Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2H)-one and Aceanthrylene-1(2H)-one Fragments Against Tumor Cell Lines
Abstract
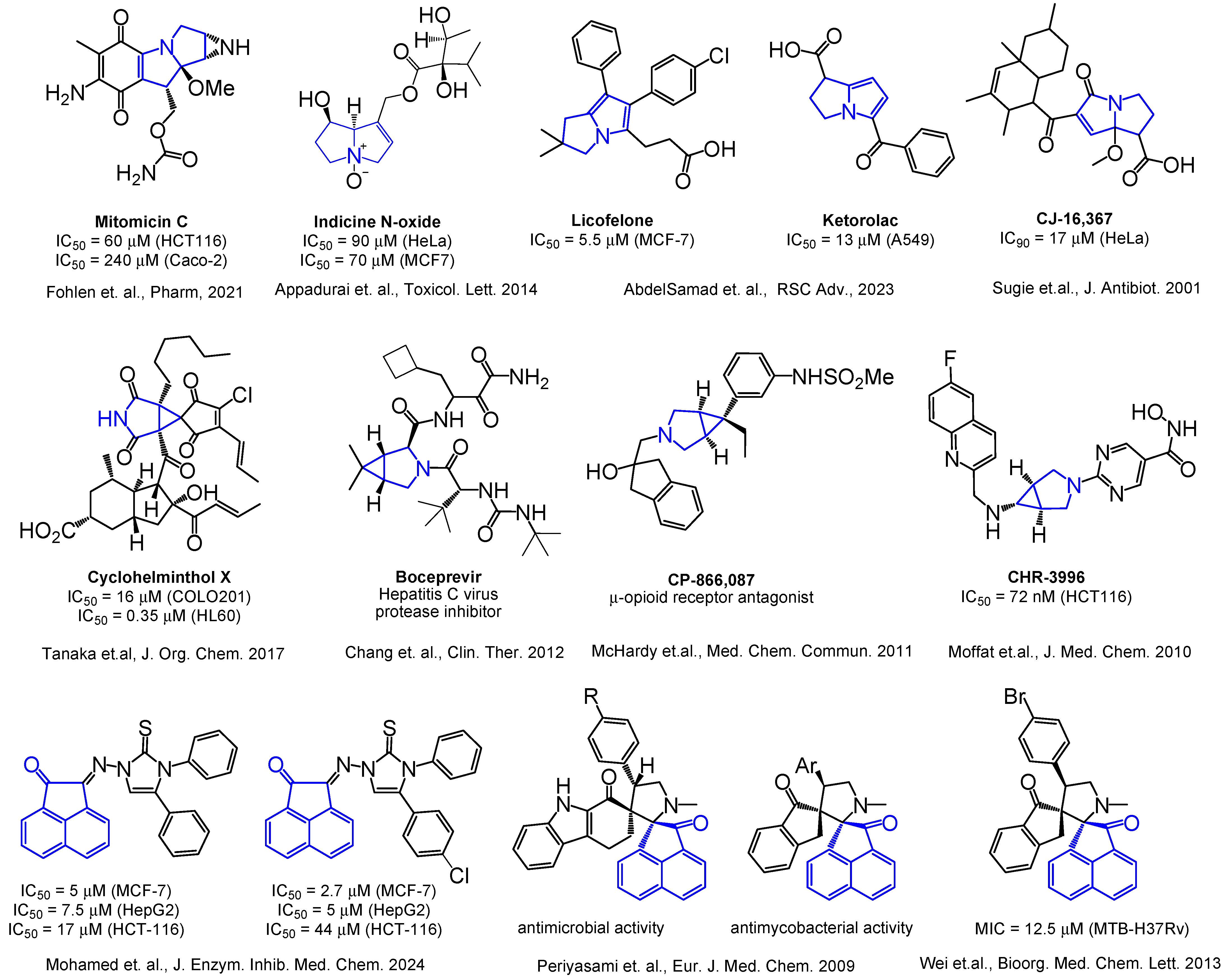
1. Introduction
2. Results and Discussion
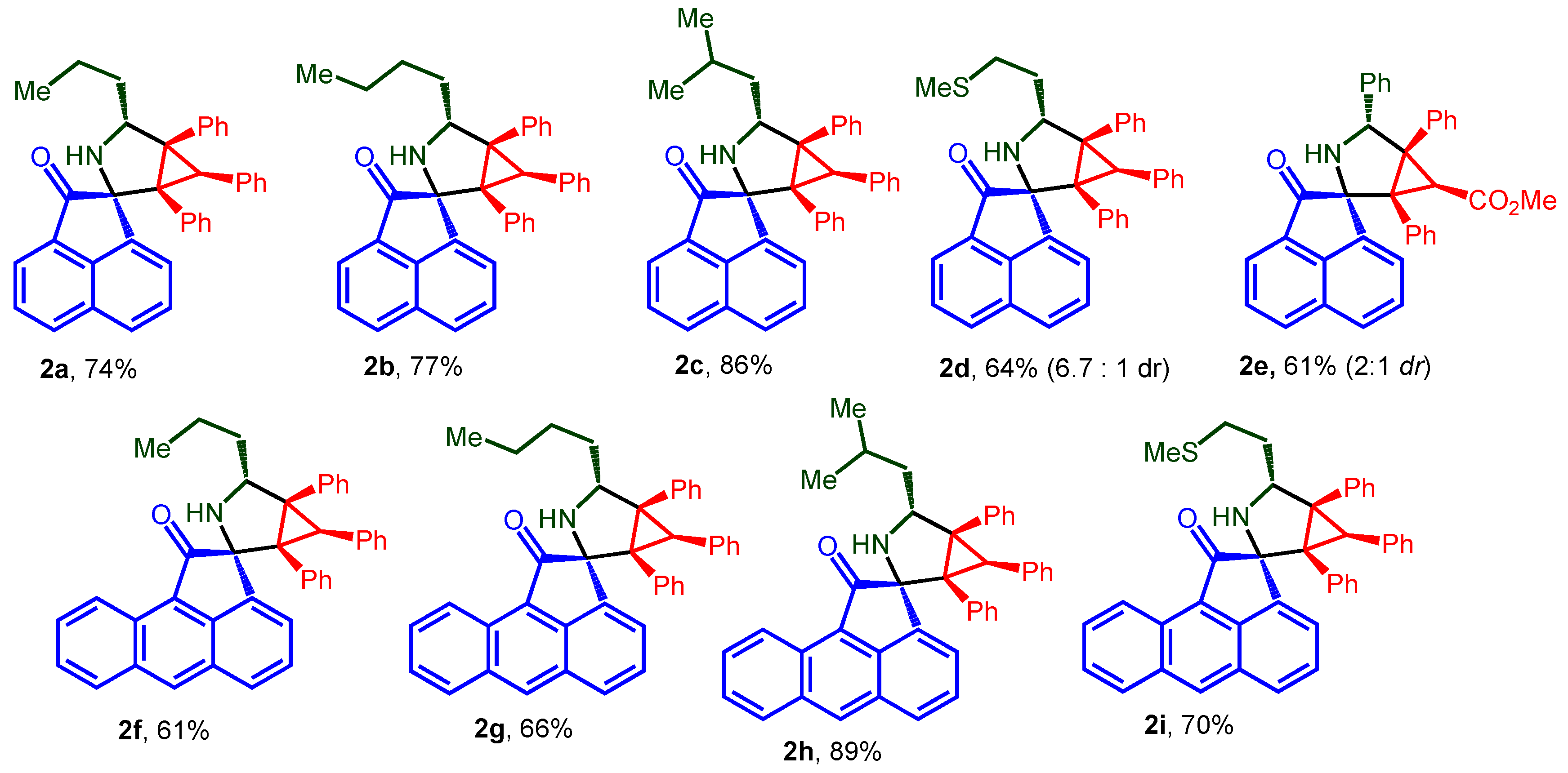
2.1. Chemistry
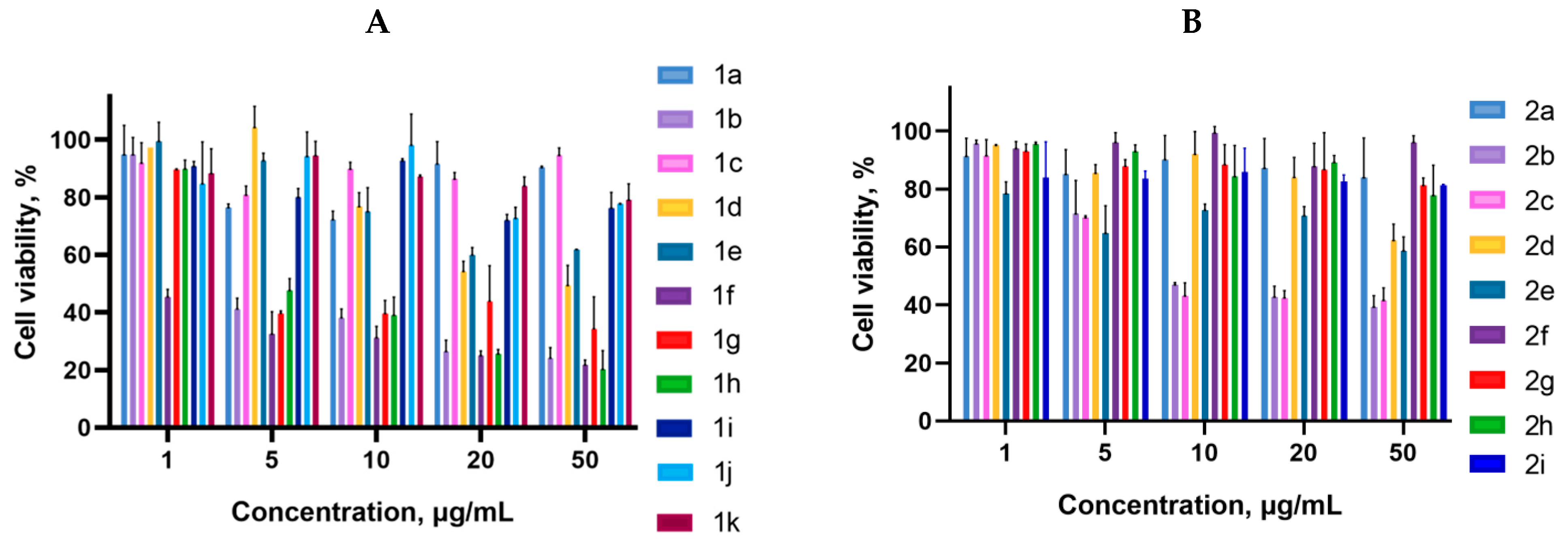
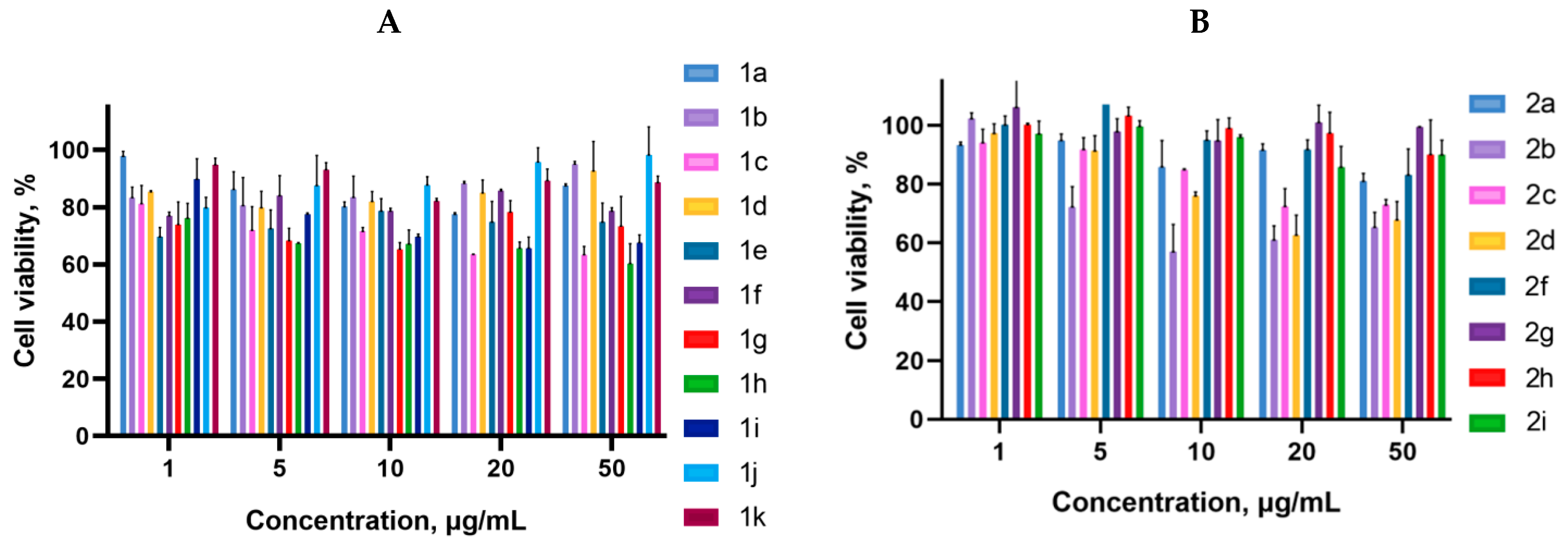
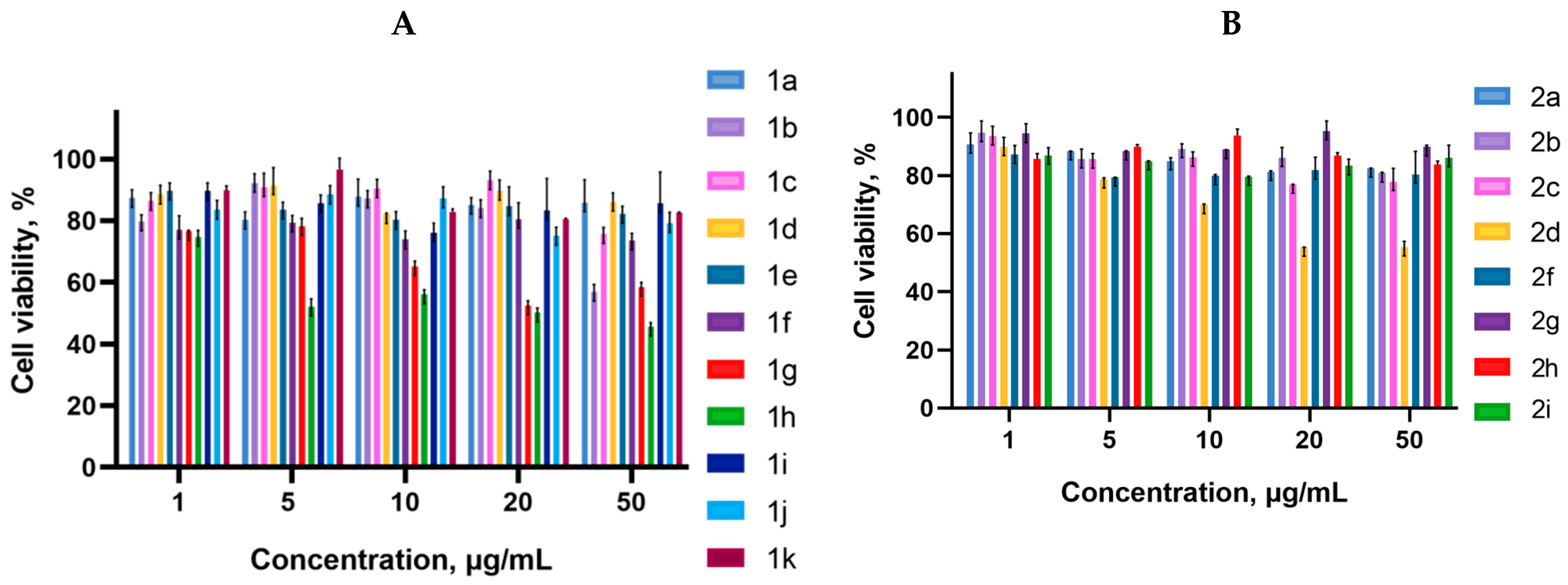
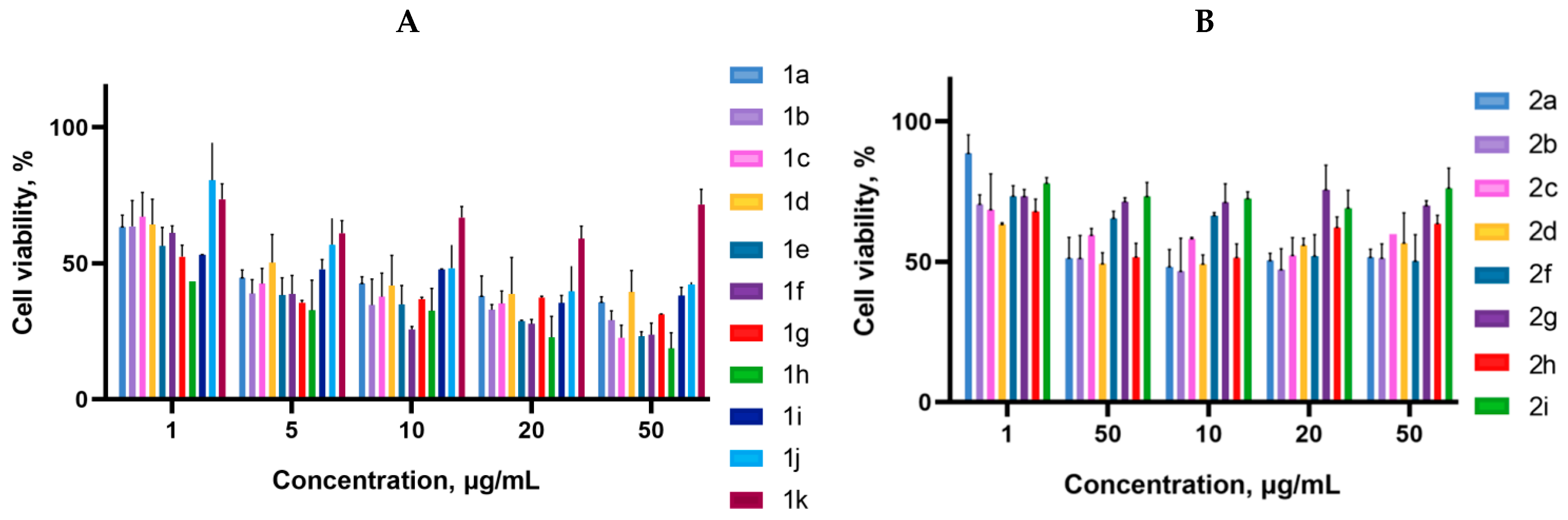
2.2. Biology
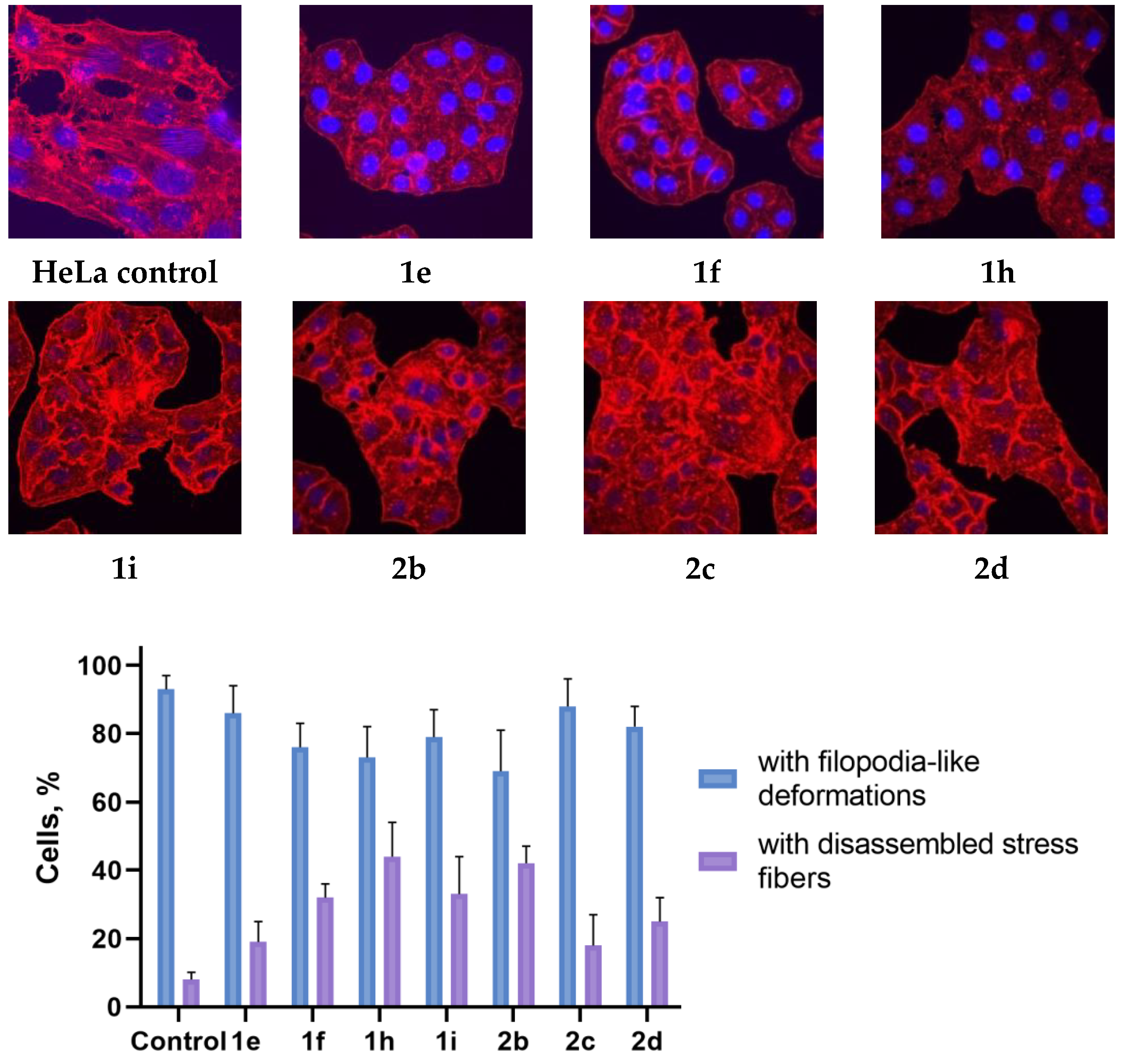
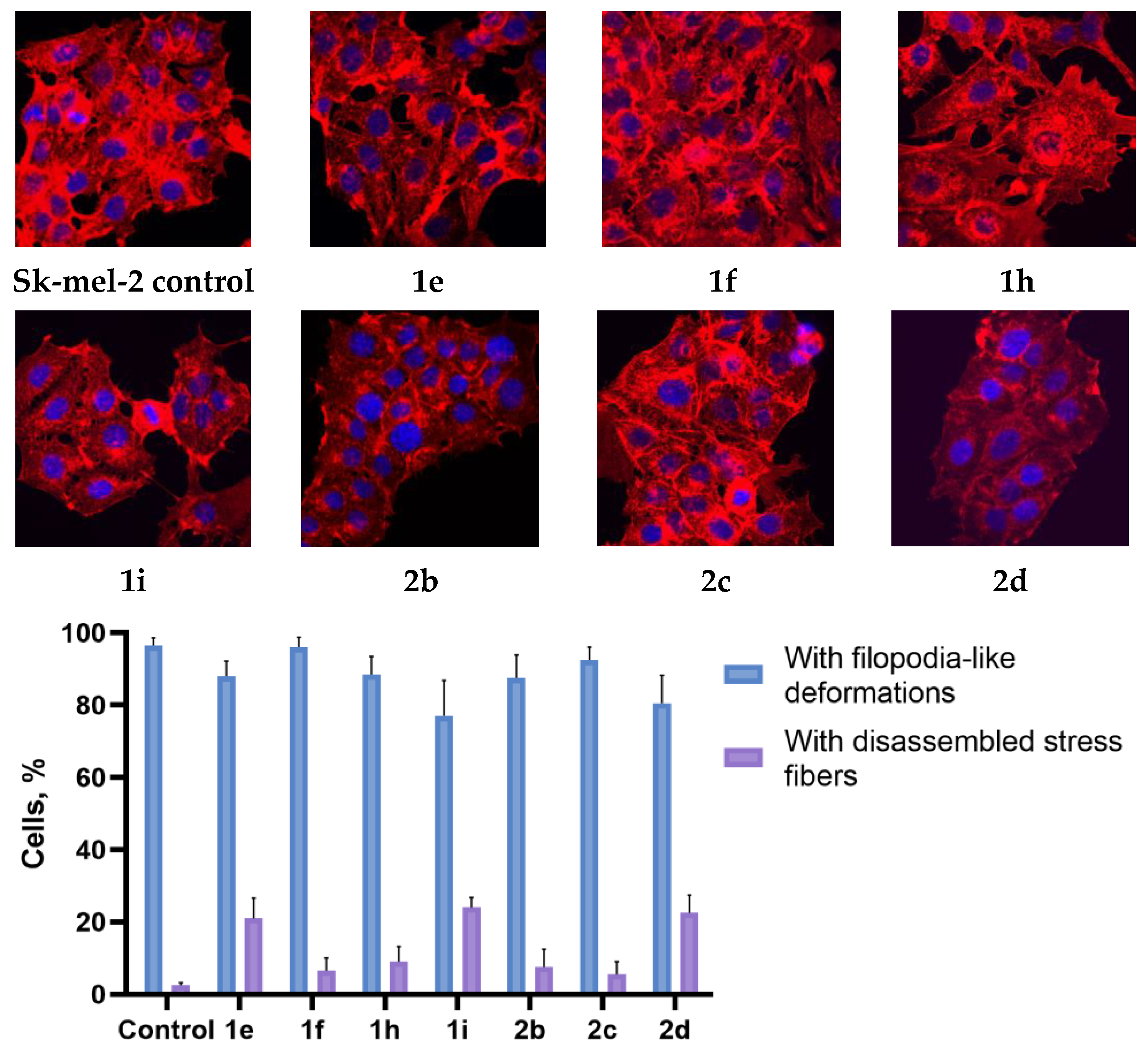
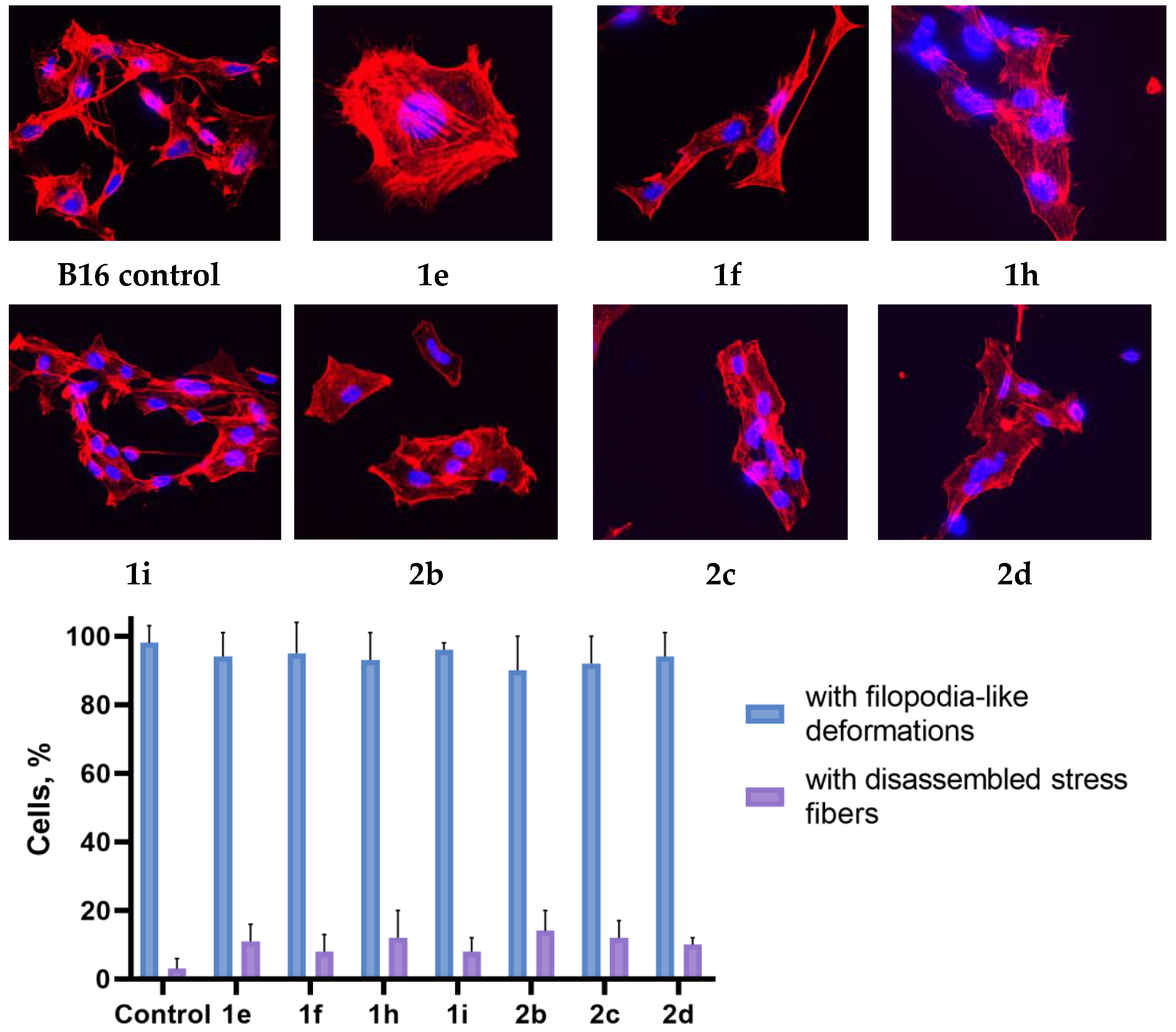
2.3. Actine Cytoskeleton Changes
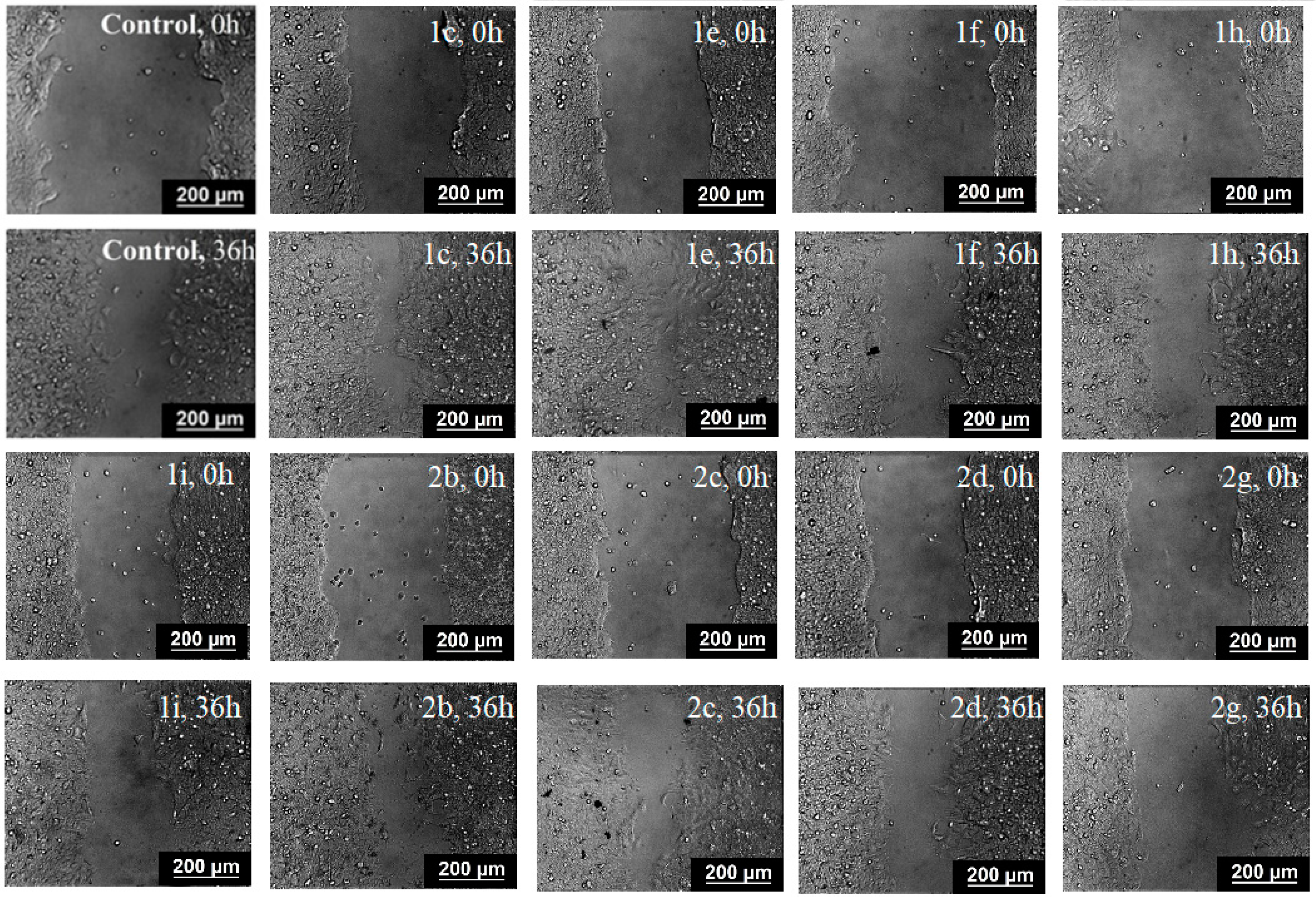
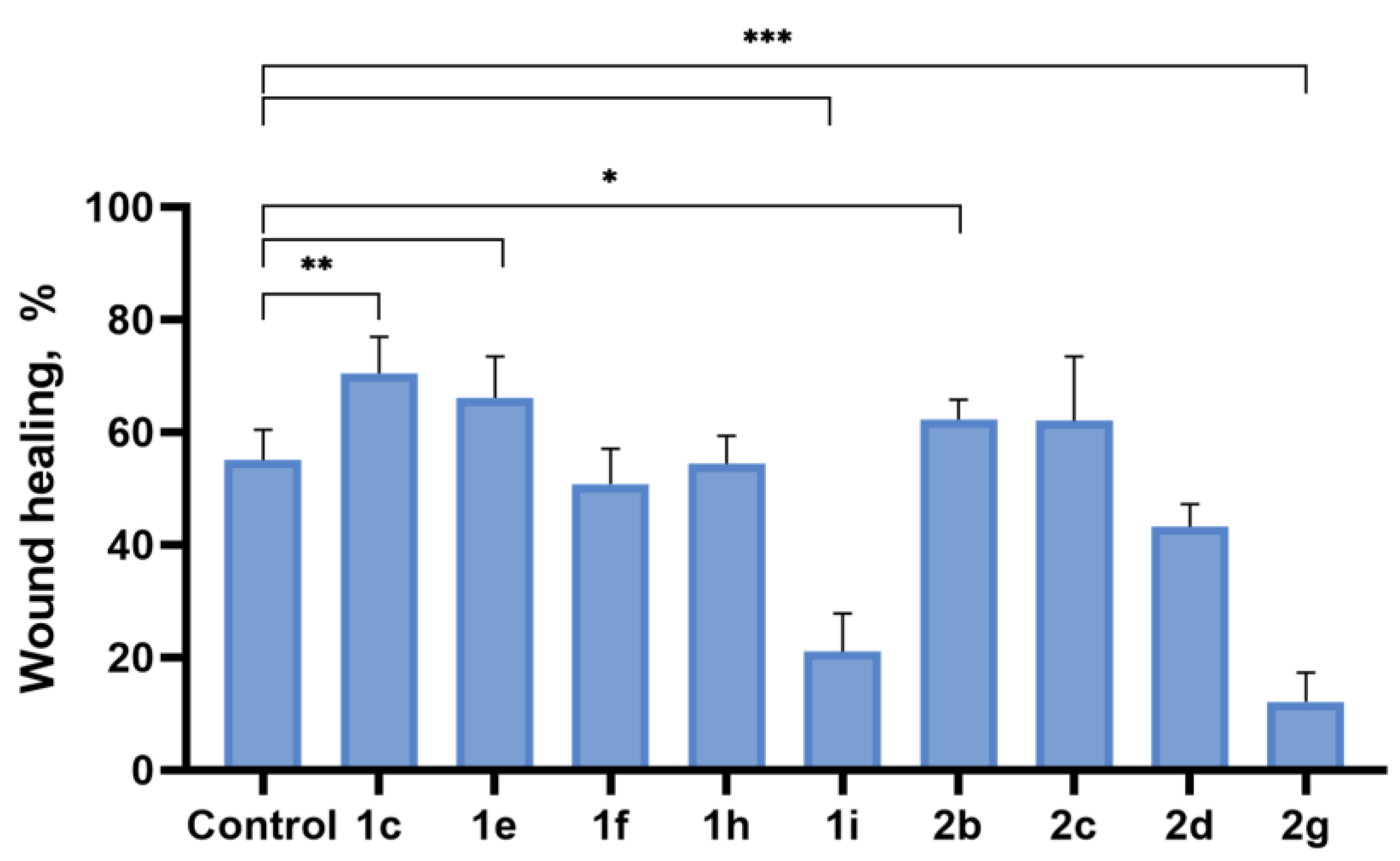
2.4. Inhibition of Cell Motility Evaluated by Scratch-Test
2.5. Mitochondrial Membrane Potential (∆Ψm) Changes
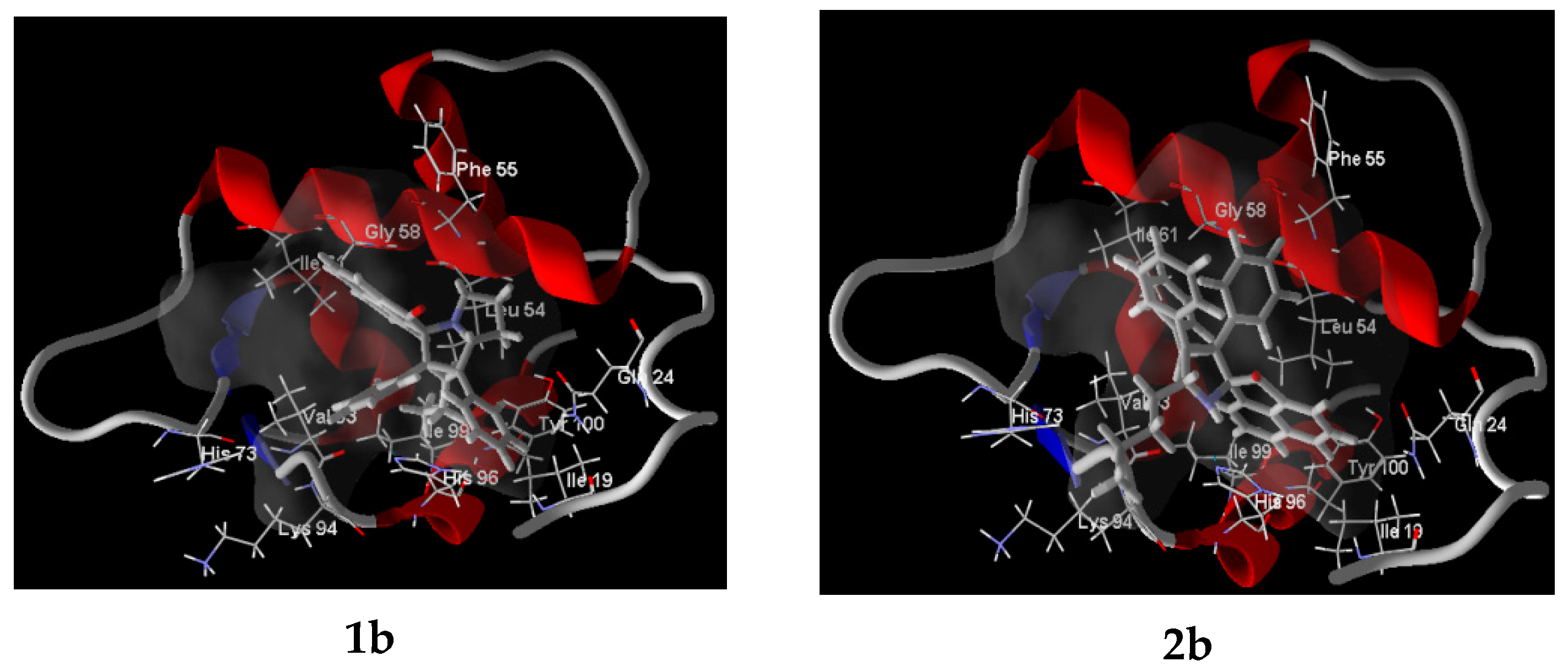
2.6. Molecular Docking
3. Materials and Methods
3.1. Chemistry
In Silico Analysis
3.2. Cell Culture and Culturing Conditions
3.3. Cell Proliferation Assay
3.4. Actin Cytoskeleton Staining
3.5. Evaluation of Cell Motility by Scratch Test
3.6. Investigation of the Mitochondrial Membrane Potential (∆Ψm) Changes Under Incubation Conditions with Synthesized Compounds
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286, 18–24. [Google Scholar] [CrossRef]
- Guo, M.; Jin, J.; Zhao, D.; Rong, Z.; Cao, L.Q.; Li, A.H.; Sun, X.Y.; Jia, L.Y.; Wang, Y.D.; Huang, L.; et al. Research advances on anti-cancer natural products. Front. Oncol. 2022, 12, 866154. [Google Scholar] [CrossRef]
- Grigalunas, M.; Brakmann, S.; Waldmann, H. Chemical evolution of natural product structure. J. Am. Chem. Soc. 2022, 144, 3314–3329. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, M.M.; Zhao, R.; Wang, D.; Ma, Y.R.; Ai, L. Plant natural products: Promising resources for cancer chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Karageorgis, G.; Foley, D.J.; Laraia, L.; Brakmann, S.; Waldmann, H. Pseudo Natural Products—Chemical Evolution of Natural Product Structure. Angew. Chem. Int. Ed. 2021, 60, 15705–15723. [Google Scholar] [CrossRef] [PubMed]
- Breugst, M.; Reissig, H.-U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Otevrel, J.; Eugui, M.; Ričko, S.; Jørgensen, K.A. Enantioselective organocatalytic cycloadditions for the synthesis of medium-sized rings. Nat. Synth. 2023, 2, 1142–1158. [Google Scholar] [CrossRef]
- Arrastia, I.; Arrieta, A.; Cossio, F.P. Application of 1,3-Dipolar Reactions between Azomethine Ylides and Alkenes to the Synthesis of Catalysts and Biologically Active Compounds. Eur. J. Org. Chem. 2018, 2018, 5889–5904. [Google Scholar] [CrossRef]
- Wang, K.-K.; Li, Y.-L.; Chen, R.; Wang, Z.-Y.; Li, N.-B.; Zhang, L.-L.; Gu, S. Substrate-Controlled Regioselectivity Switchable [3 + 2] Annulations To Access Spirooxindole Skeletons. J. Org. Chem. 2022, 87, 8158–8169. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, Q.; Zhuge, Y.; Liwei, X.; Huang, Y. One-Pot Synthesis of Cyclopropanes from Methylene Azabicyclo [3.1.0]hexanes Obtained by Formal Sequential [1 + 2]- and [2 + 3]-Cycloaddition Reaction of Prop-2-ynylsulfonium Salts and Tosylaminomethyl Enones. Adv. Synth. Catal. 2018, 360, 438–443. [Google Scholar] [CrossRef]
- Hol, W.; Van Veen, J. Pyrrolizidine alkaloids from Senecio jacobaea affect fungal growth. J. Chem. Ecol. 2002, 28, 1763–1772. [Google Scholar] [CrossRef]
- Fohlen, A.; Bordji, K.; Assenat, E.; Gongora, C.; Bazille, C.; Boulonnais, J.; Naveau, M.; Breuil, C.; Pérès, E.A.; Bernaudin, M.; et al. Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals 2021, 14, 639. [Google Scholar] [CrossRef]
- Appadurai, P.; Rathinasamy, K. Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: A mechanism for its cytotoxic activity. Toxicol. Lett. 2014, 225, 66–77. [Google Scholar] [CrossRef] [PubMed]
- AbdelSamad, A.L.; El-Saadi, M.T.; Gouda, A.M.; AboulMagd, A.M. Pyrrolizine/indolizine-bearing (un)substituted isoindole moiety: Design, synthesis, antiproliferative and MDR reversal activities, and in silico studies. RSC Adv. 2023, 13, 30753–30770. [Google Scholar] [CrossRef] [PubMed]
- Sugie, Y.; Hirai, H.; Kachi-Tonai, H.; Kim, Y.-J.; Kojima, Y.; Shiomi, Y.; Kojima, N. New Pyrrolizidinone Antibiotics CJ-16,264 and CJ-16,367. J. Antibiot. 2001, 54, 917–925. [Google Scholar] [CrossRef]
- Aboelmagd, M.; Elokely, K.; Zaki, M.A.; Said, A.; Haggag, E.G.; Ross, S.A. Anti-inflammatory of pyrrolizidine alkaloids from Heliotropium digynum. Med. Chem. Res. 2018, 27, 1066–1073. [Google Scholar] [CrossRef]
- Abbas, S.E.; Awadallah, F.M.; Ibrahim, N.A.; Gouda, A.M. Novel substituted and fused pyrrolizine derivatives: Synthesis, anti-inflammatory and ulcerogenecity studies. Eur. J. Med. Chem. 2010, 45, 482–491. [Google Scholar] [CrossRef]
- Qiao, J.; Li, Y.-S.; Zeng, R.; Liu, F.-L.; Luo, R.-H.; Huang, C.; Wang, Y.-F.; Zhang, J.; Quan, B.; Shen, C.; et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374–1378. [Google Scholar] [CrossRef]
- Schweizer, E.; Hoffmann-Röder, A.; Olsen, J.A.; Seiler, P.; Obst-Sander, U.; Wagner, B.; Kansy, M.; Banner, D.W.; Diederich, F. Multipolar interactions in the D pocket of thrombin: Large differences between tricyclic imide and lactam inhibitors. Org. Biomol. Chem. 2006, 4, 2364–2375. [Google Scholar] [CrossRef]
- Shen, P.; Guo, Y.; Wei, J.; Zhao, H.; Zhai, H.; Zhao, Y. Straightforward Synthesis of Succinimide-Fused Pyrrolizidines by A Three-Component Reaction of α-Diketone, Amino Acid, and Maleimide. Synthesis 2021, 53, 1262–1270. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Belal, A.; El-Gendy, B.E.-D.M. Pyrrolizines: Promising scaffolds for anticancer drugs. Bioorg. Med. Chem. 2014, 22, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Honmura, Y.; Uesugi, S.; Fukushi, E.; Tanaka, K.; Maeda, H.; Kimura, K.; Nehira, T.; Hashimoto, M. Cyclohelminthol X, a Hexa-Substituted Spirocyclopropane from Helminthosporium velutinum yone96: Structural Elucidation, Electronic Circular Dichroism Analysis, and Biological Properties. J. Org. Chem. 2017, 82, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Netz, N.; Opatz, T. A Modular Formal Total Synthesis of (±)-Cycloclavine. J. Org. Chem. 2016, 81, 1723–1730. [Google Scholar] [CrossRef]
- Chang, M.H.; Gordon, L.A.; Fung, H.B. Boceprevir: A protease inhibitor for the treatment of hepatitis C. Clin. Ther. 2012, 34, 2021–2038. [Google Scholar] [CrossRef]
- Lunn, G.; Banks, B.J.; Crook, R.; Feeder, N.; Pettman, A.; Sabnis, Y. Discovery and synthesis of a new class of opioid ligand having a 3-azabicyclo [3.1.0]hexane core. An example of a ‘magic methyl’ giving a 35-fold improvement in binding. Bioorg. Med. Chem. Lett. 2011, 21, 4608–4611. [Google Scholar] [CrossRef]
- McHardy, S.F.; Heck, S.D.; Guediche, S.; Kalman, M.; Allen, M.P.; Tu, M.; Bryce, D.K.; Schmidt, A.W.; Vanase-Frawley, M.; Callegari, E.; et al. Discovery of CP-866,087, a mu opioid receptor antagonist for the treatment of alcohol abuse and dependence. Med. Chem. Commun. 2011, 2, 1001–1005. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, C.; Zhu, R.; Lin, Z.; Wu, W.; Jiang, H. Synthesis of 3-azabicyclo [3.1.0]hexane derivatives via palladium-catalyzed cyclopropanation of maleimides with N-tosylhydrazones: Practical and facile access to CP-866,087. Org. Biomol. Chem. 2017, 15, 1228–1235. [Google Scholar] [CrossRef]
- Topczewski, J.J.; Cabrera, P.J.; Saper, N.I.; Sanford, M.S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 2016, 531, 220–224. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Z.; Ye, F.; Ma, J.; Xu, Z.; Bai, X.; Li, L.; Xu, L. Highly efficient desymmetrization of cyclopropenes to azabicyclo [3.1.0]hexanes with five continuous stereogenic centers by copper-catalyzed [3 + 2] cycloadditions. Org. Chem. Front. 2018, 5, 2759–2764. [Google Scholar] [CrossRef]
- Runyon, S.P.; Kormos, C.M.; Gichinga, M.G.; Mascarella, S.W.; Navarro, H.A.; Deschamps, J.R.; Imler, G.H.; Carroll, F.I. Design, synthesis, and biological evaluation of structurally rigid analogues of 4-(3-hydroxyphenyl)piperidine opioid receptor antagonists. J. Org. Chem. 2016, 81, 10383–10391. [Google Scholar] [CrossRef]
- Moffat, D.; Patel, S.; Day, F.; Belfield, A.; Donald, A.; Rowlands, M.; Wibawa, J.; Brotherton, D.; Stimson, L.; Clark, V.; et al. Discovery of 2-(6-{[(6-Fluoroquinolin-2-yl)methyl]amino}bicyclo [3.1.0]hex-3-yl)-N-hydroxypyrimidine-5-carboxamide (CHR-3996), a Class I Selective Orally Active Histone Deacetylase Inhibitor. J. Med. Chem. 2010, 53, 8663–8678. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Meilandt, W.J.; Erickson, R.I.; Chen, J.; Deshmukh, G.; Estrada, A.A.; Fuji, R.N.; Gibbons, P.; Gustafson, A.; Harris, S.F.; et al. Selective Inhibitors of Dual Leucine Zipper Kinase (DLK, MAP3K12) with Activity in a Model of Alzheimer’s Disease. J. Med. Chem. 2017, 60, 8083–8102. [Google Scholar] [CrossRef] [PubMed]
- Skepper, C.K.; Armstrong, D.; Balibar, C.J.; Bauer, D.; Bellamacina, C.; Benton, B.M.; Bussiere, D.; De Pascale, G.; De Vicente, J.; Dean, C.R.; et al. Topoisomerase Inhibitors Addressing Fluoroquinolone Resistance in Gram-Negative Bacteria. J. Med. Chem. 2020, 63, 7773–7816. [Google Scholar] [CrossRef]
- Komine, T.; Kojima, A.; Asahina, Y.; Saito, T.; Takano, H.; Shibue, T.; Fukuda, Y. Synthesis and Structure−Activity Relationship Studies of Highly Potent Novel Oxazolidinone Antibacterials. J. Med. Chem. 2008, 51, 6558–6562. [Google Scholar] [CrossRef] [PubMed]
- Kneller, D.W.; Li, H.; Phillips, G.; Weiss, K.L.; Zhang, Q.; Arnould, M.A.; Jonsson, C.B.; Surendranathan, S.; Parvathareddy, J.; Blakeley, M.P.; et al. Covalent narlaprevir- and boceprevir-derived hybrid inhibitors of SARS-CoV-2 main protease. Nat. Commun. 2022, 13, 2268. [Google Scholar] [CrossRef]
- Appel, N.M.; Li, S.H.; Holmes, T.H.; Acri, J.B. Dopamine D3 receptor antagonist (GSK598809) potentiates the hypertensive effects of cocaine in conscious, freely-moving dogs. J. Pharmacol. Exp. Ther. 2015, 354, 484–492. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, B.; Wang, Y.; Sun, J.; Ma, X.; Wang, G.; Fu, S.; Lin, C.; Li, Y. Three new acenaphthene derivatives from rhizomes of Musabasjoo and their cytotoxic activity. Nat. Prod. Res. 2019, 35, 1307–1312. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Alshammari, M.B.; Aly, A.A.; Sadek, K.U.; Ahmad, A.; Aziz, E.A.; El-Yazbi, A.F.; El-Agroudy, E.J.; Abdelaziz, M.E. New imidazole-2-thiones linked to acenaphythylenone as dual DNA intercalators and topoisomerase II inhibitors: Structural optimization, docking, and apoptosis studies. J. Enzym. Inhib. Med. Chem. 2024, 39, 2311818. [Google Scholar] [CrossRef]
- Periyasami, G.; Raghunathan, R.; Surendiran, G.; Mathivanan, N. Regioselective synthesis and antimicrobial screening of novel ketocarbazolodispiropyrrolidine derivatives. Eur. J. Med. Chem. 2009, 44, 959–966. [Google Scholar] [CrossRef]
- Wei, A.C.; Ali, M.A.; Yoon, Y.K.; Ismail, R.; Choon, T.S.; Kumar, R.S. A facile three-component [3+2]-cycloaddition for the regioselective synthesis of highly functionalised dispiropyrrolidines acting as antimycobacterial agents. Bioorg. Med. Chem. Lett. 2013, 23, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Filatov, A.S.; Knyazev, N.A.; Ryazantsev, M.N.; Suslonov, V.V.; Larina, A.G.; Molchanov, A.P.; Kostikov, R.R.; Boitsov, V.M.; Stepakov, A.V. A highly diastereoselective one-pot three-component 1,3-dipolar cycloaddition of cyclopropenes with azomethine ylides generated from 11H-indeno [1,2-b]-quinoxalin-11-ones. Org. Chem. Front. 2018, 5, 595–605. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Shmakov, S.V.; Bogdanov, A.A.; Ryazantsev, M.N.; Shtyrov, A.A.; Starova, G.L.; Molchanov, A.P.; Larina, A.G.; Boitsov, V.M.; et al. Concise Synthesis of Tryptanthrin Spiro Analogues with In Vitro Antitumor Activity Based on One-Pot, Three-Component 1,3-Dipolar Cycloaddition of Azomethine Ylides to Cyclopropenes. Synthesis 2019, 51, 713–729. [Google Scholar] [CrossRef]
- Pronina, Y.; Filatov, A.; Shmakov, S.; Selivanov, S.; Kryukova, M.; Spiridonova, D.; Ponyaev, A.; Stepakov, A.; Boitsov, V. Highly Efficient Synthesis of Spiro [1-azabicyclo [3.2.0]heptane] Frameworks via [3+2]-Cycloaddition. J. Org. Chem. 2025; online. [Google Scholar] [CrossRef]
- Pronina, Y.A.; Viktorov, N.B.; Selivanov, S.I.; Kornev, A.A.; Ponyaev, A.I.; Boitsov, V.M.; Stepakov, A.V. Organocatalytic Diastereoselective Synthesis of Spiro [3-azabicyclo [3.1.0]hexanes] via 1,3-Dipolar Cycloaddition of Azomethine Ylides with Cyclopropenes. Russ. J. Gen. Chem. 2024, 94, 804–823. [Google Scholar] [CrossRef]
- Filatov, A.S.; Selivanov, S.I.; Shmakov, S.V.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. An Experimental and Theoretical Study of the 1,3-Dipolar Cycloaddition of Alloxan-Derived Azomethine Ylides to Cyclopropenes. Synthesis 2022, 54, 1803–1816. [Google Scholar] [CrossRef]
- Wang, S.; Filatov, A.S.; Lozovskiy, S.V.; Shmakov, S.V.; Khoroshilova, O.V.; Larina, A.G.; Selivanov, S.I.; Boitsov, V.M.; Stepakov, A.V. Construction of Spiro [3-azabicyclo [3.1.0]hexanes] via 1,3-Dipolar Cycloaddition of 1,2-Diphenylcyclopropenes to Ninhydrin-Derived Azomethine Ylides. Synthesis 2021, 53, 2114–2132. [Google Scholar] [CrossRef]
- Filatov, A.S.; Khoroshilova, O.V.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of bis-spirocyclic derivatives of 3-azabicyclo [3.1.0]hexane via cyclopropene cycloadditions to the stable azomethine ylide derived from Ruhemann’s purple. Beilstein J. Org. Chem. 2022, 18, 769–780. [Google Scholar] [CrossRef]
- Kornev, A.A.; Shmakov, S.V.; Ponyaev, A.I.; Stepakov, A.V.; Boitsov, V.M. Study of Cytotoxicity of Spiro-Fused [3-Azabicyclo [3.1.0]hexane]oxindoles and Cyclopropa[a]pyrrolizidine-oxindoles Against Tumor Cell Lines. Pharmaceuticals 2024, 17, 1582. [Google Scholar] [CrossRef]
- Latypova, D.K.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Knyazev, N.A.; Boitsov, V.M. Identification of Spiro-Fused Pyrrolo [3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation. Int. J. Mol. Sci. 2021, 22, 11997. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, N.A.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Boitsov, V.M.; Filatova, N.A. Identification of Spiro-Fused 3-Azabicyclo [3.1.0]hexane]oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast. Int. J. Mol. Sci. 2021, 22, 8264. [Google Scholar] [CrossRef] [PubMed]
- Stepakov, A.V.; Pronina, Y.A.; Filatov, A.S.; Selivanov, S.I.; Kornev, A.A.; Kryukova, M.A.; Ponyaev, A.I.; Boitsov, V.M. Diastereoselective 1,3-dipolar cycloaddition of cyclopropenes to acenaphthenequinone azomethine ylides. Tetrahedron 2024, 151, 133792. [Google Scholar] [CrossRef]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell. Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef]
- Sousa-Squiavinato, A.C.M.; Rocha, M.R.; Barcellos-de-Souza, P.; de Souza, W.F.; Morgado-Diaz, J.A. Cofilin-1 signaling mediates epithelial-mesenchymal transition by promoting actin cytoskeleton reorganization and cell-cell adhesion regulation in colorectal cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front. Cell. Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin Enhances Cell Stiffness and Decreases Invasiveness Rate in Prostate Cancer Cells by Actin Accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef]
- Memmel, S.; Sisario, D.; Zöller, C.; Fiedler, V.; Katzer, A.; Heiden, R.; Becker, N.; Eing, L.; Ferreira, F.L.R.; Zimmermann, H.; et al. Migration Pattern, Actin Cytoskeleton Organization and Response to PI3K-, MTOR-, and Hsp90-Inhibition of Glioblastoma Cells with Different Invasive Capacities. Oncotarget 2017, 8, 45298–45310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datta, A.; Deng, S.; Gopal, V.; Yap, K.C.-H.; Halim, C.E.; Lye, M.L.; Ong, M.S.; Tan, T.Z.; Sethi, G.; Hooi, S.C.; et al. Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis. Cancers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef]
- Brayford, S.; Schevzov, G.; Vos, J.; Gunning, P. The Role of the Actin Cytoskeleton in Cancer and Its Potential Use as a Therapeutic Target. In The Cytoskeleton in Health and Disease; Schatten, H., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Dugina, V.B.; Shagieva, G.S.; Kopnin, P.B. Biological Role of Actin Isoforms in Mammalian Cells. Biochemistry 2019, 84, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W.; Mannherz, H.G.; Suck, D.; Pai, E.F.; Holmes, K.C. Atomic structure of the actin:DNase I complex. Nature 1990, 347, 37–44. [Google Scholar] [CrossRef]
- Arora, A.S.; Huang, H.-L.; Singh, R.; Narui, Y.; Suchenko, A.; Hatano, T.; Heissler, S.M.; Balasubramanian, M.K.; Chinthalapudi, K. Structural insights into actin isoforms. eLife 2023, 12, e82015. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R.; Kudryashov, D.S.; Pashkov, I.; Adisetiyo, H.; Reisler, E.; Yeates, T.O. Multiple crystal structures of actin dimers and their implications for interactions in the actin filament. Biol. Crystallogr. 2008, 64, 454–465. [Google Scholar] [CrossRef]
- Anifowose, A.; Agbowuro, A.A.; Yang, X.; Wang, B. Anticancer strategies by upregulating p53 through inhibition of its ubiquitination by MDM2. Med. Chem. Res. 2020, 29, 1105–1121. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Chessari, G.; Hardcastle, I.R.; Ahn, J.S.; Anil, B.; Anscombe, E.; Bawn, R.H.; Bevan, L.D.; Blackburn, T.J.; Buck, I.; Cano, C.; et al. Structure-Based Design of Potent and Orally Active Isoindolinone Inhibitors of MDM2-p53 Protein−Protein Interaction. J. Med. Chem. 2021, 64, 4071–4088. [Google Scholar] [CrossRef]
- Breslow, R.; Chang, H.W. Triarylcyclopropenium Ions. Synthesis and Stability in the Phenyl p-Anisyl Series. J. Am. Chem. Soc. 1961, 83, 2367–2375. [Google Scholar] [CrossRef]
- Padwa, A.; Blacklock, T.J.; Getman, D.; Hatanaka, N.; Loza, R. Photochemical transformations of small ring compounds. 95. The problem of regioselectivity in the photochemical ring-opening reaction of 3-phenyl- and 3-vinyl-substituted cyclopropenes to indenes and 1,3-cyclopentadienes. J. Org. Chem. 1978, 43, 1481–1492. [Google Scholar] [CrossRef]
- Gilbertson, R.D.; Weakley, T.J.R.; Haley, M.M. Preparation, X-ray Crystal Structures, and Reactivity of Alkynylcyclopropenylium Salts. J. Org. Chem. 2000, 65, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- White, E.H.; Winter, R.E.K.; Graeve, R.; Zirngibl, U.; Friend, E.W.; Maskill, H.; Mende, U.; Kreiling, G.; Reisenauer, H.P.; Maier, G. Kleine Ringe, 33: Versuche zur Darstellung von Diphenyltetrahedran. Chem. Berichte 1981, 114, 3906–3915. [Google Scholar] [CrossRef]
- Belyy, A.Y.; Levina, A.A.; Platonov, D.N.; Salikov, R.F.; Medvedev, M.G.; Tomilov, Y.V. Synthesis of Diazanorcaradienes and 1,2-Diazepines via the Tandem [4+2]-Cycloaddition/Retro-[4+2]-Cycloaddition Reaction between Methoxycarbonylcyclopropenes and Dimethoxycarbonyltetrazine. Eur. J. Org. Chem. 2019, 2019, 4133–4138. [Google Scholar] [CrossRef]




| Compound | MW | nHBD | nHBA | Log P | nRotB | TPSA, Å2 | NViolation | Meet Lipinski Criteria | Meet Veber Criteria | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lipinski | Veber | |||||||||
| <500 | <5 | <10 | ≤5 | <10 | <140 | <1 | 0 | Yes/No | Yes/No | |
| 1c | 453.57 | 0 | 2 | 5.54 | 3 | 20.31 | 1 | 0 | Yes | Yes |
| 1e | 503.63 | 0 | 2 | 6.13 | 3 | 20.31 | 2 | 0 | No | Yes |
| 1f | 485.57 | 0 | 4 | 4.62 | 4 | 46.61 | 1 | 0 | Yes | Yes |
| 1h | 519.63 | 1 | 3 | 5.25 | 3 | 40.54 | 2 | 0 | No | Yes |
| 1i | 521.67 | 0 | 2 | 5.95 | 3 | 45.61 | 2 | 0 | No | Yes |
| 2b | 519.67 | 1 | 2 | 6.30 | 6 | 29.10 | 2 | 0 | No | Yes |
| 2c | 519.67 | 1 | 2 | 6.30 | 5 | 29.10 | 2 | 0 | No | Yes |
| 2d | 537.71 | 1 | 2 | 6.13 | 6 | 54.40 | 2 | 0 | No | Yes |
| 2f | 569.73 | 1 | 2 | 6.82 | 6 | 29.10 | 2 | 0 | No | Yes |
| Compound | HIA, % | BBB | PPB, % | CYP_2D6 Inhibition | Solubility, mg/L | Carcinogenicity (Rat/Mouse) | Mutagenicity | hERG Inhibition |
|---|---|---|---|---|---|---|---|---|
| 1c | 100 | 2.01 | 93,41 | inhibitor | 0.0036 | negative/negative | mutagen | low risk |
| 1e | 100 | 2.53 | 97.22 | inhibitor | 0.0005 | negative/negative | non-mutagen | low risk |
| 1f | 98.17 | 5.69 | 90.55 | inhibitor | 0.0224 | negative/negative | mutagen | low risk |
| 1h | 96.97 | 1.59 | 100 | inhibitor | 0.0010 | negative/negative | mutagen | low risk |
| 1i | 98.31 | 5.44 | 100 | inhibitor | 9 × 10−5 | negative/negative | non-mutagen | low risk |
| 2b | 97.18 | 10.22 | 100 | inhibitor | 4 × 10−5 | positive/negative | non-mutagen | low risk |
| 2c | 97.18 | 5.89 | 100 | inhibitor | 5 × 10−5 | negative/negative | mutagen | low risk |
| 2d | 97.64 | 0.36 | 100 | inhibitor | 0.0001 | negative/negative | non-mutagen | low risk |
| 2f | 96.85 | 9.74 | 100 | inhibitor | 7 × 10−7 | positive/negative | non-mutagen | low risk |
| Compound | IC50, μM | Compound | IC50, μM | ||||
|---|---|---|---|---|---|---|---|
| K562 | U2OS | B16 | K562 | U2OS | B16 | ||
| 1a | >40 | >40 | 15 ± 3 | 1h | 12 ± 2 | 28 ± 7 | 4 ± 1 |
| 1b | 13 ± 2 | >40 | 10 ± 2 | 1i | >40 | >40 | 13 ± 4 |
| 1c | >40 | >40 | 11 ± 2 | 1j | >40 | >40 | 20 ± 5 |
| 1d | >40 | >40 | 15 ± 4 | 2b | 27 ± 5 | >40 | 22 ± 7 |
| 1e | >40 | >40 | 7 ± 2 | 2c | 25 ± 4 | >40 | >40 |
| 1f | 4 ± 1 | >40 | 7 ± 2 | Doxorubicin | 1 ± 0.3 | 7 ± 2 | 6 ± 1 |
| 1g | 17 ± 4 | >40 | 8 ± 2 | ||||
| # | Protein PDB ID | Ligand | MolDock Score a | Rerank Score a | HBond a | Amino Acid Residue ID (Target-Binding Cleft) b |
|---|---|---|---|---|---|---|
| 1 | 8DNH | 1b | −153.95 | −97.868 | 0 | Ala 107, Arg 115, Asn 110, Cys 373, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 2 | 8DNH | 1c | −159.21 | −100.86 | 0 | Ala 107, Arg 115, Asn 110, Cys 373, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 3 | 8DNH | 1e | −160.79 | −101.22 | 0 | Arg 115, Arg 371, Asn 110, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 4 | 8DNH | 1f | −157.07 | −99.393 | −5.088 | Ala 107, Arg 115, Asn 110, Cys 373, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 5 | 8DNH | 1g | −152.95 | −98.533 | −1.274 | Ala 107, Arg 115, Asn 110, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 6 | 8DNH | 1i | −163.94 | −99.137 | 0 | Ala 107, Arg 115, Arg 371, Asn 110, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 369, Glu 194, Lys 190, Thr 193 |
| 7 | 8DNH | 2b | −177.53 | −105.71 | 0 | Ala 107, Arg 115, Arg 371, Asn 110, Cys 373, Glu 106, His 172, His 370, Ile 135, Ile 174, Leu 109, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Val 133, Val 369, Glu 194, Lys 190, Thr 193 |
| 8 | 8DNH | 2c | −172.68 | −93.067 | −2.874 | Ala 107, Ala 134, Ala 169, Arg 115, Asn 110, Cys 373, Glu 106, His 370, Ile 135, Ile 174, Leu 109, Leu 170, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Tyr 132, Val 133, Val 138, Val 162, Val 42 |
| 9 | 8DNH | 2d | −176.60 | −115.24 | −0.799 | Ala 107, Ala 134, Ala 169, Arg 115, Asn 110, Cys 373, Glu 106, His 370, Ile 135, Ile 174, Leu 109, Leu 170, Lys 112, Phe 374, Pro 108, Pro 111, Pro 171, Tyr 132, Val 133, Val 138, Val 162, Val 42 |
| # | Protein PDB ID | Ligand | MolDock Score a | Rerank Score a | HBond a |
|---|---|---|---|---|---|
| 1 | 7BJ6 | TVK* | −159.09 | −108.74 | −2.261 |
| 2 | 7BJ6 | 1b | −142.49 | −88.908 | 0 |
| 3 | 7BJ6 | 1c | −134.28 | −83.325 | 0 |
| 4 | 7BJ6 | 1e | −138.17 | −77.631 | 0 |
| 5 | 7BJ6 | 1f | −132.48 | −77.205 | 0 |
| 6 | 7BJ6 | 1g | −130.21 | −76.526 | 0 |
| 7 | 7BJ6 | 1i | −135.84 | −76.857 | 0 |
| 8 | 7BJ6 | 2b | −159.08 | −97.295 | −0.764 |
| 9 | 7BJ6 | 2c | −148.45 | −74.777 | 0 |
| 10 | 7BJ6 | 2d | −158.34 | −85.999 | 0 |
| 11 | 7BJ6 | TUZ | −163.89 | −111.47 | −2.918 |
| 12 | 7BIR | TUZ* | −166.93 | −112.48 | −3.878 |
| 13 | 7BIR | 1b | −139.12 | −85.431 | 0 |
| 14 | 7BIR | 1c | −135.18 | −81.189 | 0 |
| 15 | 7BIR | 1e | −138.72 | −72.773 | 0 |
| 16 | 7BIR | 1f | −134.55 | −69.128 | 0 |
| 17 | 7BIR | 1g | −137.06 | −82.389 | 0 |
| 18 | 7BIR | 1i | −136.86 | −68.419 | 0 |
| 19 | 7BIR | 2b | −158.62 | −96.458 | 0 |
| 20 | 7BIR | 2c | −151.68 | −81.008 | 0 |
| 21 | 7BIR | 2d | −162.68 | −94.685 | 0 |
| 22 | 7BIR | TVK | −145.61 | −96.633 | −5.341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornev, A.A.; Shmakov, S.V.; Gryschenko, A.M.; Pronina, Y.A.; Ponyaev, A.I.; Stepakov, A.V.; Boitsov, V.M. Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2H)-one and Aceanthrylene-1(2H)-one Fragments Against Tumor Cell Lines. Int. J. Mol. Sci. 2025, 26, 3474. https://doi.org/10.3390/ijms26083474
Kornev AA, Shmakov SV, Gryschenko AM, Pronina YA, Ponyaev AI, Stepakov AV, Boitsov VM. Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2H)-one and Aceanthrylene-1(2H)-one Fragments Against Tumor Cell Lines. International Journal of Molecular Sciences. 2025; 26(8):3474. https://doi.org/10.3390/ijms26083474
Chicago/Turabian StyleKornev, Anton A., Stanislav V. Shmakov, Alexandra M. Gryschenko, Yulia A. Pronina, Alexander I. Ponyaev, Alexander V. Stepakov, and Vitali M. Boitsov. 2025. "Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2H)-one and Aceanthrylene-1(2H)-one Fragments Against Tumor Cell Lines" International Journal of Molecular Sciences 26, no. 8: 3474. https://doi.org/10.3390/ijms26083474
APA StyleKornev, A. A., Shmakov, S. V., Gryschenko, A. M., Pronina, Y. A., Ponyaev, A. I., Stepakov, A. V., & Boitsov, V. M. (2025). Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2H)-one and Aceanthrylene-1(2H)-one Fragments Against Tumor Cell Lines. International Journal of Molecular Sciences, 26(8), 3474. https://doi.org/10.3390/ijms26083474






