Abstract
Metabolic diseases, including cardiovascular diseases, type 2 diabetes mellitus (T2DM), osteoporosis, and non-alcoholic fatty liver disease (NAFLD), constitute a major global health burden associated with chronic morbidity and mortality. Lactate, once considered as a metabolic byproduct, has emerged as a key regulator of cellular reprogramming through lactylation, a novel post-translational modification (PTM) that dynamically couples metabolic flux to chromatin remodeling. Lactylation exerts dual regulatory roles as a signaling molecule via GPR81/GPR4-mediated pathways and as a substrate for the covalent modification of histones and metabolic enzymes. Pathologically, chronic hyperlactatemia suppresses mitochondrial biogenesis, driving metabolic cardiomyopathy through the epigenetic silencing of oxidative metabolism genes. Conversely, exercise-induced lactate surges transiently enhance insulin sensitivity via AMPK/PGC-1α/GLUT4 signaling, resolve inflammation through GPR81-mediated M2 macrophage polarization, and restore mitochondrial function via lactylation-dependent pathways. This review delineates lactylation as a spatiotemporal rheostat: chronic dysregulation perpetuates metabolic disorders, whereas acute exercise-mediated lactylation remodels transcriptional networks to restore metabolic homeostasis. Future research should integrate multiomics to clarify lactylation’s spatiotemporal dynamics, tissue-specific thresholds, metabolism–immunity interactions, and metabolic–epigenetic crosstalk for the precision management of metabolic diseases.
1. Introduction
The escalating global burden of metabolic diseases—including cardiovascular disorders, T2DM, obesity, and NAFLD—poses a critical public health challenge, with prevalence doubling between 1990 and 2020 [1]. Although pharmacological interventions remain central to disease management, emerging evidence underscores the pivotal roles of metabolic reprogramming and epigenetic regulation in disease pathogenesis. Among these mechanisms, lactate, once dismissed as a mere glycolytic byproduct, has emerged as a pleiotropic signaling molecule that coordinates systemic energy homeostasis through receptor-mediated pathways and a novel PTM: lysine lactylation (Kla) [2,3].
Lactate’s dual role as a metabolic intermediate and a driver of epigenetic reprogramming via lactylation positions it at the nexus of immunometabolic crosstalk. Beyond its role in redox balancing, lactate drives transcriptional and functional reprogramming via lactylation, which dynamically modifies histones (e.g., H3K18la) and nonhistone substrates (e.g., PKM2 and hypoxia-inducible factor 1-alpha (HIF-1α)) to regulate inflammation, mitochondrial plasticity, and cellular differentiation [3,4,5]. Similarly, lactate–G-protein-coupled receptor (GPCR) signaling (e.g., GPR81/GPR4) modulates macrophage polarization, T cell activation, and endothelial function, with concentration-dependent effects on disease progression [6,7]. Paradoxically, physiological lactate gradients (<4.5 mM) support tissue repair and angiogenesis, whereas chronic hyperlactatemia (>5 mM) exacerbates insulin resistance and vascular remodeling, underscoring its context-dependent dual roles [8,9].
Exercise, a cornerstone of non-pharmacological intervention, remodels lactate flux and lactylation dynamics to counteract metabolic dysregulation. Transient exercise-induced hyperlactatemia (8–12 mM) enhances insulin sensitivity via AMPK/PGC-1α/GLUT4 signaling, resolves chronic inflammation through GPR81-mediated macrophage M2 polarization, and restores mitochondrial oxidative capacity [10,11]. Conversely, chronic lactate accumulation in sedentary states perpetuates pathological lactylation patterns, such as H3K18la-driven PD-1 upregulation in tumor-infiltrating lymphocytes and monocarboxylate transporter 1 (MCT1)-dependent adipocyte apoptosis [5,12]. Despite these advances, critical gaps persist in elucidating how spatiotemporal lactylation dynamics orchestrate exercise-induced metabolic adaptations and disease-specific transcriptional reprogramming, particularly in tissue-specific contexts.
This review synthesizes recent breakthroughs in lactylation biology, focusing on its mechanistic roles in glucose/lipid metabolisms, immunity regulation, and tissue remodeling across cardiometabolic disorders. We further explore exercise-mediated lactate–lactylation networks as a therapeutic axis, emphasizing their potential to rewire transcriptional landscapes and mitigate metabolic inflexibility. By delineating the interplay among lactate dynamics, epigenetic reprogramming, and exercise physiology, this work aims to bridge translational gaps and identify precision-targeted strategies for metabolic syndrome management.
2. Overview of Lactate and Metabolic Diseases
Lactate, traditionally viewed as a glycolytic byproduct, has emerged as a central hub coordinating metabolic adaptation across physiological systems [13]. Although oxidative metabolism predominantly directs pyruvate into the mitochondrial tricarboxylic acid cycle in normoxia, hypoxic stress or mitochondrial insufficiency triggers LDHA-mediated lactate generation—a process now recognized as integral to cellular redox balancing and epigenetic regulation via lactylation [14].
The skeletal muscle–liver lactate shuttle exemplifies systemic metabolic integration. High-intensity exercise induces transient muscular lactate accumulation via anaerobic glycolysis (generating ATP 100-fold faster than in oxidative phosphorylation), while endurance training enhances hepatic Cori cycle activity—converting lactate to glucose at energy costs equivalent to six ATPs per molecule recycled [2].
Within the central nervous system (CNS), the astrocyte–neuron lactate shuttle (ANLS) operates as a neurovascular coupling mechanism [15]. Cognitive activation triggers astrocytic glycogenolysis, producing lactate, which fuels neuronal long-term potentiation via monocarboxylate transporter 2 (MCT2)-dependent uptake [16]. Recent studies have revealed that this metabolic coupling extends to immunity regulation: microglial GPR81 receptors sense extracellular lactate gradients, suppressing neuroinflammation through CREB-mediated anti-inflammatory cytokine production [17,18]. Notably, neuronal activity induces histone lactylation at plasticity-related gene loci [19], suggesting that lactate may directly bridge energy metabolism and epigenetic memory formation.
Gut-microbiota-derived D-lactate adds another dimension to lactate’s systemic signaling. Commensal bacteria generate stereospecific D-lactate isomers that strengthen intestinal barrier functions through complementary mechanisms: HDAC3 lactylation in colonic epithelia enhances tight junction assembly [20,21]. However, dysbiosis-induced D-lactate overproduction may paradoxically impair barrier integrity via excessive matrix metalloproteinase activation [22], illustrating the delicate balance in lactate-mediated mucosal homeostasis.
The pathophysiological duality of lactate manifests most strikingly in immunometabolic crosstalk. Physiological lactate gradients support tissue repair through HIF-1α/VEGF-driven angiogenesis [23] and PPARα-mediated fatty acid oxidation. Conversely, chronic lactate accumulation creates an immunosuppressive niche via two synergistic pathways: (1) M2 macrophage polarization through PKM2 lactylation [4] and (2) PD-1 upregulation in tumor-infiltrating lymphocytes via histone H3K18 lactylation [5,6].
2.1. Intercellular Signaling
It has been reviewed that the lactate shuttle theory (LST) elucidates the process of lactate transport between cells, which has energy transfer and signaling functions [2]. Lactate is transported across cells via monocarboxylate transporters (MCTs), which regulate various physiological and pathological cellular processes [23]. According to Felmlee et al., MCT belongs to the solute carrier (SLC) transporter protein family, which consists of 14 different proteins (MCT1-MCT14) [24] (Table 1). These proteins facilitate the transport of monocarboxylic acids, such as lactate, pyruvate, and ketone bodies, across cell membranes [25].

Table 1.
Functional description table of MCT subtypes.
In contemporary scientific discourse, lactate is recognized as a signaling molecule capable of exerting its biological functions through three major classes of G-protein-coupled receptors (GPCRs) [34]. The primary known lactate receptors include GPR81, GPR132, and the proton-sensing GPR4. GPR81 exhibits tissue-specific expression patterns, with high abundance in adipocytes, neurons, and myeloid cells, playing pivotal roles in both physiological and pathological processes [23]. Adipocyte GPR81 activation inhibits hormone-sensitive lipase (HSL) phosphorylation via cAMP/PKA signaling, thereby reducing lipolysis at physiological lactate concentrations [8]. In immunity regulation, GPR81 exerts anti-inflammatory effects by blocking IκBα degradation to prevent NF-κB nuclear translocation and disrupting ASC oligomerization required for NLRP3 inflammasome assembly [35]. GPR81 agonists suppress fasting plasma free-fatty-acid levels in rodents and improve insulin sensitivity in mouse models of insulin resistance and diabetes, highlighting its therapeutic potential [36].
GPR132 is a lactate-sensitive GPCR that plays dual roles in metabolic diseases by modulating energy metabolism and inflammatory responses [37]. This leads to the suppression of lipolysis and promotion of lipogenesis in adipocytes while driving anti-inflammatory M2 polarization in macrophages, thereby improving insulin sensitivity [37].
GPR4, a proton-sensing GPCR highly expressed in vascular endothelial cells, orchestrates vascular inflammation through pH-dependent activation cascades [38]. At pathological pH levels, lactate synergizes with protons to stabilize GPR4-β–arrestin complexes, amplifying downstream signaling in various pathological states, including inflammation and ischemia [7]. GPR4 also induces ER stress and apoptosis in endothelial cells and promotes angiogenesis by regulating endothelial cells’ tubule formation, migration, and proliferation under acidic conditions [39]. Additionally, lactate activates dendritic cells and modulates the immune response through paracrine signaling [7]. Emerging evidence reveals crosstalk between lactate receptors and epigenetic modifications. Fam172a modulates POMC neuronal activity through H4K12-lactylation-dependent chromatin remodeling at neuropeptide gene loci (AgRP and POMC), with hypothalamic H4K12la levels correlating inversely with bodyweight in diet-induced obesity models [40].
2.2. Lactate Regulates Inflammation and Immune Responses
Lactate has been shown to modulate the functions of immune cells via multiple pathways [41]. For instance, lactate inhibits the activation of proinflammatory macrophages and promotes their conversion to an anti-inflammatory phenotype, thereby mitigating the inflammatory response [42]. Additionally, lactate inhibits metabolic reprogramming and proinflammatory responses in macrophages via receptors such as GPR81 [43,44]. Moreover, studies have shown that lactate can inhibit T-cell overactivation, thereby exerting an immunomodulatory effect on chronic inflammation [6].
Lactate exhibits a dual role in the inflammatory microenvironment. It serves as an energy source to support the metabolic needs of immune cells and regulates gene expression and protein function via mechanisms such as lactate modification, thereby influencing the intensity and duration of the inflammatory response [3]. Moreover, lactate contributes to tissue repair by regulating immune cell functions and promoting a balance between inflammation and repair [45]. In the context of inflammation, lactate accumulation can inhibit mitochondrial function, thereby prompting a rapid shift toward glycolysis to maintain cellular energy production and ensure cellular survival and functions [45].
Lactate significantly influences disease development and therapeutic efficacy. Lactate’s roles in the microenvironments of chronic inflammatory diseases have led to novel therapeutic concepts [46]. For instance, modulating lactate metabolism and regulating lactate levels have been proposed as potential therapeutic strategies for inflammatory diseases [46].
In summary, lactate has multiple critical roles in organismal metabolism, both as a glycolysis end product and as a signaling molecule regulating intercellular signaling, inflammation, and immune responses via GPCRs. The lactate shuttle hypothesis indicates that lactate regulates cellular physiological and pathological processes via the intercellular shuttle process of MCTs. Additionally, lactate fulfills a dual role in the inflammatory microenvironment by serving as an energy source for immune cells and regulating gene expression and protein function via lactate modification, thereby impacting the intensity and duration of inflammatory responses.
2.3. Association of Lactate with Metabolic Diseases
Lactate exhibits concentration-dependent duality in metabolic regulation, with its pathophysiological impact determined by temporal–spatial dynamics and receptor activation thresholds.
2.3.1. T2DM
Abnormal lactate levels are associated with dysregulated glycemic control, affecting insulin sensitivity [47]. In the management of T2DM, it is imperative to monitor lactate levels and adjust the intervention program accordingly. A study found that patients with T2DM exhibited slightly elevated blood lactate levels [48]. These slightly elevated lactate levels may be associated with a relative deficiency in insulin secretion, leading to decreased mitochondrial pyruvate utilization and increased glycolysis, especially when blood glucose is elevated, potentially resulting in increased blood lactate production [49]. Slightly elevated lactate levels have been shown to exacerbate insulin resistance and be detrimental to long-term glycemic control [12].
Exercise constitutes a pivotal element in the management of T2DM [30]. Exercise interventions modulate lactate metabolism through:
Acute effects: Post-exercise lactate elevation, typically reaching levels of 8–12 mM, is associated with enhanced insulin sensitivity. This occurs via the activation of the AMPK/PGC-1α pathway, which mediates the translocation of glucose transporter type 4 (GLUT4) [50,51]. AMP-activated protein kinase (AMPK), an energy-sensing enzyme, is activated in response to exercise-induced metabolic stress, including increases in lactate levels [52]. Activated AMPK then phosphorylates downstream targets [53]. PGC-1α, a key regulator of mitochondrial biogenesis and energy metabolism, is also regulated by AMPK [54]. GLUT4 is a glucose transporter protein, and its translocation to the cell membrane allows for increased glucose uptake into cells, thereby enhancing insulin sensitivity [55].
Chronic adaptation: Regular moderate-intensity aerobic and resistance exercises have been shown to reduce blood glucose levels, decrease insulin resistance, improve lactate metabolism, and increase the body’s tolerance to lactate in patients with T2DM [56].
2.3.2. Obesity
Lactate is a double-edged sword in adipose remodeling. Obesity, a complex metabolic disorder, is intricately linked to dysregulated lactate metabolism. Studies have demonstrated that adipose tissue hypoxia contributes to elevated lactate levels in obese individuals [57,58]. Dynamic fluctuations in the lactate concentration critically influence metabolic homeostasis and disease progression through multifaceted mechanisms.
Slightly elevated lactate levels (<4.5 mM) can have beneficial effects on metabolic regulation through three pathways: (1) Enhanced glucose metabolism: Lactate stimulates glucose oxidation, ameliorating glucose–lipid metabolic imbalances [57]; (2) Suppression of lipolysis: It downregulates adipose tissue lipolysis, thereby reducing free-fatty-acid release [59]; and (3) Maintenance of adipocyte homeostasis: Lactate prevents pathological adipocyte hypertrophy and functional impairment [60]. Furthermore, lactate activates the GPR81 receptor to promote extracellular efflux [61], mitigating intracellular accumulation. This mechanism inhibits adipocyte apoptotic signaling and inflammatory cytokine secretion, ultimately enhancing systemic insulin sensitivity.
At concentrations exceeding 4.5 mM, lactate paradoxically exacerbates metabolic disturbances by aggravating systemic inflammation and insulin resistance [62] and disrupting adipose tissue functionality by impairing the thermogenic and lipolytic balance [63].
Adipose tissue is the central hub of lactate homeostasis. Adipose tissue accounts for >50% of the resting-state lactate production in humans [64], establishing its pivotal role in systemic lactate dynamics. Mechanistically, the disruption of monocarboxylate transporter 1 (MCT1) in adipocytes induces intracellular lactate accumulation, triggering apoptotic cascades and proinflammatory cytokine release [12,65]. These pathological changes exacerbate obesity-associated insulin resistance, highlighting the tissue-specific regulatory importance of lactate flux and providing evidence that lactate is a key mediator of obesity-related insulin resistance [66]. The pharmacological modulation of adipose lactate transport, particularly through MCT1 or GPR81 targeting, may represent a novel therapeutic strategy for metabolic dysfunction in obesity.
Consequently, lactate plays multifaceted and pivotal roles in a broad spectrum of metabolic diseases, and fluctuations in its concentration have substantial impacts on disease progression and therapeutic outcomes. Therefore, in the management of metabolic diseases, it is imperative to monitor lactate levels and adjust intervention programs accordingly.
3. Mechanisms of Lactylation in Metabolic Diseases
3.1. Lactylation-Dependent Gene Regulation
Lactylation, a recently characterized metabolite-driven post-translational modification, regulates gene expressions through dual mechanisms.
3.1.1. Direct Transcriptional Control
Pan-histone profiling may identify multiple conserved lactylation sites, with H3K18la strongly correlating with transcriptional activation [3]. Neural stem cells (NSCs) exhibit reduced neurogenesis via H4K12la-mediated p53 stabilization, highlighting lactylation’s role in cellular plasticity [67].
3.1.2. Indirect Chromatin Remodeling
Lactylation modulates transcription factors and enzymes to reshape chromatin landscapes. For example, YY1 lactylation increases in retinal microglia, promoting the activation of microglia and transcription of inflammatory genes, thus aggravating autoimmune uveitis [68].
3.1.3. Dynamic Interactions Between Lactylation and Other Epigenetic Modifications
As a newly emerging PTM, lactylation exhibits dynamic interaction mechanisms, with classical modifications, such as acetylation and methylation, in the epigenetic regulatory network. It has been found that lactylation and acetylation have highly similar distribution characteristics in the genome, sharing some modification enzyme systems (e.g., p300 and HDAC1-3) and regulating gene expressions through the competitive modification of lysine residues. In the process of liver fibrosis, hexokinase-2 (HK2) inhibits the transcriptional activation of fibrosis-suppressing genes by promoting lactylation at histone H3K18 sites rather than acetylation, which reveals the competitive substitution mechanism of lactylation at acetylation modification sites [69,70]. This competitive relationship may be because of substrate bias shifts induced by changes in the metabolic microenvironment—when intracellular lactate levels rise, lactoacyl-CoA production increases, prompting the modifying enzyme to catalyze lactylation over acetylation [71]. However, the interaction between the two is significantly cell-type dependent: in anoxic HeLa cells, the lactylation level increases as the acetylation level decreases, while in LPS-activated macrophages, the two synergize to promote HMGB1 modification and exosome release [72,73], suggesting that metabolic reprogramming can restore the modification network’s balance by regulating the substrate’s availability and enzymes’ kinetic parameters.
There is a synergistic regulation between lactylation and methylation. In the process of DNA damage repair, H2BK123ub (the ubiquitination modification of histone H2B) was found to promote the methylations of H3K4, H3K46, and H3K79, which are essential for DNA damage repair. At the same time, some studies have shown that there is a correlation between lactylation and methylation such that in some cases, lactylation may affect the level of the methylation modification, but its specific mechanism needs to be further studied [74].
As the hub of the metabolism–epigenetic network, lactylation enables the dynamic coupling of the cellular metabolic state and gene expression program. During the polarization of macrophages, lactate produced by enhanced glucose metabolism drives phenotypic transition to the repair type by promoting histone lactylation in the promoter region of repair genes, such as Arg1 [72]. This metabolic signal transduction depends on the homeostasis of lactoacyl-CoA. When metabolic parameters, such as glucose and oxygen levels in the microenvironment, change, the lactylation modification level can respond sensitively and then reprogram the gene expression profile by regulating the chromatin accessibility and transcription factor activity [71,75]. It is worth noting that the hub role of the lactylation network is reflected not only in its regulation of classical PTM but also in its ability to integrate multidimensional biological information as a “metabolic sensor”; through competitive inhibition, collaborative enhancement, and other mechanisms, lactylation modifications translate metabolic flux changes into precise epigenetic instructions. Finally, the precise control of the cellular fate determination and pathophysiological processes is realized.
3.2. The Roles of Lactylation in Inflammation and Immune Responses
Lactylation serves as a metabolic checkpoint in inflammatory responses through dual regulatory mechanisms:
Macrophage Polarization: The lactylation of PKM2 at K62 promotes M2 polarization by enhancing tetramer formation, resolving inflammation via anti-cytokine production [4].
T-Cell Functional Reprogramming: In rheumatoid arthritis, lactate has been shown to prolong chronic inflammation by regulating T-cell function and promoting the production of inflammatory factors [76].
Inflammatory Microenvironment Crosstalk: Collectively, the critical roles of lactate homeostasis, lactate shuttling, and lactylation in acute and chronic inflammatory responses are highlighted by the inflammatory microenvironment [45].
3.3. Interaction of Lactylation with Metabolic Pathways
3.3.1. Lactylation and Glucose Metabolism
Lactylation establishes a bidirectional loop between glycolysis and epigenetics. Lactate-derived lactyl-CoA facilitates nuclear translocation, activating glycolytic genes (e.g., PKM2) to amplify the glucose flux [77]. Neuronal lactate shuttling via LDH1 sustains synaptic ATP during hypoxia, illustrating lactylation’s role in compartmentalized energy metabolism [78]. This metabolic flexibility suggests that lactylation may serve as an evolutionarily conserved mechanism for optimizing energy substrate utilization under varying oxygen tensions. Systemically, lactylation fine tunes insulin sensitivity through PI3K/Akt pathway modulation, linking cellular metabolism to whole-body glucose homeostasis [79]. Lactylation, with its dual regulatory capacity of simultaneously governing cellular metabolism and systemic responses, is positioned as a critical node in the pathogenesis of metabolic diseases.
3.3.2. Lactylation and Lipid Metabolism
Lactylation synchronizes lipogenesis with glycolytic NADPH production. Hepatic FASN lactylation may potentially stabilize its catalytic domain and contribute to lipid droplet biogenesis in NAFLD [5,80]. Paradoxically, mitochondrial CPT1A enhances fatty acid oxidation acutely but promotes lipid storage chronically, demonstrating context-dependent regulation [81]. These opposing effects underscore lactylation’s role as a metabolic buffer system.
3.3.3. Lactylation and Energy Metabolism
In hypoxia, lactylation shifts energy production from oxidative phosphorylation (OXPHOS) to glycolysis via PDH inhibition, preserving the redox balance [82]. In cardiomyocytes, acute lactylation stabilizes HIF-1α during ischemia, enhancing glycolytic gene expression to maintain ATP production [23]. Conversely, chronic hyperlactatemia suppresses mitochondrial biogenesis, driving metabolic cardiomyopathy through the epigenetic silencing of oxidative metabolism genes [83].
3.4. Tissue-Specific Lactylation Dynamics
3.4.1. Liver: NASH and Liver Fibrosis
Studies have shown that lactylation can regulate the expressions of glycolysis-related genes in liver cells and affect energy metabolism in the liver [3,84,85,86]. For example, E3 ubiquitin ligase (TRIM56) slows NAFLD progression by ubiquitinating FASN, a key protein in fatty acid synthesis. TRIM56 is downregulated in human NAFLD liver tissue and diet-induced mouse NAFLD liver tissue, while its gene level remains unchanged, and changes in TRIM56 protein expression occur only in liver cells [87]. These findings shed light on the potential role of lactate modification in the development of NASH, possibly by regulating the transcriptions and protein translations of specific genes involved in the progression of the disease. In liver fibrosis, lactylation affects the liver structure by regulating collagen expression. Studies have shown that HK2-induced lactate promotes histone lactylation, which controls stellate cellular activation and leads to liver fibrosis. The stellate-cell-specific or systemic deletion of HK2 to inhibit H3K18la can mitigate stellate cellular activation and liver fibrosis [69,70,88] (Table 2).
3.4.2. Heart: Ischemic Remodeling
Lactylation plays a crucial role in enhancing post-infarction repair in patients with metabolic disorders, such as diabetes, complicated by myocardial infarction. This is achieved through histone lactylation (e.g., VEGFA) and macrophage modulation, which help to establish immunity homeostasis and activate the cardiac repair process in a timely manner [89] (Table 2). Exercise-induced MECP2K271la may attenuate atherosclerosis in patients with metabolic disorders by suppressing vascular adhesion molecules (e.g., ICAM-1) and promoting anti-inflammatory pathways (Table 2). This finding provides a potential therapeutic approach for metabolic-syndrome-related cardiovascular diseases [90,91,92].
3.4.3. Bone: Osteoporosis
Lactate modification activates osteogenesis-related genes, including the collagen type I alpha 2 chain (COL1A2), cartilage oligomeric matrix protein (COMP), ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) [93], and transcription factor 7-like 2 (TCF7L2) [94]. These genes are essential for osteoblast differentiation and functions. Conversely, it inhibits osteoclastogenesis through LDHA suppression, reducing bone resorption [95,96]. Consequently, this dual regulation maintains bone homeostasis, with dysregulation contributing to osteoporosis.

Table 2.
Lactylation sites of the key metabolic organs.
Table 2.
Lactylation sites of the key metabolic organs.
| Organ | Target(s) | Mechanism(s) of Action | Reference(s) |
|---|---|---|---|
| Liver | H3K18la and H3K9la | Studies have shown that HK2-induced lactate promotes histone lactylation, which controls stellate cellular activation and leads to liver fibrosis. The stellate-cell-specific or systemic deletion of HK2 to inhibit H3K18la can mitigate stellate cellular activation and liver fibrosis. The inhibition of H3K9la can inhibit HCC development. | [69,70,88] |
| Heart | VEGFA, H3K9la, and MECP2K271la | Histone lactylation (e.g., VEGFA) helps to establish immunity homeostasis and activate the cardiac repair process in a timely manner. A feedback loop between H3K9la and HDAC2 drives VEGF-induced angiogenesis. Exercise-induced MECP2K271la may attenuate atherosclerosis in patients with metabolic disorders by suppressing vascular adhesion molecules (e.g., ICAM-1) and promoting anti-inflammatory pathways. | [89,90,91,92] |
| Adipose | APOC2-K70la | Lactate stabilizes APOC2 and promotes extracellular lipolysis by enhancing lactylation at the K70 site. | [97] |
| Brain | SNAP91 | Lactate-mediated lactylation of synaptosomal SNAP91 in the prefrontal cortex enhances synaptic plasticity, mitigating anxiety-like behaviors. | [98] |
In summary, lactylation integrates metabolic flux with epigenetic and immunity regulations, acting as a spatiotemporal rheostat in metabolic diseases. Future studies should prioritize tissue-specific thresholds and the therapeutic targeting of lactylation dynamics to address disease-specific metabolic inflexibility (Figure 1).
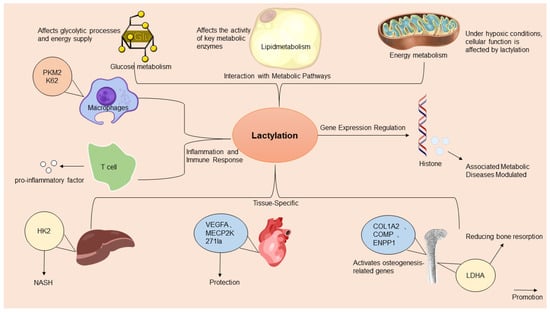
Figure 1.
A comprehensive overview of the multifaceted mechanisms of action of lactylation in metabolic diseases. Lactylation, a significant epigenetic modification, influences metabolic diseases by regulating gene expressions, metabolic pathways, inflammation, tissue-specific lactylation dynamics, and immune responses. It directly affects gene transcription by modifying histones and transcription factors. Adapted from SciDraw (www.scidraw.io) under CC BY 4.0.
4. Lactate–Lactylation-Mediated Regulation of Exercise in Metabolic Diseases
The dynamic interplay among exercise, lactate metabolism, and lactylation modifications serves as a pivotal axis in understanding exercise-induced protection against metabolic disorders. During high-intensity exercise, rapid glycolysis generates the accumulation of transient lactate (5–10 mM) [8], which functions as both a metabolic intermediate and a signaling molecule. This lactate surge activates GPR81, a lactate-sensitive receptor, to stimulate angiogenesis [99], promote adipose tissue browning [100] (Figure 2), and enhance immunity modulation by shifting macrophage polarization from proinflammatory M1 to anti-inflammatory M2 phenotypes [11,101] (Figure 2). Conversely, moderate-intensity exercise (2–4.5 mM) improves the mitochondrial oxidative capacity and reduces chronic lactate overproduction, thereby enhancing metabolic flexibility and lactate clearance. Systematic reviews highlight that regular moderate-intensity exercise lowers baseline blood lactate levels and increases the lactate threshold in T2DM patients, improving their exercise tolerance and glycemic control [102]. Critically, exercise-induced lactate dynamics regulate lactylation, where lactate-derived acyl groups modulate protein functions. Lactylation fine tunes metabolic enzymes, such as PDH and LDHA, balancing glycolysis and oxidative phosphorylation [103]; stabilizes hypoxia-inducible factor HIF-1α to adapt to oxygen level fluctuations [81]; and suppresses inflammatory gene transcription in adipose tissue, thereby alleviating obesity-related metabolic dysfunction [90].
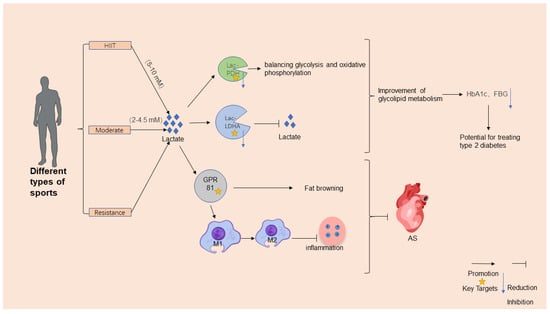
Figure 2.
Mechanisms for exercise’s regulatory effects on metabolic diseases. Exercise positively regulates metabolic diseases by enhancing lactate metabolism and lactylation. High-intensity or hypoxic exercise increases the muscles’ production of lactate, which is converted to glucose via hepatic gluconeogenesis. Lactylation, modified by exercise, impacts glucose and energy metabolisms, influencing metabolic disease development. Exercise protects against metabolic diseases through mechanisms such as improved insulin sensitivity, enhanced lipid metabolism, reduced inflammation, and optimized mitochondrial and nervous system functions. Adapted from SciDraw (www.scidraw.io) under CC BY 4.0.
Exercise further combats metabolic diseases through multilayered mechanisms. Aerobic exercise, particularly high-intensity interval training (HIIT), enhances insulin sensitivity by upregulating mitochondrial biogenesis and glucose transporter GLUT-4 expression [104], while moderate-intensity aerobic exercise boosts free-fatty-acid oxidation, reducing plasma triglyceride levels and improving lipid profiles [105]. Resistance training activates the PI3K/Akt pathway to enhance insulin signaling and the muscles’ glucose uptake [106] while also increasing muscle mass to elevate the basal metabolic rate and caloric expenditure [107]. Both exercise modalities reduce systemic inflammation by lowering proinflammatory cytokine levels (e.g., TNF-α and IL-6) and promoting anti-inflammatory macrophage polarization [4,108].
Notably, exercise-induced lactate not only modulates peripheral metabolism but also exerts neuroprotective effects. For instance, the lactate-mediated lactylation of synaptosomal SNAP91 in the prefrontal cortex enhances synaptic plasticity, mitigating anxiety-like behaviors [98] (Table 2), while lactate–lactylation networks regulate immunity–metabolism crosstalk in cardiovascular diseases via mitochondrial modulation [3]. However, lactate dynamics display context-dependent effects. Emerging evidence reveals that excessive exercise induces muscle-derived lactate-rich small extracellular vesicles (sEVs) containing FBXO2 and lactylated SORBS3 proteins. These sEVs activate hepatic stellate cells through systemic circulation, triggering pro-fibrotic transformation and inflammatory pathway activation, ultimately accelerating hepatic fibrogenesis [109]. This pathological process contrasts sharply with the transient lactylation events during moderate exercise, highlighting the importance of exercise intensity modulation.
Clinically, chronic hyperlactatemia in diabetes [110] and hypoxia [111] exacerbates insulin resistance through sustained pathological lactylation, whereas controlled exercise interventions lower HbA1c, improve lipid profiles [112], and enhance mitochondrial biogenesis [113]. The differential outcomes underscore lactate’s role as a metabolic rheostat: physiological fluctuations promote homeostasis, while chronic elevation or acute overload drives pathology. Targeting lactylation dynamics and sEV generation may, thus, provide novel therapeutic strategies for exercise-associated organ injury.
There were significant physiological/pathological differences in the dynamic characteristics and metabolic regulation of lactate modification [114]. During acute stimuli, such as exercise, a surge in lactate levels can rapidly induce lactate modification of both histone (e.g., H3K18) and non-histone (e.g., PKM2) proteins, with the peak appearing within 30 min and rapidly fading with prolonged lactate metabolism. This short-term modification enhances insulin sensitivity by enhancing glycolysis [90]. Conversely, long-term modifications, triggered by persistently high lactylation in chronic diseases, inhibit mitochondrial biogenesis genes, leading to mitochondrial dysfunction and exacerbating insulin resistance. The study suggests that lactate modification has a time threshold effect: the transient modification of an acute stimulus has a metabolic protective effect, while continuous modification turns to pathological processes. At present, it is necessary to analyze its dynamic regulation mechanism with multiomic technology to reveal the key nodes of metabolic homeostasis transformation.
5. Potential of Lactylation in the Treatment of Metabolic Diseases
5.1. Potential of Lactylation as a Therapeutic Target
Emerging evidence positions protein lactylation as a pivotal post-translational modification with profound implications for metabolic disease pathogenesis and therapeutic development. The dynamic regulation of histone lactylation through NSC lactate homeostasis establishes a critical interface between cellular metabolism and tissue regeneration. Mechanistically, this process modulates the MDM2-p53 signaling axis [67], suggesting that lactylation serves as a metabolic rheostat by coordinating cellular proliferation and metabolic adaptation. This regulatory paradigm extends beyond histones, as lactylation-induced conformational changes in the chromatin architecture [115] create an epigenetic landscape that fine tunes transcriptional programs governing glucose and lipid metabolisms.
The immunometabolic dimension of lactylation reveals intriguing therapeutic possibilities. In macrophage polarization dynamics, lactate-mediated PKM2 activation exerts dual regulatory effects: the suppression of the Warburg metabolism and induction of pro-reparative phenotypic switching [4]. This metabolic checkpoint mechanism demonstrates how lactylation coordinates inflammatory resolution through metabolic reprogramming. Notably, exercise-induced immunomodulation appears to exploit this pathway through macrophage polarization toward M2 anti-inflammatory phenotypes, suggesting endogenous mechanisms for lactylation pathway regulation [90].
The pathological implications of lactylation are evident in metabolic diseases. Abnormal lactylation in key proteins related to metabolic disorders may lead to the dysregulation of metabolic pathways and exacerbate the progression of metabolic diseases. For example, in certain cases of diabetes, the aberrant lactylation of specific proteins might contribute to the development of diabetic complications by affecting glucose metabolism and insulin signaling. Conversely, histone lactylation (e.g., VEGFA) and macrophage regulation help to establish immunity homeostasis and activate the cardiac repair process in a timely manner [89]. These contrasting pathophysiological roles underscore the context-dependent nature of lactylation signaling.
Therapeutic development requires addressing three key challenges: (1) the tissue-specific regulation of the lactate flux to modulate lactylation patterns, (2) the development of enzymes targeting specific lactylation sites, and (3) the temporal control of lactylation dynamics during disease progression. Current evidence suggests that combinatorial approaches targeting both lactate metabolism and post-translational modification machinery may yield synergistic therapeutic effects across metabolic, inflammatory, and cardiovascular disorders.
5.2. Clinical Applications of Lactylation Modifiers
The development of lactylation modifiers represents a paradigm shift in targeting lactate-centric pathologies through the dual modulation of the metabolic flux and epigenetic reprogramming. Current therapeutic strategies focus on three principal axes: (1) systemic lactate metabolism regulation, (2) site-specific lactylation intervention, and (3) lactate-receptor-signaling modulation.
Metabolic Homeostasis Restoration: Pharmacological lactate modulators demonstrate therapeutic efficacy by rewiring the cellular energy metabolism. As a PDK inhibitor, DCA can activate PDH by inhibiting PDK2, promoting pyruvate to enter the tricarboxylic acid cycle, and reducing lactylation accumulation, thereby improving energy metabolism disorders and oxidative stress in cerebral ischemia reperfusion (I/R) injury [116] (Figure 3). However, the specificity of its mechanism of action and potential off-target effects require careful evaluation. First of all, there are many isoenzymes in the PDK family (PDK1-4), which only emphasize the effective inhibition of DCA by PDK2, without clarifying its specific effects on other isoenzymes, which may lead to non-targeted effects. For example, the key role of PDK1 in cardiometabolic functions, if inhibited, may interfere with tissue-specific metabolic balance. Second, the activation of PDH by DCA may indirectly affect the acetylation modification network. If DCA changes intracellular acetyl-CoA levels through metabolic reprogramming, it may interfere with the acetylation status of histone or non-histone proteins, thereby affecting gene expressions or protein functions. In addition, studies have confirmed that DCA exerts antioxidant effects through the Nrf2 pathway, but whether Nrf2 activation is entirely dependent on the PDK2-PDH axis is unclear. The DCA protective effect disappeared after ML385 inhibited Nrf2, suggesting that Nrf2 is the core downstream target, but the molecular coupling mechanism between PDH activation and Nrf2 signaling still needs to be further analyzed [117]. In conclusion, although DCA plays a neuroprotective role through the PDK2-PDH-Nrf2 axis, its potential effects on metabolic modifications other than lactylation (e.g., acetylation) and multi-target regulation need to be further validated to ensure its therapeutic specificity and avoid off-target risks.
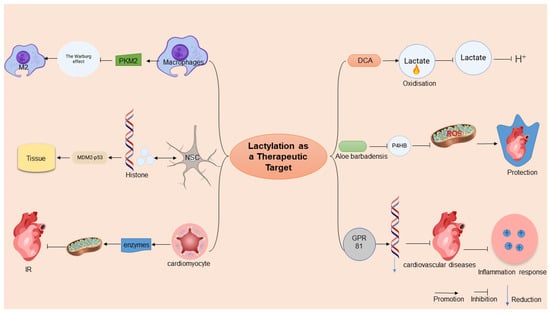
Figure 3.
Potential of lactylation in the treatment of metabolic diseases. This figure illustrates the therapeutic potential of lactylation in metabolic diseases. Lactylation modifies histone conformations, influencing gene expressions and serving as an anti-inflammatory target by altering macrophage phenotypes and mitochondrial functions. In clinical applications, lactylation modifiers, like sodium dichloroacetate (DCA) and aloe vera rhodopsin, target lactate metabolism, reducing acidosis and myocardial injury. Adapted from SciDraw (www.scidraw.io) under CC BY 4.0.
Precision Targeting of Lactylation Sites: Emerging evidence highlights the therapeutic potential of direct lactylation site modulation. Aloin, a natural anthraquinone, exhibits cardioprotective effects by specifically inhibiting P4HB lactylation at lysine 311, thereby suppressing NDP52-mediated mitophagy and mitigating radiation-induced cardiac injury [118] (Figure 3). However, although studies have focused on the regulation of lactylation, its potential off-target effects need to be carefully discussed. First, both lactylation and acetylation target lysine residues and may share a part of the enzymatic system, and aloin’s intervention in the lactylation pathway may indirectly interfere with the kinetic balance of acetylation modification, for example, through the competitive occupation of lysine sites or by affecting the metabolic availability of cofactors (e.g., NAD+) [119]. Second, the stabilizing effect of aloin on GOT2-mediated kynurenine metabolism may influence the microenvironment of other lysine-modified substrates through metabolic reprogramming, thereby affecting hydroxylation or demethylation processes. Although the effect of aloin on acetylation has not been directly reported in the literature, its extensive regulation of mitochondrial functions, ROS metabolism, and inflammatory pathways suggests that its role may go beyond lactylation, and its specificity to other post-translational modifications (e.g., acetylation and ubiquitination) needs to be further tested to fully evaluate its therapeutic potential and risks.
Receptor-Mediated Immunometabolic Modulation: Lactate–GPR81 signaling-axis manipulation offers a novel immunoregulatory strategy. In septic models, lactate-mediated GPR81 activation orchestrates systemic anti-inflammatory responses through the coordinated downregulation of NF-κB-dependent proinflammatory networks, concurrently improving cardiac function and microcirculatory perfusion [120]. This receptor-targeted approach demonstrates the dual advantage of metabolic regulation and immune cell phenotypic modulation.
5.3. Future Directions in Lactylation-Targeted Therapeutics
The dynamic nature of lactylation as a metabolic–epigenetic interface presents both opportunities and challenges for next-generation therapeutic development. Three strategic frontiers will likely dominate this field:
The Systems Biology of Lactylation Networks: The metabolic reprogramming of lactylation may comprehensively regulate mitochondrial functions by affecting lactate modification levels of various key enzymes of the TCA cycle and other metabolic pathways, thereby alleviating myocardial ischemia–reperfusion injury to a certain extent and playing important roles in the occurrence and development of metabolic diseases; contrasting evidence shows that P4HB lactylation exacerbates radiation-induced cardiotoxicity via dysregulated mitophagy [118] (Figure 2). Such context-dependent effects demand the systematic characterization of the “lactylome” across disease states, integrating metabolomic flux analysis with single-cell epigenetic profiling to identify targetable nodal points.
Chronotherapeutic Modulation Strategies: Future interventions must account for lactylation’s dual temporal dimensions: acute metabolic signaling versus chronic epigenetic remodeling. The development of stimuli-responsive lactylation modulators—activated by disease-specific metabolites or oxidative stress—could enable the dynamic control of this modification. Clinical translation requires addressing three-dimensional heterogeneity: (1) spatial: tissue-specific lactylation thresholds (e.g., cardiac vs. hepatic systems), (2) temporal: stage-dependent modification patterns (early inflammation vs. chronic fibrosis), and (3) individual: genetic/epigenetic variants influencing the lactate metabolic capacity. Emerging technologies, like CRISPR-based lactylation editing and AI-driven multiomic prediction models, could enable personalized regimen design. The case of aloin-mediated P4HB lactylation inhibition [118] exemplifies how natural compound libraries may yield selective pharmacophores for specific lactylation sites.
6. Conclusions and Future Perspectives
Lactylation has emerged as a pivotal regulatory mechanism that dynamically integrates metabolic flux with epigenetic landscapes, offering unprecedented insights into the pathogenesis of metabolic diseases. Emerging evidence reveals its dual regulatory capacity: transient exercise-induced lactylation enhances insulin sensitivity and mitochondrial plasticity through AMPK/PGC-1α/GLUT4 signaling, while chronic hyperlactatemia suppresses mitochondrial biogenesis, driving metabolic diseases through the epigenetic silencing of oxidative metabolism genes. This dichotomy underscores lactylation’s role as a spatiotemporal rheostat, where its effects are contingent upon the metabolic context and tissue-specific thresholds. For instance, hepatic H3K9la promotes HCC development, while a feedback loop between H3K9la and HDAC2 drives VEGF-induced angiogenesis. Such organ specificity highlights the need for precision-targeted interventions to exploit lactylation’s therapeutic potential without disrupting physiological processes, such as neuronal plasticity or immunity surveillance.
Future research should focus on integrating multiomic approaches to clarify lactylation’s spatiotemporal dynamics across organs, especially its tissue-specific thresholds, interactions with metabolism–immunity networks, and hierarchical regulation of metabolic–epigenetic crosstalk, to achieve translational breakthroughs in metabolic disease management.
Author Contributions
P.S. provided the direction and coordination of this manuscript. G.C. collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. J.L. collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. Y.G. reviewed and made revisions to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32171130) to P.S.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Yu, T.; Gao, M.; Liu, D.; Zhang, J.; Lu, C.; Chen, X.; Zhang, X.; Liu, Y. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int. J. Biol. Sci. 2022, 18, 6210–6225. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, S.; Sun, J.; Zhao, Y.; Liu, C. Lactate and lactylation in cardiovascular diseases: Current progress and future perspectives. Metabolism 2024, 158, 155957. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLOS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Manoharan, I.; Prasad, P.D.; Thangaraju, M.; Manicassamy, S. Lactate-Dependent Regulation of Immune Responses by Dendritic Cells and Macrophages. Front. Immunol. 2021, 12, 691134. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Cui, Q.; Guo, B.; Ding, W.; Liu, J.; Quan, L.; Li, X.; Xie, P.; Jin, L.; et al. Activation of GPR81 by lactate drives tumour-induced cachexia. Nat. Metab. 2024, 6, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, N.; Gong, Z.; Zhou, W.; Ku, Y.; Chen, Y. Lactate and lysine lactylation of histone regulate transcription in cancer. Heliyon 2024, 10, e38426. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Huang, C.; Lin, D. Lactate Activates AMPK Remodeling of the Cellular Metabolic Profile and Promotes the Proliferation and Differentiation of C2C12 Myoblasts. Int. J. Mol. Sci. 2022, 23, 13996. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Kopach, O.; Braga, A.; Nizari, S.; Hosford, P.S.; Sagi-Kiss, V.; Hadjihambi, A.; Konstantinou, C.; Esteras, N.; Del Arroyo, A.G.; et al. Adenosine signalling to astrocytes coordinates brain metabolism and function. Nature 2024, 632, 139–146. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, M.; Wang, S.; Chen, L.; Li, Z.; Li, C.; Cao, P.; Chen, Y. Lactate Is a Key Mediator That Links Obesity to Insulin Resistance via Modulating Cytokine Production from Adipose Tissue. Diabetes 2022, 71, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Jeanson, Y.; Portais, J.-C.; Galinier, A.; Ader, I.; Casteilla, L.; Carrière, A. Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective. Front. Physiol. 2021, 12, 689747. [Google Scholar] [CrossRef]
- Sun, P.; Ma, L.; Lu, Z. Lactylation: Linking the Warburg effect to DNA damage repair. Cell Metab. 2024, 36, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Kambe, Y.; Kurihara, T.; Miyata, A. [Astrocyte-neuron lactate shuttle, the major effector of astrocytic PACAP signaling for CNS functions]. Nihon Yakurigaku Zasshi 2018, 151, 239–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, X.; Zhang, R.; Wei, C.; Gao, Y.; Yu, Y.; Wang, L.; Jiang, J.; Zhang, X.; Li, J.; Chen, X. MCT2 overexpression promotes recovery of cognitive function by increasing mitochondrial biogenesis in a rat model of stroke. Anim. Cells Syst. 2021, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Wang, H.; Liu, L.; Li, W.; Xie, P. The regulatory effects of lactic acid on neuropsychiatric disorders. Discov. Ment. Health 2022, 2, 8. [Google Scholar] [CrossRef]
- Yang, C.; Pan, R.-Y.; Guan, F.; Yuan, Z. Lactate metabolism in neurodegenerative diseases. Neural Regen. Res. 2024, 19, 69–74. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef] [PubMed]
- Pujada, A.; Walter, L.; Patel, A.; Bui, T.A.; Zhang, Z.; Zhang, Y.; Denning, T.L.; Garg, P. Matrix metalloproteinase MMP9 maintains epithelial barrier function and preserves mucosal lining in colitis associated cancer. Oncotarget 2017, 8, 94650–94665. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Singh, M.; Afonso, J.; Sharma, D.; Gupta, R.; Kumar, V.; Rani, R.; Baltazar, F.; Kumar, V. Targeting monocarboxylate transporters (MCTs) in cancer: How close are we to the clinics? Semin. Cancer Biol. 2023, 90, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, J.; He, Z.; Wang, G.; Wang, Y.; Zhao, D.; Wang, Z.; Luo, C.; Tian, C.; Jiang, Q. Monocarboxylate Transporter 1 in Brain Diseases and Cancers. Curr. Drug Metab. 2019, 20, 855–866. [Google Scholar] [CrossRef]
- Huang, T.; Feng, Q.; Wang, Z.; Li, W.; Sun, Z.; Wilhelm, J.; Huang, G.; Vo, T.; Sumer, B.D.; Gao, J. Tumor-Targeted Inhibition of Monocarboxylate Transporter 1 Improves T-Cell Immunotherapy of Solid Tumors. Adv. Healthc Mater. 2021, 10, e2000549. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, B.; Hashimi, S.M.; Alqosaibi, A.I.; Alqurashi, N.; Alhazmi, S. Targeting the monocarboxylate transporter MCT2 and lactate dehydrogenase A LDHA in cancer cells with FX-11 and AR-C155858 inhibitors. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6605–6617. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Gan, R.; Liu, Z.; Deng, Y. Identification and validation of lactate metabolism-related genes in oxygen-induced retinopathy. Sci. Rep. 2023, 13, 13319. [Google Scholar] [CrossRef]
- Zhang, L.; Xin, C.; Wang, S.; Zhuo, S.; Zhu, J.; Li, Z.; Liu, Y.; Yang, L.; Chen, Y. Lactate transported by MCT1 plays an active role in promoting mitochondrial biogenesis and enhancing TCA flux in skeletal muscle. Sci. Adv. 2024, 10, eadn4508. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Narumi, K.; Furugen, A.; Iseki, K. Transport function, regulation, and biology of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol. Ther. 2021, 226, 107862. [Google Scholar] [CrossRef]
- Ruppert, P.M.; Kersten, S. Mechanisms of hepatic fatty acid oxidation and ketogenesis during fasting. Trends Endocrinol. Metab. 2024, 35, 107–124. [Google Scholar] [CrossRef]
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Thyroid hormone transporters—Functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cai, M.; Liu, Y.; Liu, B.; Xue, X.; Ji, R.; Bian, X.; Lou, S. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J. Cell. Physiol. 2020, 235, 8938–8950. [Google Scholar] [CrossRef]
- Wallenius, K.; Thalén, P.; Björkman, J.-A.; Johannesson, P.; Wiseman, J.; Böttcher, G.; Fjellström, O.; Oakes, N.D. Involvement of the metabolic sensor GPR81 in cardiovascular control. JCI Insight. 2017, 2, e92564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Dou, X.-D.; Cheng, J.; Gao, M.-X.; Xu, G.-F.; Ding, W.; Ding, J.-H.; Li, Y.; Wang, S.-H.; Ji, Z.-W.; et al. Functional screening and rational design of compounds targeting GPR132 to treat diabetes. Nat. Metab. 2023, 5, 1726–1746. [Google Scholar] [CrossRef]
- Krewson, E.A.; Sanderlin, E.J.; Marie, M.A.; Akhtar, S.N.; Velcicky, J.; Loetscher, P.; Yang, L.V. The Proton-Sensing GPR4 Receptor Regulates Paracellular Gap Formation and Permeability of Vascular Endothelial Cells. iScience 2020, 23, 100848. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Cai, H.; Ma, H.; Zhao, D.; Zhang, X.; Li, Z.; Wang, S.; Wang, J.; Liu, R.; et al. Human GPR4 and the Notch signaling pathway in endothelial cell tube formation. Mol. Med. Rep. 2016, 14, 1235–1240. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, B.; Zhang, H.; Zhang, L.; Zhang, R.; Li, L.; Zhang, Y.; Hu, C. Histone lactylation mediated by Fam172a in POMC neurons regulates energy balance. Nat. Commun. 2024, 15, 10111. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y. Lactate: A multifunctional signaling molecule. Yeungnam Univ. J. Med. 2021, 38, 183–193. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Fan, M.; Tu, F.; Wang, X.; Ha, T.; Williams, D.L.; Li, C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-kappaB Activation via GPR81-Mediated Signaling. Front. Immunol. 2020, 11, 587913. [Google Scholar] [CrossRef]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Shanmugam, A.; Swafford, D.; Suryawanshi, A.; Bhattacharjee, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Kurago, Z.B.; Thangaraju, M.; et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J. Immunol. 2018, 200, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Z.; Yang, L.; Li, W.; Wang, Y.; Kong, Z.; Miao, J.; Chen, Y.; Bian, Y.; Zeng, L. Emerging roles of lactate in acute and chronic inflammation. Cell Commun. Signal. 2024, 22, 276. [Google Scholar] [CrossRef]
- Jiang, R.; Ren, W.-J.; Wang, L.-Y.; Zhang, W.; Jiang, Z.-H.; Zhu, G.-Y. Targeting Lactate: An Emerging Strategy for Macrophage Regulation in Chronic Inflammation and Cancer. Biomolecules 2024, 14, 1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dai, Z.; Cooper, D.E.; Kirsch, D.G.; Locasale, J.W. Quantitative Analysis of the Physiological Contributions of Glucose to the TCA Cycle. Cell Metab. 2020, 32, 619–628.e21. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, X.; Chen, J.; Zeng, Y.; Guo, M.; Tan, X.; Wang, Y.; Wang, P.; Yan, P.; Lei, Y.; et al. Development of Serum Lactate Level-Based Nomograms for Predicting Diabetic Kidney Disease in Type 2 Diabetes Mellitus Patients. Diabetes Metab. Syndr. Obes. 2024, 17, 1051–1068. [Google Scholar] [CrossRef]
- Maschari, D.; Saxena, G.; Law, T.D.; Walsh, E.; Campbell, M.C.; Consitt, L.A. Lactate-induced lactylation in skeletal muscle is associated with insulin resistance in humans. Front. Physiol. 2022, 13, 951390. [Google Scholar] [CrossRef]
- Kjobsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef]
- Knudsen, J.R.; Steenberg, D.E.; Hingst, J.R.; Hodgson, L.R.; Henriquez-Olguin, C.; Li, Z.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.; Verkade, P.; et al. Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation. Mol. Metab. 2020, 39, 100998. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B. The Energy Sensor AMPK: Adaptations to Exercise, Nutritional and Hormonal Signals; Springer: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase—A journey from 1 to 100 downstream targets. Biochem. J. 2022, 479, 2327–2343. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Cai, B.; Nie, Q. PGC-1alpha affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genom. 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Yin, T.C.; Van Vranken, J.G.; Srivastava, D.; Mittal, A.; Buscaglia, P.; Moore, A.E.; Verdinez, J.A.; Graham, A.E.; Walsh, S.A.; Acevedo, M.A.; et al. Insulin sensitization by small molecules enhancing GLUT4 translocation. Cell Chem. Biol. 2023, 30, 933–942.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zheng, F.; Xie, K.-L.; Xie, M.-R.; Jiang, L.-J.; Cai, Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther. Nucleic Acids 2019, 18, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef]
- DE-Cleva, R.; Cardia, L.; Vieira-Gadducci, A.; Greve, J.M.; Santo, M.A. Lactate Can Be a Marker of Metabolic Syndrome in Severe Obesity? Arq. Bras. Cir. Dig. 2021, 34, e1579. [Google Scholar] [CrossRef]
- Rooney, K.; Trayhurn, P. Lactate and the GPR81 receptor in metabolic regulation: Implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br. J. Nutr. 2011, 106, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, X.; Zhang, Z.; Chen, J.; Wang, F.; Wang, L.; Liu, J. Moderate l-lactate administration suppresses adipose tissue macrophage M1 polarization to alleviate obesity-associated insulin resistance. J. Biol. Chem. 2022, 298, 101768. [Google Scholar] [CrossRef]
- Peng, X.; He, Z.; Yuan, D.; Liu, Z.; Rong, P. Lactic acid: The culprit behind the immunosuppressive microenvironment in hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189164. [Google Scholar] [CrossRef]
- Godinjak, A.; Jusufovic, S.; Rama, A.; Iglica, A.; Zvizdic, F.; Kukuljac, A.; Tancica, I.; Rozajac, S. Hyperlactatemia and the Importance of Repeated Lactate Measurements in Critically Ill Patients. Med Arch. 2017, 71, 404–407. [Google Scholar] [CrossRef]
- Guo, B.; Shu, H.; Luo, L.; Liu, X.; Ma, Y.; Zhang, J.; Liu, Z.; Zhang, Y.; Fu, L.; Song, T.; et al. Lactate Conversion by Lactate Dehydrogenase B Is Involved in Beige Adipocyte Differentiation and Thermogenesis in Mice. Nutrients 2023, 15, 4846. [Google Scholar] [CrossRef] [PubMed]
- Krycer, J.R.; Quek, L.-E.; Francis, D.; Fazakerley, D.J.; Elkington, S.D.; Diaz-Vegas, A.; Cooke, K.C.; Weiss, F.C.; Duan, X.; Kurdyukov, S.; et al. Lactate production is a prioritized feature of adipocyte metabolism. J. Biol. Chem. 2020, 295, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Nieto, A.; McDonald, P. Inhibition of the Monocarboxylate Transporter 1 (MCT1) Promotes 3T3-L1 Adipocyte Proliferation and Enhances Insulin Sensitivity. Int. J. Mol. Sci. 2022, 23, 1901. [Google Scholar] [CrossRef]
- Yao, Z.; Liang, S.; Chen, J.; Zhang, H.; Chen, W.; Li, H. Dietary Lactate Intake and Physical Exercise Synergistically Reverse Brown Adipose Tissue Whitening to Ameliorate Diet-Induced Obesity. J. Agric. Food Chem. 2024, 72, 25286–25297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, Z.; Qi, H.; Luo, X.; Wang, M.; Du, Z.; Guo, W. Lactate shuttling links histone lactylation to adult hippocampal neurogenesis in mice. Dev. Cell, 2025; Online ahead of print. [Google Scholar]
- Huang, J.; Wang, X.; Li, N.; Fan, W.; Li, X.; Zhou, Q.; Liu, J.; Li, W.; Zhang, Z.; Liu, X.; et al. YY1 Lactylation Aggravates Autoimmune Uveitis by Enhancing Microglial Functions via Inflammatory Genes. Adv. Sci. 2024, 11, e2308031. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423.e8. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.; Zhan, Y. H3K18 lactylation accelerates liver fibrosis progression through facilitating SOX9 transcription. Exp. Cell Res. 2024, 440, 114135. [Google Scholar] [CrossRef] [PubMed]
- Raju, C.; Sankaranarayanan, K. Insights on post-translational modifications in fatty liver and fibrosis progression. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167659. [Google Scholar] [CrossRef]
- Wu, H.; Huang, H.; Zhao, Y. Interplay between metabolic reprogramming and post-translational modifications: From glycolysis to lactylation. Front. Immunol. 2023, 14, 1211221. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm (2020) 2023, 4, e292. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, X.; Zhao, P.; Chen, C.; Xu, G.; Ke, X. Potential of lactylation as a therapeutic target in cancer treatment (Review). Mol. Med. Rep. 2025, 31, 91. [Google Scholar] [CrossRef] [PubMed]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4(+) T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055–1074.e8. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Q.; Hu, Y.; He, B.; Cao, T.; Tang, Y.; Zhou, X.P.; Lan, X.P.; Liu, S.Q. Advances in the interaction of glycolytic reprogramming with lactylation. Biomed. Pharmacother. 2024, 177, 116982. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Wu, X.-F.; Xu, J.-Y.; Xu, X. Lactate-mediated lactylation in human health and diseases: Progress and remaining challenges. J. Adv. Res. 2024; In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, A.; Oh, K.-J.; Lee, S.C.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Z.; Li, Z.; Wang, Y.; Wu, N.; Sun, H.; Zhou, Z.; Hu, Q. Lactylation: The novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenetics 2024, 16, 72. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Li, L.; Chen, L. Involvement of the Warburg effect in non-tumor diseases processes. J. Cell. Physiol. 2018, 233, 2839–2849. [Google Scholar] [CrossRef]
- Lin, J.; Ren, J. Lactate-induced lactylation and cardiometabolic diseases: From epigenetic regulation to therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167247. [Google Scholar] [CrossRef]
- Sun, W.; Jia, M.; Feng, Y.; Cheng, X. Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy 2023, 19, 3240–3241. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Wu, J.; Chi, Q.; Wang, S.; Liu, W.; Yang, L.; Song, G.; Pan, L.; Xu, K.; Wang, C. Lactylome Analysis Unveils Lactylation-Dependent Mechanisms of Stemness Remodeling in the Liver Cancer Stem Cells. Adv. Sci. 2024, 11, e2405975. [Google Scholar] [CrossRef]
- Yao, S.; Chai, H.; Tao, T.; Zhang, L.; Yang, X.; Li, X.; Yi, Z.; Wang, Y.; An, J.; Wen, G.; et al. Role of lactate and lactate metabolism in liver diseases (Review). Int. J. Mol. Med. 2024, 54, 59. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, X.; Wang, S.; Xu, M.; Fang, T.; Ma, X.; Chen, M.; Fu, J.; Guo, J.; Tian, S.; et al. TRIM56 protects against nonalcoholic fatty liver disease by promoting the degradation of fatty acid synthase. J. Clin. Investig. 2024, 134, e166149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, J.; Huang, H.; Liu, L.; Ren, L.; Hu, J.; Jiang, X.; Zheng, Y.; Xu, L.; Zhong, F.; et al. The m(6)A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis. Cell Death Dis. 2024, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, W.; Wang, X.; Mang, G.; Chen, J.; Yan, X.; Tong, Z.; Yang, Q.; Wang, M.; Chen, L.; et al. Histone Lactylation Boosts Reparative Gene Activation Post–Myocardial Infarction. Circ. Res. 2022, 131, 893–908. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhang, M.; Li, X.; Yang, X.; Huang, T.; Ban, Y.; Li, Y.; Li, Q.; Zheng, Y.; et al. Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the Ereg/MAPK signalling pathway. Atherosclerosis 2023, 375, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, B.; Yang, T.; Zhang, W.; Mei, Z. Lactylation modification in cardio-cerebral diseases: A state-of-the-art review. Ageing Res. Rev. 2024, 104, 102631. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Yu, J.; Zhang, X.; Ran, S.; Wang, S.; Ye, W.; Luo, Z.; Li, X.; Hao, Y.; et al. Lactate metabolism and lactylation in cardiovascular disease: Novel mechanisms and therapeutic targets. Front. Cardiovasc. Med. 2024, 11, 1489438. [Google Scholar] [CrossRef]
- Raychaudhuri, D.; Singh, P.; Chakraborty, B.; Hennessey, M.; Tannir, A.J.; Byregowda, S.; Natarajan, S.M.; Trujillo-Ocampo, A.; Im, J.S.; Goswami, S. Histone lactylation drives CD8(+) T cell metabolism and function. Nat. Immunol. 2024, 25, 2140–2151. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Dong, Y.; Sun, L.V.; Zheng, Y. Lactate promotes H3K18 lactylation in human neuroectoderm differentiation. Cell. Mol. Life Sci. 2024, 81, 459. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, Z.; Ren, H.; Zhang, Z.; Fu, Y.; Li, L.; Shen, Z.; Li, T.; Tang, S.; et al. PGC-1alpha/LDHA signaling facilitates glycolysis initiation to regulate mechanically induced bone remodeling under inflammatory microenvironment. Bone 2024, 185, 117132. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, X.; Lee, W.-C.; Shi, Y.; Li, B.; Abel, E.D.; Jiang, D.; Huang, W.; Long, F. Increased glycolysis mediates Wnt7b-induced bone formation. FASEB J. 2019, 33, 7810–7821. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Wang, Y.; Liu, M.; Zhang, Y.; Feng, T.; Xiao, C.; Song, H.; Miao, R.; Xu, L.; et al. Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis. Adv. Sci. 2024, 11, e2406333. [Google Scholar] [CrossRef]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392.e33. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Yao, Z.; Liang, S.; Chen, J.; Dai, Y.; Zhang, H.; Li, H.; Chen, W. A Combination of Exercise and Yogurt Intake Protects Mice against Obesity by Synergistic Promotion of Adipose Browning. J. Agric. Food Chem. 2024, 72, 13906–13917. [Google Scholar] [CrossRef]
- Chang, J.W.; Tang, C.-H. The role of macrophage polarization in rheumatoid arthritis and osteoarthritis: Pathogenesis and therapeutic strategies. Int. Immunopharmacol. 2024, 142 Pt A, 113056. [Google Scholar] [CrossRef]
- Zhao, T.; Le, S.; Freitag, N.; Schumann, M.; Wang, X.; Cheng, S. Effect of Chronic Exercise Training on Blood Lactate Metabolism Among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 652023. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, D. Lactylation constrains OXPHOS under hypoxia. Cell Res. 2024, 34, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, D.; Lund, M.T.; Scheuer, C.M.; Dehlbæk, M.S.; Dideriksen, S.G.; Abildskov, C.V.; Christensen, K.K.; Dohlmann, T.L.; Larsen, S.; Vigelsø, A.H.; et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 2018, 222, e13009. [Google Scholar] [CrossRef]
- Doewes, R.I.; Gharibian, G.; Zadeh, F.A.; Zaman, B.A.; Vahdat, S.; Akhavan-Sigari, R. An Updated Systematic Review on the Effects of Aerobic Exercise on Human Blood Lipid Profile. Curr. Probl. Cardiol. 2023, 48, 101108. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise performance and health: Role of GLUT4. Free. Radic. Biol. Med. 2024, 224, 479–483. [Google Scholar] [CrossRef]
- Scarpelli, M.C.; Bergamasco, J.G.A.; Godwin, J.S.; Mesquita, P.H.C.; Chaves, T.S.; Silva, D.G.; Bittencourt, D.; Dias, N.F.; Junior, R.A.M.; Filho, P.C.C.; et al. Resistance training-induced changes in muscle proteolysis and extracellular matrix remodeling biomarkers in the untrained and trained states. Eur. J. Appl. Physiol. 2024, 124, 2749–2762. [Google Scholar] [CrossRef]
- Son, W.H.; Park, H.-T.; Jeon, B.H.; Ha, M.-S. Moderate intensity walking exercises reduce the body mass index and vascular inflammatory factors in postmenopausal women with obesity: A randomized controlled trial. Sci. Rep. 2023, 13, 20172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, R.; Guo, Y.; Hu, B.; Xie, L.; An, Y.; Wen, J.; Liu, Z.; Zhou, M.; Kuang, W.; et al. Muscle-derived small extracellular vesicles induce liver fibrosis during overtraining. Cell Metab. 2025, 37, 824–841.e8. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, M.; Ren, M.; Li, C.; Zheng, H.; Gao, H. Metabolomic Analysis Identifies Lactate as an Important Pathogenic Factor in Diabetes-associated Cognitive Decline Rats. Mol. Cell. Proteom. 2018, 17, 2335–2346. [Google Scholar] [CrossRef]
- Yang, L.; Gilbertsen, A.; Xia, H.; Benyumov, A.; Smith, K.A.; Herrera, J.A.; Racila, E.; Bitterman, P.B.; Henke, C.A. Hypoxia enhances IPF mesenchymal progenitor cell fibrogenicity via the lactate/GPR81/HIF1alpha pathway. JCI Insight. 2023, 8, e163820. [Google Scholar] [CrossRef]
- Heo, J.; No, M.; Cho, J.; Choi, Y.; Cho, E.; Park, D.; Kim, T.; Kim, C.; Seo, D.Y.; Han, J.; et al. Moderate aerobic exercise training ameliorates impairment of mitochondrial function and dynamics in skeletal muscle of high-fat diet-induced obese mice. FASEB J. 2021, 35, e21340. [Google Scholar] [CrossRef] [PubMed]
- Allard, N.A.; Janssen, L.; Aussieker, T.; Stoffels, A.A.; Rodenburg, R.J.; Assendelft, W.J.; Thompson, P.D.; Snijders, T.; Hopman, M.T.; Timmers, S. Moderate Intensity Exercise Training Improves Skeletal Muscle Performance in Symptomatic and Asymptomatic Statin Users. J. Am. Coll Cardiol. 2021, 78, 2023–2037. [Google Scholar] [CrossRef]
- Jing, F.; Zhu, L.; Zhang, J.; Zhou, X.; Bai, J.; Li, X.; Zhang, H.; Li, T. Multi-omics reveals lactylation-driven regulatory mechanisms promoting tumor progression in oral squamous cell carcinoma. Genome Biol. 2024, 25, 272. [Google Scholar] [CrossRef]
- Wu, D.; Spencer, C.B.; Ortoga, L.; Zhang, H.; Miao, C. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024, 74, 103194. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Mo, Y.; Li, R.; Huang, S.; Zhang, A.; Ni, X.; Dai, Q.; Wang, J. DCA Protects against Oxidation Injury Attributed to Cerebral Ischemia-Reperfusion by Regulating Glycolysis through PDK2-PDH-Nrf2 Axis. Oxid Med. Cell. Longev. 2021, 2021, 5173035. [Google Scholar] [CrossRef] [PubMed]
- Skorja, M.N.; Dolinar, K.; Miš, K.; Matkovič, U.; Bizjak, M.; Pavlin, M.; Podbregar, M.; Pirkmajer, S. Suppression of Pyruvate Dehydrogenase Kinase by Dichloroacetate in Cancer and Skeletal Muscle Cells Is Isoform Specific and Partially Independent of HIF-1? Int. J. Mol. Sci. 2021, 22, 8610. [Google Scholar] [CrossRef]
- Ouyang, F.; Li, Y.; Wang, H.; Liu, X.; Tan, X.; Xie, G.; Zeng, J.; Zeng, G.; Luo, Q.; Zhou, H.; et al. Aloe Emodin Alleviates Radiation-Induced Heart Disease via Blocking P4HB Lactylation and Mitigating Kynurenine Metabolic Disruption. Adv. Sci. 2024, 11, e2406026. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2022, 13, 877–919. [Google Scholar] [CrossRef]
- Madaan, A.; Nadeau-Vallée, M.; Rivera, J.C.; Obari, D.; Hou, X.; Sierra, E.M.; Girard, S.; Olson, D.M.; Chemtob, S. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am. J. Obstet. Gynecol. 2017, 216, 60.e1–60.e17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).