The Activity of Phytotherapic Extracts Combined in a Unique Formulation Alleviates Oxidative Stress and Protects Mitochondria Against Atorvastatin-Induced Cardiomyopathy
Abstract
1. Introduction
2. Results
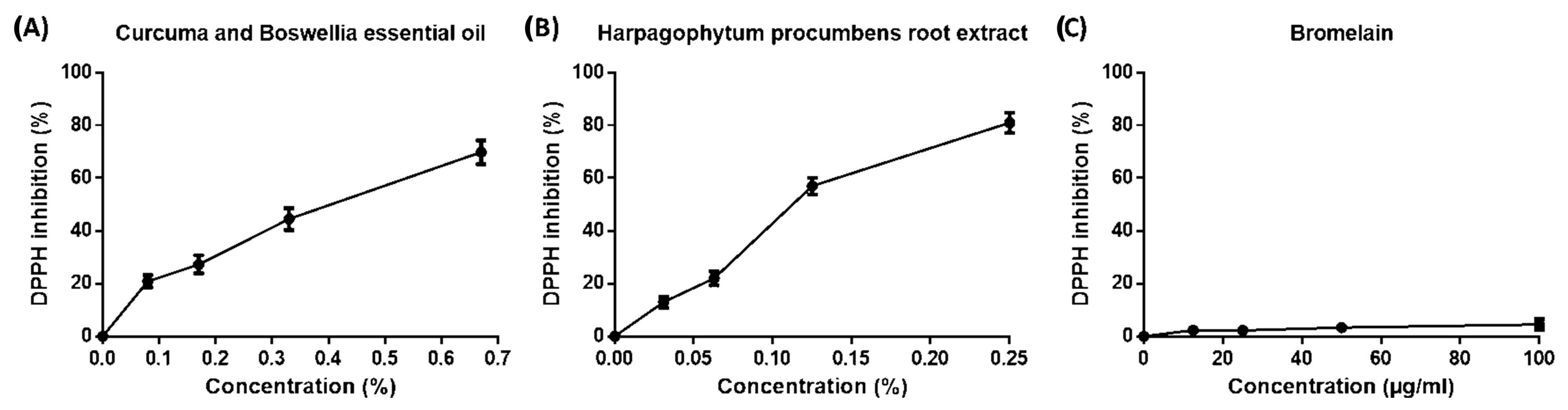
2.1. Scavenging Capacity of Phytotherapic Compounds by DPPH Assay
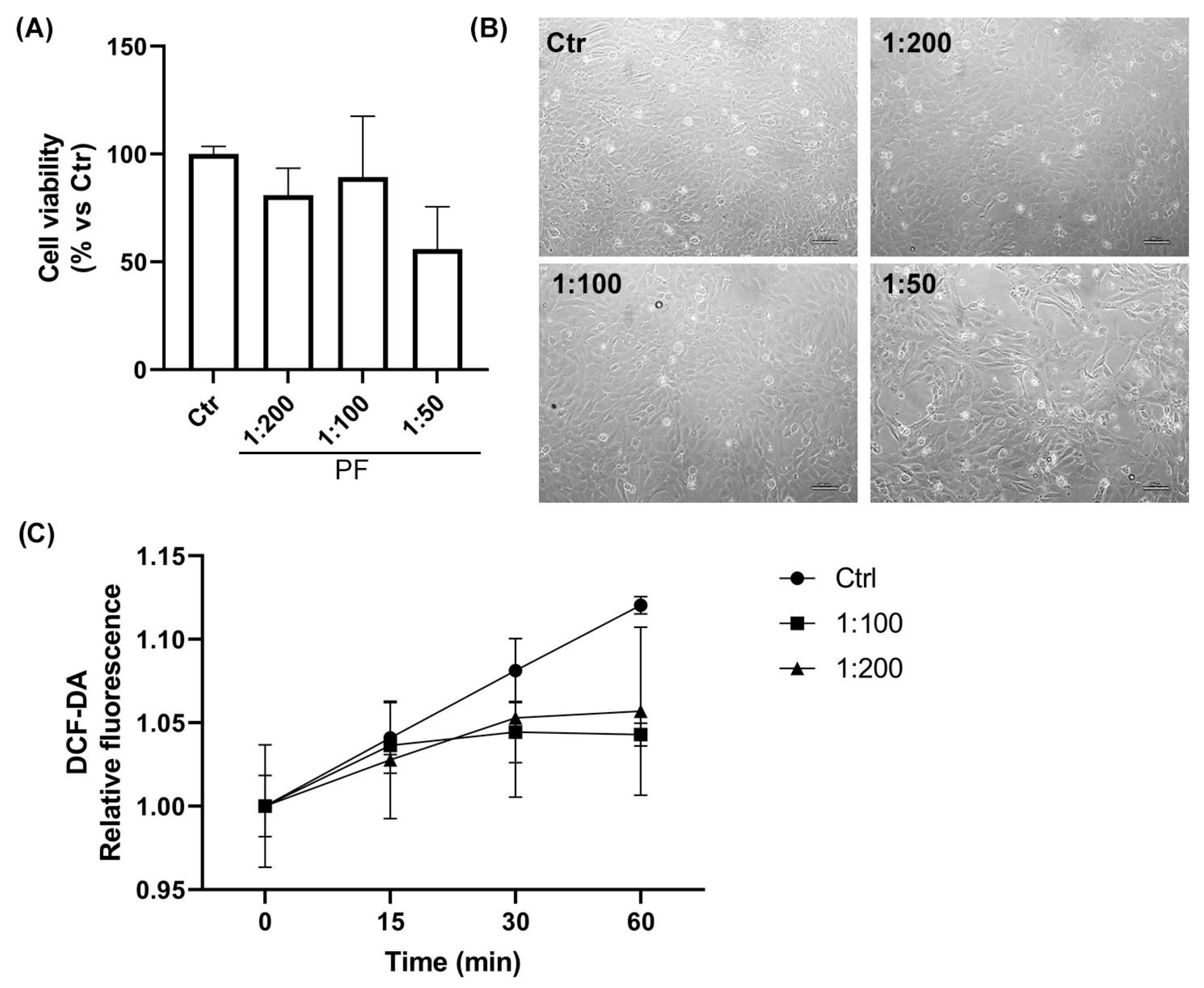
2.2. Assessment of the Cellular Compatibility of the Phytotherapic Formulation (PF): Dose–Response Study in AC16 Cell Line
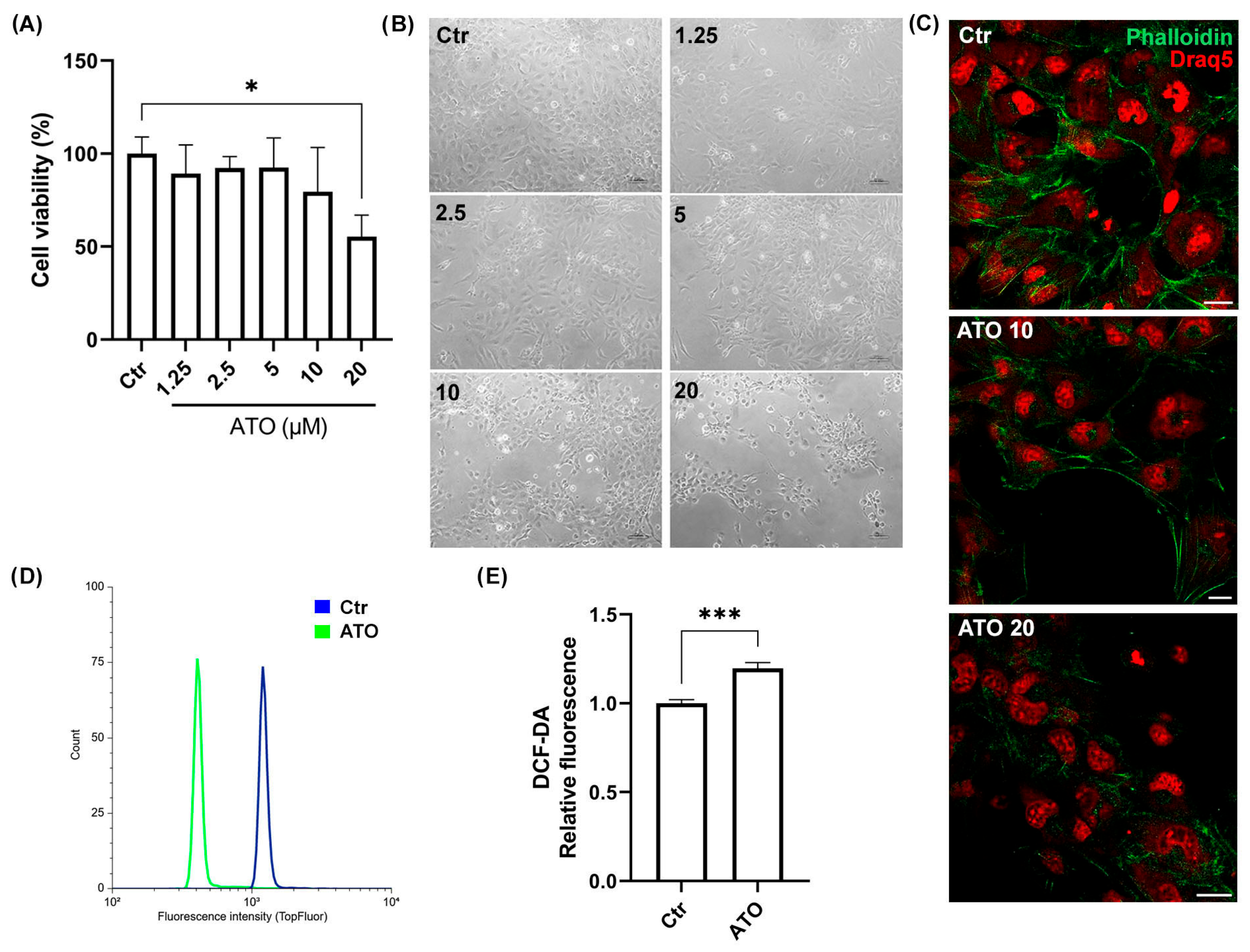
2.3. Atorvastatin Induces AC16 Cell Damage in a Dose-Dependent Manner
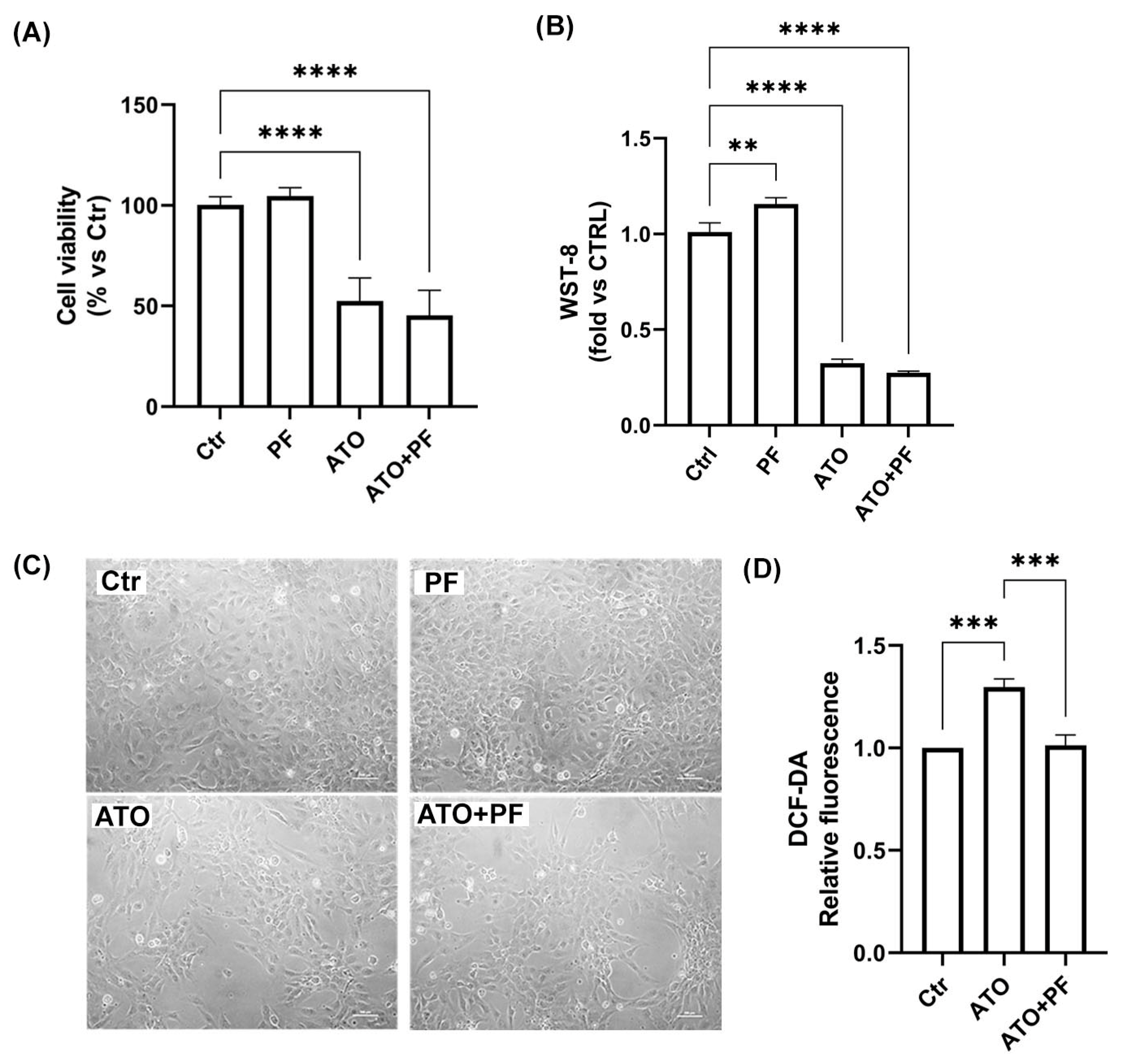
2.4. Effect of the Phytotherapic Formulation on Cell Viability and ROS Production in ATO-Treated Cells
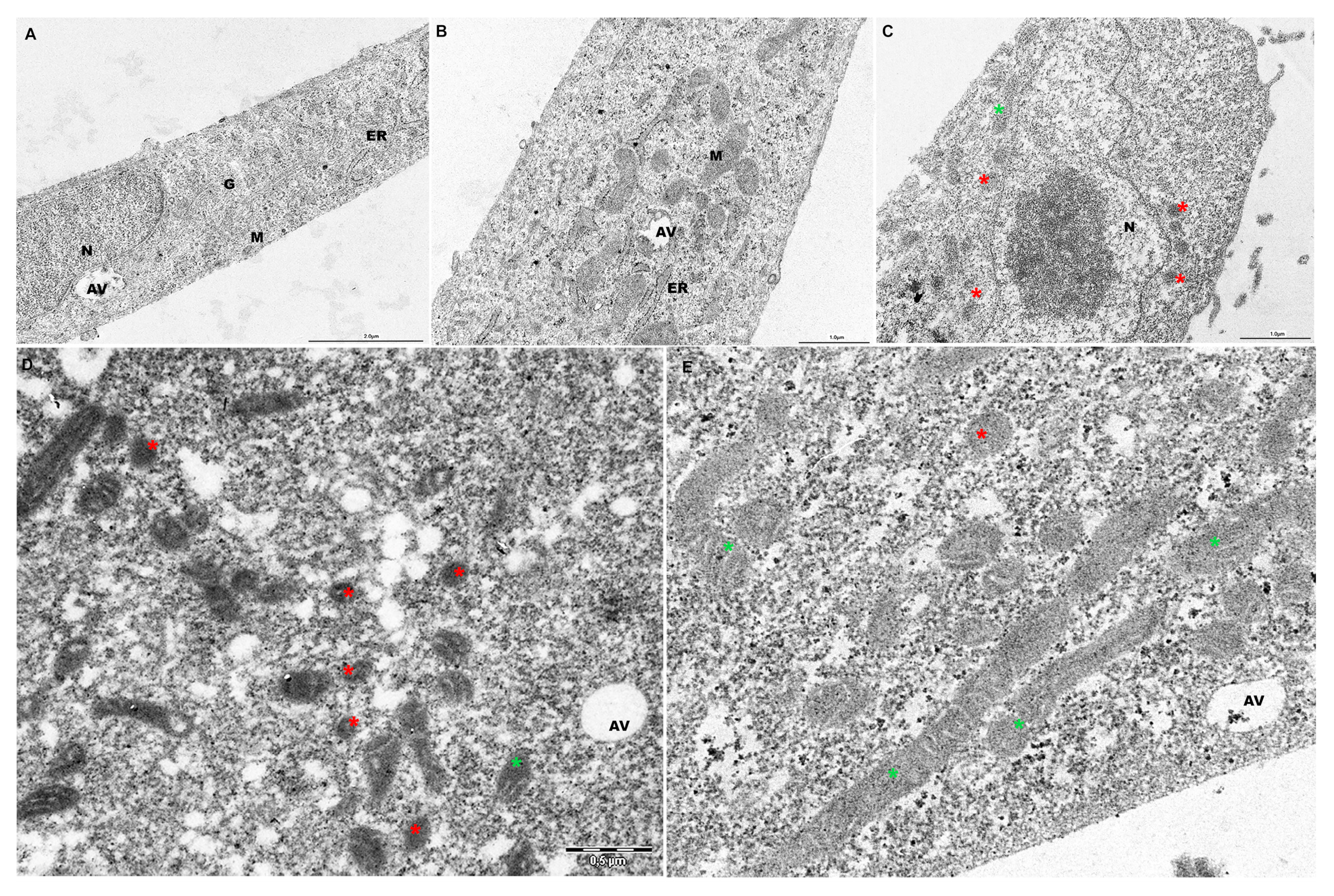
2.5. PF Preserves Mitochondrial Ultrastructure in ATO-Treated Cells
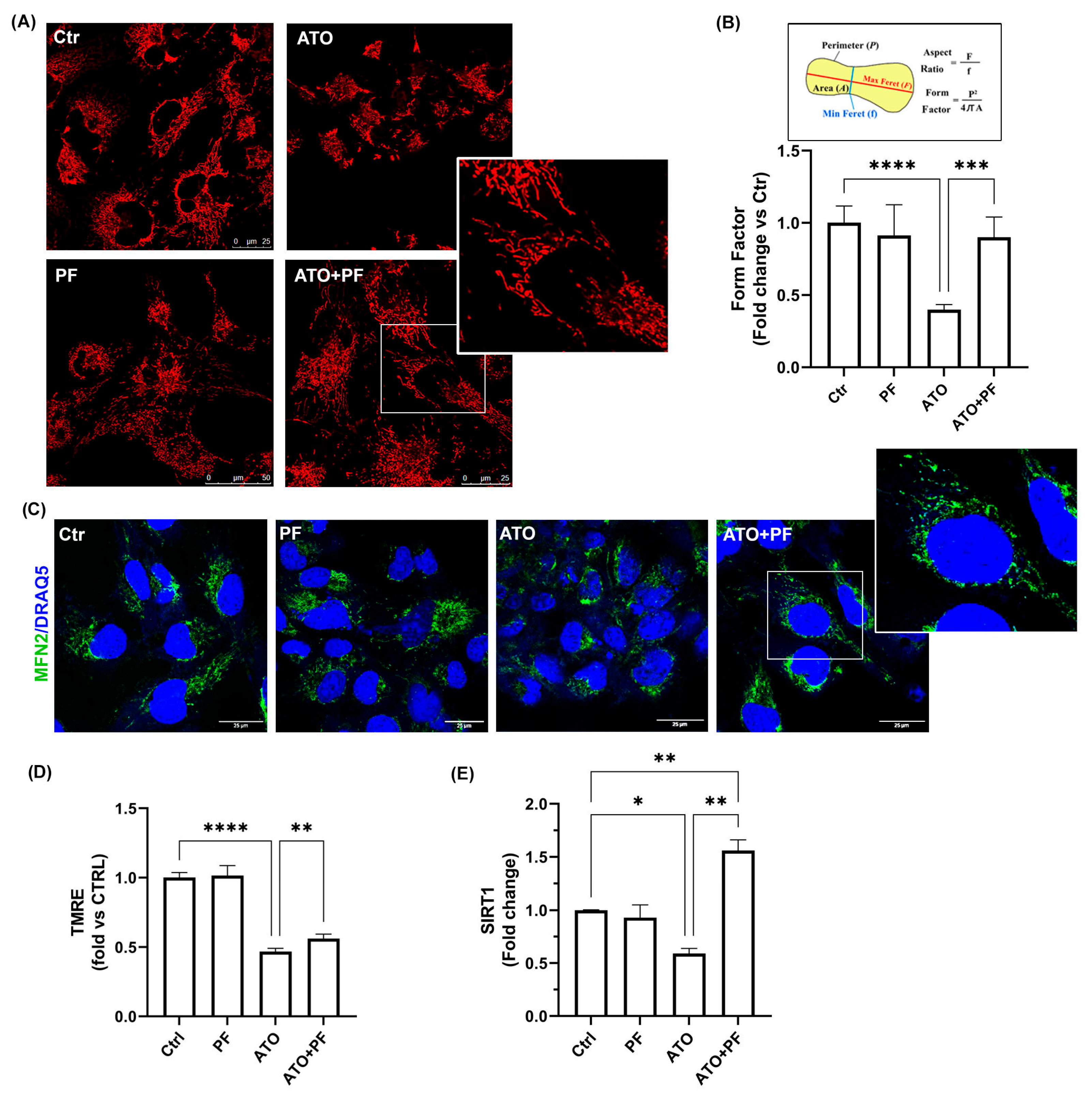
2.6. PF Reverses Mitochondrial Dysfunction Induced by Atorvastatin by Improving Mitochondrial Reshaping
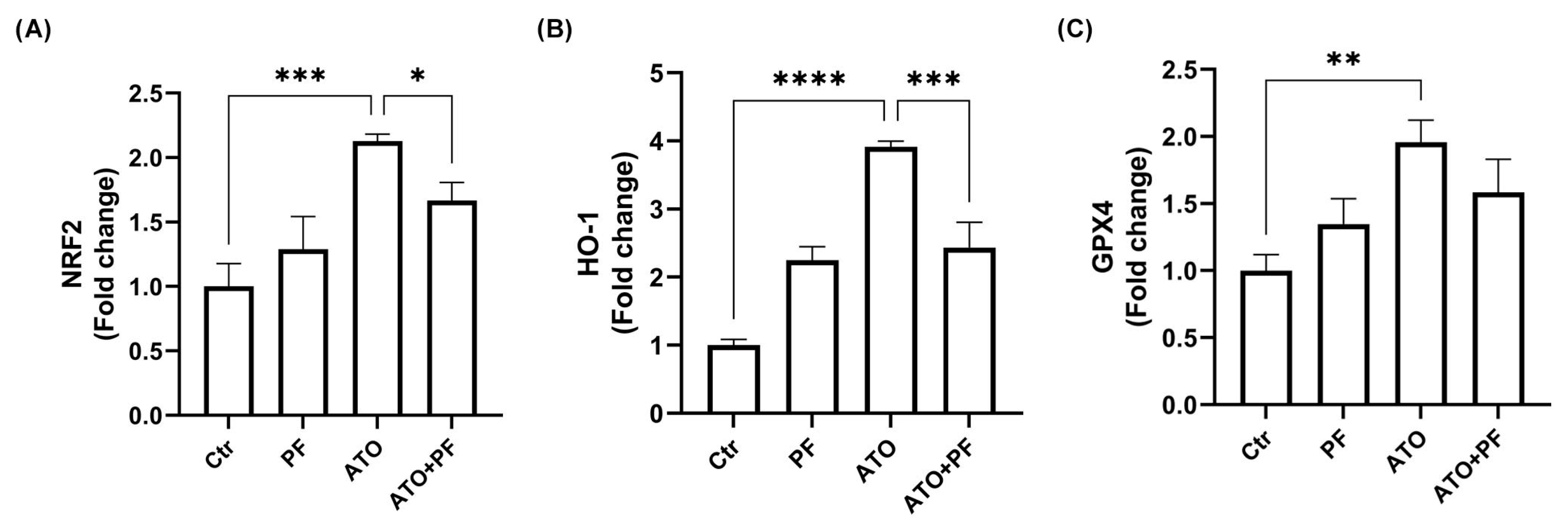
2.7. PF Restores Nrf2/HO-1/GPX4 Pathway Involved in Myotoxicity Induced by ATO
3. Discussion
4. Materials and Methods
4.1. Phytotherapic Formulation Composition
4.2. DPPH Test
4.3. Cell Culture
4.4. In Vitro Model of Atorvastatin-Induced Myopathy and Csell Treatments
4.5. Trypan Blue Test
4.6. Cell Counting Kit-8 Assay
4.7. DCF-DA Assay
4.8. Tali Image-Based Cytometric Analysis
4.9. TMRE Assay
4.10. Mitochondria Evaluation by MitoTracker Deep Red (MTDR) Staining
4.11. Transmission Electron Microscopy (TEM)
4.12. Real-Time PCR
4.13. Phalloidin Immunofluorescence Analysis
4.14. MFN2 Immunofluorescence
4.15. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PF | Phytotherapic formulation (Curcuma and Boswellia essential oils, Harpagophytum procumbens root, and Bromelain) |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| GPX4 | Glutathione peroxidase 4 |
| ETC | Electron transport chain |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl hydrate |
| DCFH-DA | 2’,7’-dichlorofluorescein diacetate |
| MTDR | MitoTracker Deep Red |
| TMRE | Tetramethylrhodamine, ethyl ester |
| MMP | Mitochondrial membrane potential |
| FF | Form factor |
| MFN2 | Mitofusin-2 |
| SIRT1 | Sirtuin 1 |
| ATO | Atorvastatin |
| TEM | Transmission electron microscopy |
References
- Istvan, E.S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Bianconi, V.; Pirro, M.; Jamialahmadi, T.; Sahebkar, A. Statins Attenuate Fibrotic Manifestations of Cardiac Tissue Damage. Curr. Mol. Pharmacol. 2021, 14, 782–797. [Google Scholar] [CrossRef]
- Sathasivam, S. Statin induced myotoxicity. Eur. J. Intern. Med. 2012, 23, 317–324. [Google Scholar] [CrossRef]
- Tariq, S.; Goriparthi, L.; Ismail, D.; Tonpouwo, G.K.; Thapa, M.; Khalid, K.; Cooper, A.C.; Jean-Charles, G. Correlates of Myopathy in Diabetic Patients Taking Statins. Cureus 2023, 15, e37708. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef]
- Kaufmann, P.; Török, M.; Zahno, A.; Waldhauser, K.M.; Brecht, K.; Krähenbühl, S. Krähenbühl, Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. 2006, 63, 2415–2425. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Guo, J.; Ares, I.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Anadón, A.; Wang, X.; Martínez, M.-A. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol. Ther. 2019, 195, 54–84. [Google Scholar] [CrossRef]
- Al-Shalchi, R.F.; Mohammad, F.K. Oxidative Stress-Induced Adverse Effects of Three Statins Following Single or Repetitive Treatments in Mice. Cureus 2024, 16, e51433. [Google Scholar] [CrossRef]
- Mthembu, S.X.; Mazibuko-Mbeje, S.E.; Silvestri, S.; Orlando, P.; Nkambule, B.B.; Muller, C.J.; Tiano, L.; Dludla, P.V. Prolonged exposure to simvastatin affects coenzyme Q9/10 status leading to impaired mitochondrial respiratory capacity and reduced viability of cultured cardiac cells. Toxicol. In Vitro 2025, 106, 106052. [Google Scholar] [CrossRef]
- Godoy, J.C.; Niesman, I.R.; Busija, A.R.; Kassan, A.; Schilling, J.M.; Schwarz, A.; Alvarez, E.A.; Dalton, N.D.; Drummond, J.C.; Roth, D.M.; et al. Atorvastatin, but not pravastatin, inhibits cardiac Akt/mTOR signaling and disturbs mitochondrial ultrastructure in cardiac myocytes. FASEB J. 2019, 33, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Collinson, P.; Kiely, P. Unexpected Troponin Elevation in a Patient Treated with Atorvastatin. J. Appl. Lab. Med. 2020, 5, 798–801. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-xCT/GPx4 Axis. Front. Cell Dev. Biol. 2022, 10, 806081. [Google Scholar] [CrossRef]
- Kuethe, F.; Sigusch, H.; Bornstein, S.; Hilbig, K.; Kamvissi, V.; Figulla, H. Apoptosis in patients with dilated cardiomyopathy and diabetes: A feature of diabetic cardiomyopathy? Horm. Metab. Res. 2007, 39, 672–676. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, M.; Wang, S.; Wang, J.; Guo, Y.; Tang, Y.; Gu, J. Molecular mechanisms of doxorubicin-induced cardiotoxicity: Novel roles of sirtuin 1-mediated signaling pathways. Cell. Mol. Life Sci. 2021, 78, 3105–3125. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, S.S.; Singh, S.; Dabur, R. Natural products: Potential therapeutic agents to prevent skeletal muscle atrophy. Eur. J. Pharmacol. 2022, 925, 174995. [Google Scholar] [CrossRef]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxidative Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somers, T.; Siddiqi, S.; Morshuis, W.J.; Russel, F.G.M.; Schirris, T.J.J. Schirris, Statins and Cardiomyocyte Metabolism, Friend or Foe? J. Cardiovasc. Dev. Dis. 2023, 10, 417. [Google Scholar] [CrossRef]
- Pal, S.; Ghosh, M.; Ghosh, S.; Bhattacharyya, S.; Sil, P.C. Atorvastatin induced hepatic oxidative stress and apoptotic damage via MAPKs, mitochondria, calpain and caspase12 dependent pathways. Food Chem. Toxicol. 2015, 83, 36–47. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Stockwell, Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- TVanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Vandenabeele, Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid with Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- Hil, E.F.H.-V.D.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 23. [Google Scholar] [CrossRef]
- Okon, E.; Kukula-Koch, W.; Jarzab, A.; Halasa, M.; Stepulak, A.; Wawruszak, A. Advances in Chemistry and Bioactivity of Magnoflorine and Magnoflorine-Containing Extracts. Int. J. Mol. Sci. 2020, 21, 1330. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Wen, R.; Liu, C.-F.; Zhang, T.-N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. Abramov, The emerging role of Nrf2 in mitochondrial function. Free. Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Dodson, M.; Schmidlin, C.J.; Liu, P.; Zhang, D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020, 27, 436–447. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Bartolucci, G.; Campana, R.; Salucci, S.; Benedetti, S.; Caprioli, G.; Maggi, F.; Sagratini, G.; Vittori, S.; Lucarini, S. Quantification of 2- and 3-isopropylmalic acids in forty Italian wines by UHPLC-MS/MS triple quadrupole and evaluation of their antimicrobial, antioxidant activities and biocompatibility. Food Chem. 2020, 321, 126726. [Google Scholar] [CrossRef]
- Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Oxidative stress and apoptosis induction in human thyroid carcinoma cells exposed to the essential oil from Pistacia lentiscus aerial parts. PLoS ONE 2017, 12, e0172138. [Google Scholar] [CrossRef]
- Benedetti, S.; Nasoni, M.G.; Luchetti, F.; Palma, F. New insights into the cytotoxic effects of Thymus vulgaris essential oil on the human triple-negative breast cancer cell line MDA-MB-231. Toxicol. Vitr. 2023, 93, 105705. [Google Scholar] [CrossRef]
| Gene Target | FWD Primer Sequence (5′ → 3′) | REV Primer Sequence (5′ → 3′) |
|---|---|---|
| GPX4 | CTTCCCGTGTAACCAGTTCG | TCACGCAGATCTTGCTGAAC |
| HO-1 | ATGACACCAAGGACCAGAGC | GTGTAAGGACCCATCGGAGA |
| Nrf2 | AAACCAGTGGATCTGCCAAC | ACGTAGCCGAAGAAACCTCA |
| SIRT1 | CCGGATTTGAAGAATGTTGG | ATCTGCTCCTTTGCCACTCT |
| GAPDH | AGGTCGGAGTCAACGGAT | TCCTGGAAGATGGTGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasoni, M.G.; Benedetti, S.; Bargagni, E.; Burattini, S.; Osman, R.; Battistelli, M.; Luchetti, F. The Activity of Phytotherapic Extracts Combined in a Unique Formulation Alleviates Oxidative Stress and Protects Mitochondria Against Atorvastatin-Induced Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 4917. https://doi.org/10.3390/ijms26104917
Nasoni MG, Benedetti S, Bargagni E, Burattini S, Osman R, Battistelli M, Luchetti F. The Activity of Phytotherapic Extracts Combined in a Unique Formulation Alleviates Oxidative Stress and Protects Mitochondria Against Atorvastatin-Induced Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(10):4917. https://doi.org/10.3390/ijms26104917
Chicago/Turabian StyleNasoni, Maria Gemma, Serena Benedetti, Erik Bargagni, Sabrina Burattini, Riham Osman, Michela Battistelli, and Francesca Luchetti. 2025. "The Activity of Phytotherapic Extracts Combined in a Unique Formulation Alleviates Oxidative Stress and Protects Mitochondria Against Atorvastatin-Induced Cardiomyopathy" International Journal of Molecular Sciences 26, no. 10: 4917. https://doi.org/10.3390/ijms26104917
APA StyleNasoni, M. G., Benedetti, S., Bargagni, E., Burattini, S., Osman, R., Battistelli, M., & Luchetti, F. (2025). The Activity of Phytotherapic Extracts Combined in a Unique Formulation Alleviates Oxidative Stress and Protects Mitochondria Against Atorvastatin-Induced Cardiomyopathy. International Journal of Molecular Sciences, 26(10), 4917. https://doi.org/10.3390/ijms26104917










