The E3 Ubiquitin Ligase PRAJA1: A Key Regulator of Synaptic Dynamics and Memory Processes with Implications for Alzheimer’s Disease
Abstract
1. Introduction
2. Results
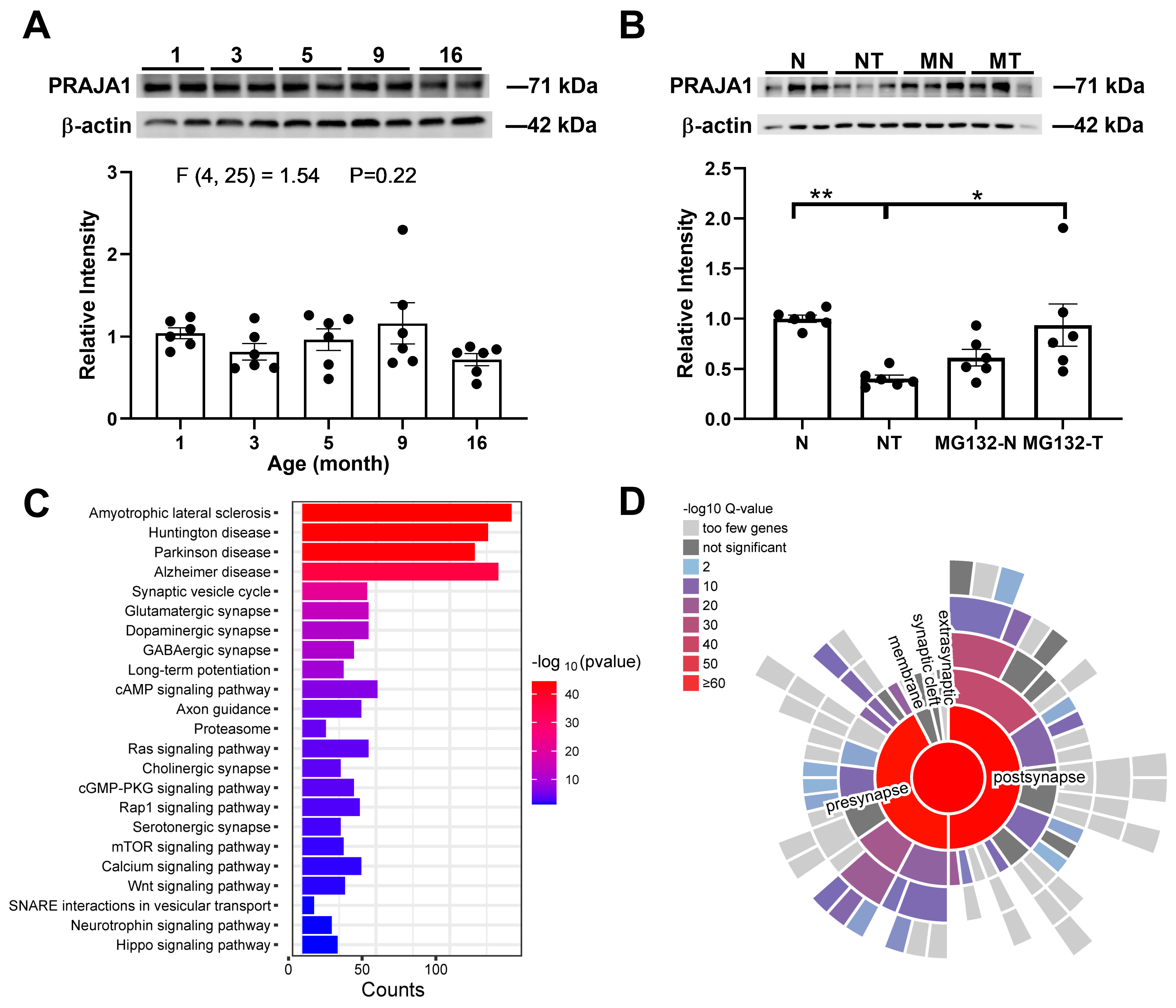
2.1. PRAJA1 Expression and Activity-Dependent Regulation in the Hippocampus
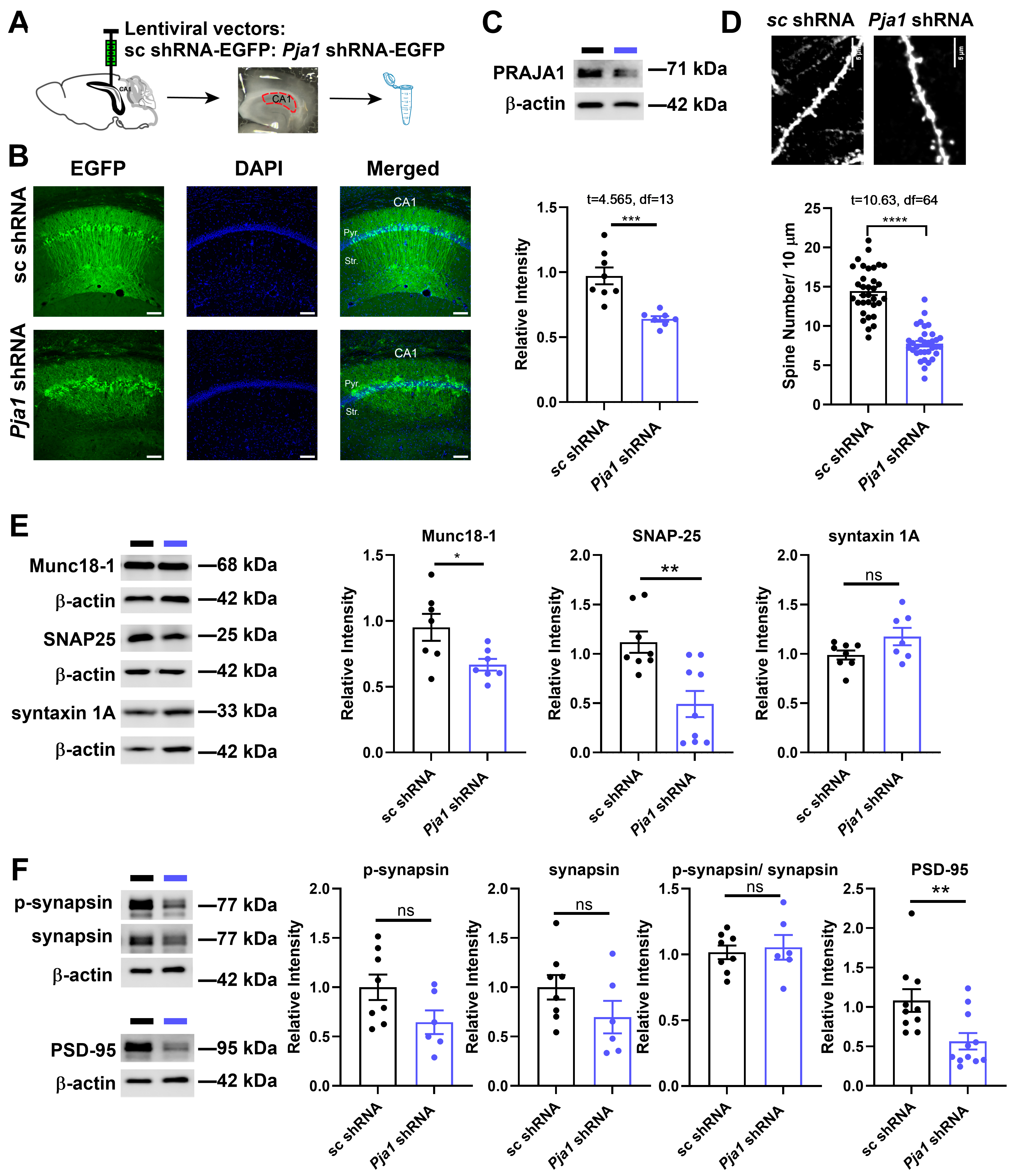
2.2. PRAJA1 Deficiency in CA1 Disrupts Synaptic Structure and Function
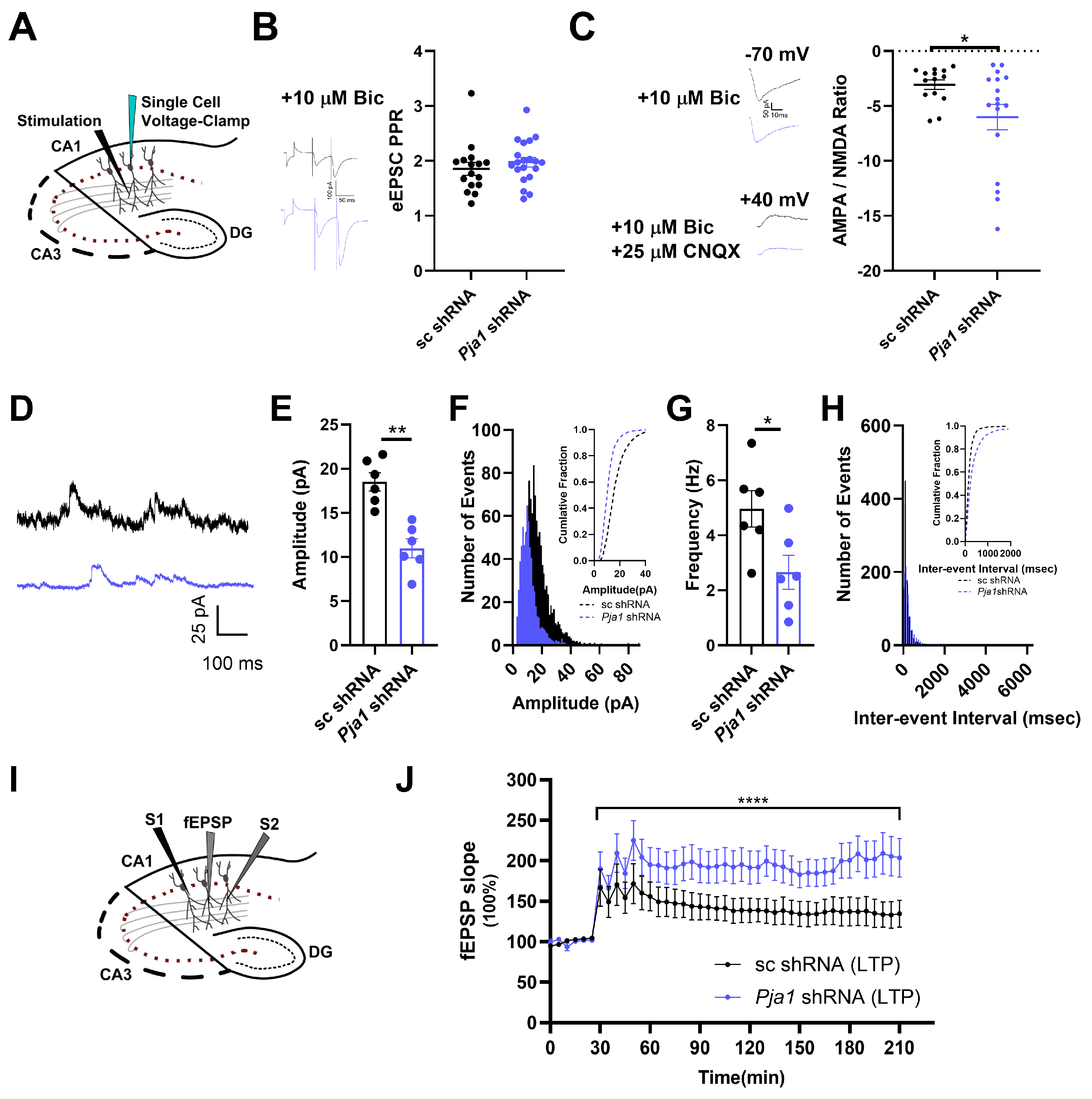
2.3. PRAJA1 Deficiency in CA1 Region Disrupts Glutamatergic and GABAergic Synaptic Transmission, Leading to Enhanced LTP
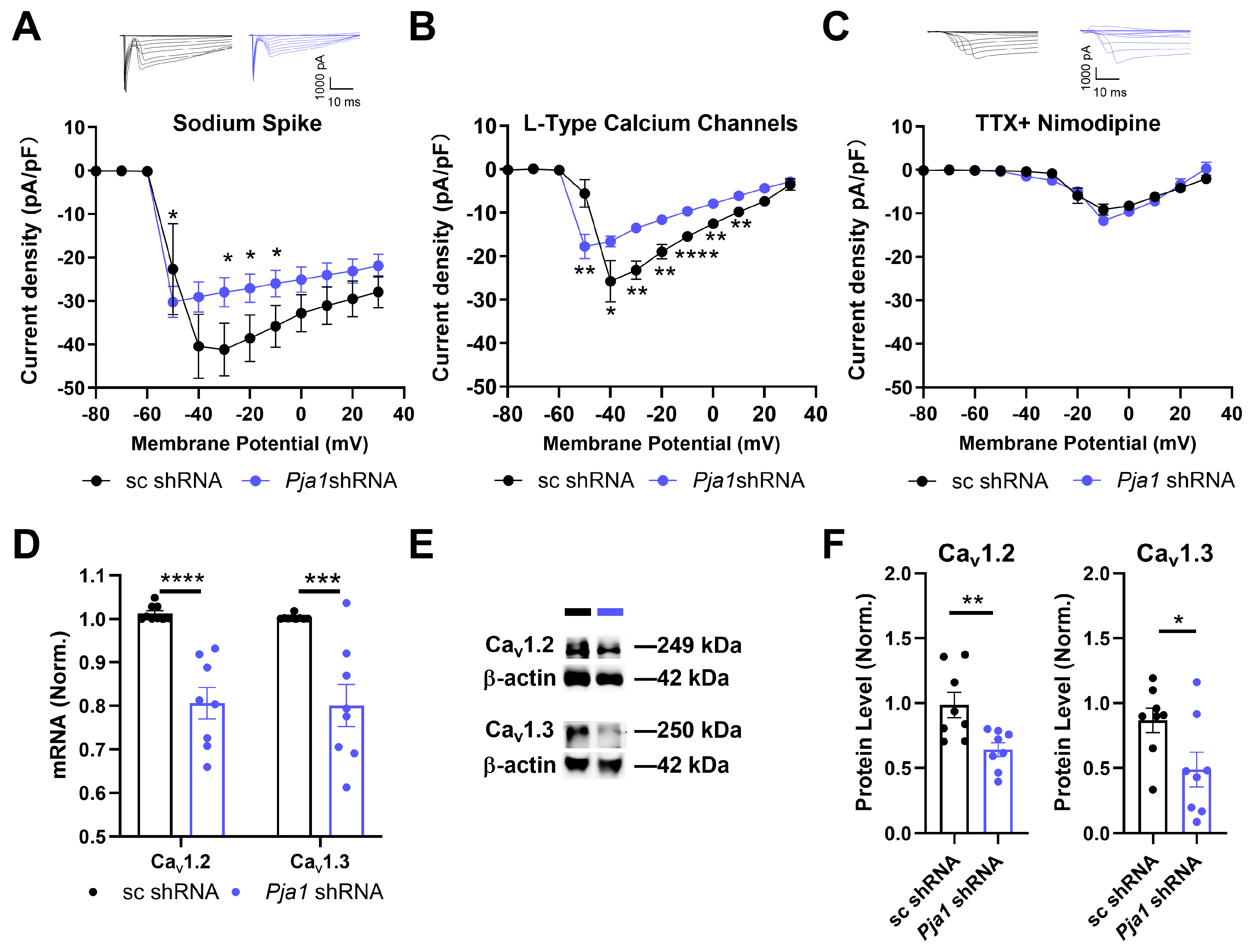
2.4. PRAJA1 Regulates Neuronal Excitability and Voltage-Gated Channel Expression
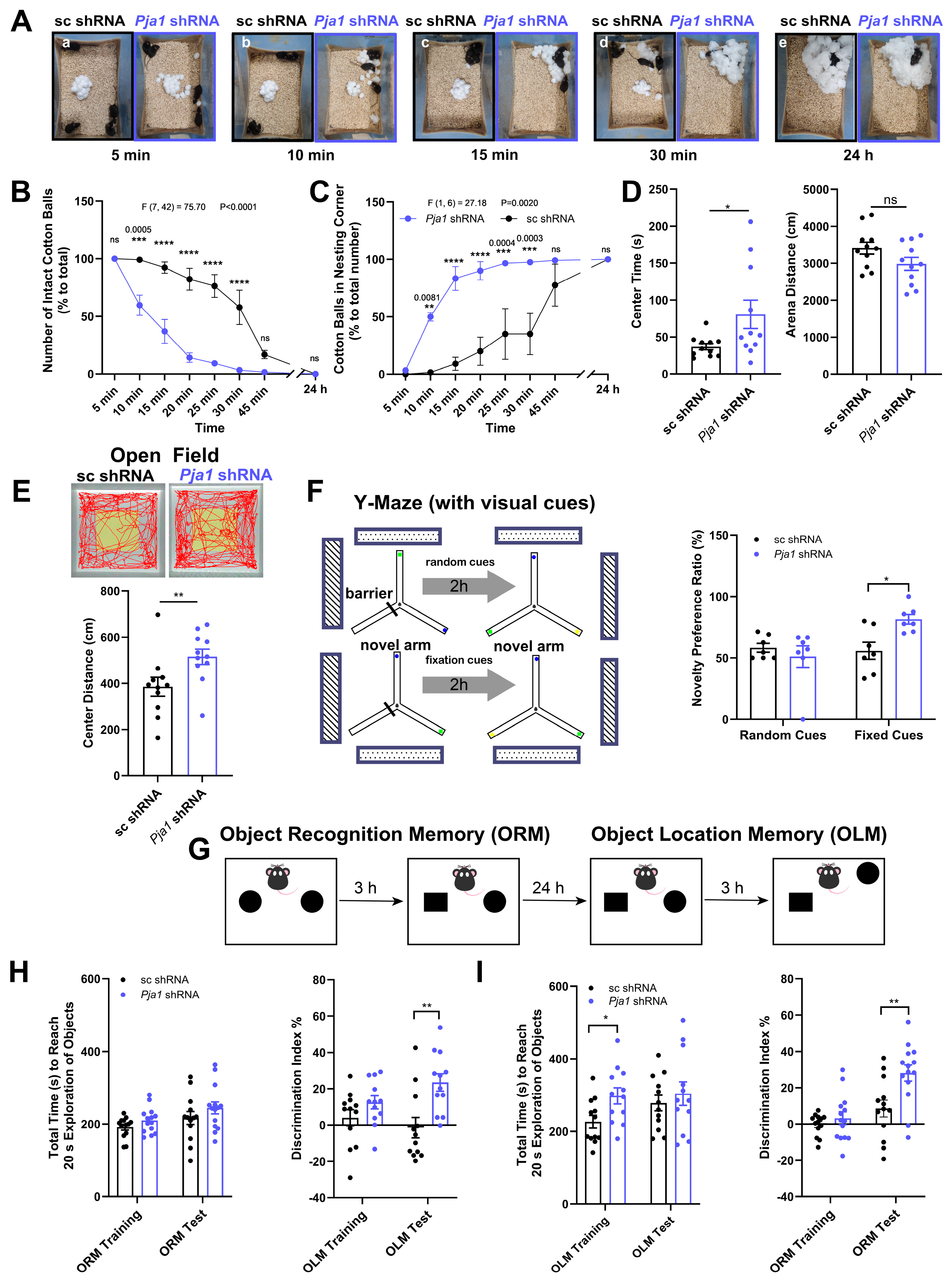
2.5. PRAJA1 Deficiency Enhances Specific Aspects of Learning and Memory
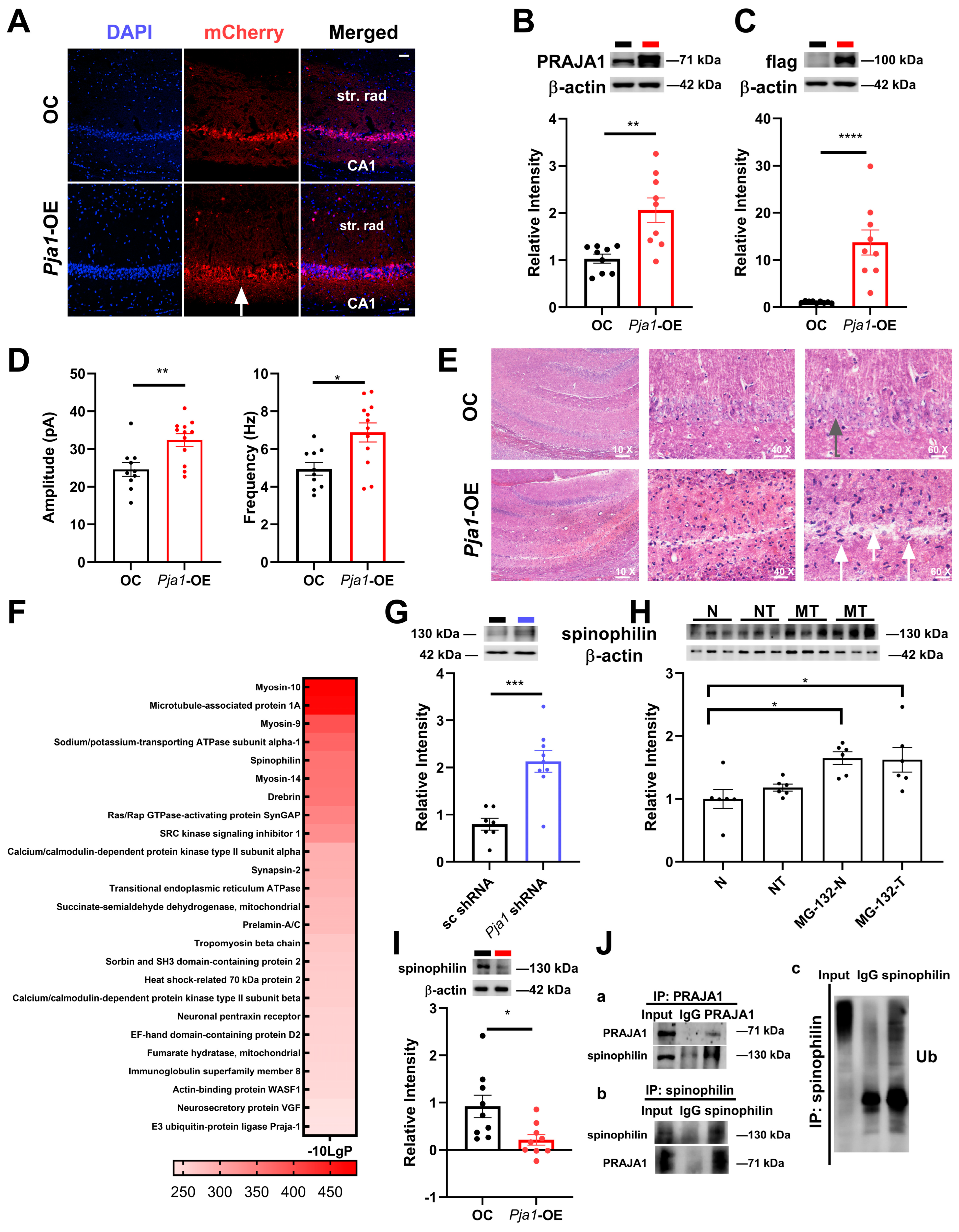
2.6. Consequences of Pja1 Overexpression in the Hippocampal CA1 Region
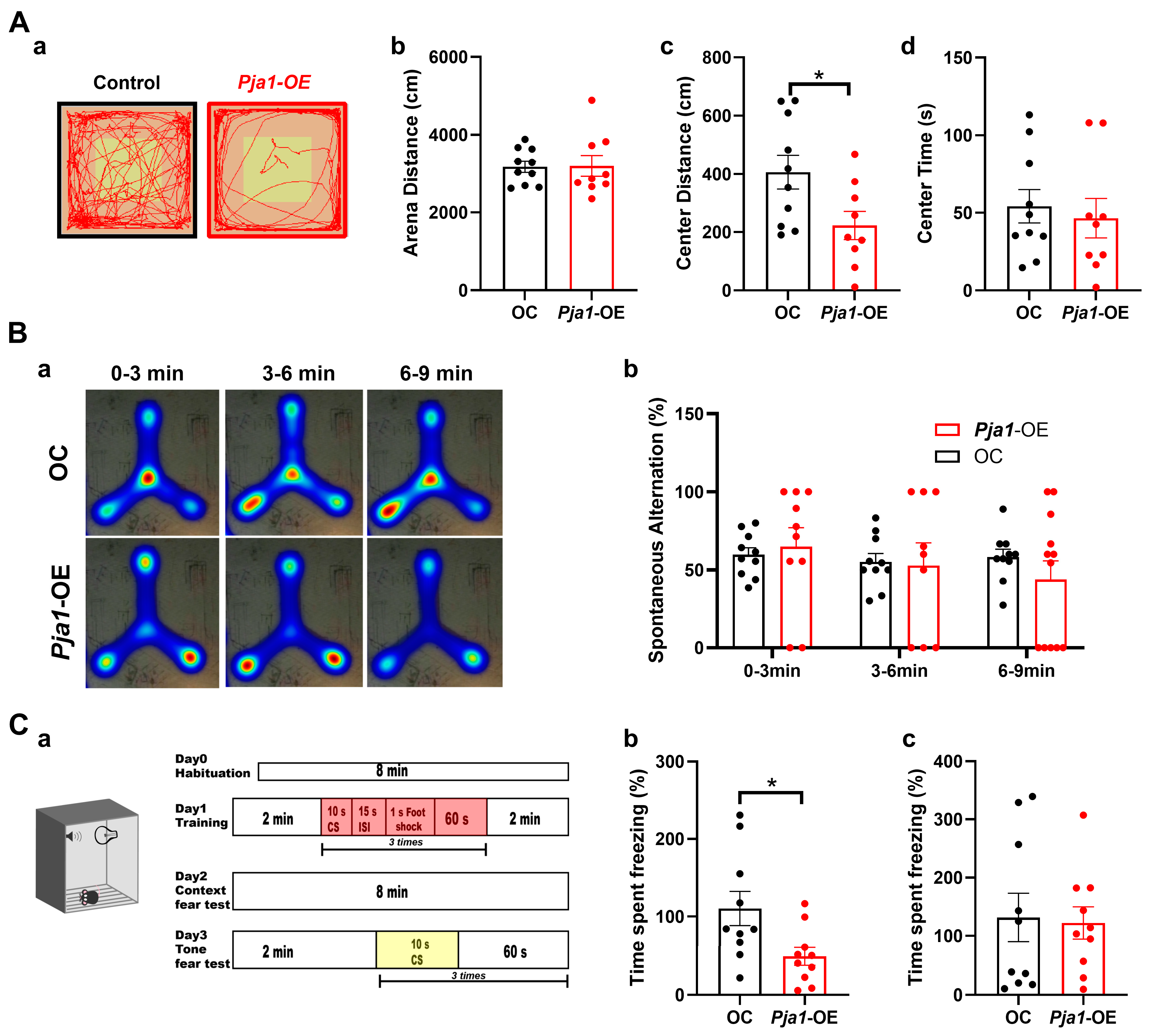
2.7. Behavioral Consequences of PRAJA1 Overexpression in the Hippocampal CA1 Region
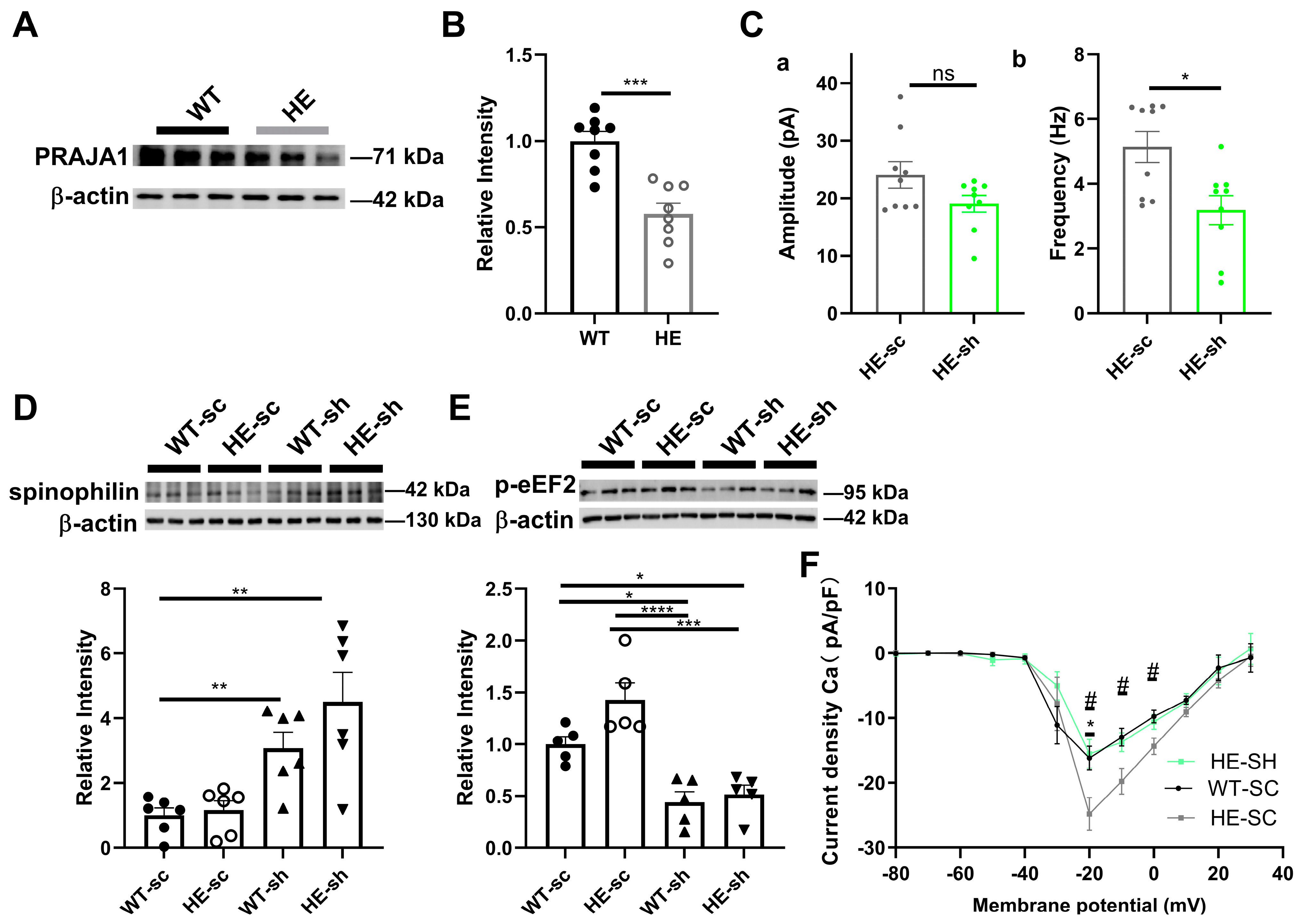
2.8. Exploring the Role of PRAJA1 in an Alzheimer’s Disease-like Animal Model
3. Discussion
3.1. Pja1 Regulates Synaptic Protein Expression and Density
3.2. Pja1 Critically Influences Synaptic Transmission and Neuronal Excitability
3.3. Pja1 Acts as a Constraint on Specific Hippocampus-Dependent Memory Processes
3.4. Pja1 and Spinophilin: A Critical Link to Alzheimer’s Disease Pathogenesis
3.5. Translational Relevance of PRAJA1
3.6. Unresolved Questions and Future Directions
4. Materials and Methods
4.1. Animals
4.2. Stereotaxic Intrahippocampal Injections
4.3. Reverse Transcription Quantitative Polymerase Chain Reaction
4.4. Western Blot Analysis
4.5. Immunofluorescence and Von Kossa’s Staining
4.6. Coimmunoprecipitationh
4.7. Coimmunoprecipitation with Subsequent Mass Spectrometry
4.8. Field Potential Recordings
4.9. Whole-Cell Voltage-Clamp Recordings
4.10. Nest-Building Behavior
4.11. Open Field Test
4.12. Y-Maze Test and Spatial Novelty Y-Maze Test
4.13. Object Recognition Test and Object Location Test
4.14. Trace Fear Conditioning Task
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| eEPSC | evoked excitatory postsynaptic current |
| fEPSP | field excitatory postsynaptic potential |
| LTP | long-term potentiation |
| mIPSCs | miniature inhibitory postsynaptic currents |
| OLM | Object location memory |
| ORM | Object recognition memory |
| PPR | paired-pulse ratio |
| STET | strong high-frequency stimulation |
| UPS | ubiquitin-proteasome system |
References
- Hegde, A.N. Proteolysis, synaptic plasticity and memory. Neurobiol. Learn. Mem. 2017, 138, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Cali, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Murata, T.; Suzuki, E.; Ito, S.; Sawatsubashi, S.; Zhao, Y.; Yamagata, K.; Tanabe, M.; Fujiyama, S.; Kimura, S.; Ueda, T.; et al. RNA-binding protein hoip accelerates polyQ-induced neurodegeneration in Drosophila. Biosci. Biotechnol. Biochem. 2008, 72, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, T.T.; Xie, G.G.; Zhu, X.Q.; Wang, Y. Ubiquitin ligase TRIM32 promotes dendrite arborization by mediating degradation of the epigenetic factor CDYL. FASEB J. 2022, 36, e22087. [Google Scholar] [CrossRef]
- Pavlou, M.A.S.; Colombo, N.; Fuertes-Alvarez, S.; Nicklas, S.; Cano, L.G.; Marin, M.C.; Goncalves, J.; Schwamborn, J.C. Expression of the Parkinson’s Disease-Associated Gene Alpha-Synuclein is Regulated by the Neuronal Cell Fate Determinant TRIM32. Mol. Neurobiol. 2017, 54, 4257–4270. [Google Scholar] [CrossRef]
- Mishra, L.; Tully, R.E.; Monga, S.P.; Yu, P.; Cai, T.; Makalowski, W.; Mezey, E.; Pavan, W.J.; Mishra, B. Praja1, a novel gene encoding a RING-H2 motif in mouse development. Oncogene 1997, 15, 2361–2368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, N. Ubiquitin System. Int. J. Mol. Sci. 2018, 19, 1080. [Google Scholar] [CrossRef]
- Stork, O.; Welzl, H. Memory formation and the regulation of gene expression. Cell. Mol. Life Sci. 1999, 55, 575–592. [Google Scholar] [CrossRef]
- Shin, J.; Mishra, V.; Glasgow, E.; Zaidi, S.; Chen, J.; Ohshiro, K.; Chitti, B.; Kapadia, A.A.; Rana, N.; Mishra, L.; et al. PRAJA is overexpressed in glioblastoma and contributes to neural precursor development. Genes Cancer 2017, 8, 640–649. [Google Scholar] [CrossRef]
- Consalvi, S.; Brancaccio, A.; Dall’Agnese, A.; Puri, P.L.; Palacios, D. Praja1 E3 ubiquitin ligase promotes skeletal myogenesis through degradation of EZH2 upon p38alpha activation. Nat. Commun. 2017, 8, 13956. [Google Scholar] [CrossRef]
- Sasaki, A.; Masuda, Y.; Iwai, K.; Ikeda, K.; Watanabe, K. A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J. Biol. Chem. 2002, 277, 22541–22546. [Google Scholar] [CrossRef]
- Loch, C.M.; Eddins, M.J.; Strickler, J.E. Protein microarrays for the identification of praja1 e3 ubiquitin ligase substrates. Cell Biochem. Biophys. 2011, 60, 127–135. [Google Scholar] [CrossRef]
- Teuber, J.; Mueller, B.; Fukabori, R.; Lang, D.; Albrecht, A.; Stork, O. The ubiquitin ligase Praja1 reduces NRAGE expression and inhibits neuronal differentiation of PC12 cells. PLoS ONE 2013, 8, e63067. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Feng, B.; Ma, X.; Zhang, K.; Yang, F.; Liu, Z.; Yang, L.; Yue, J.; Lu, L.; et al. PJA1 mediates the effects of astrocytic GPR30 on learning and memory in female mice. J. Clin. Investig. 2023, 133, e165812. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Karmakar, S.; Prasad, M.; Mandal, A.K. Praja1 ubiquitin ligase facilitates degradation of polyglutamine proteins and suppresses polyglutamine-mediated toxicity. Mol. Biol. Cell 2021, 32, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Watabe, K.; Kato, Y.; Sakuma, M.; Murata, M.; Niida-Kawaguchi, M.; Takemura, T.; Hanagata, N.; Tada, M.; Kakita, A.; Shibata, N. Praja1 RING-finger E3 ubiquitin ligase suppresses neuronal cytoplasmic TDP-43 aggregate formation. Neuropathology 2020, 40, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Wieland, I.; Weidner, C.; Ciccone, R.; Lapi, E.; McDonald-McGinn, D.; Kress, W.; Jakubiczka, S.; Collmann, H.; Zuffardi, O.; Zackai, E.; et al. Contiguous gene deletions involving EFNB1, OPHN1, PJA1 and EDA in patients with craniofrontonasal syndrome. Clin. Genet. 2007, 72, 506–516. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Raveau, M.; Miyake, N.; Sudo, G.; Tsurusaki, Y.; Watanabe, T.; Sugaya, Y.; Tatsukawa, T.; Mazaki, E.; et al. A recurrent PJA1 variant in trigonocephaly and neurodevelopmental disorders. Ann. Clin. Transl. Neurol. 2020, 7, 1117–1131. [Google Scholar] [CrossRef]
- Stork, O.; Stork, S.; Pape, H.C.; Obata, K. Identification of genes expressed in the amygdala during the formation of fear memory. Learn. Mem. 2001, 8, 209–219. [Google Scholar] [CrossRef]
- Stein, T.D.; Anders, N.J.; DeCarli, C.; Chan, S.L.; Mattson, M.P.; Johnson, J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J. Neurosci. 2004, 24, 7707–7717. [Google Scholar] [CrossRef]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234e214. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, D.F.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.L.; Bi, R.; Yao, Y.G. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer’s disease. Alzheimers Dement. 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Zhang, D.F.; Fan, Y.; Xu, M.; Wang, G.; Wang, D.; Li, J.; Kong, L.L.; Zhou, H.; Luo, R.; Bi, R.; et al. Complement C7 is a novel risk gene for Alzheimer’s disease in Han Chinese. Natl. Sci. Rev. 2019, 6, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.P.; Prinslow, E.A.; Rizo, J. Munc18-1 is crucial to overcome the inhibition of synaptic vesicle fusion by alphaSNAP. Nat. Commun. 2019, 10, 4326. [Google Scholar] [CrossRef]
- Kadkova, A.; Murach, J.; Ostergaard, M.; Malsam, A.; Malsam, J.; Lolicato, F.; Nickel, W.; Sollner, T.H.; Sorensen, J.B. SNAP25 disease mutations change the energy landscape for synaptic exocytosis due to aberrant SNARE interactions. Elife 2024, 12, RP88619. [Google Scholar] [CrossRef]
- Verhage, M.; Sorensen, J.B. SNAREopathies: Diversity in Mechanisms and Symptoms. Neuron 2020, 107, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yan, Z.; Ferreira, A.; Tomizawa, K.; Liauw, J.A.; Zhuo, M.; Allen, P.B.; Ouimet, C.C.; Greengard, P. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA. 2000, 97, 9287–9292. [Google Scholar] [CrossRef]
- Lu, Y.M.; Mansuy, I.M.; Kandel, E.R.; Roder, J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron 2000, 26, 197–205. [Google Scholar] [CrossRef]
- Sarkar, S.N.; Huang, R.Q.; Logan, S.M.; Yi, K.D.; Dillon, G.H.; Simpkins, J.W. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2008, 105, 15148–15153. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Quinn, J.F.; Harris, C.; Frahler, K.; Wadsworth, T.L.; Woltjer, R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011, 41, 62–70. [Google Scholar] [CrossRef]
- Simakova, O.; Arispe, N.J. Early and late cytotoxic effects of external application of the Alzheimer’s Abeta result from the initial formation and function of Abeta ion channels. Biochemistry 2006, 45, 5907–5915. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.Y.; Hyman, B.T.; Bacskai, B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Coon, A.L.; Wallace, D.R.; Mactutus, C.F.; Booze, R.M. L-type calcium channels in the hippocampus and cerebellum of Alzheimer’s disease brain tissue. Neurobiol. Aging 1999, 20, 597–603. [Google Scholar] [CrossRef]
- Campbell, L.W.; Hao, S.Y.; Thibault, O.; Blalock, E.M.; Landfield, P.W. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J. Neurosci. 1996, 16, 6286–6295. [Google Scholar] [CrossRef]
- Choi, J.; Chandrasekaran, K.; Demarest, T.G.; Kristian, T.; Xu, S.; Vijaykumar, K.; Dsouza, K.G.; Qi, N.R.; Yarowsky, P.J.; Gallipoli, R.; et al. Brain diabetic neurodegeneration segregates with low intrinsic aerobic capacity. Ann. Clin. Transl. Neurol. 2014, 1, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Ciernia, A.; Wood, M.A. Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 2014, 69, 8.31.1–8.31.17. [Google Scholar] [CrossRef] [PubMed]
- Haettig, J.; Stefanko, D.P.; Multani, M.L.; Figueroa, D.X.; McQuown, S.C.; Wood, M.A. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn. Mem. 2011, 18, 71–79. [Google Scholar] [CrossRef]
- Mehta, B.; Snellman, J.; Chen, S.; Li, W.; Zenisek, D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron 2013, 77, 516–527. [Google Scholar] [CrossRef]
- Gosrani, S.P.; Jester, H.M.; Zhou, X.; Ryazanov, A.G.; Ma, T. Repression of eEF2 kinase improves deficits in novel object recognition memory in aged mice. Neurobiol. Aging 2020, 95, 154–160. [Google Scholar] [CrossRef]
- Beckelman, B.C.; Yang, W.; Kasica, N.P.; Zimmermann, H.R.; Zhou, X.; Keene, C.D.; Ryazanov, A.G.; Ma, T. Genetic reduction of eEF2 kinase alleviates pathophysiology in Alzheimer’s disease model mice. J. Clin. Investig. 2019, 129, 820–833. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, Y.; Sun, W.; Yu, Y.; Wei, N.; Du, R.; Yang, Y.; Liang, T.; Wang, X.L.; Ou, C.H.; et al. Spinophilin modulates pain through suppressing dendritic spine morphogenesis via negative control of Rac1-ERK signaling in rat spinal dorsal horn. Neurobiol. Dis. 2021, 152, 105302. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.W.; Watkins, D.S.; Shah, N.R.; Pennington, T.; Hens, B.; Qi, G.; Doud, E.H.; Mosley, A.L.; Atwood, B.K.; Baucum, A.J., 2nd. Spinophilin Limits Metabotropic Glutamate Receptor 5 Scaffolding to the Postsynaptic Density and Cell Type Specifically Mediates Excessive Grooming. Biol. Psychiatry 2023, 93, 976–988. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Sun, M.K.; Alkon, D.L. PKC epsilon activation prevents synaptic loss, Abeta elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 2011, 31, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Christoffel, D.; Rocher, A.B.; Bouras, C.; Kovari, E.; Perl, D.P.; Morrison, J.H.; Herrmann, F.R.; Haroutunian, V.; Giannakopoulos, P.; et al. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: Relationship with the progression of Alzheimer’s disease. Neurobiol. Aging 2008, 29, 1296–1307. [Google Scholar] [CrossRef]
- Gao, J.; Hu, X.D.; Yang, H.; Xia, H. Distinct Roles of Protein Phosphatase 1 Bound on Neurabin and Spinophilin and Its Regulation in AMPA Receptor Trafficking and LTD Induction. Mol. Neurobiol. 2018, 55, 7179–7186. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Chen, C.Y.; Li, Z.; Martin, A.R.; Bryois, J.; Ma, X.; Gaspar, H.; Ikeda, M.; Benyamin, B.; Brown, B.C.; et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019, 51, 1670–1678. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, C.; Zhuang, Y.; Chen, Y.; Behnisch, T. Direct Medial Entorhinal Cortex Input to Hippocampal CA3 Is Crucial for eEF2K Inhibitor-Induced Neuronal Oscillations in the Mouse Hippocampus. Front. Cell. Neurosci. 2020, 14, 24. [Google Scholar] [CrossRef]
- Li, D.; Jing, D.; Liu, Z.; Chen, Y.; Huang, F.; Behnisch, T. Enhanced Expression of Secreted alpha-Klotho in the Hippocampus Alters Nesting Behavior and Memory Formation in Mice. Front. Cell. Neurosci. 2019, 13, 133. [Google Scholar] [CrossRef]
- Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H.; Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006, 1, 3166–3173. [Google Scholar] [CrossRef]
- Lu, Q.; Murakami, C.; Murakami, Y.; Hoshino, F.; Asami, M.; Usuki, T.; Sakai, H.; Sakane, F. 1-Stearoyl-2-docosahexaenoyl-phosphatidic acid interacts with and activates Praja-1, the E3 ubiquitin ligase acting on the serotonin transporter in the brain. FEBS Lett. 2020, 594, 1787–1796. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Zhang, W.; Chen, Y.; Li, C.; Zhao, F.; Behnisch, T. Positive Regulatory Domain I-binding Factor 1 Mediates Peripheral Nerve Injury-induced Nociception in Mice by Repressing Kv4.3 Channel Expression. Anesthesiology 2021, 134, 435–456. [Google Scholar] [CrossRef]
- Yun, D.; Zhuang, Y.; Kreutz, M.R.; Behnisch, T. The role of 19S proteasome associated deubiquitinases in activity-dependent hippocampal synaptic plasticity. Neuropharmacology 2018, 133, 354–365. [Google Scholar] [CrossRef]
- Weng, W.; Li, D.; Peng, C.; Behnisch, T. Recording Synaptic Plasticity in Acute Hippocampal Slices Maintained in a Small-volume Recycling-, Perfusion-, and Submersion-type Chamber System. J. Vis. Exp. 2018, 131, e55936. [Google Scholar] [CrossRef]
- Jing, D.; Li, D.; Peng, C.; Chen, Y.; Behnisch, T. Role of microtubules in late-associative plasticity of hippocampal Schaffer collateral-CA1 synapses in mice. Neurobiol. Learn. Mem. 2019, 163, 107038. [Google Scholar] [CrossRef]
- Ting, J.T.; Daigle, T.L.; Chen, Q.; Feng, G. Acute brain slice methods for adult and aging animals: Application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 2014, 1183, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, M.; Vakhitova, J.V.; Yamidanov, R.S.; Salimgareeva, M.; Seredenin, S.B.; Behnisch, T. The effects of ladasten on dopaminergic neurotransmission and hippocampal synaptic plasticity in rats. Neuropharmacology 2007, 53, 601–608. [Google Scholar] [CrossRef]
- Behnisch, T.; Wilsch, V.W.; Balschun, D.; Reymann, K.G. The role of group II metabotropic glutamate receptors in hippocampal CA1 long-term potentiation in vitro. Eur. J. Pharmacol. 1998, 356, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, Y.; Lin, D.; Zheng, P.; Zhang, P.; Hu, M.; Wang, Q.; Pan, W.; Yang, X.; Hu, T.; et al. beta-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020, 8, 143. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Joca, L.; Zuloaga, D.G.; Raber, J.; Siegel, J.A. Long-term effects of early adolescent methamphetamine exposure on depression-like behavior and the hypothalamic vasopressin system in mice. Dev. Neurosci. 2014, 36, 108–118. [Google Scholar] [CrossRef]
- Nogueira Neto, J.D.; de Almeida, A.A.; da Silva Oliveira, J.; Dos Santos, P.S.; de Sousa, D.P.; de Freitas, R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Hong, X.; Liu, J.; Zhu, G.; Zhuang, Y.; Suo, H.; Wang, P.; Huang, D.; Xu, J.; Huang, Y.; Yu, M.; et al. Parkin overexpression ameliorates hippocampal long-term potentiation and beta-amyloid load in an Alzheimer’s disease mouse model. Hum. Mol. Genet. 2014, 23, 1056–1072. [Google Scholar] [CrossRef]
- Clement, Y.; Calatayud, F.; Belzung, C. Genetic basis of anxiety-like behaviour: A critical review. Brain Res. Bull. 2002, 57, 57–71. [Google Scholar] [CrossRef]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Zhao, F.; Wang, C.F.; Zhang, X.M.; Xiao, Y.; Zhou, F.; Wu, M.N.; Zhang, J.; Qi, J.S.; Yang, W. Xestospongin C, a Reversible IP3 Receptor Antagonist, Alleviates the Cognitive and Pathological Impairments in APP/PS1 Mice of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Li, A.; Li, X.Y.; Zhou, Y.; Li, X.; He, Z.; Wang, L.; Reilly, J.; Tan, Z.; Xiao, Z.Y.; et al. Trans-urocanic acid facilitates spatial memory, implications for Alzheimer’s disease. Physiol. Behav. 2022, 252, 113827. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, F.K.; Pulford, L.J.; Barkus, C.; Liao, F.; Portelius, E.; Webb, R.; Chavez-Gutierrez, L.; Cleverley, K.; Noy, S.; Sheppard, O.; et al. Trisomy of human chromosome 21 enhances amyloid-beta deposition independently of an extra copy of APP. Brain 2018, 141, 2457–2474. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Binder, S.; Baier, P.C.; Molle, M.; Inostroza, M.; Born, J.; Marshall, L. Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiol. Learn. Mem. 2012, 97, 213–219. [Google Scholar] [CrossRef]
- Raza, S.A.; Albrecht, A.; Caliskan, G.; Muller, B.; Demiray, Y.E.; Ludewig, S.; Meis, S.; Faber, N.; Hartig, R.; Schraven, B.; et al. HIPP neurons in the dentate gyrus mediate the cholinergic modulation of background context memory salience. Nat. Commun. 2017, 8, 189. [Google Scholar] [CrossRef]
- Han, C.J.; O’Tuathaigh, C.M.; van Trigt, L.; Quinn, J.J.; Fanselow, M.S.; Mongeau, R.; Koch, C.; Anderson, D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA. 2003, 100, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Burman, M.A.; Simmons, C.A.; Hughes, M.; Lei, L. Developing and validating trace fear conditioning protocols in C57BL/6 mice. J. Neurosci. Methods 2014, 222, 111–117. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward | Reverse |
|---|---|---|
| Cav1.2 | 5′-GAGACAATGTGTGGAATATGCC-3′ | 3′-GTAGGTAGAGTTGACCACGTAC-5′ |
| Cav1.3 | 5′-GCACAGATGAAGCCAAAAGTAA-3′ | 3′-CGTTCTCACCGTTTGAATCAAT-5′ |
| Gapdh | 5′-AGGTCGGTGTGAACGGATTTG-3′ | 3′-TGTAGACCATGTAGTTGAGGTCA-5′ |
| Gabra1 | 5′-CACCATGAGGTTGACCGTGA-3′ | 3′-CTACAACCACTGAACGGGCT-5′ |
| Gabrb1 | 5′-CATAGACATGGTCTCGGAAG-3′ | 3′-GTCAGCTACTCTGTTGTCAA-5′ |
| Gabrb2 | 5′-ATTTGGTGGCTCAAACGGTC-3′ | 3′-GAGATTTCCTCACCAGCAGGA-5′ |
| Gabrg2 | 5′-GGAGCCGGCATCAAATCATC-3′ | 3′-CTTTTGGCTTGTGAAGCCTGG-5′ |
| Gabrd | 5′-ATACACCATGACTGTGTTCC-3′ | 3′-TAGGCGGATAAGCTTGTTTT-5′ |
| Grin1 | 5′-CATCGGACTTCAGCTAATCA-3′ | 3′-GTCCCCATCCTCATTGAATT-5′ |
| Grin2a | 5′-GGCTACAGAGACTTCATCAG-3′ | 3′-ATCCAGAAGAAATCGTAGCC-5′ |
| Grin2b | 5′-TTAACAACTCCGTACCTGTG-3′ | 3′-TGGAACTTCTTGTCACTCAG-5′ |
| Gria1 | 5′-GCCTTAATCGAGTTCTGCTA-3′ | 3′-GAATGGATTGCATGGACTTG-5′ |
| Gria2 | 5′-AGCCTATGAGATCTGGATGT-3′ | 3′-GAGAGAGATCTTGGCGAAAT-5′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yan, Y.; Stork, O.; Shen, R.; Behnisch, T. The E3 Ubiquitin Ligase PRAJA1: A Key Regulator of Synaptic Dynamics and Memory Processes with Implications for Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 2909. https://doi.org/10.3390/ijms26072909
Li C, Yan Y, Stork O, Shen R, Behnisch T. The E3 Ubiquitin Ligase PRAJA1: A Key Regulator of Synaptic Dynamics and Memory Processes with Implications for Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(7):2909. https://doi.org/10.3390/ijms26072909
Chicago/Turabian StyleLi, Chuhan, Yan Yan, Oliver Stork, Ruling Shen, and Thomas Behnisch. 2025. "The E3 Ubiquitin Ligase PRAJA1: A Key Regulator of Synaptic Dynamics and Memory Processes with Implications for Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 7: 2909. https://doi.org/10.3390/ijms26072909
APA StyleLi, C., Yan, Y., Stork, O., Shen, R., & Behnisch, T. (2025). The E3 Ubiquitin Ligase PRAJA1: A Key Regulator of Synaptic Dynamics and Memory Processes with Implications for Alzheimer’s Disease. International Journal of Molecular Sciences, 26(7), 2909. https://doi.org/10.3390/ijms26072909





