Circaea mollis Siebold & Zucc. Induces Apoptosis in Colorectal Cancer Cells by Inhibiting c-Myc Through the Mediation of RPL5
Abstract
:1. Introduction
2. Results
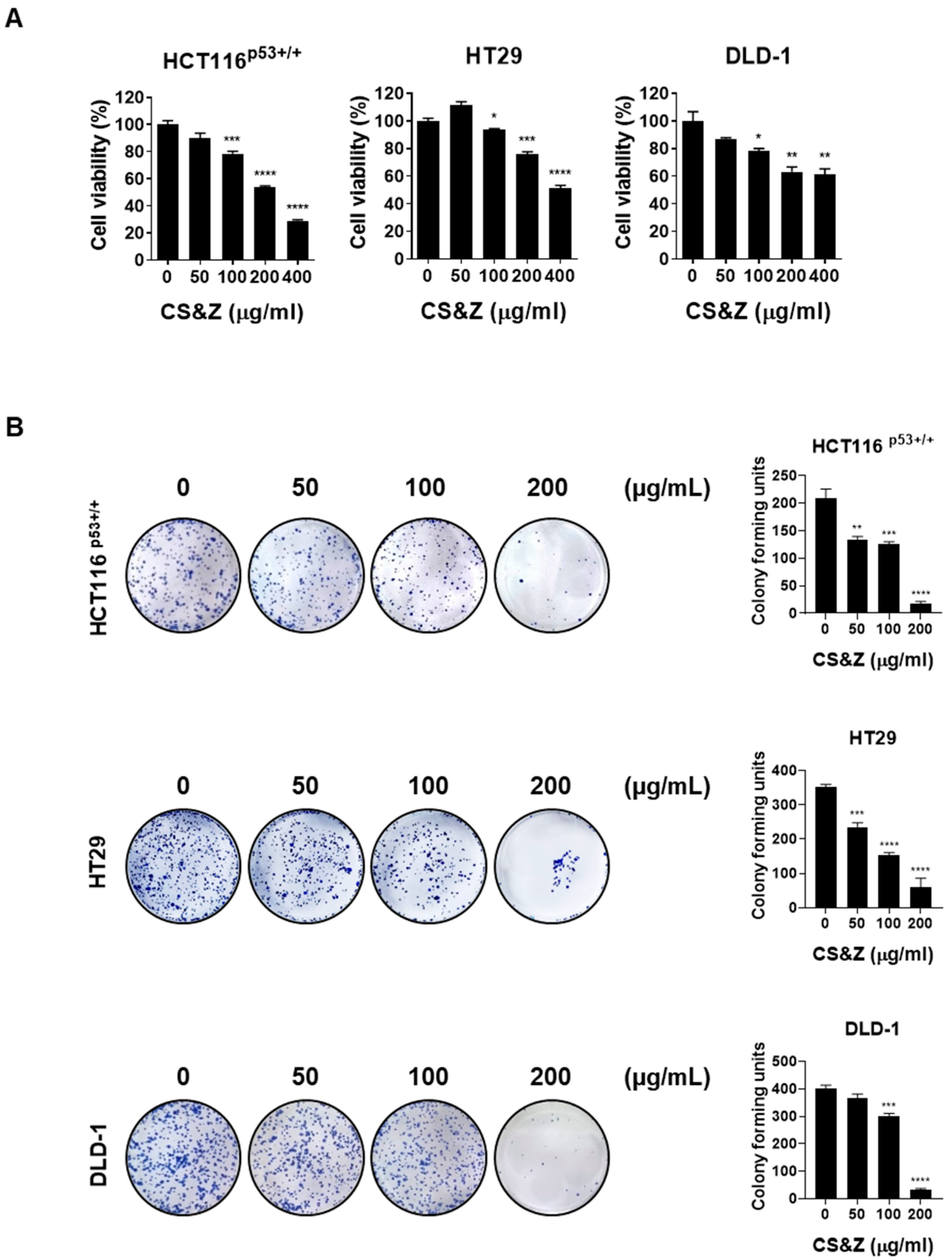
2.1. CS&Z Inhibits the Viability and Proliferation of Colorectal Cancer Cells
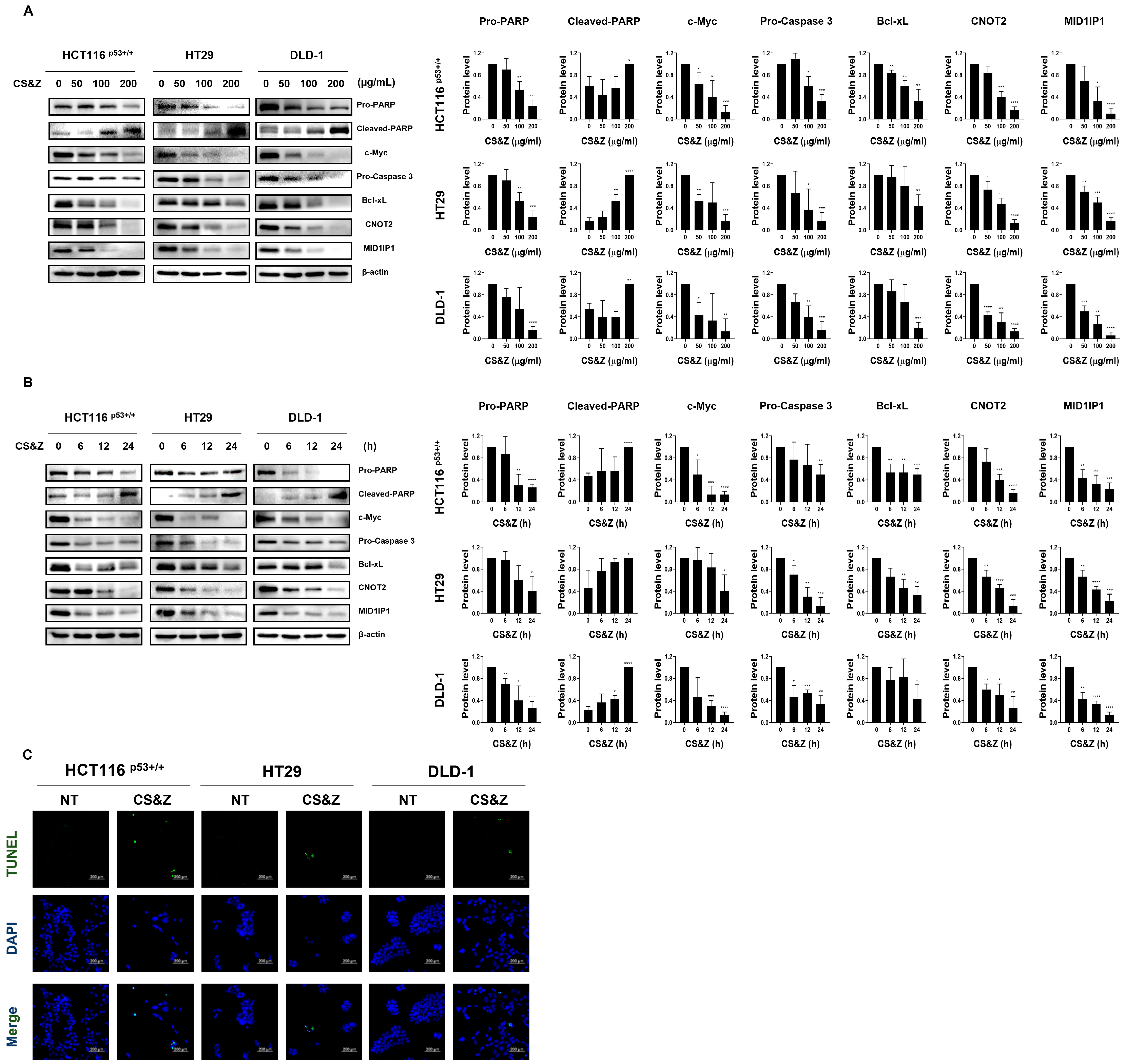
2.2. CS&Z Induces Apoptosis and Regulates Oncogenes Such as c-Myc in Colorectal Cancer Cells
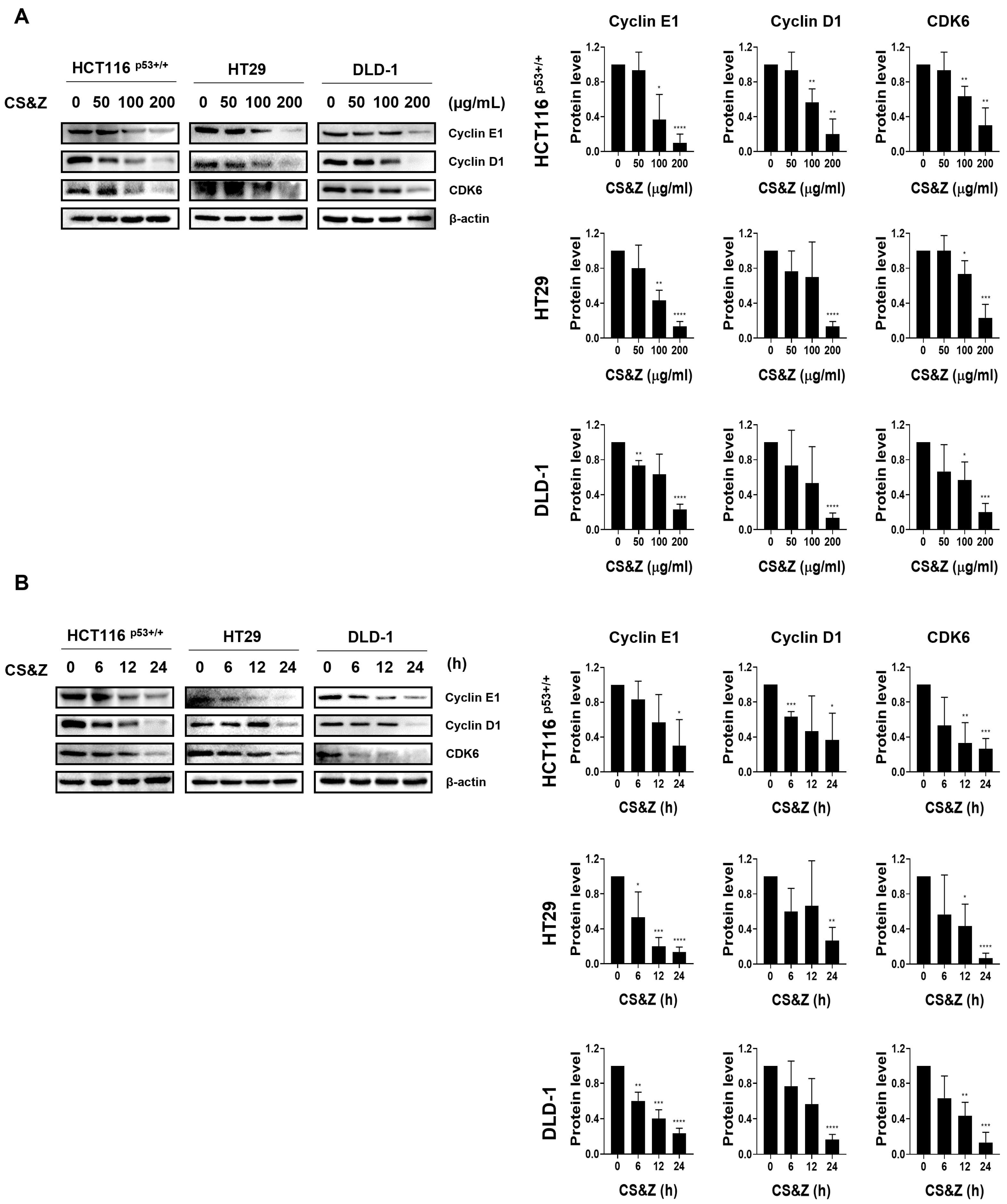
2.3. CS&Z Induces Cell Cycle Arrest in Colorectal Cancer Cells at the G1/S Stage
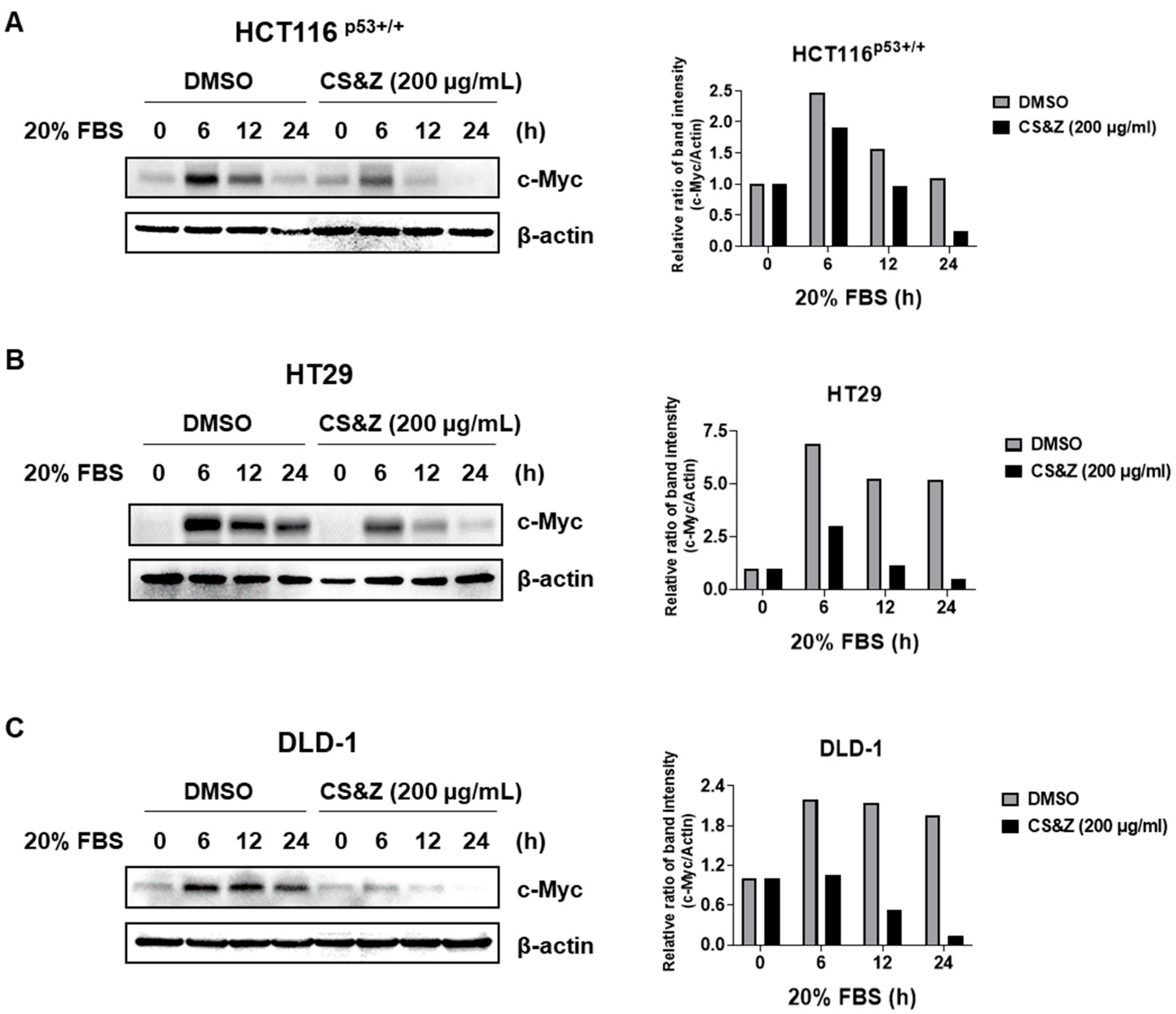
2.4. CS&Z Inhibits c-Myc Sensitivity About FBS in Colorectal Cancer Cells
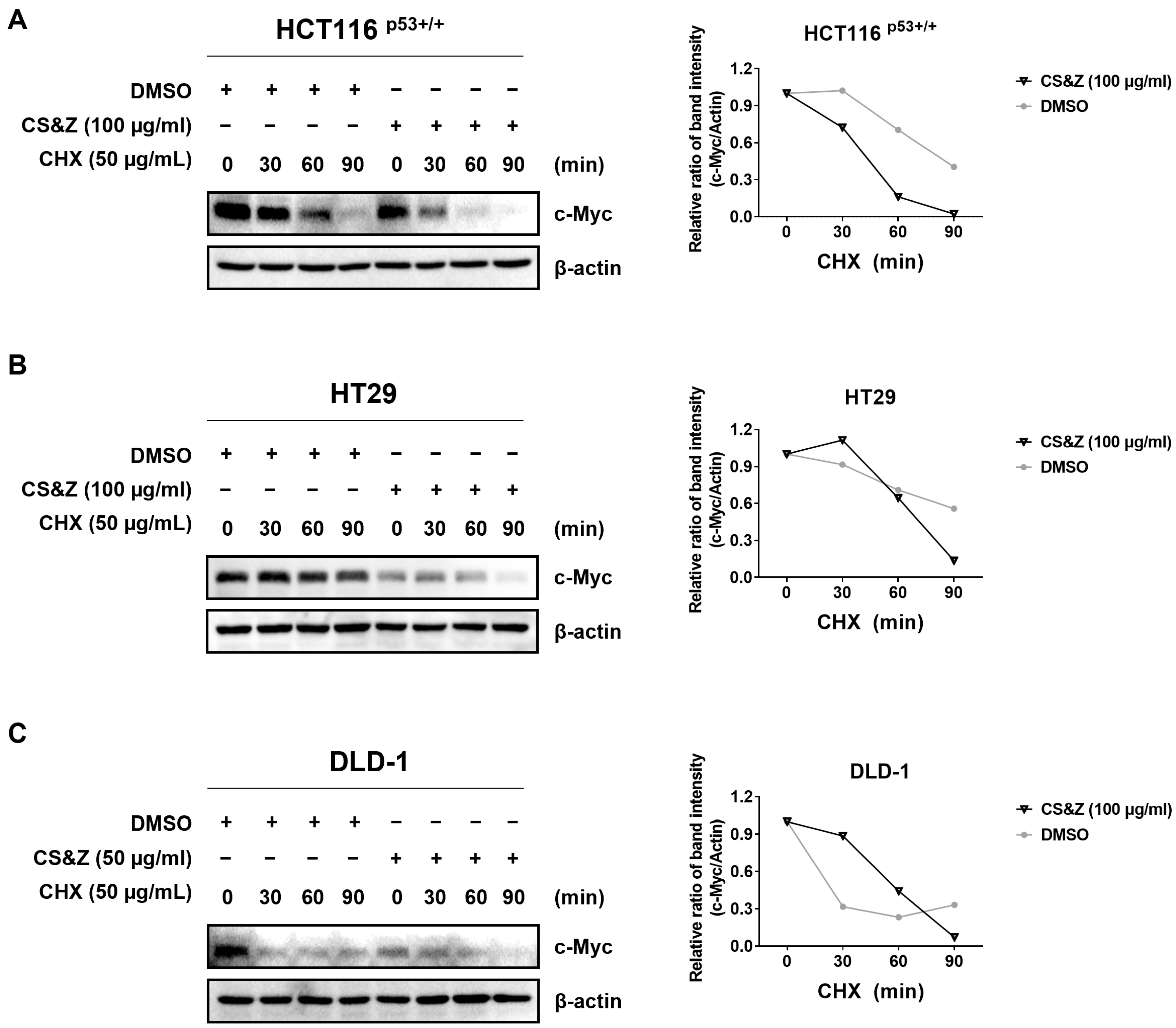
2.5. CS&Z Reduces c-Myc Stability in Colorectal Cancer Cells
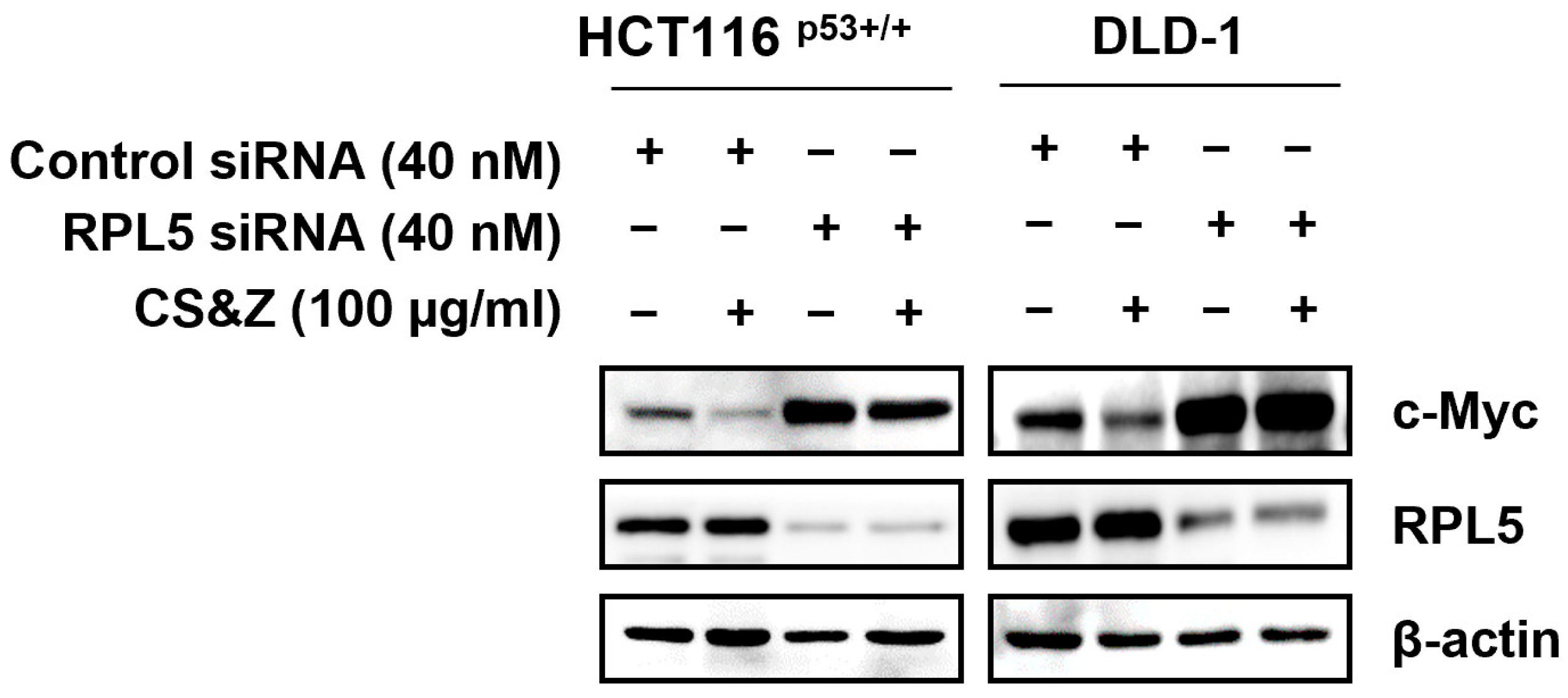
2.6. CS&Z Modulates c-Myc by Mediating RPL5 in Colorectal Cancer Cells
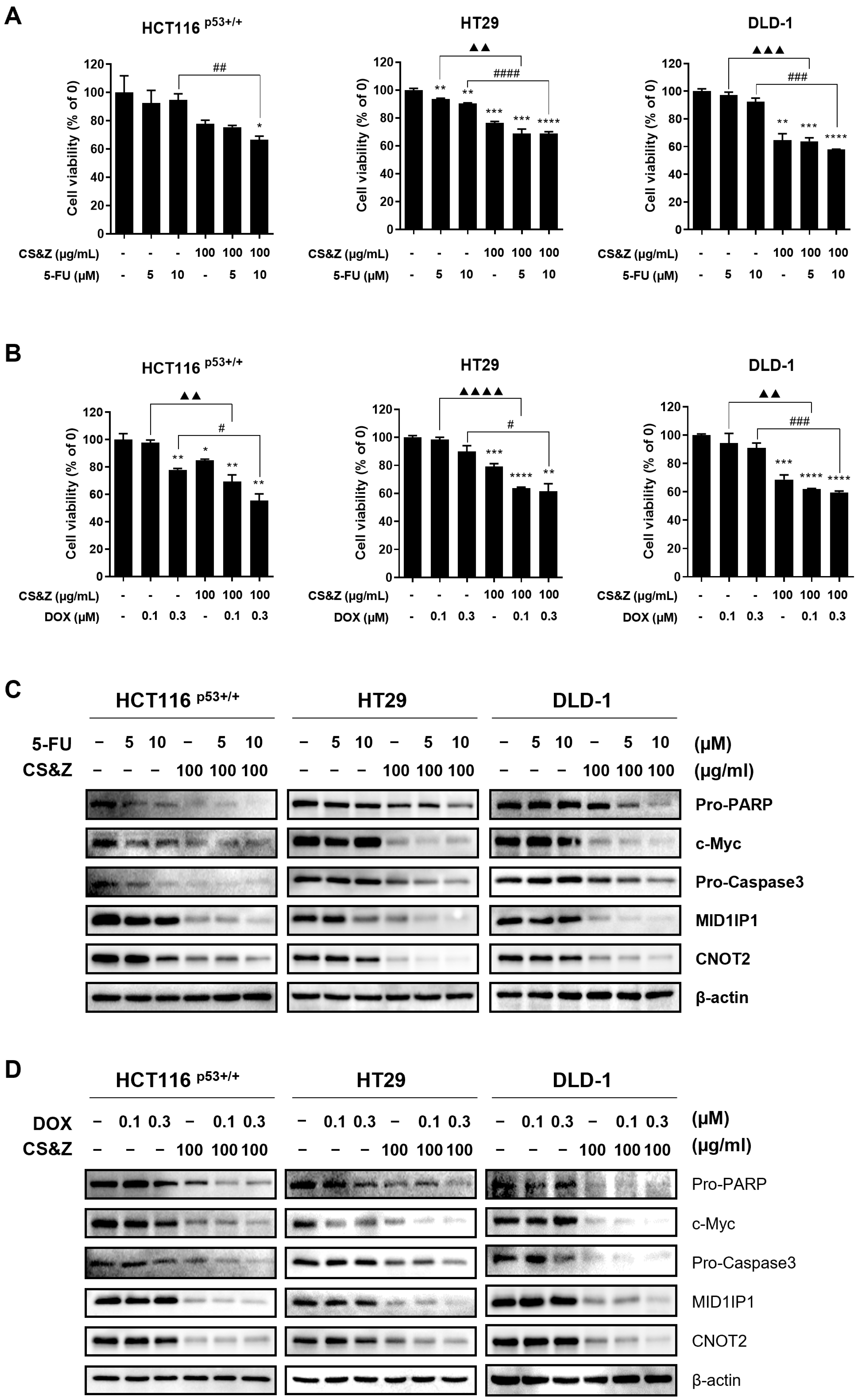
2.7. CS&Z Has a Synergic Antitumor Effect on Colorectal Cancer Cells When Co-Administered with 5-FU or DOX
2.8. LC-MS Analysis of a Methanolic Extract of CS&Z
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of a Whole-Plant Extract of Circaea mollis
4.3. Liquid Chromatography–Mass Spectrometry (LC-MS)
4.4. Cell Lines
4.5. Cytotoxicity Assay
4.6. Colony Formation Assay
4.7. Western Blotting
4.8. c-Myc Stability Assay Using Cycloheximide
4.9. Serum Stimulation
4.10. Terminal Deoxynucleotidyl Transferase Nick-End-Labeling Assay
4.11. siRNA Transfection
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS&Z | Circaea mollis Siebold & Zucc. |
| DOX | Doxorubicin |
| DMSO | Dimethyl sulfoxide |
| CRC | Colorectal cancer |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| 5-FU | 5-fluorouracil |
| Bcl-xL | Bcl-extra-large |
| RPL5 | Ribosomal protein L5 |
| CDK6 | Cyclin-dependent kinase 6 |
| FBS | Fetal bovine serum |
| LC-MS | Liquid chromatography–mass spectrometry |
| CHX | Cycloheximide |
| TUNEL | Terminal deoxynucleotidyl transferase nick-end labeling |
| CNOT | CCR4-NOT |
| PARP | Poly (adenosine diphosphate-ribose) polymerase |
| KHIDI | Korea Health Industry Development Institute |
| AMPK | AMP-activated protein kinase |
References
- Zhang, C.; Stampfl-Mattersberger, M.; Ruckser, R.; Sebesta, C. Colorectal cancer. Wien. Med. Wochenschr. 2023, 173, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef]
- Tang, J.; Yan, T.; Bao, Y.; Shen, C.; Yu, C.; Zhu, X.; Tian, X.; Guo, F.; Liang, Q.; Liu, Q.; et al. LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat. Commun. 2019, 10, 3499. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Yan, H.; Talty, R.; Johnson, C.H. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023, 33, 185–188. [Google Scholar] [CrossRef]
- Wooster, A.L.; Girgis, L.H.; Brazeale, H.; Anderson, T.S.; Wood, L.M.; Lowe, D.B. Dendritic cell vaccine therapy for colorectal cancer. Pharmacol. Res. 2021, 164, 105374. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Ko, H.M.; Jee, W.; Park, D.-i.; Kim, K.-I.; Jung, J.H.; Jang, H.-J. The Antitumor Effect of Timosaponin A3 through c-Myc Inhibition in Colorectal Cancer Cells and Combined Treatment Effect with 5-FU or Doxorubicin. Int. J. Mol. Sci. 2022, 23, 11900. [Google Scholar] [CrossRef]
- Jee, W.; Ko, H.M.; Park, D.-I.; Park, Y.-R.; Park, S.-M.; Kim, H.; Na, Y.-C.; Jung, J.H.; Jang, H.-J. Momordicae Semen inhibits migration and induces apoptotic cell death by regulating c-Myc and CNOT2 in human pancreatic cancer cells. Sci. Rep. 2023, 13, 12800. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, W.; Qin, Q.; Yang, X.; Yang, Y.; Liu, H.; Lu, W.; Gu, S.; Cao, X.; Feng, D.; et al. E3 ubiquitin ligase MAGI3 degrades c-Myc and acts as a predictor for chemotherapy response in colorectal cancer. Mol. Cancer 2022, 21, 151. [Google Scholar] [CrossRef]
- Park, S.M.; Jee, W.; Park, Y.R.; Kim, H.; Na, Y.C.; Jung, J.H.; Jang, H.J. Euonymus sachalinensis Induces Apoptosis by Inhibiting the Expression of c-Myc in Colon Cancer Cells. Molecules 2023, 28, 3473. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Jee, W.; Lee, D.; Jang, H.J.; Jung, J.H. Ophiopogonin D increase apoptosis by activating p53 via ribosomal protein L5 and L11 and inhibiting the expression of c-Myc via CNOT2. Front. Pharmacol. 2022, 13, 974468. [Google Scholar] [CrossRef]
- Park, J.E.; Jung, J.H.; Lee, H.-J.; Sim, D.Y.; Im, E.; Park, W.Y.; Shim, B.S.; Ko, S.-G.; Kim, S.-H. Ribosomal protein L5 mediated inhibition of c-Myc is critically involved in sanggenon G induced apoptosis in non-small lung cancer cells. Phytother. Res. 2021, 35, 1080–1088. [Google Scholar] [CrossRef]
- Wyżewski, Z.; Świtlik, W.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. The Role of Bcl-xL Protein in Viral Infections. Int. J. Mol. Sci. 2021, 22, 1956. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, D.; Ko, H.M.; Jang, H.-J. Inhibition of CNOT2 Induces Apoptosis via MID1IP1 in Colorectal Cancer Cells by Activating p53. Biomolecules 2021, 11, 1492. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Westfall, M.D.; Pietenpol, J.A. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Shawky, E.M.; Elgindi, M.R.; Ibrahim, H.A.; Baky, M.H. The potential and outgoing trends in traditional, phytochemical, economical, and ethnopharmacological importance of family Onagraceae: A comprehensive review. J. Ethnopharmacol. 2021, 281, 114450. [Google Scholar] [CrossRef]
- Xie, L.; Wagner, W.L.; Ree, R.H.; Berry, P.E.; Wen, J. Molecular phylogeny, divergence time estimates, and historical biogeography of Circaea (Onagraceae) in the Northern Hemisphere. Mol. Phylogenetics Evol. 2009, 53, 995–1009. [Google Scholar] [CrossRef]
- Park, J.H.; Son, Y.J.; Lee, C.H.; Nho, C.W.; Yoo, G. Circaea mollis Siebold & Zucc. Alleviates postmenopausal osteoporosis in a mouse model via the BMP-2/4/Runx2 pathway. BMC Complement. Med. Ther. 2020, 20, 123. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.; Li, J.; Guan, Y.; Huang, J.; Wang, Z.; Zhang, Z.; Zhang, W.; Guo, J.; Li, J.; et al. Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc. (whole plant) in rodents. J. Ethnopharmacol. 2018, 225, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.M.; Zhou, X.; Gatignol, A.; Lu, H. Ribosomal proteins L5 and L11 co-operatively inactivate c-Myc via RNA-induced silencing complex. Oncogene 2014, 33, 4916–4923. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Hu, X.; Feng, J.; Wang, H.; Xiong, F. Protective effects of cynaroside on oxidative stress in retinal pigment epithelial cells. J. Biochem. Mol. Toxicol. 2019, 33, e22352. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, Z.; Sun, W.; Li, Z.; Cai, H.; Zhao, E.; Cui, H. Effects of Cynaroside on Cell Proliferation, Apoptosis, Migration and Invasion though the MET/AKT/mTOR Axis in Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 12125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Tan, X.; Liu, J.; Zhi, Y.; Yi, L.; Bai, S.; Du, Q.; Li, Q.X.; Dong, Y. Isoorientin Inhibits Inflammation in Macrophages and Endotoxemia Mice by Regulating Glycogen Synthase Kinase 3β. Mediat. Inflamm. 2020, 2020, 8704146. [Google Scholar] [CrossRef]
- Li, H.; Yuan, L.; Li, X.; Luo, Y.; Zhang, Z.; Li, J. Isoorientin Attenuated the Pyroptotic Hepatocyte Damage Induced by Benzo[a]pyrene via ROS/NF-κB/NLRP3/Caspase-1 Signaling Pathway. Antioxidants 2021, 10, 1275. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xiao, H.; Wu, W.; Wang, Y.; Liu, X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem. Toxicol. 2013, 53, 62–68. [Google Scholar] [CrossRef]
- Li, C.; Cai, C.; Zheng, X.; Sun, J.; Ye, L. Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Biosci. Biotechnol. Biochem. 2020, 84, 774–779. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A Review on Medicinal Properties of Orientin. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules 2019, 9, 418. [Google Scholar] [CrossRef]
- Yang, C.Z.; Wang, S.H.; Zhang, R.H.; Lin, J.H.; Tian, Y.H.; Yang, Y.Q.; Liu, J.; Ma, Y.X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; Mansour, M.R. Therapeutic targeting of “undruggable” MYC. EBioMedicine 2022, 75, 103756. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Walter, H.S.; Dyer, M.J.S. Targeting anti-apoptotic BCL2 family proteins in haematological malignancies—From pathogenesis to treatment. Br. J. Haematol. 2017, 178, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Na, Y.C.; Chung, W.S.; Jang, H.J. Apoptosis and G2/M cell cycle arrest induced by a timosaponin A3 from Anemarrhena asphodeloides Bunge on AsPC-1 pancreatic cancer cells. Phytomedicine 2019, 56, 48–56. [Google Scholar] [CrossRef]

| No. | Compound | R.T (min) | Mass | Molecular Formula | Experimental Mass (m/z) | Selected Ion Species |
|---|---|---|---|---|---|---|
| 1 | Galuteolin Isoorientin Orientin Astragalin | 6.062 | 448.1006 | C21H20O11 | (+) 449.0662 (−) 447.0973 | (M + H)+ (M − H)− |
| 2 | Vitexin | 6.360 | 432.1056 | C21H20O10 | (+) 433.1038 (−) 431.1003 | (M + H)+ (M − H)− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-M.; Kim, N.; Park, Y.-R.; Kim, S.W.; Jung, J.H.; Na, Y.-C.; Kwon, D.; Kim, H.; Jang, H.-J. Circaea mollis Siebold & Zucc. Induces Apoptosis in Colorectal Cancer Cells by Inhibiting c-Myc Through the Mediation of RPL5. Int. J. Mol. Sci. 2025, 26, 4664. https://doi.org/10.3390/ijms26104664
Park S-M, Kim N, Park Y-R, Kim SW, Jung JH, Na Y-C, Kwon D, Kim H, Jang H-J. Circaea mollis Siebold & Zucc. Induces Apoptosis in Colorectal Cancer Cells by Inhibiting c-Myc Through the Mediation of RPL5. International Journal of Molecular Sciences. 2025; 26(10):4664. https://doi.org/10.3390/ijms26104664
Chicago/Turabian StylePark, So-Mi, Nanyeong Kim, Ye-Rin Park, Seok Woo Kim, Ji Hoon Jung, Yun-Cheol Na, Daeho Kwon, Hyungsuk Kim, and Hyeung-Jin Jang. 2025. "Circaea mollis Siebold & Zucc. Induces Apoptosis in Colorectal Cancer Cells by Inhibiting c-Myc Through the Mediation of RPL5" International Journal of Molecular Sciences 26, no. 10: 4664. https://doi.org/10.3390/ijms26104664
APA StylePark, S.-M., Kim, N., Park, Y.-R., Kim, S. W., Jung, J. H., Na, Y.-C., Kwon, D., Kim, H., & Jang, H.-J. (2025). Circaea mollis Siebold & Zucc. Induces Apoptosis in Colorectal Cancer Cells by Inhibiting c-Myc Through the Mediation of RPL5. International Journal of Molecular Sciences, 26(10), 4664. https://doi.org/10.3390/ijms26104664








