Deciphering the Causes of IbfA-Mediated Abortive Infection in the P22-like Phage UAB_Phi20
Abstract
:1. Introduction
2. Results
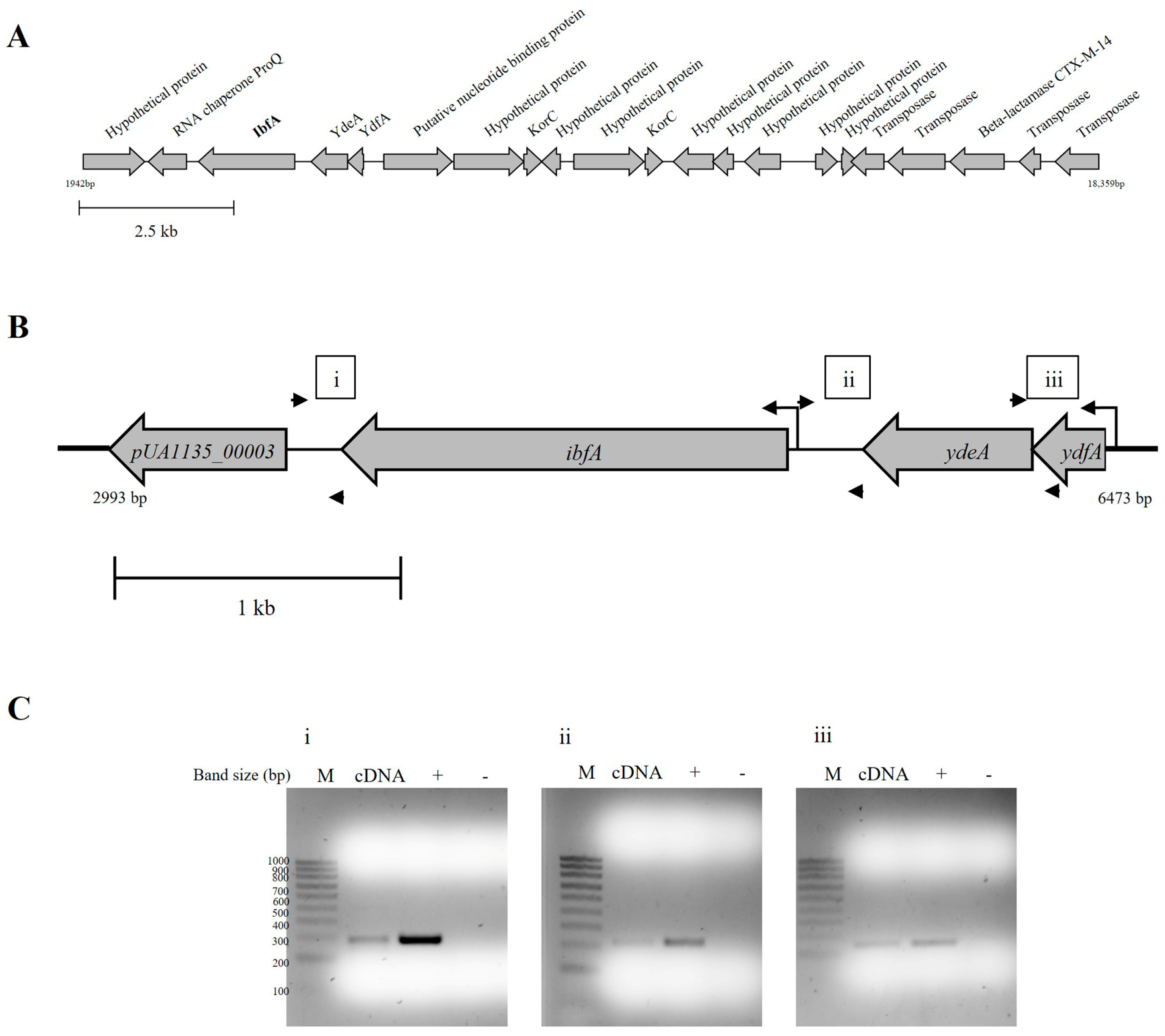
2.1. Unravelling the Plasmid Region That Disrupts the UAB_Phi20 Multiplicative Cycle
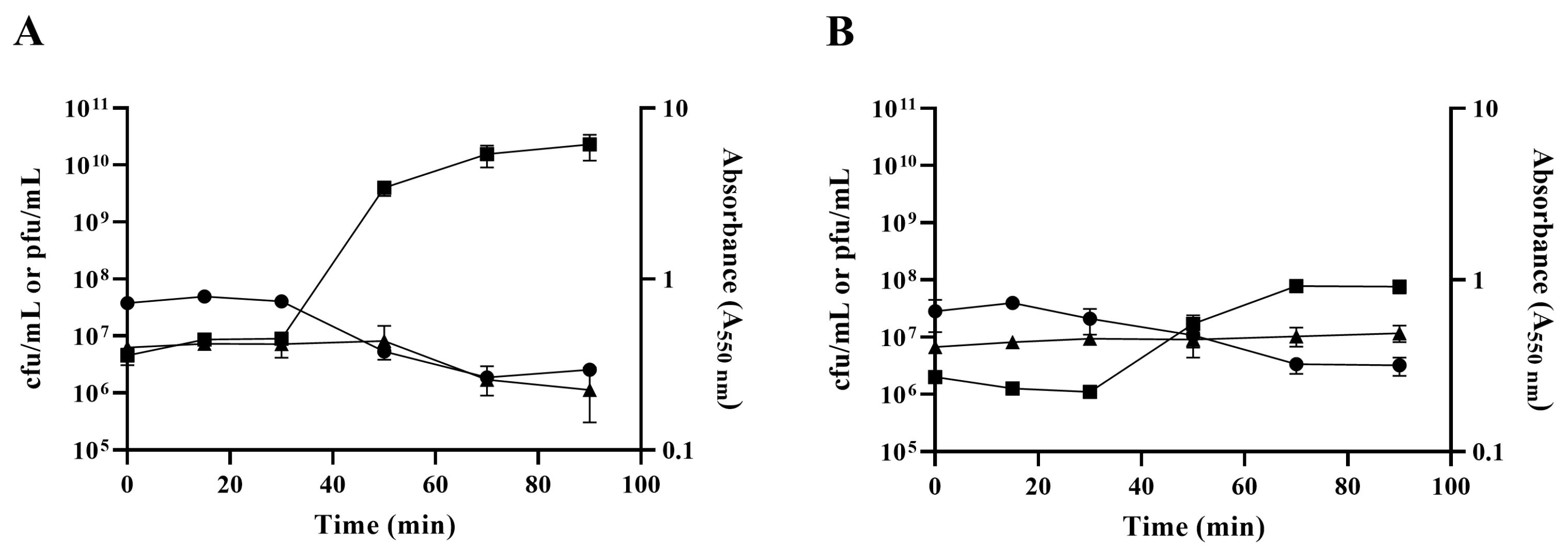
2.2. ibfA Impacts Phage Productivity Without Impairing Bacterial Growth Kinetics
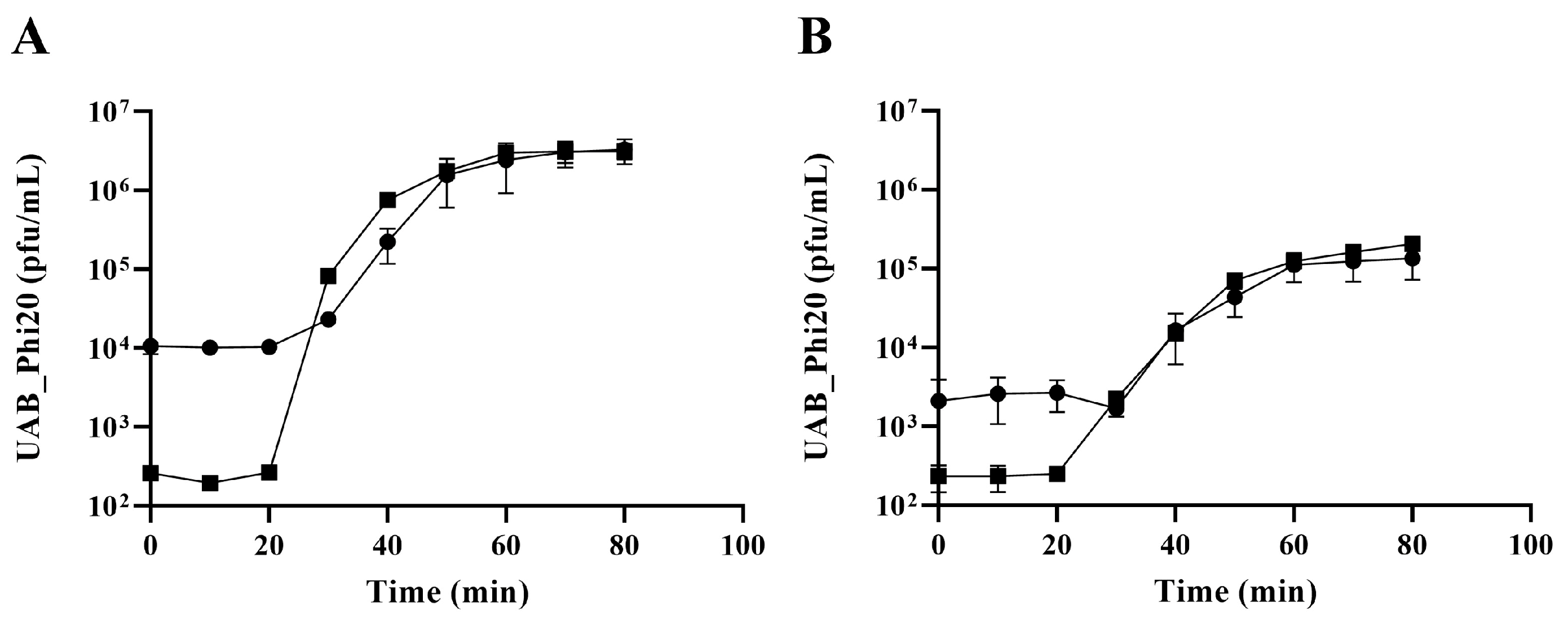
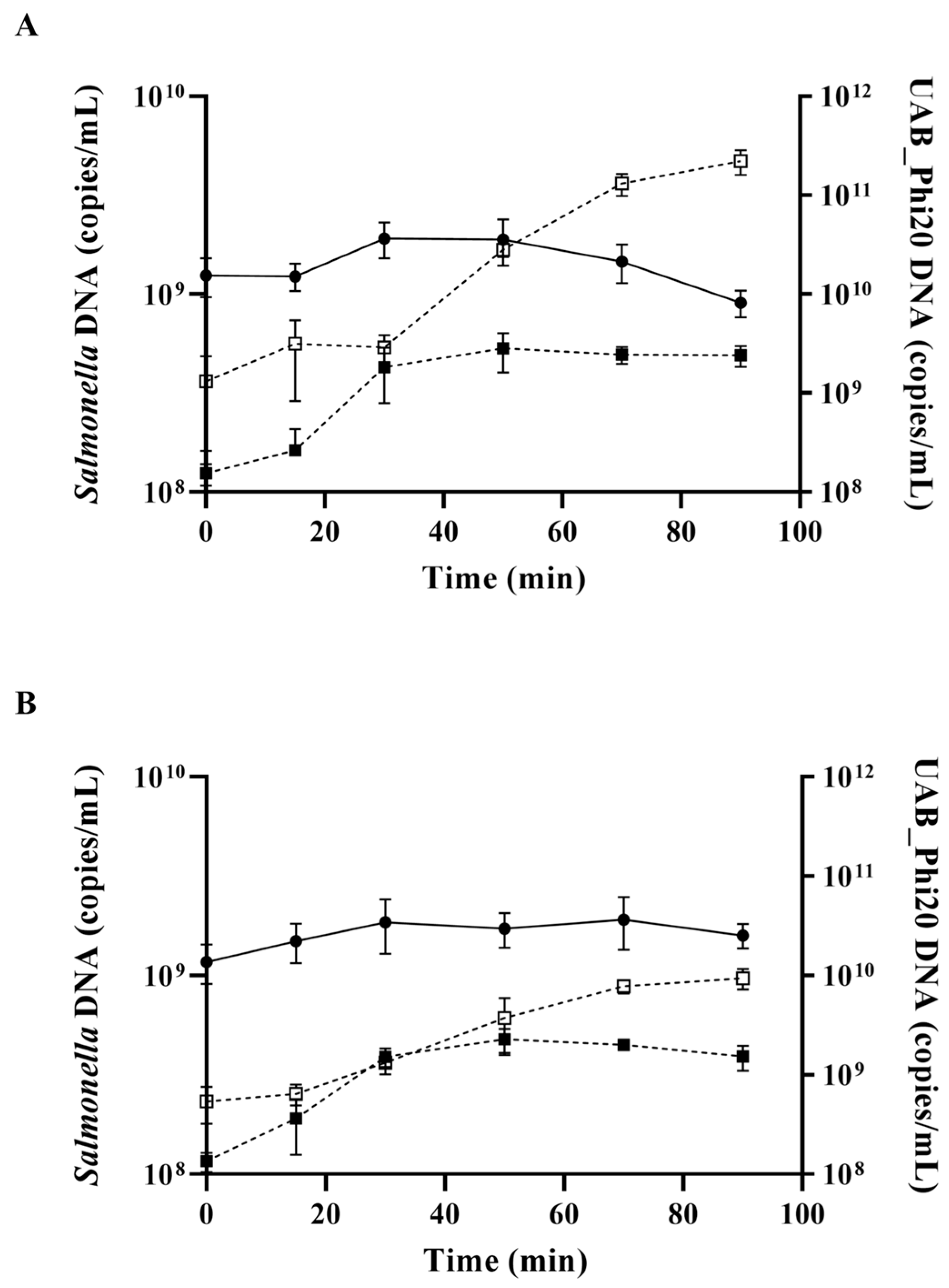
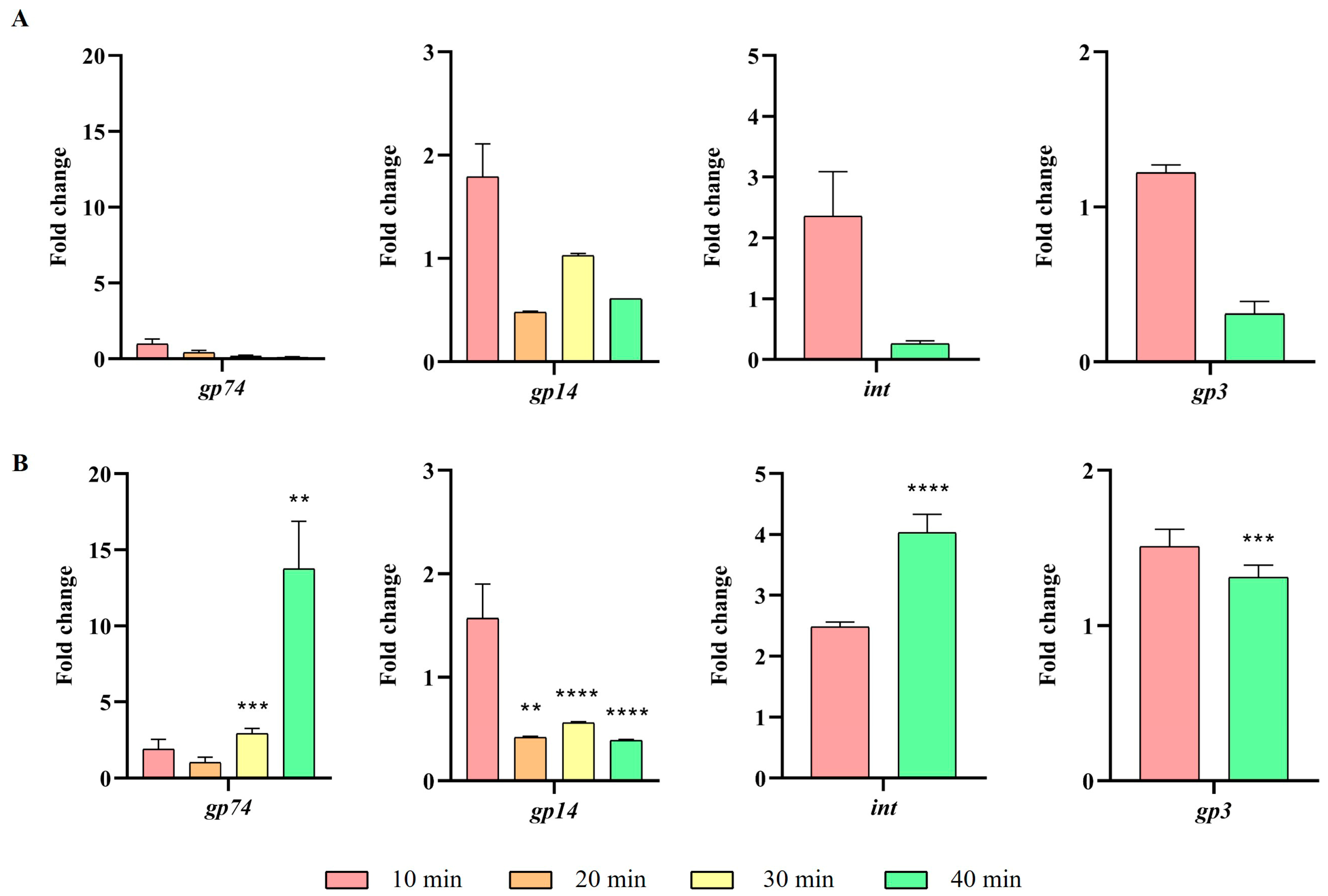
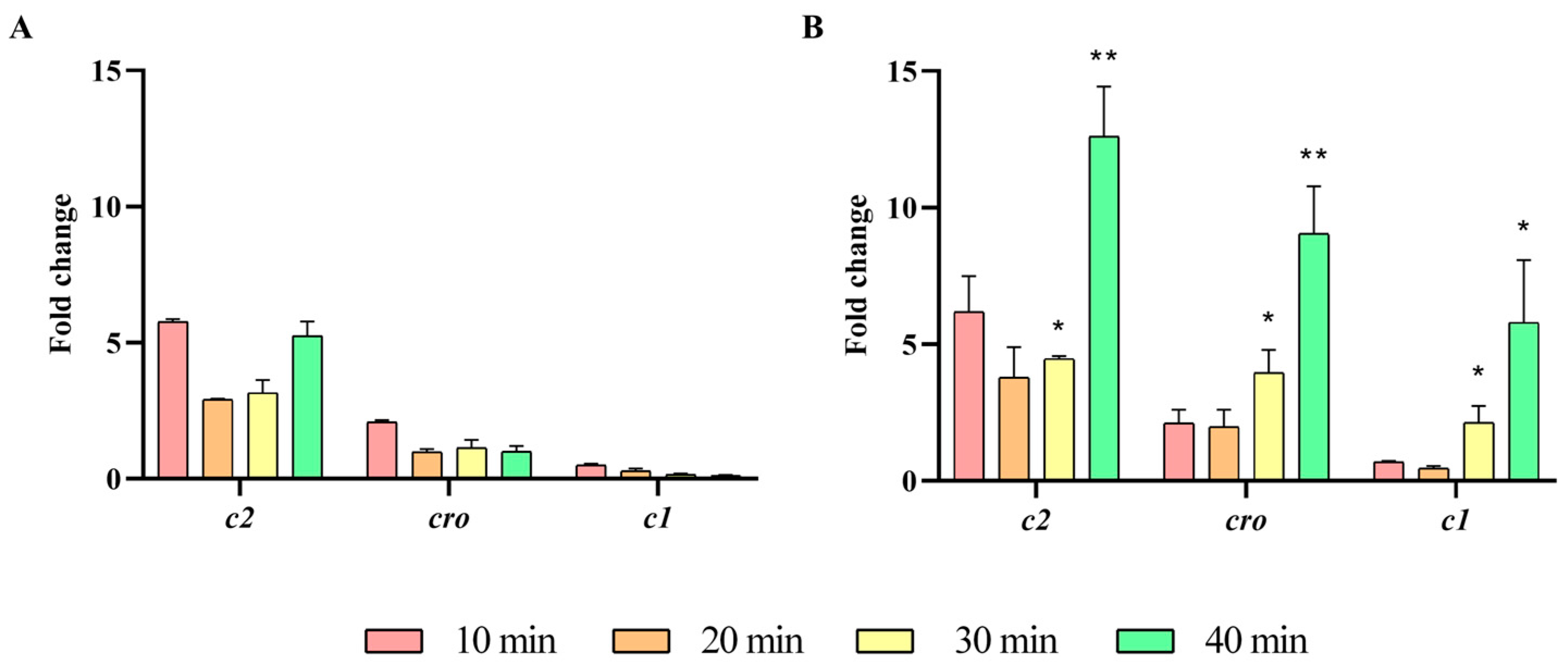
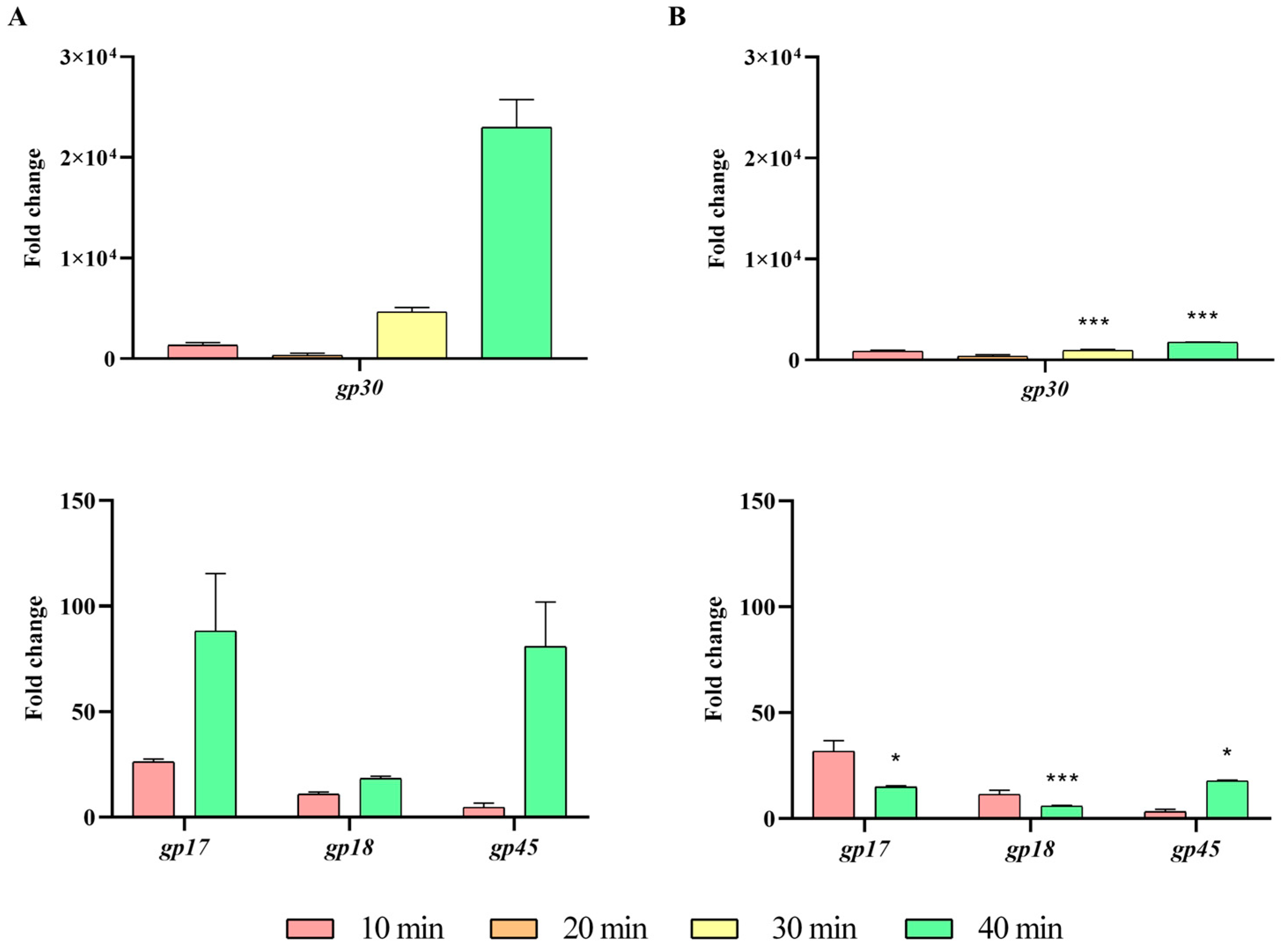
2.3. Impact of the ibfA Gene on the Multiplicative Cycle of UAB_Phi20 Bacteriophage
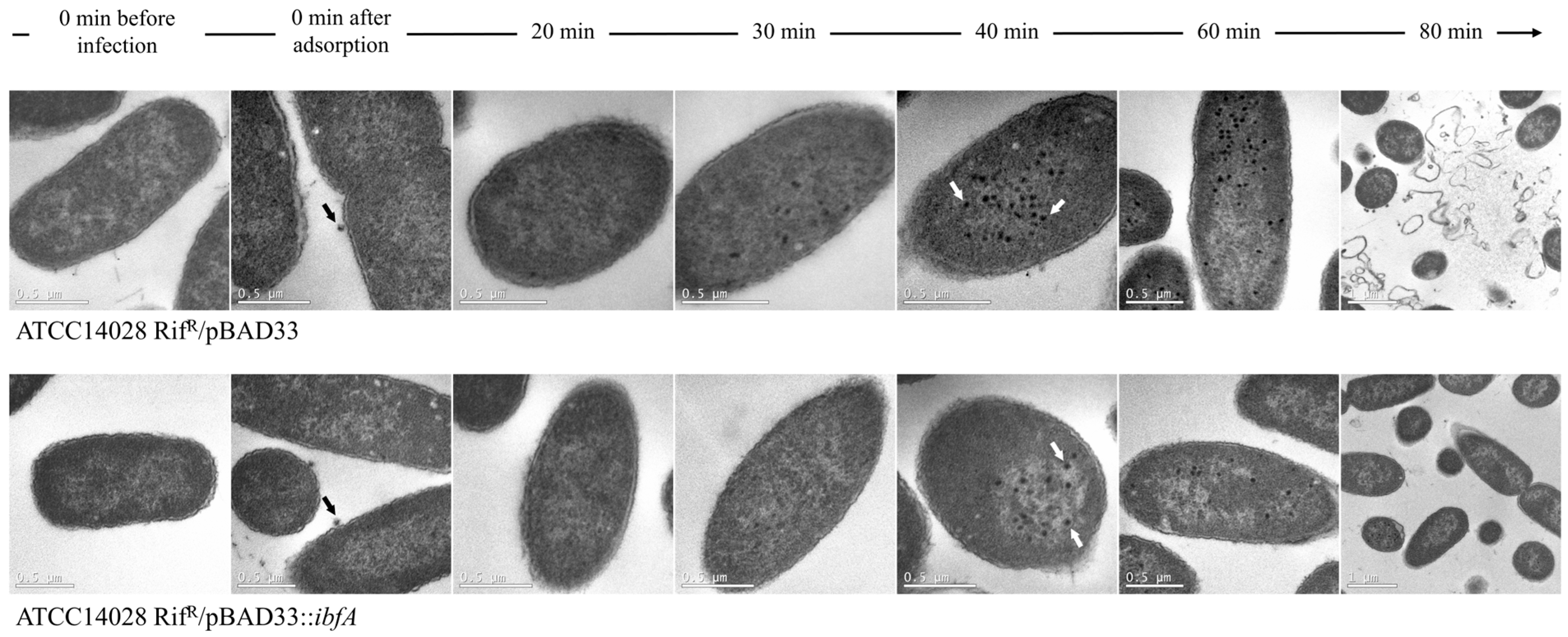
2.4. Visualization of UAB_Phi20 Virions by Electron Microscopy
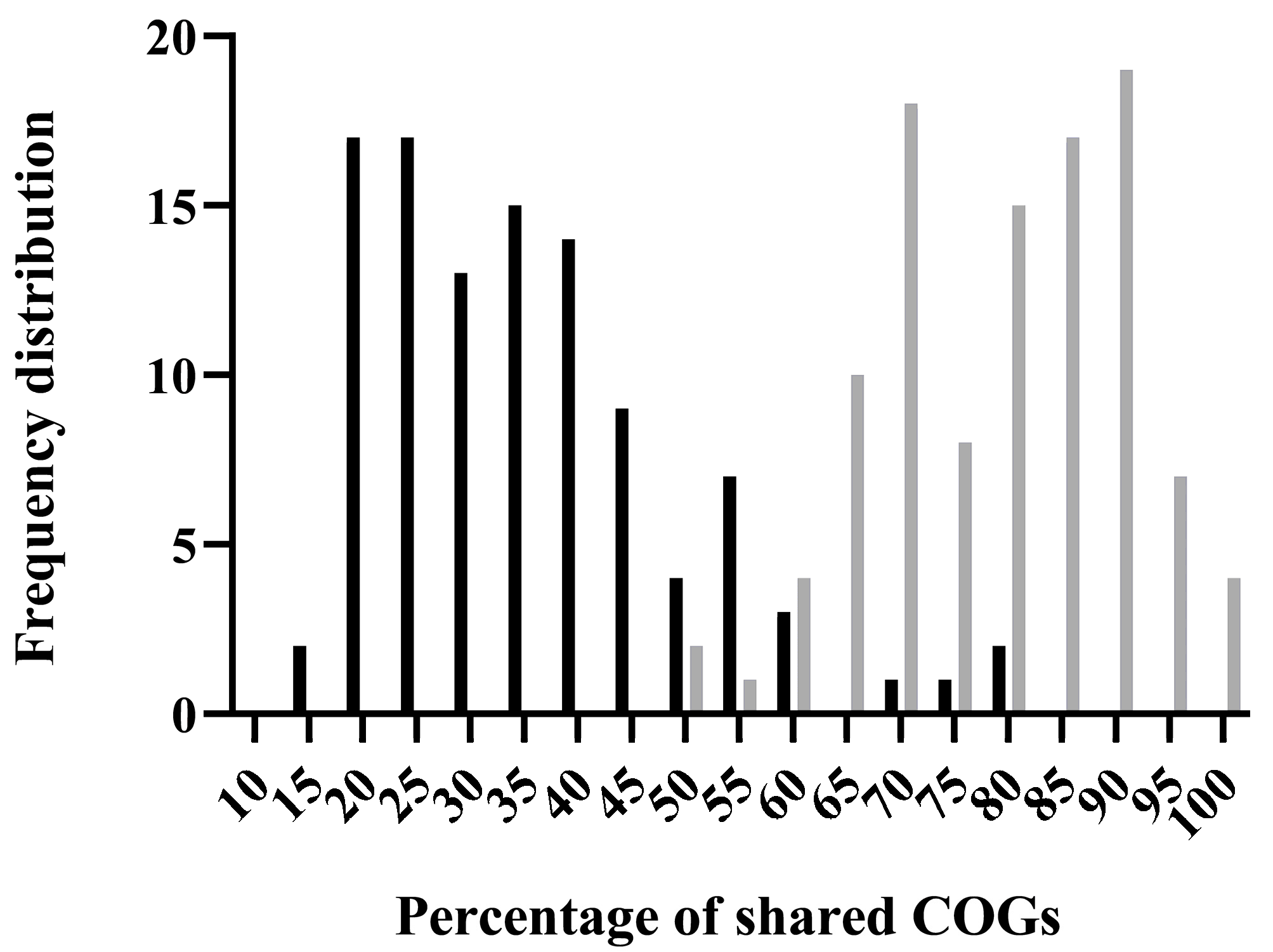
2.5. Distribution of ibfA-Related Genes in Prokaryotic Genomes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Phage Lysate Production
4.3. Bacterial Growth Kinetics
4.4. EOP, Phage Adsorption Constant, Efficiency of Center of Infection (ECOI), and Burst Size Determination
4.5. One-Step Growth
4.6. Phage Infection Kinetics
4.7. Deletion of ibfA Through One-Step Inactivation
4.8. Transcriptional Analysis of ibfA Operon
4.9. Cloning of ibfA onto the Vector pBAD33
4.10. Replication of UAB_Phi20 Genome
4.11. Gene Expression Quantification
4.12. Transmission Electron Microscopy (TEM) of Infected Salmonella Cells
4.13. Bioinformatic Studies
4.14. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscopy |
| TOPRIM | Topoisomerase-primase |
| RM | Restriction-modification |
| PgI | Phage growth limitation |
| BREX | Bacteriophage exclusion |
| DISARM | Defense island associated with RM |
| pAgos | Prokaryotic argonautes |
| RADAR | Restriction by an adenosine deaminase acting on RNA |
| CRISPR-Cas | Clustered regularly interspaced short palindromic repeats and associated proteins |
| pVips | Prokaryotic viperins |
| Abi | Abortive infection |
| TA | Toxin-antitoxin |
| Rif | Rifampicin |
| dsDNA | Double-stranded DNA |
| PLSDB | NCBI plasmid database |
| COG | Clusters of orthologous groups |
| EOP | Efficiency of plating |
| Sar | small antisense RNA |
| Hma | Helicase, methylase, ATPase |
| ECOI | Efficiency of center of infection |
| PES | Polyether sulfone |
| COI | Center of infection |
| KCN | Potassium cyanide |
| qPCR | Quantitative real-time PCR |
References
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the prokaryotic virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Parikka, K.J.; Le Romancer, M.; Wauters, N.; Jacquet, S. Deciphering the virus-to-prokaryote ratio (VPR): Insights into virus–host relationships in a variety of ecosystems. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Georjon, H.; Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 2023, 21, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, P. Overview of phage defense systems in bacteria and their applications. Int. J. Mol. Sci. 2024, 25, 13316. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef]
- Bondy-Denomy, J.; Qian, J.; Westra, E.R.; Buckling, A.; Guttman, D.S.; Davidson, A.R.; Maxwell, K.L. Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 2016, 10, 2854–2866. [Google Scholar] [CrossRef]
- Tock, M.R.; Dryden, D.T.F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005, 8, 466–472. [Google Scholar] [CrossRef]
- Chinenova, T.A.; Mkrtumian, N.M.; Lomovskaia, N.D. Genetic characteristics of a new phage resistance trait in Streptomyces coelicolor A3(2). Genetika 1982, 18, 1945–1952. [Google Scholar]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015, 34, 169–183. [Google Scholar] [CrossRef]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 2018, 3, 90–98. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; van der Oost, J.; Koonin, E.V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 2009, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Altae-Tran, H.; Böhning, F.; Makarova, K.S.; Segel, M.; Schmid-Burgk, J.L.; Koob, J.; Wolf, Y.I.; Koonin, E.V.; Zhang, F. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 2020, 369, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luo, X.; Li, P.; Li, Z.; Ye, F.; Liu, S.; Gao, P. Molecular basis of RADAR anti-phage supramolecular assemblies. Cell 2023, 186, 999–1012.e20. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The biology of CRISPR-Cas: Backward and forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef]
- Bernheim, A.; Millman, A.; Ofir, G.; Meitav, G.; Avraham, C.; Shomar, H.; Rosenberg, M.M.; Tal, N.; Melamed, S.; Amitai, G.; et al. Prokaryotic viperins produce diverse antiviral molecules. Nature 2021, 589, 120–124. [Google Scholar] [CrossRef]
- Dy, R.L.; Przybilski, R.; Semeijn, K.; Salmond, G.P.C.; Fineran, P.C. A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism. Nucleic Acids Res. 2014, 42, 4590–4605. [Google Scholar] [CrossRef]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Cohen, D.; Melamed, S.; Millman, A.; Shulman, G.; Oppenheimer-Shaanan, Y.; Kacen, A.; Doron, S.; Amitai, G.; Sorek, R. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 2019, 574, 691–695. [Google Scholar] [CrossRef]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359, eaar4120. [Google Scholar] [CrossRef]
- Ofir, G.; Herbst, E.; Baroz, M.; Cohen, D.; Millman, A.; Doron, S.; Tal, N.; Malheiro, D.B.A.; Malitsky, S.; Amitai, G.; et al. Antiviral activity of bacterial TIR domains via immune signalling molecules. Nature 2021, 600, 116–120. [Google Scholar] [CrossRef]
- Johnson, A.G.; Wein, T.; Mayer, M.L.; Duncan-Lowey, B.; Yirmiya, E.; Oppenheimer-Shaanan, Y.; Amitai, G.; Sorek, R.; Kranzusch, P.J. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 2022, 375, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Bernheim, A.; Stokar-Avihail, A.; Fedorenko, T.; Voichek, M.; Leavitt, A.; Oppenheimer-Shaanan, Y.; Sorek, R. Bacterial retrons function in anti-phage defense. Cell 2020, 183, 1551–1561.e12. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Leavitt, A.; Doron, S.; Bernheim, A.; Hör, J.; Garb, J.; Bechon, N.; Brandis, A.; Lopatina, A.; et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 2022, 30, 1556–1569.e5. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef]
- López-Pérez, J.; Otero, J.; Sánchez-Osuna, M.; Erill, I.; Cortés, P.; Llagostera, M. Impact of mutagenesis and lateral gene transfer processes in bacterial susceptibility to phage in food biocontrol and phage therapy. Front. Cell Infect. Microbiol. 2023, 13, 1266685. [Google Scholar] [CrossRef]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef]
- Bardina, C.; Colom, J.; Spricigo, D.A.; Otero, J.; Sánchez-Osuna, M.; Cortés, P.; Llagostera, M. Genomics of three new bacteriophages useful in the biocontrol of Salmonella. Front. Microbiol. 2016, 7, 545. [Google Scholar] [CrossRef]
- Hiley, L.; Graham, R.M.A.; Jennison, A.V. Characterisation of IncI1 plasmids associated with change of phage type in isolates of Salmonella enterica serovar Typhimurium. BMC Microbiol. 2021, 21, 92. [Google Scholar] [CrossRef]
- Anderson, E.S.; Lewis, M.J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature 1965, 208, 843–849. [Google Scholar] [CrossRef]
- Hughes, V.; Meynell, G.G. The contribution of plasmid and host genes to plasmid-mediated interference with phage growth. Genet. Res. 1977, 30, 179–185. [Google Scholar] [CrossRef]
- Chevallereau, A.; Westra, E.R. Bacterial immunity: Mobile genetic elements are hotspots for defence systems. Curr. Biol. 2022, 32, R923–R926. [Google Scholar] [CrossRef]
- Rousset, F.; Depardieu, F.; Miele, S.; Dowding, J.; Laval, A.L.; Lieberman, E.; Garry, D.; Rocha, E.P.C.; Bernheim, A.; Bikard, D.P. Phages and their satellites encode hotspots of antiviral systems. Cell Host Microbe 2022, 30, 740–753.e5. [Google Scholar] [CrossRef]
- Beamud, B.; Benz, F.; Bikard, D. Going viral: The role of mobile genetic elements in bacterial immunity. Cell Host Microbe 2024, 32, 804–819. [Google Scholar] [CrossRef]
- Sampei, G.I.; Furuya, N.; Tachibana, K.; Saitou, Y.; Suzuki, T.; Mizobuchi, K.; Komano, T. Complete genome sequence of the incompatibility group I1 plasmid R64. Plasmid 2010, 64, 92–103. [Google Scholar] [CrossRef]
- Payne, L.J.; Meaden, S.; Mestre, M.R.; Palmer, C.; Toro, N.; Fineran, P.C.; Jackson, S.A. PADLOC: A web server for the identification of antiviral defence systems in microbial genomes. Nucleic Acids Res. 2022, 50, W541–W550. [Google Scholar] [CrossRef]
- Payne, L.J.; Hughes, T.C.D.; Fineran, P.C.; Jackson, S.A. New antiviral defences are genetically embedded within prokaryotic immune systems. bioRxiv 2024. bioRxiv:01.29.577857. [Google Scholar] [CrossRef]
- Solovyev, V.; Salamov, A. Automatic annotation of microbial genomes and metagenomic sequences. In Metagenomics and Its Applications in Agriculture, Biomedicine, and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 61–78. [Google Scholar]
- Akritidou, K.; Thurtle-Schmidt, B.H. OLD family nuclease function across diverse anti-phage defense systems. Front. Microbiol. 2023, 14, 1268820. [Google Scholar] [CrossRef]
- Dot, E.W.; Thomason, L.C.; Chappie, J.S. Everything OLD is new again: How structural, functional, and bioinformatic advances have redefined a neglected nuclease family. Mol. Microbiol. 2023, 120, 122–140. [Google Scholar] [CrossRef]
- Strunnikov, A.V. SMC complexes in bacterial chromosome condensation and segregation. Plasmid 2006, 55, 135–144. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef] [PubMed]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef] [PubMed]
- Niault, T.; van Houte, S.; Westra, E.; Swarts, D.C. Evolution and ecology of anti-defence systems in phages and plasmids. Curr. Biol. 2025, 35, R32–R44. [Google Scholar] [CrossRef] [PubMed]
- Getz, L.J.; Maxwell, K.L. Diverse Antiphage Defenses Are Widespread Among Prophages and Mobile Genetic Elements. Annu. Rev. Virol. 2024, 11, 343–362. [Google Scholar] [CrossRef]
- Furuichi, T.; Komano, T.; Nisioka, T. Physical and genetic analyses of the Inc-I alpha plasmid R64. J. Bacteriol. 1984, 158, 997–1004. [Google Scholar] [CrossRef]
- Uemura, H.; Mizobuchi, K. Inhibition of growth of bacteriophage BF23 by the ColIb plasmid: Identification of the ibfA and ibfB genes of the ColIb plasmid. Mol. Gen. Genet. 1982, 185, 13–20. [Google Scholar] [CrossRef]
- Payne, L.J.; Todeschini, T.C.; Wu, Y.; Perry, B.J.; Ronson, C.W.; Fineran, P.C.; Nobrega, F.L.; Jackson, S.A. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res. 2021, 49, 10868–10878. [Google Scholar] [CrossRef]
- Deep, A.; Liang, Q.; Enustun, E.; Pogliano, J.; Corbett, K.D. Architecture and infection-sensing mechanism of the bacterial PARIS defense system. Nature 2024, 634, 432–439. [Google Scholar] [CrossRef]
- Antine, S.P.; Johnson, A.G.; Mooney, S.E.; Leavitt, A.; Mayer, M.L.; Yirmiya, E.; Amitai, G.; Sorek, R.; Kranzusch, P.J. Structural basis of Gabija anti-phage defence and viral immune evasion. Nature 2024, 625, 360–365. [Google Scholar] [CrossRef]
- Rousset, F.; Yirmiya, E.; Nesher, S.; Brandis, A.; Mehlman, T.; Itkin, M.; Malitsky, S.; Millman, A.; Melamed, S.; Sorek, R. A conserved family of immune effectors reveals global viral defense mechanisms. Science 2023, 379, 256–259. [Google Scholar] [CrossRef]
- Shim, H.; Shivram, H.; Lei, S.; Doudna, J.A.; Banfield, J.F. Diverse ATPase proteins in mobilomes constitute a large potential sink for prokaryotic host ATP. Front. Microbiol. 2021, 12, 691847. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Susskind, M.M.; Botstein, D. Molecular genetics of bacteriophage P22. Microbiol. Rev. 1978, 42, 385–413. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Pfarr, D.; Strickler, J.; Rosenberg, M. Characterization of the transcription activator protein C1 of bacteriophage P22. J. Biol. Chem. 1992, 267, 14388–14397. [Google Scholar] [CrossRef]
- Botstein, D.; Lew, K.K.; Jarvik, V.; Swanson, C.A. Role of Antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J. Mol. Biol. 1975, 91, 439–452. [Google Scholar] [CrossRef]
- Levine, M.; Truesdell, S.; Ramakrishnan, T.; Bronson, M.J. Dual control of lysogeny by bacteriophage P22: An antirepressor locus and its controlling elements. J. Mol. Biol. 1975, 91, 421–438. [Google Scholar] [CrossRef]
- Susskind, M.M.; Botstein, D. Mechanism of action of Salmonella phage P22 antirepressor. J. Mol. Biol. 1975, 98, 413–424. [Google Scholar] [CrossRef]
- Wu, T.H.; Liao, S.M.; McClure, W.R.; Susskind, M.M. Control of gene expression in bacteriophage P22 by a small antisense RNA. II. Characterization of mutants defective in repression. Genes. Dev. 1987, 1, 204–212. [Google Scholar] [CrossRef]
- Susskind, M.M.; Youderian, P. Transcription in vitro of the bacteriophage P22 antirepressor gene. J. Mol. Biol. 1982, 154, 427–447. [Google Scholar] [CrossRef]
- Susskind, M.M. A new gene of bacteriophage P22 which regulates synthesis of antirepressor. J. Mol. Biol. 1980, 138, 685–713. [Google Scholar] [CrossRef]
- Hussain, F.A.; Dubert, J.; Elsherbini, J.; Murphy, M.; VanInsberghe, D.; Arevalo, P.; Kauffman, K.; Rodino-Janeiro, B.K.; Gavin, H.; Gomez, A.; et al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science 2021, 374, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Leipe, D.D.; Koonin, E.V. Toprim--a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998, 26, 4205–4213. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [CrossRef]
- Kropinski, A.M. Measurement of the rate of attachment of bacteriophage to cells. Methods Mol. Biol. 2009, 501, 151–155. [Google Scholar] [CrossRef]
- Moineau, S.; Durmaz, E.; Pandian, S.; Klaenhammerl, T.R. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 1993, 59, 208–212. [Google Scholar] [CrossRef]
- Kropinski, A.M. Practical advice on the one-step growth curve. Methods Mol. Biol. 2018, 1681, 41–47. [Google Scholar] [CrossRef]
- Doermann, A.H. The intracellular growth of bacteriophages. I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J. Gen. Physiol. 1952, 35, 645–656. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Chaveroche, M.K.; Ghigo, J.M.; D’Enfert, C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000, 28, E97. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Ritalahti, K.M.; Amos, B.K.; Sung, Y.; Wu, Q.; Koenigsberg, S.S.; Löffler, F.E. Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl. Environ. Microbiol. 2006, 72, 2765–2774. [Google Scholar] [CrossRef]
- Brandão, A.; Pires, D.P.; Coppens, L.; Voet, M.; Lavigne, R.; Azeredo, J. Differential transcription profiling of the phage LUZ19 infection process in different growth media. RNA Biol. 2021, 18, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Tellmann, G. The E-Method: A highly accurate technique for gene-expression analysis. Nat. Methods 2006, 3, i–ii. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Abby, S.S.; Néron, B.; Ménager, H.; Touchon, M.; Rocha, E.P.C. MacSyFinder: A program to mine genomes for molecular systems with an application to CRISPR-Cas systems. PLoS ONE 2014, 9, e110726. [Google Scholar] [CrossRef]
- Tesson, F.; Hervé, A.; Mordret, E.; Touchon, M.; D’Humières, C.; Cury, J.; Bernheim, A. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 2022, 13, 2561. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. ProgressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A genomic perspective on protein families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–269. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Coppens, L.; Wicke, L.; Lavigne, R. SAPPHIRE.CNN: Implementation of dRNA-seq-driven, species-specific promoter prediction using convolutional neural networks. Comput. Struct. Biotechnol. J. 2022, 20, 4969–4974. [Google Scholar] [CrossRef]
- Söding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2005, 21, 951–960. [Google Scholar] [CrossRef]

| Strain | EOP 2 |
|---|---|
| ATCC14028 RifR | 1 |
| IC5 1 | 0 |
| IC5 Δ ibfA | 0.94 ± 0.13 |
| IC5 Δ pUA1135_00003, ibfA, ydeA, ydfA | 0.65 ± 0.07 |
| Strain | EOP 1 |
|---|---|
| IC5 Δ ibfA/pBAD33 | 1 |
| IC5 Δ ibfA/pBAD33::ibfA | 0.05 ± 0.00 |
| IC5 Δ pUA1135_00003, ibfA, ydeA, ydfA/pBAD33::pUA1135_00003, ibfA, ydeA, ydfA | 0.01 ± 0.00 |
| ibfA Gene | 40 min | 60 min | 80 min | |
|---|---|---|---|---|
| Mean of virions/cell section ± SD | − | 31 ± 3.6 | 27 ± 3.7 | 28 ± 4.3 |
| + | 16 ± 3.7 | 7 ± 3.5 | 7 ± 2.9 | |
| Percentage (%) of cell sections with virions vs. total of recorded sections | − | 32.9 | 88.8 | 8.5 |
| + | 2.3 | 3.6 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Pérez, J.; Cortés, P.; Campoy, S.; Erill, I.; Llagostera, M. Deciphering the Causes of IbfA-Mediated Abortive Infection in the P22-like Phage UAB_Phi20. Int. J. Mol. Sci. 2025, 26, 4918. https://doi.org/10.3390/ijms26104918
López-Pérez J, Cortés P, Campoy S, Erill I, Llagostera M. Deciphering the Causes of IbfA-Mediated Abortive Infection in the P22-like Phage UAB_Phi20. International Journal of Molecular Sciences. 2025; 26(10):4918. https://doi.org/10.3390/ijms26104918
Chicago/Turabian StyleLópez-Pérez, Júlia, Pilar Cortés, Susana Campoy, Ivan Erill, and Montserrat Llagostera. 2025. "Deciphering the Causes of IbfA-Mediated Abortive Infection in the P22-like Phage UAB_Phi20" International Journal of Molecular Sciences 26, no. 10: 4918. https://doi.org/10.3390/ijms26104918
APA StyleLópez-Pérez, J., Cortés, P., Campoy, S., Erill, I., & Llagostera, M. (2025). Deciphering the Causes of IbfA-Mediated Abortive Infection in the P22-like Phage UAB_Phi20. International Journal of Molecular Sciences, 26(10), 4918. https://doi.org/10.3390/ijms26104918






