaques in the APPswe/ PSEN1dE9 mouse model of Alzheimer’s disease. Neurochem. Res. 2017, 42, 1589–1598. [Google Scholar] [CrossRef]
- Neuner, S.M.; Wilmott, L.A.; Hoffmann, B.R.; Mozhui, K.; Kaczorowski, C.C. Hippocampal proteomics defines pathways associated with memory decline and resilience in normal aging and Alzheimer’s disease mouse models. Behav. Brain Res. 2017, 322 Pt B, 288–298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ojo, J.O.; Crynen, G.; Algamal, M.; Vallabhaneni, P.; Leary, P.; Mouzon, B.; Reed, J.M.; Mullan, M.; Crawford, F. Unbiased Proteomic Approach Identifies Pathobiological Profiles in the Brains of Preclinical Models of Repetitive Mild Traumatic Brain Injury, Tauopathy, and Amyloidosis. ASN Neuro 2020, 12, 1759091420914768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hinteregger, B.; Loeffler, T.; Flunkert, S.; Neddens, J.; Bayer, T.A.; Madl, T.; Hutter-Paier, B. Metabolic, Phenotypic, and Neuropathological Characterization of the Tg4-42 Mouse Model for Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 1151–1168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Füzesi, M.V.; Muti, I.H.; Berker, Y.; Li, W.; Sun, J.; Habbel, P.; Nowak, J.; Xie, Z.; Cheng, L.L.; Zhang, Y. High Resolution Magic Angle Spinning Proton NMR Study of Alzheimer’s Disease with Mouse Models. Metabolites 2022, 12, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morello, G.; Guarnaccia, M.; La Cognata, V.; Latina, V.; Calissano, P.; Amadoro, G.; Cavallaro, S. Transcriptomic Analysis in the Hippocampus and Retina of Tg2576 AD Mice Reveals Defective Mitochondrial Oxidative Phosphorylation and Recovery by Tau 12A12mAb Treatment. Cells 2023, 12, 2254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toomey, C.E.; Heywood, W.E.; Evans, J.R.; Lachica, J.; Pressey, S.N.; Foti, S.C.; Al Shahrani, M.; D’Sa, K.; Hargreaves, I.P.; Heales, S.; et al. Mitochondrial dysfunction is a key pathological driver of early stage Parkinson’s. Acta Neuropathol. Commun. 2022, 10, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, A.N.; Choi, I.Y.; Lee, P.; Sullivan, D.K.; Burns, J.M.; Swerdlow, R.H.; Kelly, E.; Taylor, M.K. Creatine monohydrate pilot in Alzheimer’s: Feasibility, brain creatine, and cognition. Alzheimers Dement. 2025, 11, e70101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dennis, N.A.; Browndyke, J.N.; Stokes, J.; Need, A.; Burke, J.R.; Welsh-Bohmer, K.A.; Cabeza, R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010, 6, 303–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, S.; Dowell, N.G.; Tabet, N.; King, S.L.; Hutton, S.B.; Rusted, J.M. Disrupted neural activity patterns to novelty and effort in young adult APOE-e4 carriers performing a subsequent memory task. Brain Behav. 2017, 7, e00612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandon, J.A.; Farmer, B.C.; Williams, H.C.; Johnson, L.A. APOE and Alzheimer’s Disease: Neuroimaging of Metabolic and Cerebrovascular Dysfunction. Front. Aging Neurosci. 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wenneberg, S.B.; Block, L.; Sörbo, A.; Naredi, S.; Oras, J.; Hendén, P.L.; Ljungqvist, J.; Liljencrantz, J.; Hergès, H.O. Long-term outcomes after aneurysmal subarachnoid hemorrhage: A prospective observational cohort study. Acta Neurol. Scand. 2022, 146, 525–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanterna, L.A.; Rigoldi, M.; Tredici, G.; Biroli, F.; Cesana, C.; Gaini, S.M.; Dalprà, L. APOE influences vasospasm and cognition of noncomatose patients with subarachnoid hemorrhage. Neurology 2005, 64, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.D.; Sun, X.C.; Zhang, J.H. The role of apolipoprotein e in the pathological events following subarachnoid hemorrhage: A review. Acta Neurochir. Suppl. 2011, 110 Pt 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mesis, R.G.; Wang, H.; Lombard, F.W.; Yates, R.; Vitek, M.P.; Borel, C.O.; Warner, D.S.; Laskowitz, D.T. Dissociation between vasospasm and functional improvement in a murine model of subarachnoid hemorrhage. Neurosurg. Focus 2006, 21, E4. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Alzheimer’s disease: What treatments could be rolled out in the next few years? BMJ 2024, 387, q2477. [Google Scholar] [CrossRef] [PubMed]
- Tobeh, N.S.; Bruce, K.D. Emerging Alzheimer’s disease therapeutics: Promising insights from lipid metabolism and microglia-focused interventions. Front. Aging Neurosci. 2023, 15, 1259012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddi Sree, R.; Kalyan, M.; Anand, N.; Mani, S.; Gorantla, V.R.; Sakharkar, M.K.; Song, B.J.; Chidambaram, S.B. Newer Therapeutic Approaches in Treating Alzheimer’s Disease: A Comprehensive Review. ACS Omega 2025, 10, 5148–5171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimers Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snow, W.M.; Cadonic, C.; Cortes-Perez, C.; Adlimoghaddam, A.; Roy Chowdhury, S.K.; Thomson, E.; Anozie, A.; Bernstein, M.J.; Gough, K.; Fernyhough, P.; et al. Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease. Nutrients 2020, 12, 3589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brewer, G.J.; Wallimann, T.W. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000, 74, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Phongpreecha, T.; Gajera, C.R.; Liu, C.C.; Vijayaragavan, K.; Chang, A.L.; Becker, M.; Fallahzadeh, R.; Fernandez, R.; Postupna, N.; Sherfield, E.; et al. Single-synapse analyses of Alzheimer’s disease implicate pathologic tau, DJ1, CD47, and ApoE. Sci. Adv. 2021, 7, eabk0473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Currais, A.; Raschke, W.; Maher, P. CMS121, a novel drug candidate for the treatment of Alzheimer’s Disease and age-related dementia. J. Alzheimers Dis. 2024, 101, S179–S192. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Komisarow, J.M.; Wang, H.; Laskowitz, D.T. Therapeutic development of apolipoprotein E mimetics for acute brain injury: Augmenting endogenous responses to reduce secondary injury. Neurotherapeutics 2020, 17, 475–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laskowitz, D.T.; Van Wyck, D.W. ApoE mimetic peptides as therapy for traumatic brain injury. Neurotherapeutics 2023, 20, 1496–1507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Wyck, D.; Kolls, B.J.; Wang, H.; Cantillana, V.; Maughan, M.; Laskowitz, D.T. Prophylactic treatment with CN-105 improves functional outcomes in a murine model of closed head injury. Exp. Brain Res. 2022, 240, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Troy, J.; Nowacki, N.; Komisarow, J.; Swisher, C.B.; Tucker, K.; Hatton, K.; Babi, M.A.; Worrall, B.B.; Andrews, C.; et al. CN-105 in participants with acute supratentorial intracerebral hemorrhage (CATCH) trial. Neurocrit. Care 2022, 36, 216–225, Erratum in Neurocrit. Care 2022, 37, 610. https://doi.org/10.1007/s12028-022-01553-9. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, L.; Peng, Y.; Shen, Q.; Chen, K.; Fang, B.; Li, W. Effects of ketogenic diet on cognitive function of patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Nutr. Health Aging 2024, 28, 100306. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Theodoridis, X.; Gkouskou, K.K.; Evangeliou, A.; Dardiotis, E.; Bogdanos, D.P. To Keto or Not to Keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on Alzheimer Disease. Adv. Nutr. 2020, 11, 1583–1602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sano, M.; Bell, K.L.; Galasko, D.; Galvin, J.E.; Thomas, R.G.; van Dyck, C.H.; Aisen, P. A randomized, doubleblind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011, 77, 556–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldman, H.H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; DeMicco, D.A.; et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, B.; Craig, D.; Bullock, R.; Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016, 146, 3160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.; Mayer, C.L.; Morelli, D.; Millard, S.P.; Raskind, W.H.; Petrie, E.C.; Cherrier, M.; Fagan, A.M.; Raskind, M.A.; Peskind, E.R. Effect of simvastatin on CSF Alzheimer disease biomarkers in cognitively normal adults. Neurology 2017, 89, 1251–1255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schultz, B.G.; Patten, D.K.; Berlau, D.J. The role of statins in both cognitive impairment and protection against dementia: A tale of two mechanisms. Transl. Neurodegener. 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burns, D.K.; Alexander, R.C.; Welsh-Bohmer, K.A.; Culp, M.; Chiang, C.; O’Neil, J.; Evans, R.M.; Harrigan, P.; Plassman, B.L.; Burke, J.R.; et al. Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer’s disease (TOMMORROW): A prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021, 20, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Craft, S.; Landreth, G.; Linnamägi, U.; Sawchak, S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: Results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrington, C.; Sawchak, S.; Chiang, C.; Davies, J.; Donovan, C.; Saunders, A.M.; Irizarry, M.; Jeter, B.; Zvartau-Hind, M.; van Dyck, C.H.; et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: Two phase 3 studies. Curr. Alzheimer Res. 2011, 8, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Thal, L.J.; Amrein, R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int. Clin. Psychopharmacol. 2003, 18, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Crapper McLachlan, D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308, Erratum in Lancet 1991, 337, 1618.. [Google Scholar] [CrossRef] [PubMed]
- Gleason, A.; Bush, A.I. Iron and Ferroptosis as Therapeutic Targets in Alzheimer’s Disease. Neurotherapeutics 2021, 18, 252–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayton, S.; Barton, D.; Brew, B.; Brodtmann, A.; Clarnette, R.; Desmond, P.; Devos, D.; Ellis, K.A.; Fazlollahi, A.; Fradette, C.; et al. Deferiprone in Alzheimer Disease: A randomized clinical trial. JAMA Neurol. 2025, 82, 11–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alzheimer’s Research UK. UK-Wide Study into Dementia Blood Tests Recruits First Participants. Available online: https://www.alzheimersresearchuk.org/news/uk-wide-study-into-dementia-blood-tests-recruits-first-participants/ (accessed on 24 July 2025).
- Chinopoulos, C. The mystery of extramitochondrial proteins lysine succinylation. Int. J. Mol. Sci. 2021, 22, 6085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and mechanisms of oxidative stress-last 20 years of research with an emphasis on kidney damage and renal transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidant stress: An overview. Curr. Protoc. Toxicol. 2005, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.S.; Galano, J.M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Helmschrodt, C.; Becker, S.; Schröter, J.; Hecht, M.; Aust, G.; Thiery, J.; Ceglarek, U. Fast LC-MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin. Chim. Acta 2013, 425, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shi, M.; Guo, Z.; Brisbon, W.; Hoover, R.; Yang, H. Different cytotoxic injuries induced by lysophosphatidylcholine and 7-ketocholesterol in mouse endothelial cells. Endothelium 2006, 13, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M. 8-oxoguanine and 8-oxodeoxyguanosine biomarkers of oxidative DNA damage: A review on HPLC-ECD determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henriksen, T.; Weimann, A.; Larsen, E.L.; Poulsen, H.E. Quantification of 8-oxo-7,8-dihydro-2′-deoxyguanosine and 8-oxo-7,8-dihydro-guanosine concentrations in urine and plasma for estimating 24-h urinary output. Free Radic. Biol. Med. 2021, 172, 350–357. [Google Scholar] [CrossRef] [PubMed]
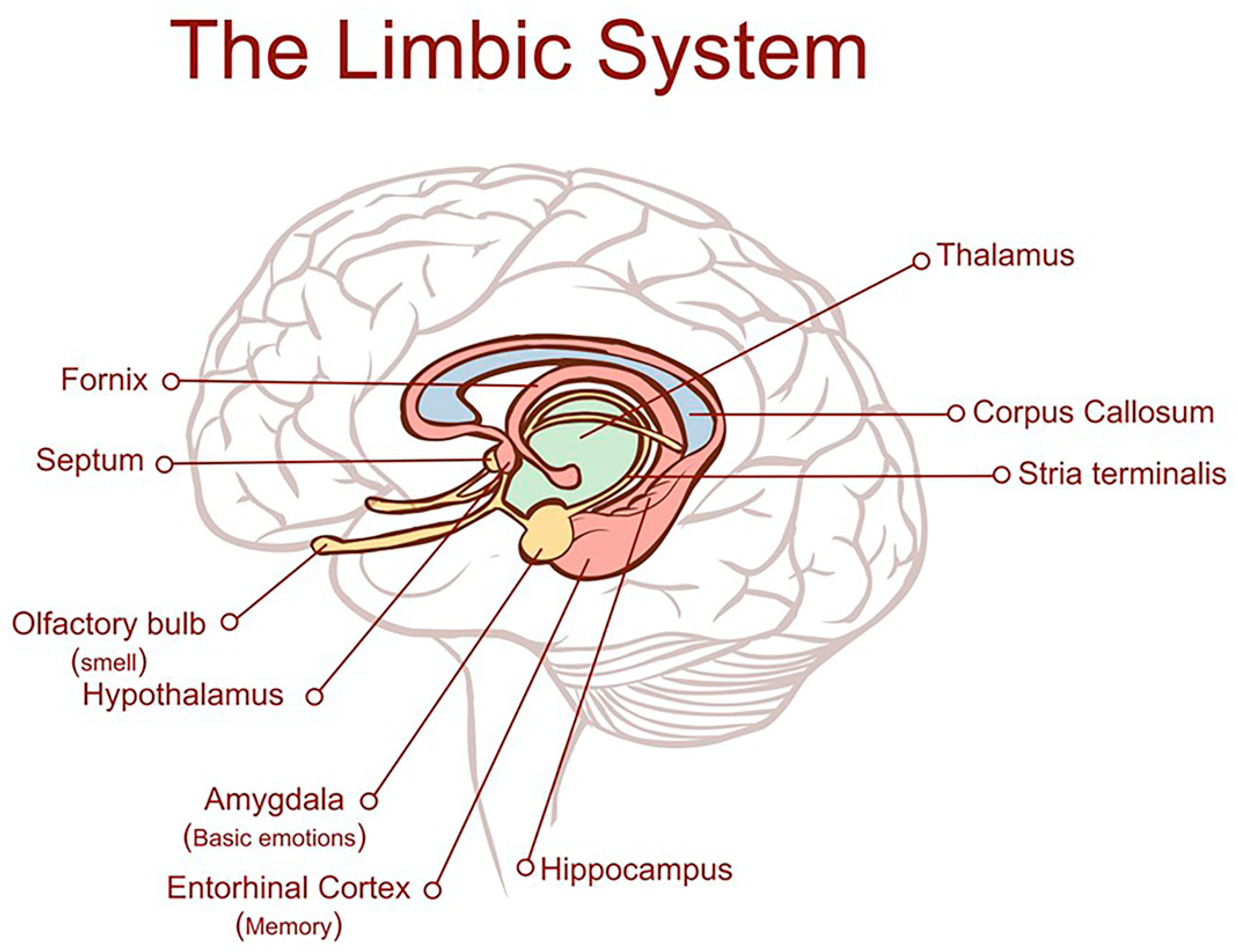
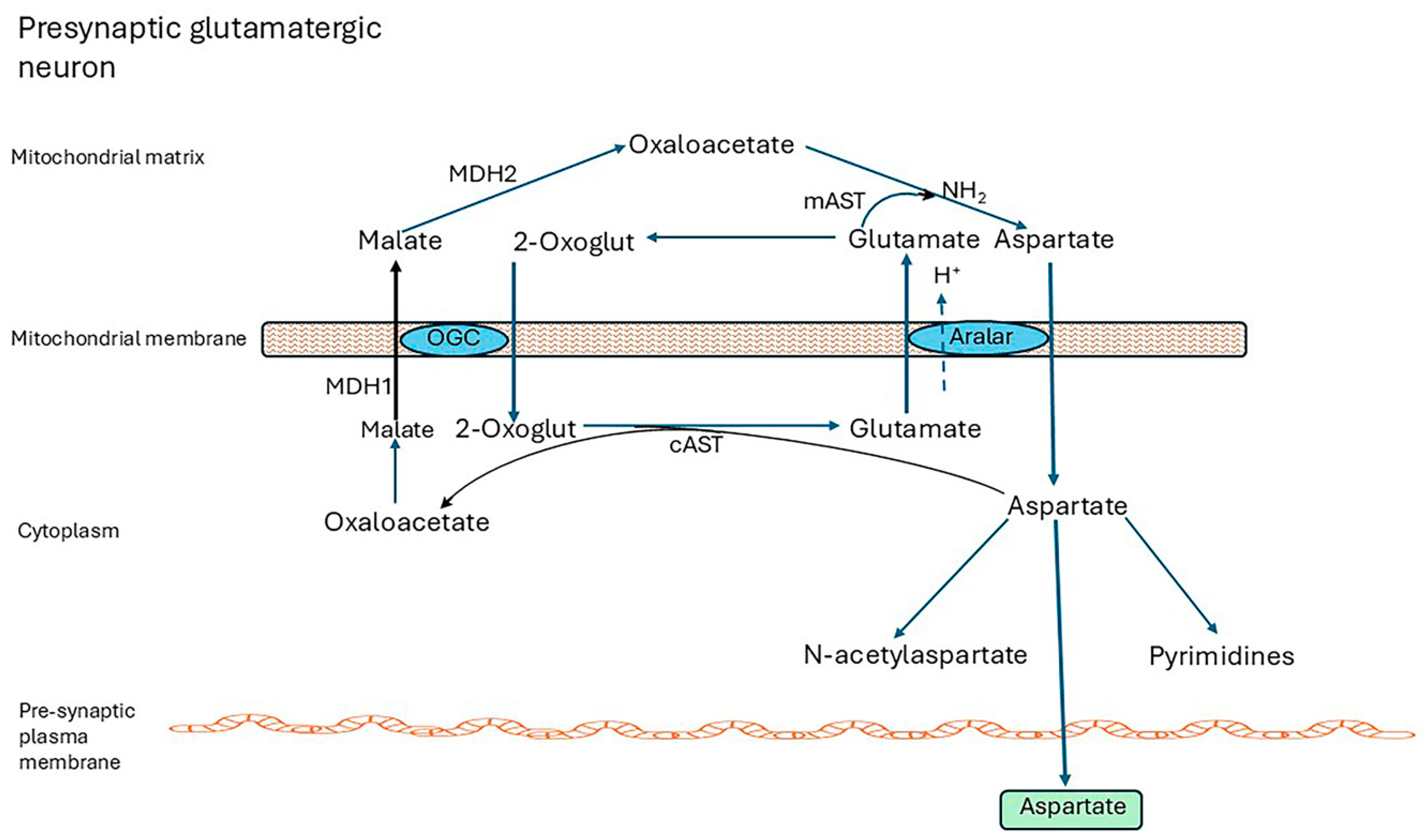

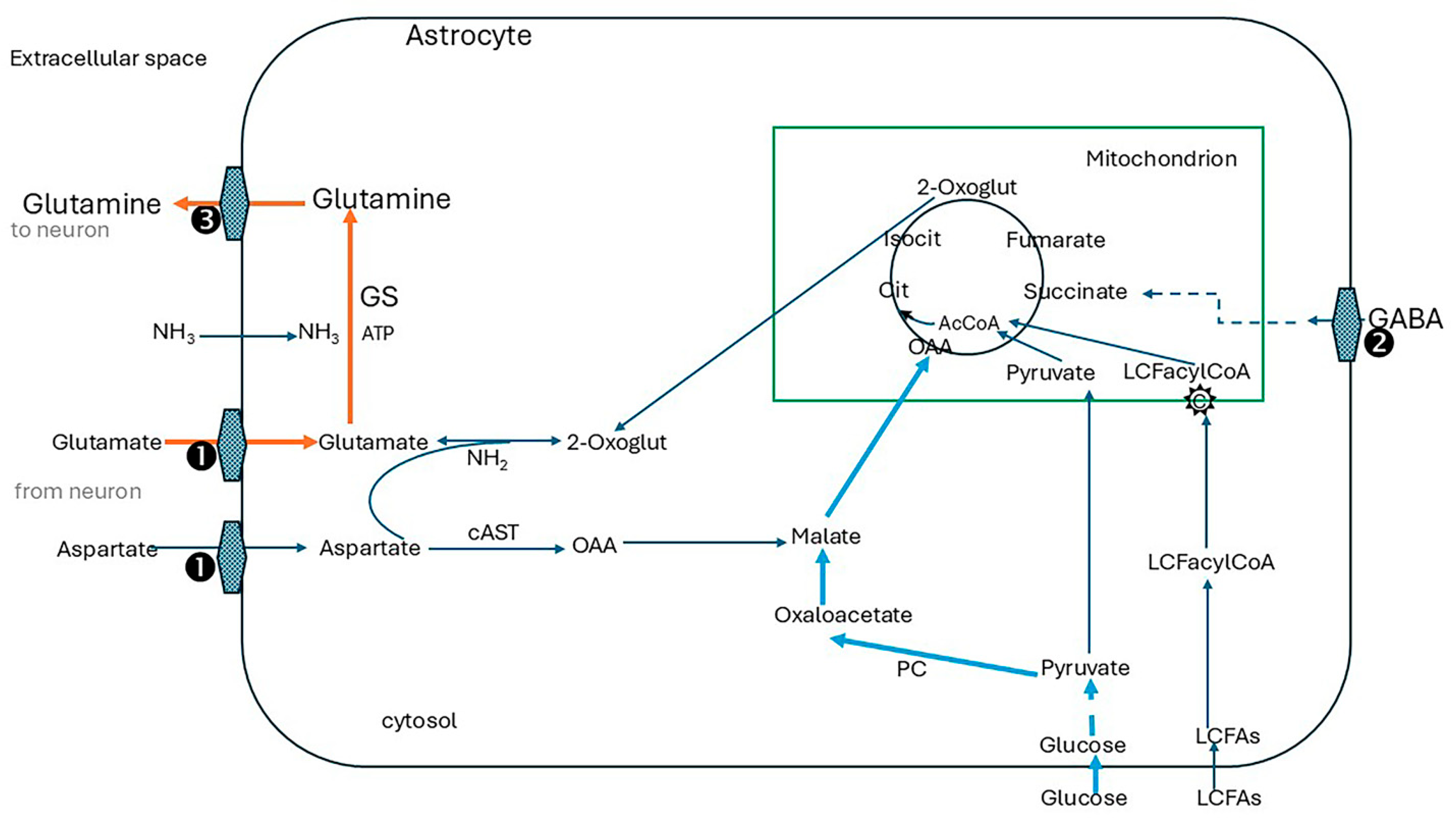
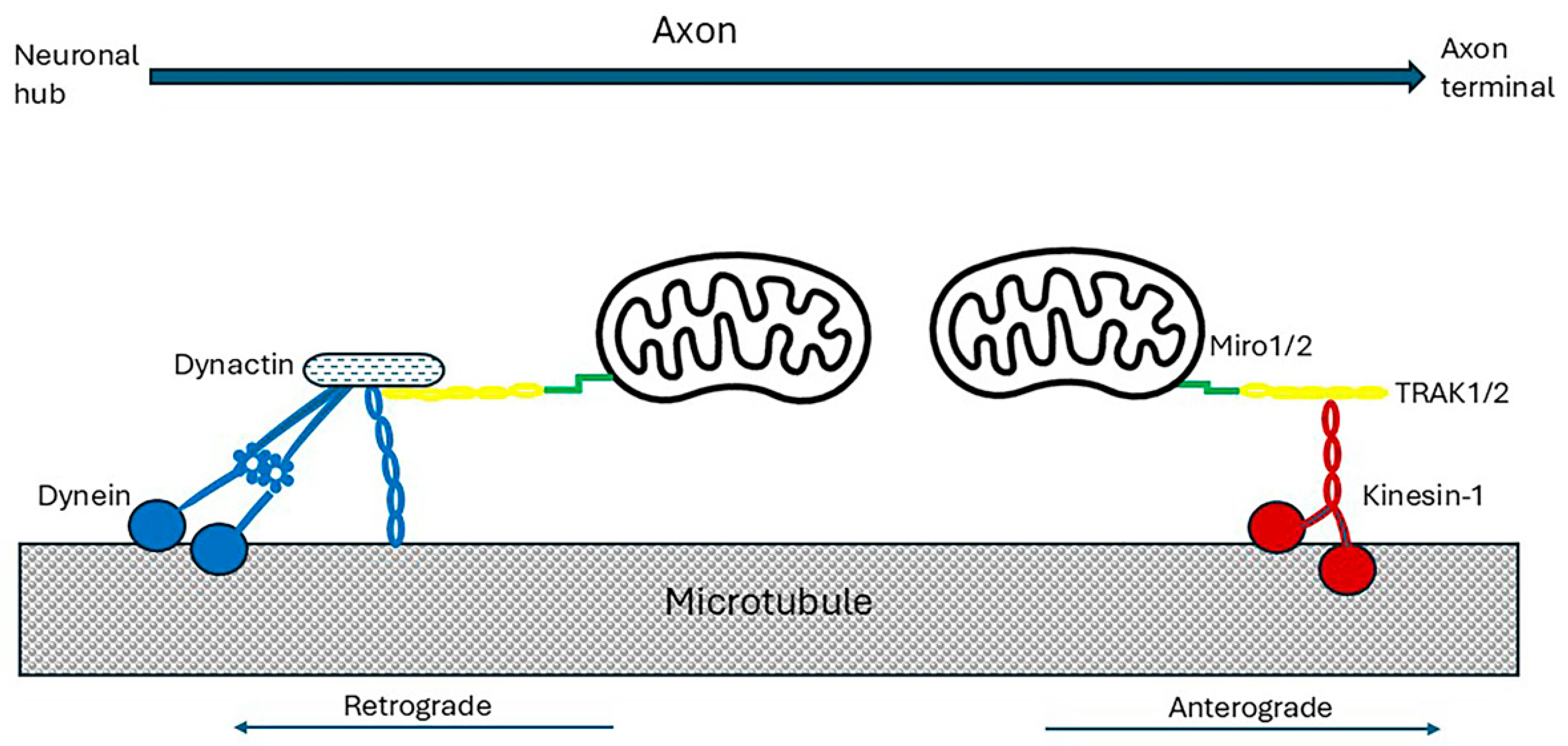

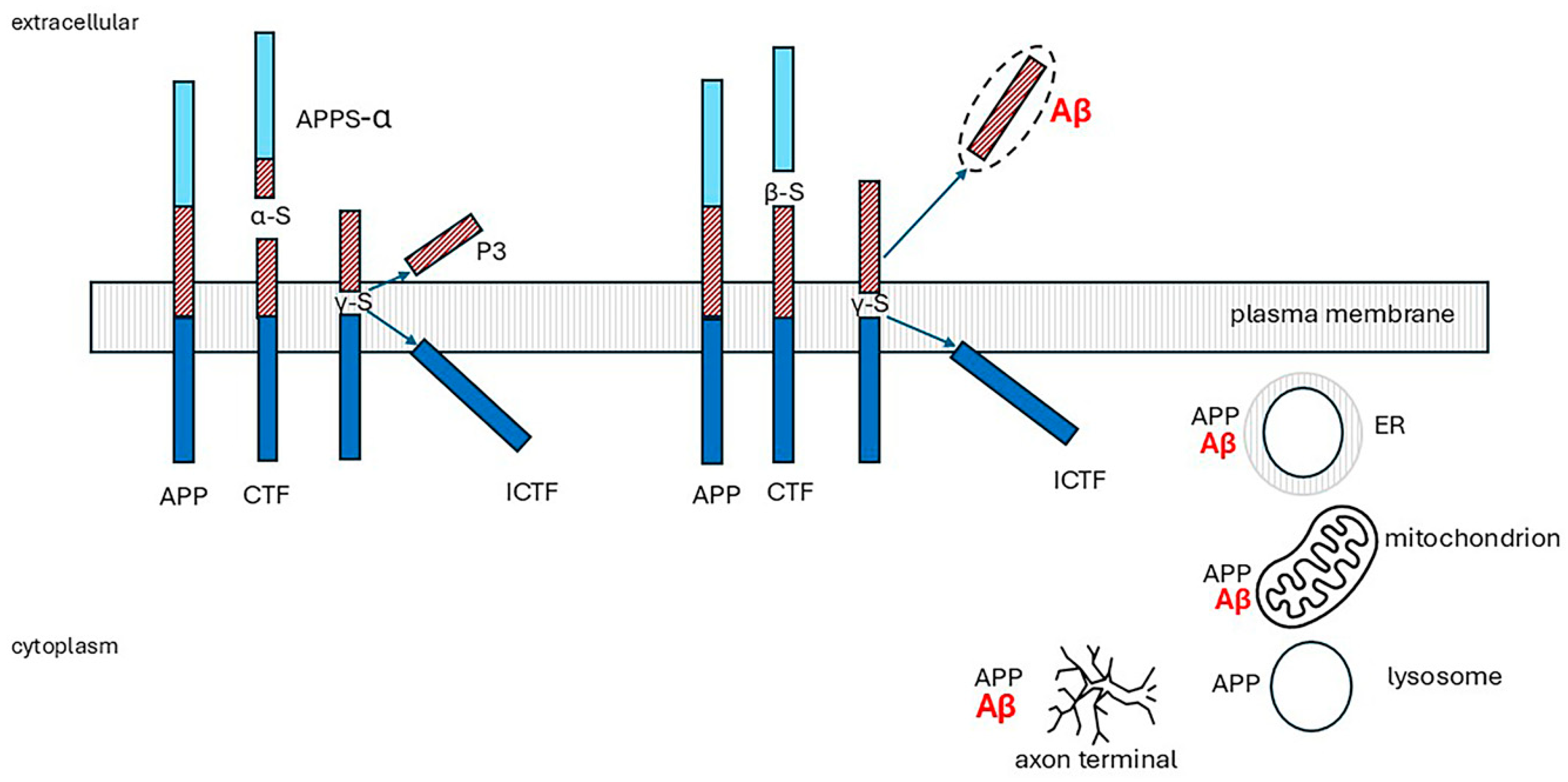
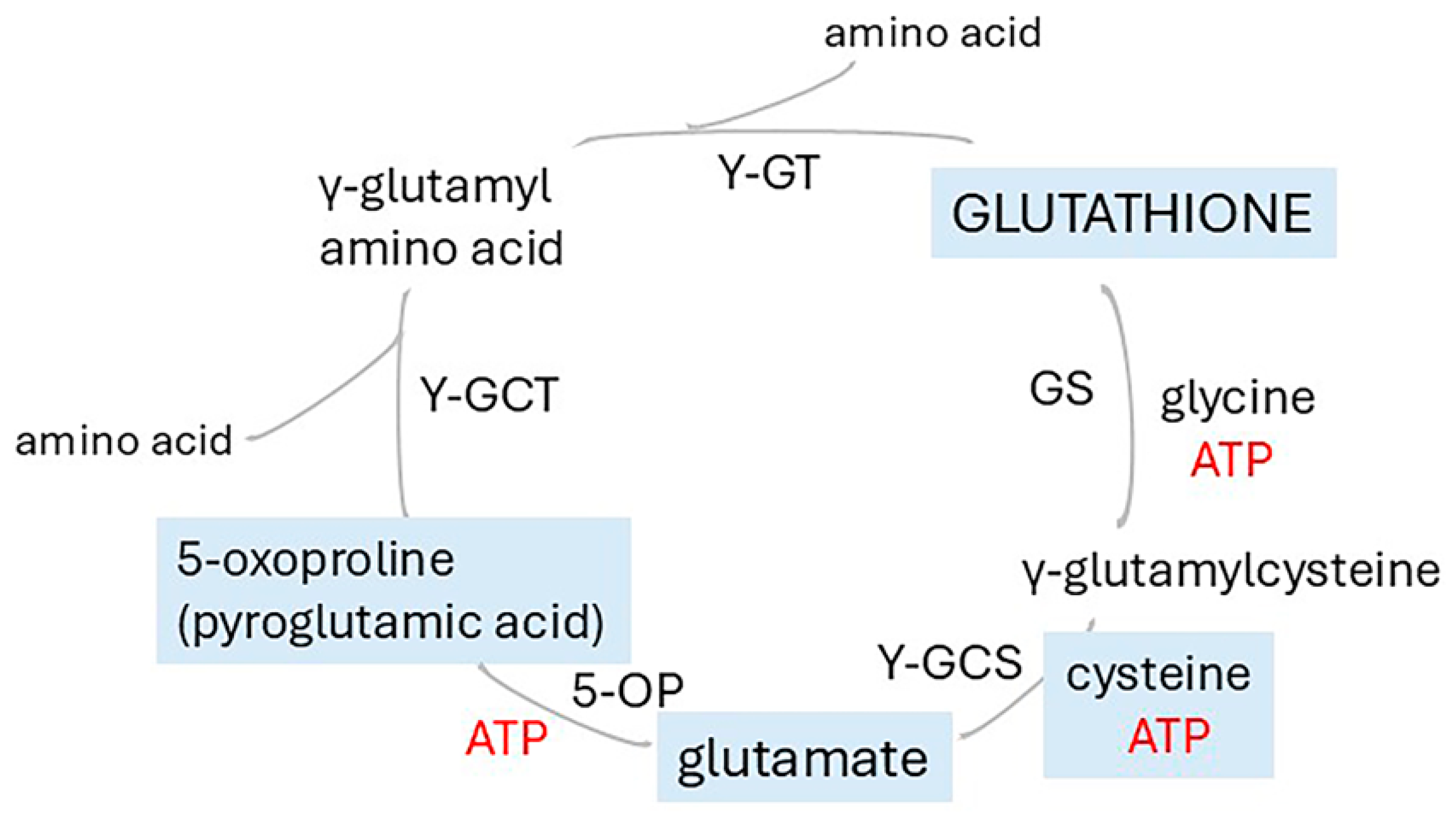
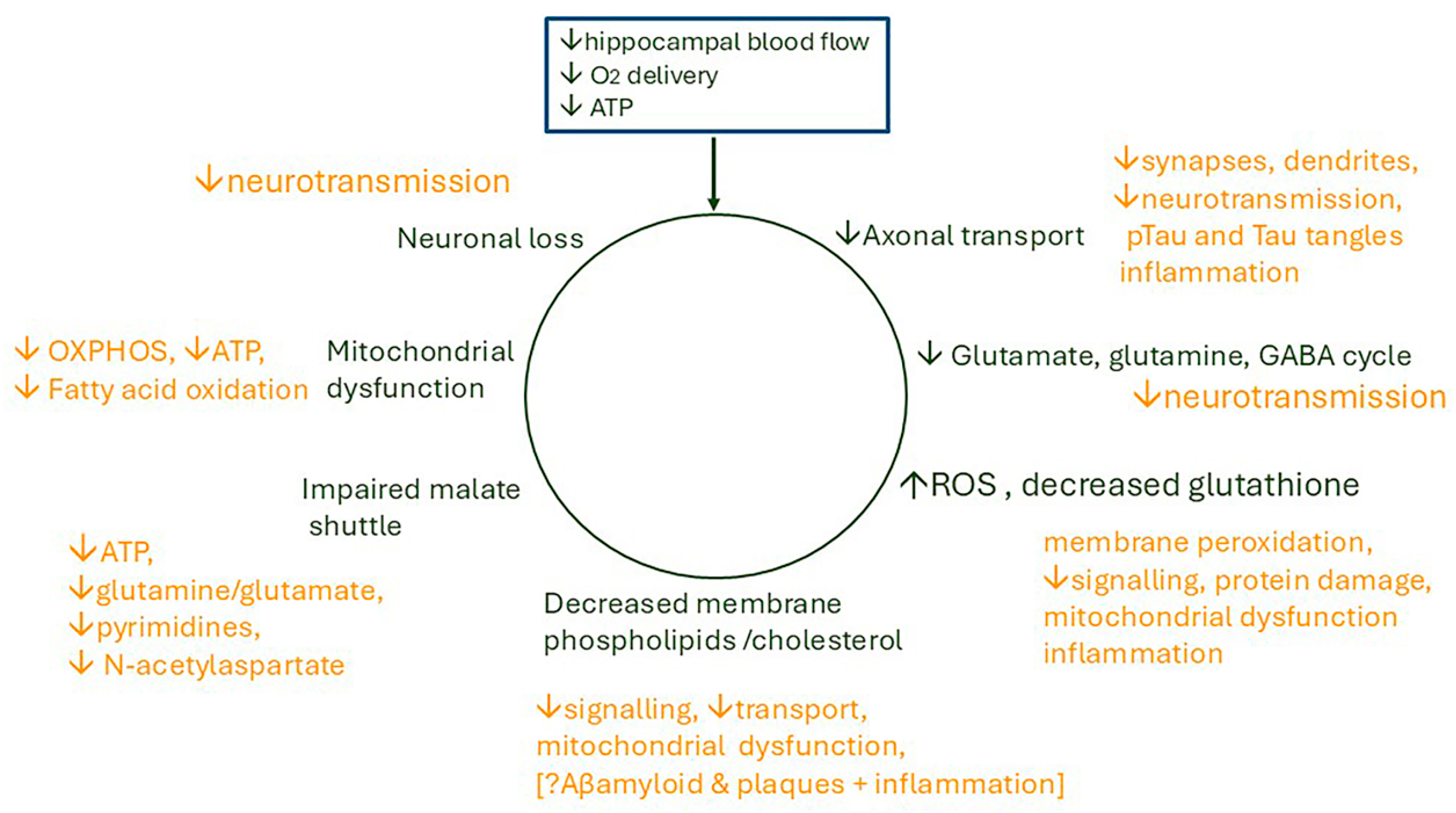
| Factor |
|---|
| Age |
| Overweight in midlife, but not late-life overweight or obesity [10] |
| High blood cholesterol; familial hypercholesterolaemia [10] |
| Low HDL cholesterol [10] |
| Persistent hypertension-midlife [10] |
| Smoking [11] |
| Chronic stress [11] |
| Depression [11] |
| Alcohol-heavy intake or abstinence [12] |
| Poor sleep [11] |
| Recurrent hypoxia-sleep apnoea, Chronic obstructive pulmonary disease COPD [13] |
| Hyperammonaemia (potential risk) [14,15] |
| Genes and polymorphisms |
| APOE4 [16] |
| TREM2 Triggering Receptor Expressed on Myeloid cells 2 [11] |
| MT-RNR2 Humanin, Mitochondrially encoded 16S RNA rs2854128—African Americans (not Europeans) [17] |
| SLC25A22 Solute Carrier Family 25 Member 22 (Glutamate/H(+) Symporter 1) [18] |
| ABCA1 ATP-binding cassette subfamily A member 1 loss of function [19] |
| ABCA7 ATP-binding cassette subfamily A member 7 numerous polymorphisms [20,21] |
| SREBP-2 Sterol Regulatory Element Binding Transcription Factor 2 [22] |
| MBOAT1 Membrane-Bound Glycerophospholipid O-Acyltransferase 1 (proposed risk) [23] |
| PICALM phosphatidylinositol binding clathrin assembly protein rs3851179 [24] |
| Mutations in genes for amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) rare early-onset AD [25] |
| Signal of Low ATP | Sensor/Mediator | Actions to Increase ATP Supply | Actions to Decrease ATP Consumption |
|---|---|---|---|
| Raised AMP/ATP or ADP/ATP ratio | AMP-activated protein kinase (AMPK) [52,57,58,59] | ↑ Glucose transporters 1 and 4 (GLUT 1/4) | ↓ Fatty acid synthesis |
| ↑ Glycolysis | ↓ Cholesterol synthesis | ||
| ↑ Fatty acid uptake by mitochondria | ↓ Triacylglycerol phosphate synthesis | ||
| ↑ Mitochondrial biogenesis | ↓ Transcription of lipogenic enzymes | ||
| ↑ Mitochondrial fission | ↓ Glycogen synthesis | ||
| ↑ Mitochondrial ADP/ATP translocase (ANT) activity | ↓ rRNA synthesis | ||
| ↑Autophagy and mitophagy | ↓ Protein synthesis | ||
| ↑NAD+/NADH ratio and inhibits Sirtuin 1 | |||
| Raised AMP/ATP or ADP/ATP ratio? via AMPK activation | Mitochondrial ADP/ATP translocase [73,74,75,76] | Increased mitochondrial ADP uptake and ATP release | |
| Low NAD+/NADH ratio in hypoxia | Sirtuin 1 (SIRT1) (down-regulated) [66,67] | With down-regulation, ↑ acetylation and activation of HIF1α | |
| Raised AMP/ATP ratio + Ca2+ release through stress hormone stimulation | Mitochondrial Ca2+-regulated ATP-Mg2+/phosphate carrier SLC25A25 [73] | ↑ ATP-Mg2+ release from mitochondria | |
| Oxygen depletion (hypoxia) | HIF-1α † [86,87,88,89] | ↑Erythropoietin stimulates red blood cell production, ↑VEGF (vascular endothelial growth factor) increases angiogenesis and increases O2 delivery. ↑ Glucose transporters 1 and 3 (GLUT 1/3) | Increases conversion of extracellular ATP to adenosine which inhibits the NMDA receptor and glutamate signalling [111] |
| Oxygen depletion (hypoxia) | HummR [86,89] | ↑ axonal mitochondrial transport | |
| Uncertain at present | Mitochondrial-derived peptides/nuclear-encoded mitochondrial peptides [17] | ||
| ↑ AMP | Phosphofructokinase [57,58] | ↑ Glucose catabolism | |
| ↑ AMP | Glycogen phosphorylase [57,58] | ↑ Glucose release from glycogen | |
| ↑AMP | Fructose 1,6-bisphosphatase (inhibited) [57,58] | ↓ gluconeogenesis |
| Study | Main Findings | Reference | |
|---|---|---|---|
| Human studies | |||
| 1 | Atherosclerosis of Circle of Willis arteries in AD | Number of stenoses and stenosis index in AD > controls; correlated with plaque, NFTs, white matter rarefaction, Braak stage | Roher AE, Esh C, Kokjohn T, et al., 2003 [281] |
| 2 | Atherosclerosis of cerebral arteries in AD | Stenosis of arteries and number of stenoses per individual in AD > controls—highly significant | Roher AE, Esh C, Rahman A, et al., 2004 [282] |
| 3 | Vascular hippocampal plasticity after aerobic exercise in older adults | Fitness improvement correlated with changes in hippocampal perfusion and head volume, but considerable interindividual variability in the response to physical exercise | Maass A, Düzel S, Goerke M, et al., 2005 [283] |
| 4 | Hippocampal vascularization patterns in vivo | Variable contribution of the anterior choroidal artery, the relationships between hippocampal and posterior cerebral artery patterns, and different distribution patterns in the right and left hemispheres. | Spallazzi M, Dobisch L, Becke A, et al., 2019 [272] |
| 5 | Cerebral Angioarchitectonics in AD, compared with other neurodegenerative and ischaemic lesions | temporal and fronto-parietal areas of all patients with AD, regardless of disease stage: specific changes in cerebral microcirculation which they named dyscirculatory angiopathy of Alzheimer’s type (DAAT). DAAT was not found in the controls. | Maksimovich IV, 2018 [284] |
| 6 | Effects of acute hypoxia on cerebral bioenergetics and memory. | In hypoxia, oxygen delivery was reduced in middle cerebral artery during central executive tasks and in posterior cerebral artery during memorization and recall; no effect on cerebral blood flow | Ando S, Tsukamoto H, Stacey BS, et al., 2023 [274] |
| 7 | Regional cerebral microvascular perfusion in acute and prolonged hypoxia | 2 h of hypoxia: perfusion increased in the frontal cortex and decreased in ‘default mode’ network; after 10 h, decreased blood flow in default mode network was more pronounced and widespread, hence reduced local perfusion; showed a relationship with vasoconstriction | Lawley JS, Macdonald JH, Oliver SJ, 2017 [285] |
| 8 | Effects of brain ischaemia on succinate and other metabolites | warm ischaemia ex vivo: time-dependent accumulation of succinate; other significant changes included increases in purine degradation, PUFAs, and 5-oxoproline and decreases in adenosine and acylcarnitines; Stroke model: succinate accumulated, other TCA metabolites decreased, and a dramatic decrease in ATP | Mottahedin A, Prag HA, Dannhorn A, et al., 2023 [286] |
| 9 | Association of regional cerebral perfusion in AD with Tau and amyloid | Tau-PET was associated with lower CBF in the entorhinal cortex, persisted after excluding AD dementia group, and was independent of Aβ, APOE genotype and MRI markers for small vessel disease. Amyloid-PET was associated with lower CBF in temporo-parietal regions | Rubinski A, Tosun D, Franzmeier N, et al., 2021 [260] |
| 10 | Tau deposition in entorhinal cortex related to hypoperfusion | baseline CBF was associated with tau deposition at the 6-year follow-up in the left but not the right entorhinal cortex; findings suggest that a reduction in CBF at the entorhinal cortex precedes tau deposition. | Kapadia A, Billimoria K, Desai P, et al., 2023 [4] |
| 11 | Longitudinal changes in CBF in the older hypertensive brain | Relative to controls, in the hypertensive group, rCBF decreased in prefrontal, anterior cingulate, and occipital areas over time | Beason-Held LL, Moghekar A, Zonderman AB, et al. 2007 [287] |
| Animal studies | |||
| 12 | Neurovascular coupling in the hippocampus and visual cortex | Compared with visual cortex, hippocampal arteries had a blunted response: fewer, smaller dilations. ATP production is restricted in tissues furthest from capillaries | Shaw K, Bell L, Boyd K, et al., 2021 [273] |
| 13 | Identification of leukotrienes C4 and D4 in gerbil brains after ischaemia and reperfusion. | Significant increases at 5, 10, or 15 min of ischaemia, more marked on reperfusion; highest in forebrain grey matter, undetectable in brain regions remote from ischaemic zone | Moskowitz MA, Kiwak KJ, Hekimian K, et al., 1984 [288] |
| 14 | Biochemical response to hypobaric oxygen: hippocampus, cortex, cerebellum | Compared with controls, increased lactate dehydrogenase, free radical generation, lipid peroxidation, glutamate dehydrogenase activity, and vesicular glutamate transporter expression decreased glutathione reductase, superoxide dismutase activity, reduced glutathione with increased oxidized glutathione | Hota SK, Barhwal K, Singh SB, et al., 2007 [289] |
| 15 | Hippocampal morphology following hypobaric hypoxia | Significant cell degeneration and death only in the CA3 region; damage is more noticeable with longer time following exposure | Shukitt-Hale B, Kadar T, Marlowe BE, et al., 1996 [290] |
| 16 | Oxidative stress in rat brain in hypobaric hypoxia | Significant increase in free radical production, nitric oxide, lipid peroxidation, and lactate dehydrogenase greater at 7 days than 3 days; reduced glutathione, glutathione peroxidase, glutathione reductase, and superoxide dismutase, and reduced/oxidized glutathione. Hippocampus is most susceptible. | Maiti P, Singh SB, Sharma AK, et al., 2006 [291] |
| 17 | Effect of acute hypobaric hypoxia on SOD and MDA, and mRNA expression of VEGF and HIF1-α in rat brain | Increased expression of HIF1-α and VEGF on days 1, 2, 3; significant increase in MDA, decreased SOD | Tahir MS, Almezgagi M, Zhang Y, et al. 2021 [292] |
| Study | Main Findings | Reference | |
|---|---|---|---|
| Brain | |||
| 1 | Neuronal loss of entorhinal cortex | Controls: neuronal numbers constant 60y to 90y AD: severe neuronal loss; mainly layers II and IV; loss correlated with NFTs and neuritic but not diffuse or total plaques | Gómez-Isla T, Price JL, McKeel DW Jr, et al., 1996 [1] |
| 2 | Non-targeted metabolomics to identify pathways altered in AD | Most affected pathway: Ala, Asp, Gln, Asp significant decrease, marked disturbances of malate-aspartate shuttle, glycerophospholipids, pyrimidines; increased S-adenosyl methionine, S-adenosylhomocysteine | Paglia G, Stocchero M, Cacciatore S, et al., 2016 [306] |
| 3 | Brain energy pathways in cingulate cortex of young adult APOE4 carriers without AD | Carriers: increased expression of subunits of mitochondrial complexes I, II, IV; no change in III or V; qPCR: significant small changes in NDUFB5, NDUF7, and ARRDC3 expression | Perkins M, Wolf AB, Chavira B, et al., 2016 [16] |
| 4 | Brain structural changes over 12–24 m in MRI scans | Mean annualized hippocampal volume change: AD 4.8%, controls 1.1%; AD increased neuronal loss | Ledig C, Schuh A, Guerrero R, et al., 2018 [38] |
| 5 | Investigation of gene pathways enriched in hippocampus in AD | In AD, significant changes in NF-κβ, and cGMP-PKG signalling pathways, MT1, MT2, NOTCH2, ADD3, MSX1, RAB31, key hub genes | Liang JW, Fang ZY, Huang Y, et al., 2018 [307] |
| 6 | Gene pathway analysis to find biomarkers of human brain ageing | Modules relevant to brain ageing: synaptic vesicle cycle, cGMP-PKG signalling pathway, and oxidative phosphorylation | Hu Y, Pan J, Xin Y, et al., 2018 [47] |
| 7 | Changes in proteome and phosphoproteome in AD progression (7) | Identified three proteome clusters associated with AD progression. Enriched pathways were mitochondria, mitochondrial function, and neurotrophic factor signalling | Bai B, Wang X, Li Y, et al., 2020 [308] |
| 8 | Gene pathway analysis to identify new gene and miRNA biomarkers for AD | Identified 8 genes, one of which, MBOAT1, was not previously reported, and five miRNAs | Soleimani Zakeri NS, Pashazadeh S, MotieGhader H, 2020 [23] |
| 9 | Genomic and transcriptomic analyses of hippocampus | Expression of 54 genes associated with AD; 21 were prioritized, including two novel genes Tyrosine-Protein Phosphatase Non-Receptor Type 9 (PTPN9) and Protocadherin Alpha 4 (PCDHA4); QPCTL (glutamyl cyclotransferase) and ERCC2 (excision repair 2) were significantly different from elderly controls | Liu N, Xu J, Liu H, et al., 2021 [309] |
| 10 | Investigation of co-expression networks and regulators of metabolism in AD progression | With AD progression, decreased branched-chain AAs, and short-chain acylcarnitines, increased medium- and long-chain acylcarnitines, increased expression of adiponectin protein and ATP-Binding Cassette Subfamily A Member 1 (ABCA1) and Carnitine Palmitoyltransferase 1A (CPT1A) genes in the Hippocampus and para-hippocampal gyrus | Horgusluoglu E, Neff R, Song W-M, et al., 2021 [310] |
| 11 | Identification of gene pathways in brain regions with AD pathology identified by use of three different PET scans | Results from Tau scans are most relevant. Pathways identified included mitochondrial respiration, electron transport, OXPHOS, and metabolism | Mullins R and Kapogiannis D, 2022 [311] |
| 12 | Transcriptomic analyses of hippocampal entorhinal subfields to identify regulators in AD | All 5 subfields were positively enriched in AD signalling pathways, extensive neuronal loss in all 5 regardless of AD pathology; most differentially expressed genes in EC and CA4, with a significant correlation of neuronal and astrocyte profiles, PSP (prosaposin), a key modulator of astrogliosis | Luo D, Li J, Liu H, et al., 2023 [312] |
| 13 | Changes in brain protein expression with AD progression to find proteins to predict progression of MCI to AD, using machine learning | 29 proteins provided best classification of AD and controls; 88 proteins were needed to classify AD and asymptomatic AD; predictive proteins of change with disease state were significantly enriched for sugar metabolism supporting dysregulation of energy metabolism | Tandon R, Levey AI, Lah JJ, et al., 2023 [313] |
| 14 | Association of 53 SLC25 carriers with AD | SLC25A10, SLC25A17, and SLC25A22 were identified as AD susceptibility genes; down-regulation of gene for glutamate carrier 1 (SLC25A22) is associated with accelerated hippocampal atrophy and increased hazard of dementia. Pathway analysis related SLC25A22 to defects in neuronal function | Tian J, Jia K, Wang T, et al., 2024 [18] |
| 15 | Human cortical peptidome in cognitive resilience against AD | 35 proteins were significantly associated with resilient AD (AD pathology but normal cognition) or with low cognition without AD pathology. In resilient increased ATP synthase F1 subunit delta (ATP5FLD) and cytochrome C oxidase subunit 8A (COX8A) were found. Heterogeneous Nuclear Ribonucleoprotein K (HNRNAP) was enriched in inhibitory neurons | Morgan GR and Carlyle BC, 2024 [2] |
| 16 | Comprehensive hippocampal bio-informatics study using machine learning to identify novel risk genes for AD | 27 down-regulated and 4 up-regulated genes correlated with AD stage. Higher expression of five genes was associated with decreased risk and slower progression of AD; four with higher risk and faster progression: PNMAL1, SLC39A10, GLRB, and PTPN3 | Li J, Li L, Cai S, et al., 2024 [11] |
| CSF | |||
| 17 | CSF Metabolite profiles in AD | In mild AD, compared with controls, combination of significantly increased cysteine and decreased uridine is 75% predictive of AD, with sensitivity of 75%; Cortisol increased with progression of AD in more advanced AD, i.e., increased cortisol | Czech C, Berndt P, Busch K, et al., 2012 [314] |
| 18 | Untargeted CSF metabolomics in prodromal AD with mild cognitive impairment | 94 of 294 differentially expressed metabolites were annotated; disturbance in 13 pathways was identified. Top four pathways related to bioenergetics and glucose metabolism (N-glycan, sialic acid, amino sugars, galactose); methionine, tyrosine, purine, and biopterin metabolism were also differentially activated | Hajjar I, Liu C, Jones DP et al., 2020 [315] |
| 19 | Unbiased CSF proteomics in patients with AD | Compared to non-AD groups, pyruvate kinase (PKM) and aldolase A (ALDOA) were up-regulated in AD CSF, glucose increased only in MCI; 33 peptides were differentially abundant between AD with dementia and all non-demented-AD groups, including clusters for glycolytic processes or canonical glycolysis and synaptic and immune response markers | de Geus MB, Leslie SN, Lam T, et al., 2023 [316] |
| 20 | CSF Proteome and metabolome of individuals with varying amyloid/taurine (AT) pathology and nine biomarkers of neurodegeneration and neuroinflammation | 61 proteins were significantly associated with AT category, and 636 proteins with biomarkers. Glucose and carbon metabolism pathways were enriched among amyloid- and tau-associated proteins, including malate dehydrogenase, aldolase A, and succinyl carnitine. Preliminary findings supported association of glucose metabolic dysregulation with alterations in amyloid and tau even before cognitive impairment; preliminary investigations suggested possible abnormalities in insulin signalling | Panyard DJ, McKetney J, Deming YK, et al., 2023 [317] |
| Study | Main Relevant Findings | Reference | |
|---|---|---|---|
| 21 | CSF metabolome of a rabbit model for late-onset AD with AD neuropathology induced by a high-cholesterol diet | Profiles changed with time; Aβ-like plaques were only seen at 12 weeks. Four clusters were identified in the top 95 metabolites, most at 12 weeks. At 12 weeks, decreased phospholipids, mainly phosphorylated fatty alcohols, akylacyl, or dialkyl-glycerophosphates, all potential precursors or degradation products of phospholipids including phosphatidylcholines and plasmalogens. | Liu QY, Bingham EJ, Twine SM, et al., 2012 [26] |
| 22 | Cerebral cortical and glutamine metabolism in a mouse AD model (APPswe/PSEN1dE9) | AD mice: significantly increased lactate and alanine, decreased TCA intermediates, decreased capacity for uptake and oxidative metabolism of glutamine; no change in glial acetate metabolism. | Andersen JV, Christensen SK, Aldana BI, et al., 2017 [318] |
| 23 | Hippocampal proteomic pathways associated with memory status in normal ageing and 5FXAD AD mouse model | Normal and AD mice, HDAC4 identified as regulator of memory-related proteins; top pathways associated with memory deficits in controls: OXPHOS, mitochondrial dysfunction, glutamate receptor signalling. | Neuner SM, Wilmott LA, Hoffmann BR, et al., 2017 [319] |
| 24 | Investigation for overlap in protein expression up to 15 m of normal mice following mild traumatic brain injury (TBI) aged 3 m; and non-traumatized mice with AD (PSAPP and mice expressing hTau) up to 15 m | Impaired in TBI: energy metabolism, clearance, neurotransmitter and intracellular signalling, and glial cell function. Little overlap with altered proteins in AD models. TBI and AD damage distinct processes | Ojo JO, Crynen G, Algamal M, et al., 2020 [320] |
| 25 | Characterization of Tg4-42 mouse model for AD [321] | Significant loss of hippocampal CA1 neurons. At 9 m caudate, putamen: significant decreases: GABA, glutamine, lactate: increased Aβ42, glutaminase, glutamine decarboxylase, CSF, increased neurofilament light chains (NFL) | Hinteregger B, Loeffler T, Flunkert S, et al., 2021 [321] |
| 26 | Metabolite analyses of cortex and hippocampus of a transgenic AD mouse model with high-resolution magic angle spinning NMR. | Controls: changes with age in cortex; at 9 m sex differences; at 9 m differences from AD mice in hippocampus: glutamate, glutamine, Nacetylaspartate (NAA), glycine, phosphocholine, and glycerophosphocholine. | Füzesi MV, Muti IH, Berker Y, et al. 2022 [322] |
| 27 | Investigation of mitochondrial dysfunction and effects of an antibody to a neurotoxic Tau peptide in hippocampus and retina of a mouse AD model | Decreased expression of genes involved in multiple energy-generating mitochondrial pathways including OXPHOS pathways; FA oxidation in the hippocampus and retina of Tg2576 AD mice; GSEA analysis: oxidative phosphorylation is the most down-regulated gene set in hippocampus of early symptomatic Tg2576; mitochondrial alterations were observed in AD mice significantly reverted by NH2htau antibody. | Morello G, Guarnaccia M, La Cognata V, et al., 2023 [323] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, V. Are Hippocampal Hypoperfusion and ATP Depletion Prime Movers in the Genesis of Alzheimer’s Disease? A Review of Recent Pertinent Observations from Molecular Biology. Int. J. Mol. Sci. 2025, 26, 7328. https://doi.org/10.3390/ijms26157328
Walker V. Are Hippocampal Hypoperfusion and ATP Depletion Prime Movers in the Genesis of Alzheimer’s Disease? A Review of Recent Pertinent Observations from Molecular Biology. International Journal of Molecular Sciences. 2025; 26(15):7328. https://doi.org/10.3390/ijms26157328
Chicago/Turabian StyleWalker, Valerie. 2025. "Are Hippocampal Hypoperfusion and ATP Depletion Prime Movers in the Genesis of Alzheimer’s Disease? A Review of Recent Pertinent Observations from Molecular Biology" International Journal of Molecular Sciences 26, no. 15: 7328. https://doi.org/10.3390/ijms26157328
APA StyleWalker, V. (2025). Are Hippocampal Hypoperfusion and ATP Depletion Prime Movers in the Genesis of Alzheimer’s Disease? A Review of Recent Pertinent Observations from Molecular Biology. International Journal of Molecular Sciences, 26(15), 7328. https://doi.org/10.3390/ijms26157328







