Synthesis and Biological Assessment of Eucalyptin: Magic Methyl Effects
Abstract
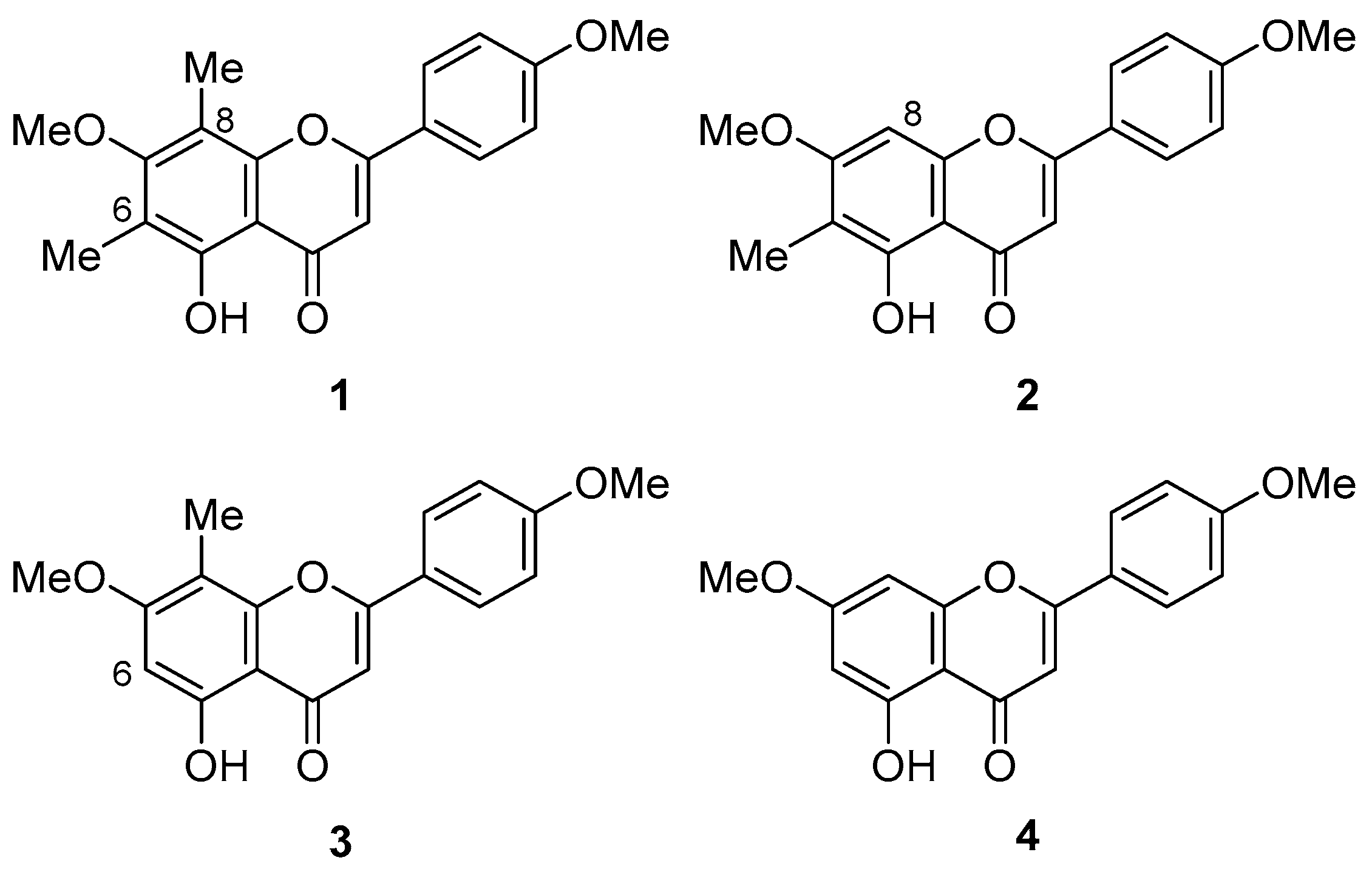
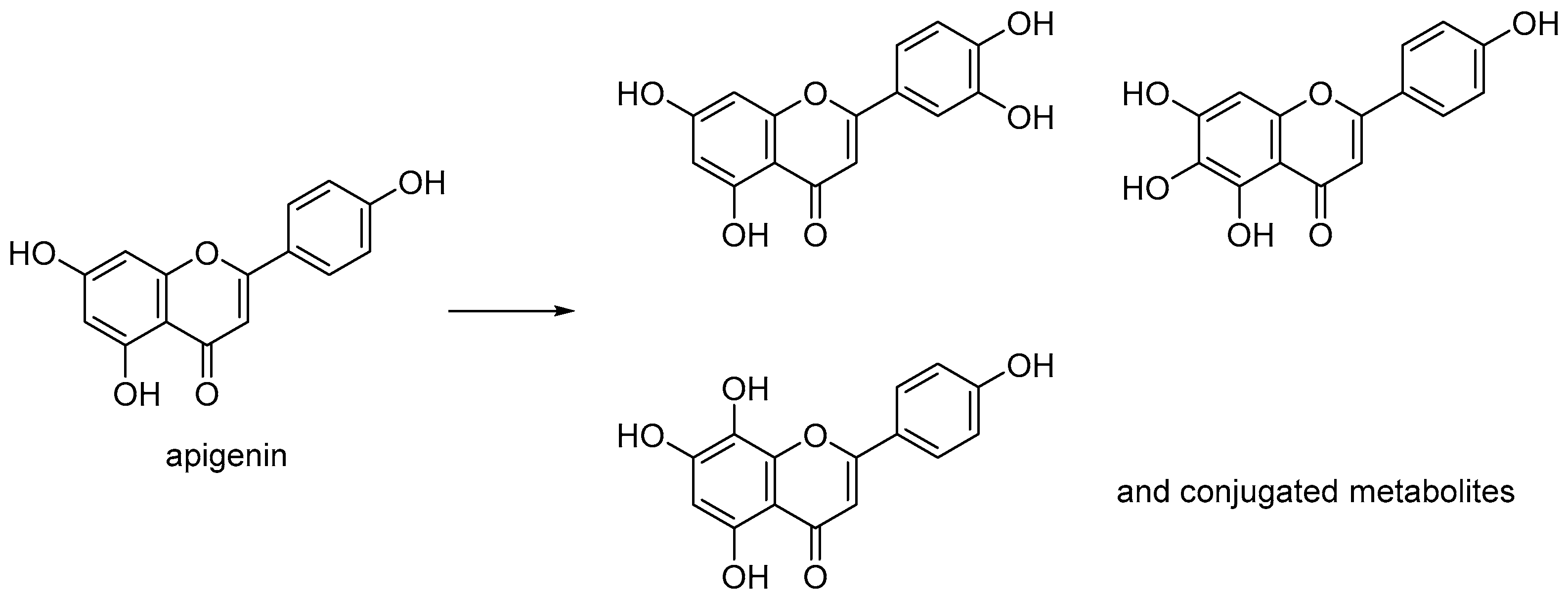
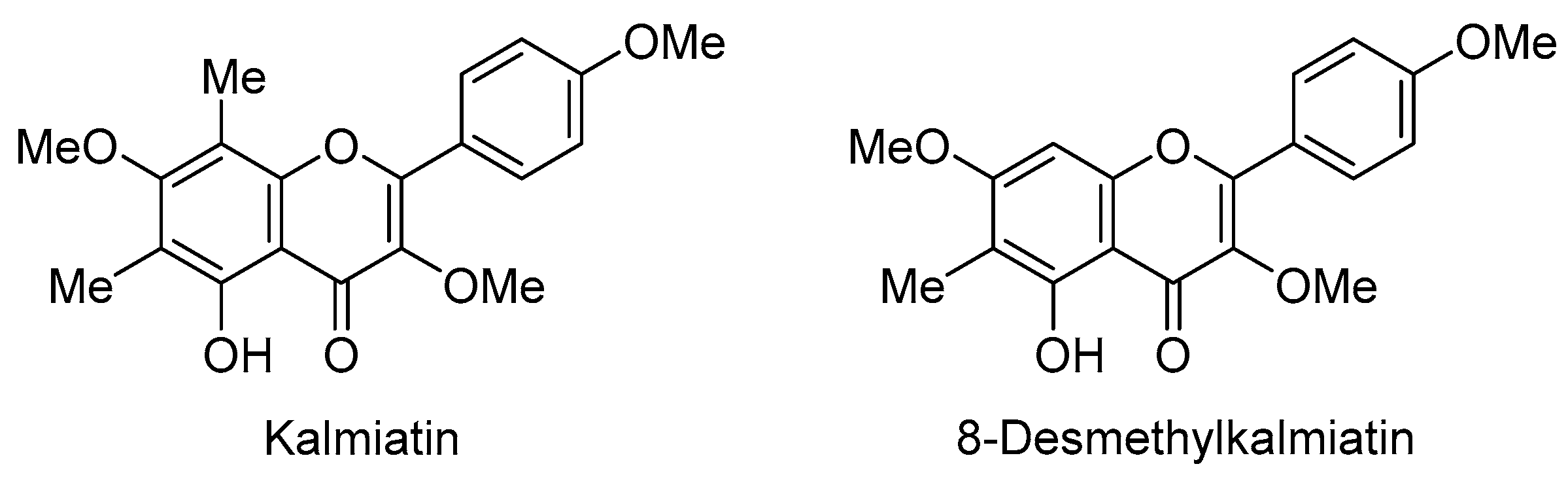
1. Introduction
2. Results and Discussion
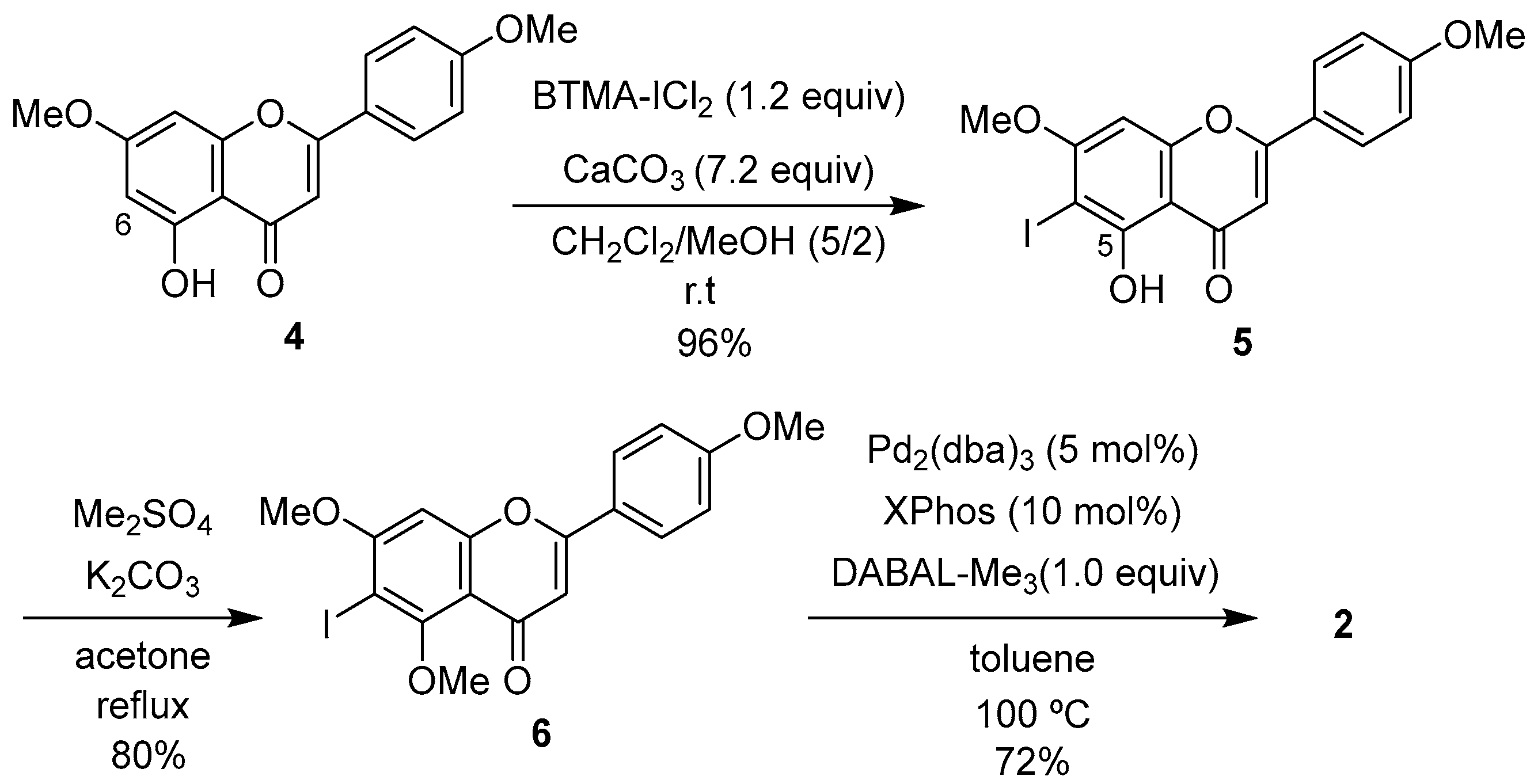
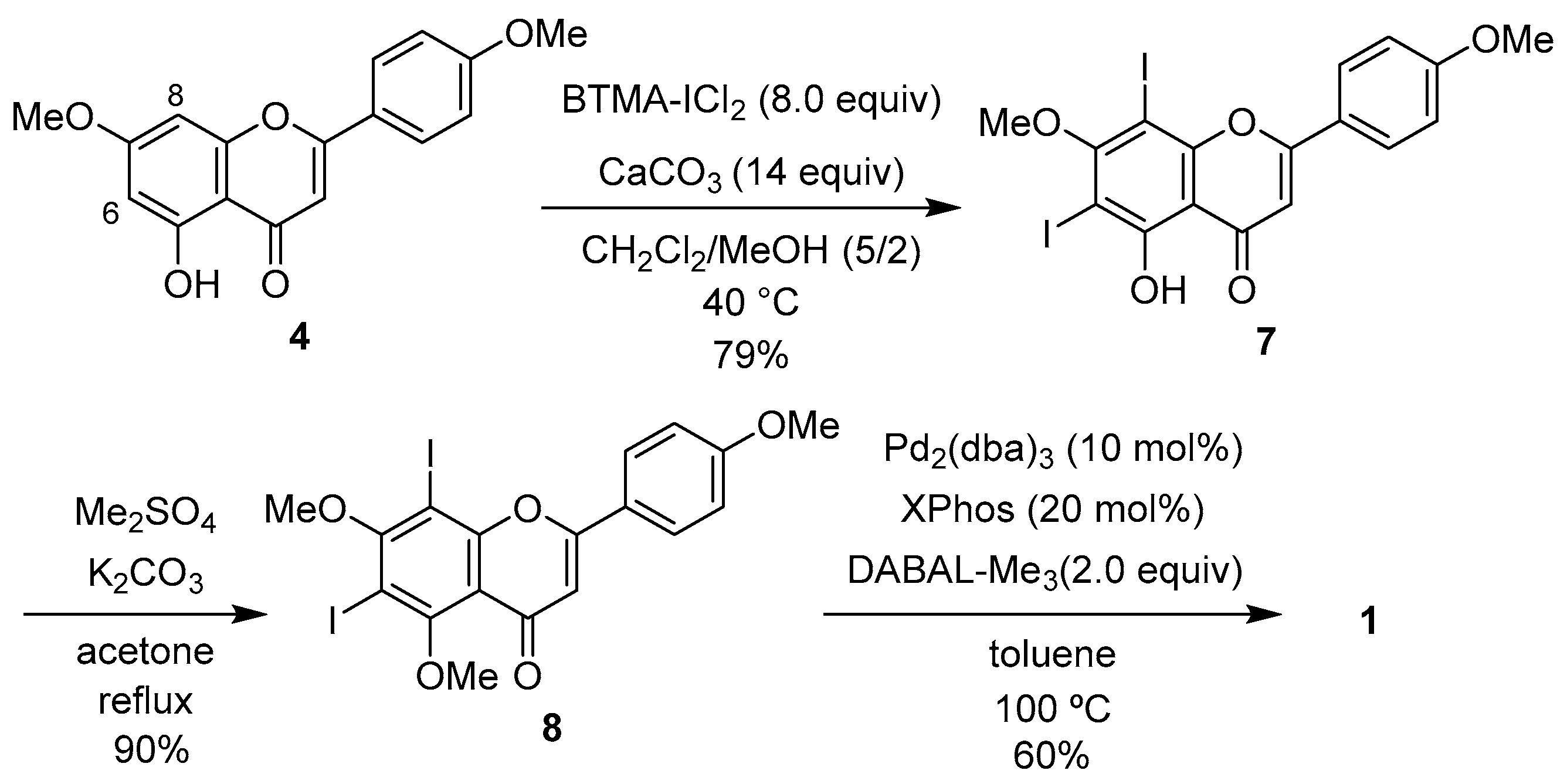
2.1. Synthesis of 1
2.2. Cytotoxic Activity of 1 and 2
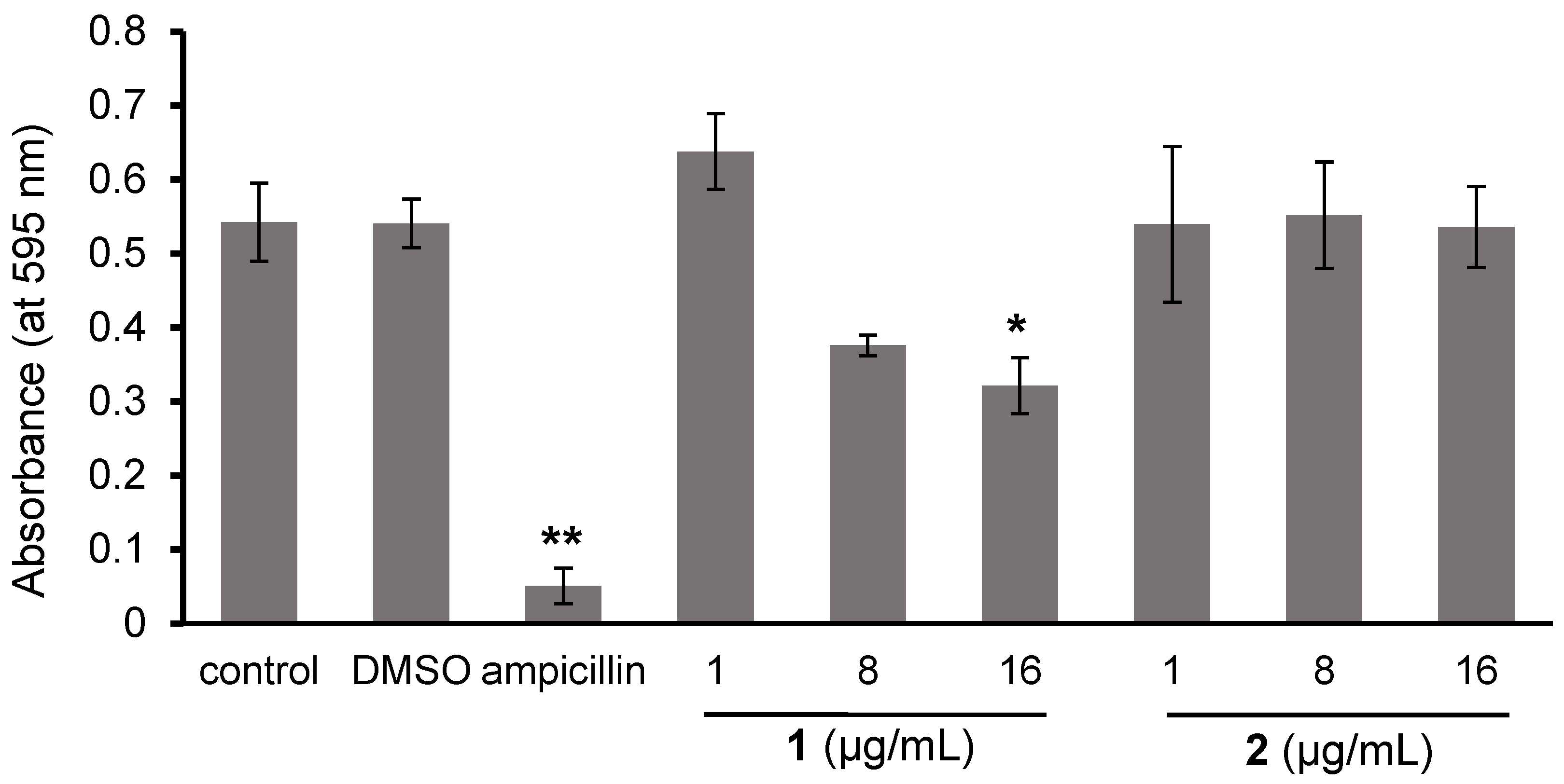
2.3. Effects of 1 and 2 on the Biofilm Formation and Growth of Cutibacterium Acnes
3. Materials and Methods
3.1. General Procedure and Materials
3.2. Chemistry
3.2.1. Synthesis of 5-Hydroxy-6,8-diiodo-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one (7)
3.2.2. Synthesis of 6,8-Diiodo-5,7-dimethoxy-2-(4-methoxyphenyl)-4H-chromen-4-one (8)
3.2.3. Synthesis of Eucalyptin (1)
3.3. Biology
3.3.1. Cell Culture
3.3.2. Cytotoxic Evaluation
3.3.3. Preparation of Modified Reinforced Clostridial Medium (RCM)
3.3.4. Bacterial Strain and Growth Conditions
3.3.5. Effects of 1 and 2 on Biofilm Formation of C. acnes
3.3.6. Effects of 1 and 2 on the Growth of C. acnes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horn, D.H.S.; Kranz, Z.H.; Lamberton, J.A. The composition of Eucalyptus and some other leaf waxes. Aust. J. Chem. 1964, 17, 464–476. Available online: https://www.publish.csiro.au/ch/ch9640464 (accessed on 18 February 2025).
- Wollenweber, E.; Kohorst, G. Epicuticular leaf flavonoids from Eucalyptus species and from Kalmia latifolia. Z. Naturforsch. C 1981, 36, 913–915. [Google Scholar] [CrossRef]
- Courtney, J.L.; Lassak, E.V.; Speirs, G.B. Leaf wax constituents of some myrtaceous species. Phytochemistry 1983, 22, 947–949. [Google Scholar] [CrossRef]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Fatema R Saber, F.R. Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 23, 112669. [Google Scholar] [CrossRef] [PubMed]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Tawila, A.M.; Sun, S.; Kim, M.J.; Omar, A.M.; Dibwe, D.F.; Ueda, J.-Y.; Toyooka, N.; Awale, S. Highly potent antiausterity agents from Callistemon citrinus and their mechanism of action against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2020, 83, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Matsuura, S. Synthesis of nuclear substituted flavonoids and allied compounds. I. Chloromethylation of acacetin 7-methyl ether. I. Isolation of two chloromethyl compounds and their derivatives. Yakugaku Zasshi 1953, 73, 481–484. [Google Scholar] [CrossRef]
- Jain, A.C.; Sarpal, P.D.; Seshadri, T.R. Synthesis of eucalyptin and 4’-demethoxyeucalyptin. Ind. J. Chem. 1966, 4, 481–483. [Google Scholar]
- Quintin, J.; Lewis, G. Regioselective 6-iodination of 5,7-dioxygenated flavones by benzyltrimethylammonium dichloroiodate. Tetrahedron Lett. 2004, 45, 3635–3638. [Google Scholar] [CrossRef]
- Asakawa, R.; Fuchiyama, K.; Ishii, Y.; Hosaka, K.; Kobayashi, A.; Shimazaki, K.; Nagasawa, J.; Tsuchida, S.; Ushida, K.; Matsubayashi, M.; et al. Synthesis and cytotoxic activities of 8- and 6-demethyleucalyptins. Biosci. Biotechnol. Biochem. 2022, 86, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzyme Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yan, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Gradolatto, A.; Canivenc-Lavier, M.-C.; Basly, J.-P.; Siess, M.-H.; Teyssier, C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug. Metab. Dispos. 2004, 32, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.d.S.M.; Franco, L.S.; Fraga, C.A.M. The magic methyl and its tricks in drug discovery and development. Pharmaceuticals 2023, 16, 1157. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Zdorovenko, E.L.; Botchkova, E.A.; Hardouin, J.; Massier, S.; Kopitsyn, D.S.; Gorbachevskii, M.V.; Kadykova, A.A.; Shashkov, A.S.; Zhurina, M.V. Composition of the biofilm matrix of Cutibacterium acnes acneic strain RT5. Front. Microbiol. 2019, 10, 456374. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Kohorst, G. Novel epicuticular leaf flavonoids from Kalmia and Gaidtheria (Ericaceae). Z. Naturforsch. C 1984, 39, 710–713. [Google Scholar] [CrossRef]

| IC50 (µM) | |||
|---|---|---|---|
| Compounds | HeLa | Jurkat | MRC-5 |
| 1 | 18.3 ± 6.4 | 4.4 ± 1.2 | >100 |
| 2 | 8.3 ± 0.5 | >100 | 23.1 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchiyama, K.; Yabuki, Y.; Yamamoto, Y.; Asakawa, R.; Matsumoto, S.; Ibayashi, Y.; Furuyama, Y.; Ohgane, K.; Kamisuki, S.; Watashi, K.; et al. Synthesis and Biological Assessment of Eucalyptin: Magic Methyl Effects. Int. J. Mol. Sci. 2025, 26, 3391. https://doi.org/10.3390/ijms26073391
Fuchiyama K, Yabuki Y, Yamamoto Y, Asakawa R, Matsumoto S, Ibayashi Y, Furuyama Y, Ohgane K, Kamisuki S, Watashi K, et al. Synthesis and Biological Assessment of Eucalyptin: Magic Methyl Effects. International Journal of Molecular Sciences. 2025; 26(7):3391. https://doi.org/10.3390/ijms26073391
Chicago/Turabian StyleFuchiyama, Kanta, Yuka Yabuki, Yuzu Yamamoto, Ryuki Asakawa, Saki Matsumoto, Yuuka Ibayashi, Yuuki Furuyama, Kenji Ohgane, Shinji Kamisuki, Koichi Watashi, and et al. 2025. "Synthesis and Biological Assessment of Eucalyptin: Magic Methyl Effects" International Journal of Molecular Sciences 26, no. 7: 3391. https://doi.org/10.3390/ijms26073391
APA StyleFuchiyama, K., Yabuki, Y., Yamamoto, Y., Asakawa, R., Matsumoto, S., Ibayashi, Y., Furuyama, Y., Ohgane, K., Kamisuki, S., Watashi, K., Matsubayashi, M., & Kuramochi, K. (2025). Synthesis and Biological Assessment of Eucalyptin: Magic Methyl Effects. International Journal of Molecular Sciences, 26(7), 3391. https://doi.org/10.3390/ijms26073391








