The Impact of Soybean Genotypes on Rhizosphere Microbial Dynamics and Nodulation Efficiency
Abstract
1. Introduction
2. Results
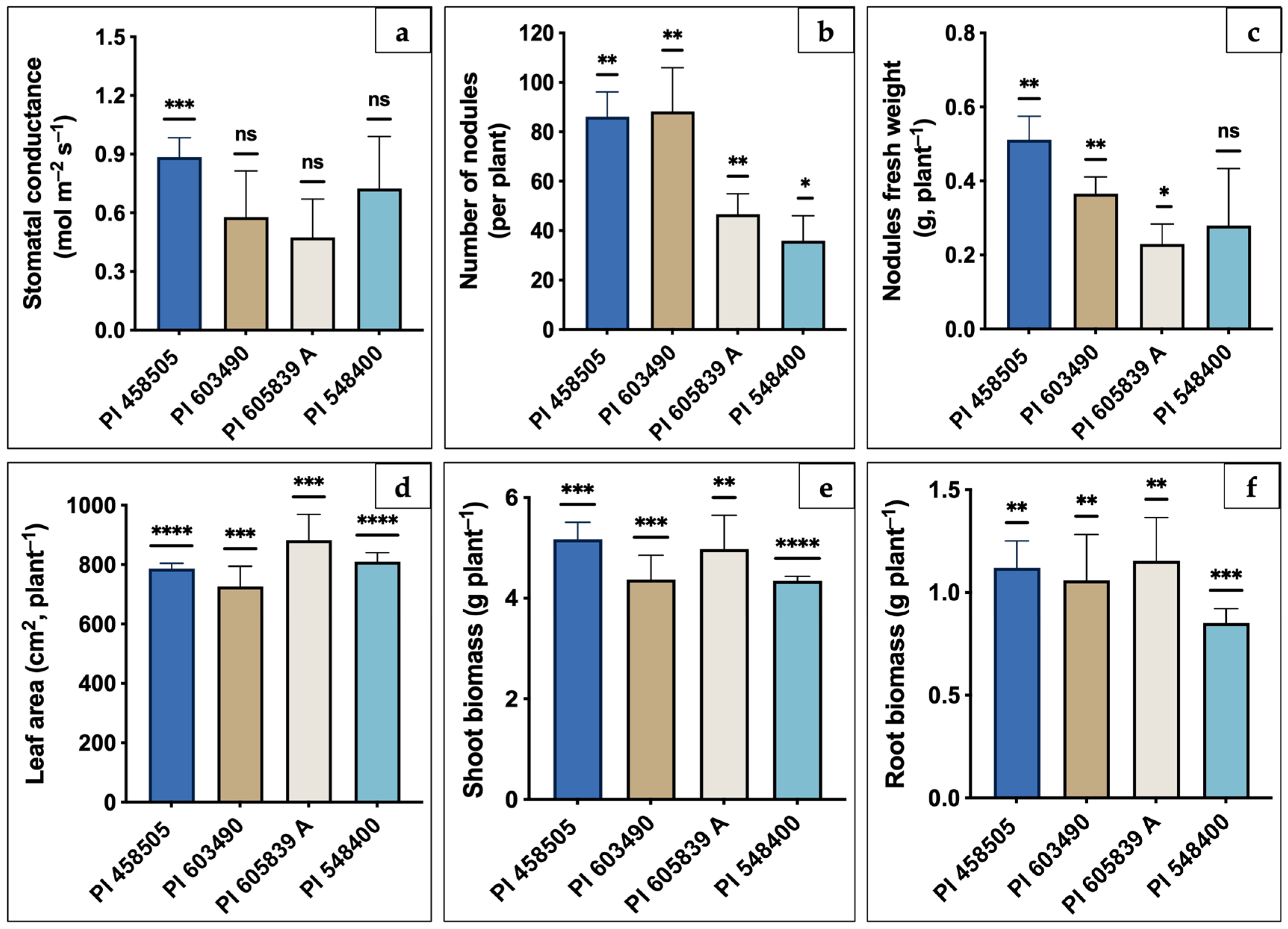
2.1. Physiological Responses of Different Soybean Genotypes
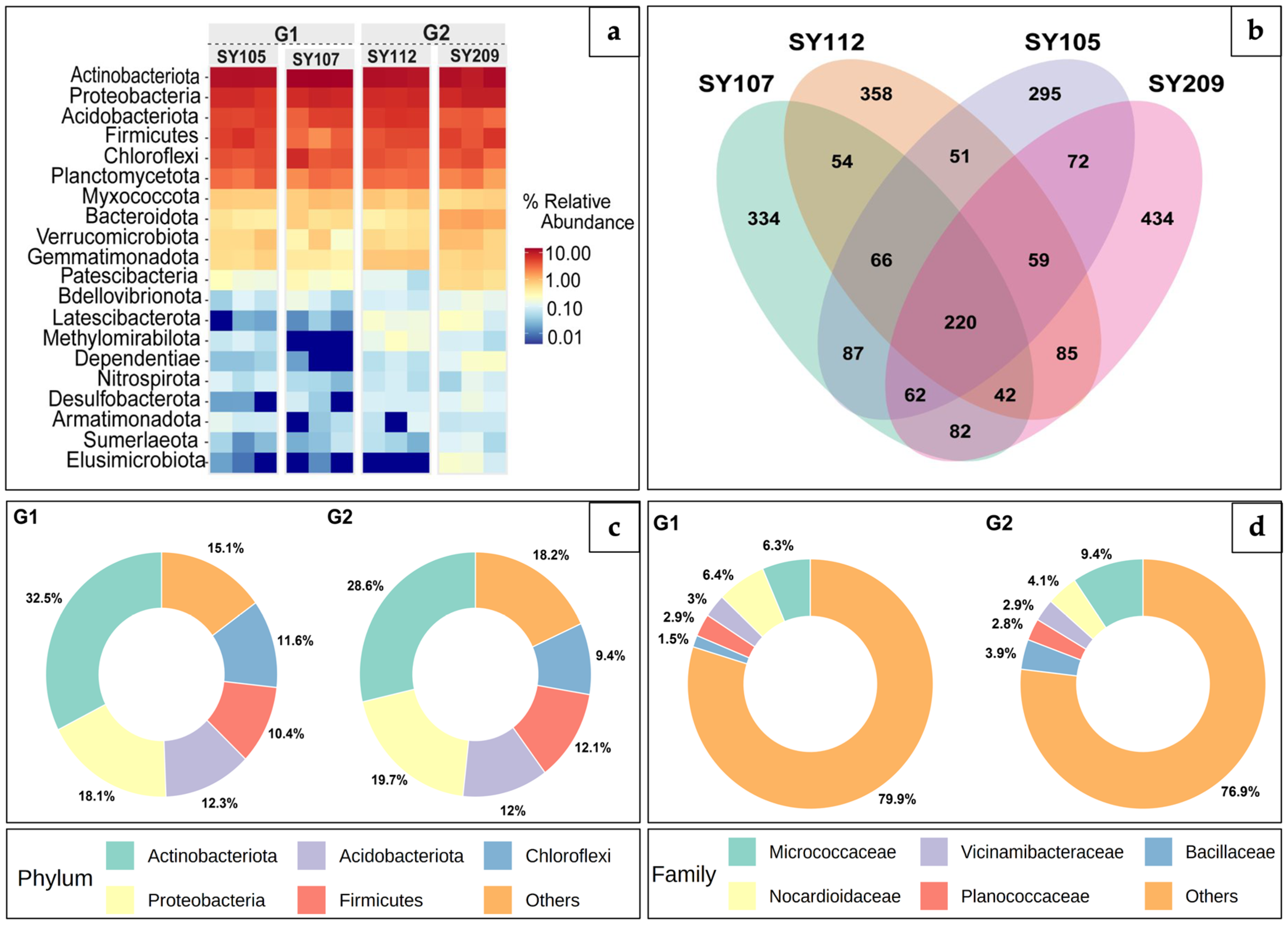
2.2. Evaluating Microbial Diversity in Soybean Rhizosphere Through Taxonomic Annotation
2.3. Differential Abundance Analysis Comparing High and Low-Nodulation Soybean Groups
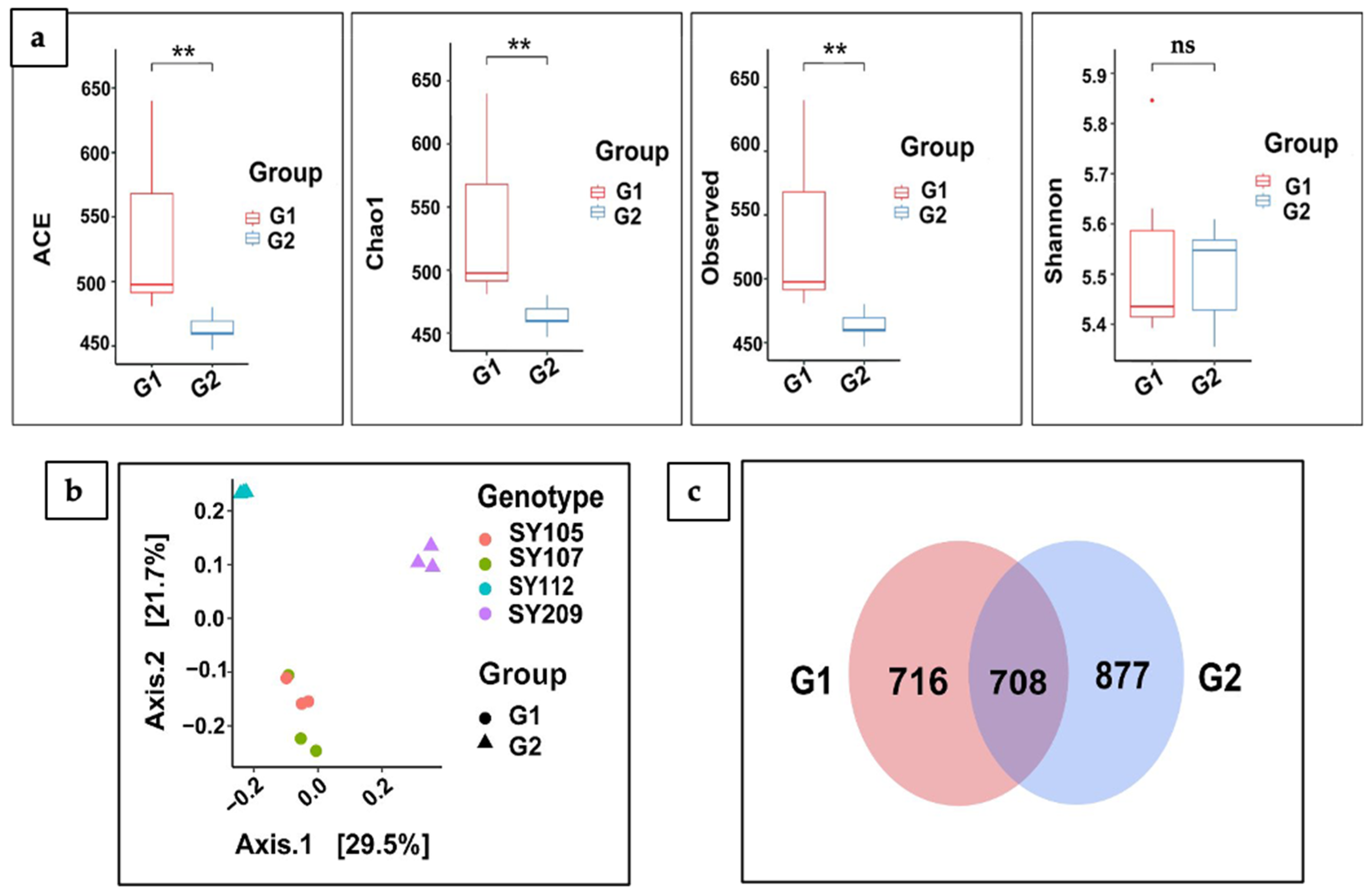
2.4. Alpha (α) and Beta (β) Diversity Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Rhizosphere Sample Collection
4.3. DNA Isolation for 16S Amplicon Sequencing
4.4. Taxonomic Abundance Analysis
4.5. Microbial Diversity Analysis
4.6. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Gouli, S.; Majeed, A.; Liu, J.; Moseley, D.; Mukhtar, M.S.; Ham, J.H. Microbiome structures and beneficial bacteria in soybean roots under field conditions of prolonged high temperatures and drought stress. Microorganisms 2024, 12, 2630. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A.; Maron, P.A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef]
- Nair, R.M.; Boddepalli, V.N.; Yan, M.R.; Kumar, V.; Gill, B.; Pan, R.S.; Wang, C.; Hartman, G.L.; Silva e Souza, R.; Somta, P. Global status of vegetable soybean. Plants 2023, 12, 609. [Google Scholar] [CrossRef]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar]
- Cvijanovic, G.; Dozet, G.; Djukic, V.; Dorcdevic, S.; Puzic, G. Microbial activity of soil during the inoculation of soya bean with symbiotic and free-living nitrogen-fixing bacteria. Afr. J. Biotechnol. 2012, 11, 590–597. [Google Scholar]
- Coşkan, A.; Doğan, K. Symbiotic nitrogen fixation in soybean. Soybean Physiol. Biochem. 2011, 307, 167–182. [Google Scholar]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Jin, Y.; He, J.; Zhu, Y.; Siddique, K.H. Nodule formation and nitrogen use efficiency are important for soybean to adapt to water and P deficit conditions. Agriculture 2022, 12, 1326. [Google Scholar] [CrossRef]
- Mohan, B.; Majeed, A.; Thingujam, D.; Burton, S.S.; Cowart, K.E.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Amplicon Sequencing Analysis of Submerged Plant Microbiome Diversity and Screening for ACC Deaminase Production by Microbes. Int. J. Mol. Sci. 2024, 25, 13330. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Liu, J.; Knight, A.J.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Bacterial Communities Associated with the Leaves and the Roots of Salt Marsh Plants of Bayfront Beach, Mobile, Alabama, USA. Microorganisms 2024, 12, 1595. [Google Scholar] [CrossRef]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar]
- Sohn, S.I.; Ahn, J.H.; Pandian, S.; Oh, Y.J.; Shin, E.K.; Kang, H.J.; Cho, W.S.; Cho, Y.S.; Shin, K.S. Dynamics of bacterial community structure in the rhizosphere and root nodule of soybean: Impacts of growth stages and varieties. Int. J. Mol. Sci. 2021, 22, 5577. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar]
- Xiong, J.; Lu, J.; Li, X.; Qiu, Q.; Chen, J.; Yan, C. Effect of rice (Oryza sativa L.) genotype on yield: Evidence from recruiting spatially consistent rhizosphere microbiome. Soil Biol. Biochem. 2021, 161, 108395. [Google Scholar]
- Liu, Y.; Han, Q.; Zhang, J.; Zhang, X.; Chen, Y.; Li, M.; Hao, Y.; Hong, Y.; Tang, R.; Ferguson, B.J.; et al. Soybean nodulation shapes the rhizosphere microbiome to increase rapeseed yield. J. Adv. Res. 2024, in press. [Google Scholar]
- Liu, F.; Hewezi, T.; Lebeis, S.L.; Pantalone, V.; Grewal, P.S.; Staton, M.E. Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol. 2019, 19, 201. [Google Scholar]
- Luo, C.; He, Y.; Chen, Y. Rhizosphere microbiome regulation: Unlocking the potential for plant growth. Curr. Res. Microb. Sci. 2024, 8, 100322. [Google Scholar]
- Dlamini, S.P.; Akanmu, A.O.; Babalola, O.O. Rhizospheric microorganisms: The gateway to a sustainable plant health. Front. Sustain. Food Syst. 2022, 6, 925802. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Moroenyane, I.; Tremblay, J.; Yergeau, É. Temporal and spatial interactions modulate the soybean microbiome. FEMS Microbiol. Ecol. 2021, 97, fiaa206. [Google Scholar]
- Yamazaki, S.; Mardani-Korrani, H.; Kaida, R.; Ochiai, K.; Kobayashi, M.; Nagano, A.J.; Fujii, Y.; Sugiyama, A.; Aoki, Y. Field multi-omics analysis reveals a close association between bacterial communities and mineral properties in the soybean rhizosphere. Sci. Rep. 2021, 11, 8878. [Google Scholar]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar]
- Sathya, A.; Vijayabharathi, R.; Gopalakrishnan, S. Plant growth-promoting actinobacteria: A new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.; Selim, S.; Zinta, G.; Mousa, A.S.; Hozzein, W.N. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Le, X.H.; Franco, C.M.; Ballard, R.A.; Drew, E.A. Isolation and characterisation of endophytic actinobacteria and their effect on the early growth and nodulation of lucerne (Medicago sativa L.). Plant Soil 2016, 405, 13–24. [Google Scholar] [CrossRef]
- Gregor, A.K.; Klubek, B.; Varsa, E.C. Identification and use of actinomycetes for enhanced nodulation of soybean co-inoculated with Bradyrhizobium japonicum. Can. J. Microbiol. 2003, 49, 483–491. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Yang, F.; Wang, S.; Ma, H.; Liang, J.; Chen, Y. Co-existence of rhizobia and diverse non-rhizobial bacteria in the rhizosphere and nodules of Dalbergia odorifera seedlings inoculated with Bradyrhizobium elkanii, Rhizobium multihospitium–like and Burkholderia pyrrocinia–like strains. Front. Microbiol. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Okubo, T.; Ikeda, S.; Kaneko, T.; Eda, S.; Mitsui, H.; Sato, S.; Tabata, S.; Minamisawa, K. Nodulation-dependent communities of culturable bacterial endophytes from stems of field-grown soybeans. Microbes Environ. 2009, 24, 253–258. [Google Scholar] [CrossRef]
- Giannelli, G.; Bisceglie, F.; Pelosi, G.; Bonati, B.; Cardarelli, M.; Antenozio, M.L.; Degola, F.; Visioli, G. Phyto-beneficial traits of rhizosphere bacteria: In vitro exploration of plant growth promoting and phytopathogen biocontrol ability of selected strains isolated from harsh environments. Plants 2022, 11, 230. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Bag, N.; Das, S.; Haokip, P.; Sharma, L. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Wen, X.; Liu, Y.; Wang, M.; Fu, Y.; Xiang, L.; Jiang, J.; Jiang, X.; Wang, F. A comparative evaluation of biochar and Paenarthrobacter sp. AT5 for reducing atrazine risks to soybeans and bacterial communities in black soil. Environ. Res. 2024, 252, 119055. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Olivares, J.; Delgado, A.; Bedmar, E.; Martínez-Molina, E. Endophytic Micromonospora from Medicago sativa are apparently not able to fix atmospheric nitrogen. Soil Biol. Biochem. 2014, 74, 201–203. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Sathya, A.; Gopalakrishnan, S. A renaissance in plant growth-promoting and biocontrol agents by endophytes. In Microbial Inoculants in Sustainable Agricultural Productivity; Research Perspectives; Springer: New Delhi, India, 2016; Volume 1, pp. 37–60. [Google Scholar]
- Nimnoi, P.; Pongsilp, N.; Lumyong, S. Co-inoculation of soybean (Glycine max) with actinomycetes and Bradyrhizobium japonicum enhances plant growth, nitrogenase activity and plant nutrition. J. Plant Nutr. 2014, 37, 432–446. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [PubMed]
- Khamna, S.; Yokota, A.; Lumyong, S. Actinomycetes isolated from medicinal plant rhizosphere soils: Diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J. Microbiol. Biotechnol. 2009, 25, 649–655. [Google Scholar]
- Nafis, A.; Raklami, A.; Bechtaoui, N.; El Khalloufi, F.; El Alaoui, A.; Glick, B.R.; Hafidi, M.; Kouisni, L.; Ouhdouch, Y.; Hassani, L. Actinobacteria from extreme niches in Morocco and their plant growth-promoting potentials. Diversity 2019, 11, 139. [Google Scholar] [CrossRef]
- Shchyogolev, S.; Burygin, G.L.; Dykman, L.A.; Matora, L. Phylogenetic and pangenomic analyses of members of the family Micrococcaceae related to a plant-growth-promoting rhizobacterium isolated from the rhizosphere of potato (Solanum tuberosum L.). Vavilov J. Genet. Breed. 2024, 28, 308. [Google Scholar]
- Bziuk, N.; Maccario, L.; Sørensen, S.J.; Schikora, A.; Smalla, K. Barley rhizosphere microbiome transplantation–a strategy to decrease susceptibility of barley grown in soils with low microbial diversity to powdery mildew. Front. Microbiol. 2022, 13, 830905. [Google Scholar]
- Lladó, S.; Žifčáková, L.; Větrovský, T.; Eichlerová, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Costa, R.M.; Costa, M.K.L.; Rocha, S.M.B.; Leite, M.R.L.; de Alcantara Neto, F.; de Souza, H.A.; de Araujo Pereira, A.P.; Melo, V.M.M.; de Medeiros, E.V.; Mendes, L.W.; et al. Soil management shapes bacterial and archaeal communities in soybean rhizosphere: Comparison of no-tillage and integrated crop-livestock systems. Rhizosphere 2024, 30, 100886. [Google Scholar]
- Zhou, X.; Zhang, Q.; Yan, Y.; Qu, J.; Zhou, J.; Zhao, J.; Zhang, J.; Cai, Z.; Dai, C.; Huang, X. Effects of soil management strategies based on different principles on soil microbial communities and the outcomes for plant health. Biol. Control 2025, 201, 105708. [Google Scholar]
- Szoboszlay, M.; White-Monsant, A.; Moe, L.A. The effect of root exudate 7, 4′-dihydroxyflavone and naringenin on soil bacterial community structure. PLoS ONE 2016, 11, e0146555. [Google Scholar]
- Tian, X.; Cao, L.; Tan, H.; Han, W.; Chen, M.; Liu, Y.; Zhou, S. Diversity of cultivated and uncultivated actinobacterial endophytes in the stems and roots of rice. Microb. Ecol. 2007, 53, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhu, G.; Qiu, H.; Li, M.; Zhang, J.; Wu, X.; Xiao, R.; Zhang, Y.; Yang, W.; Tian, B.; et al. Quality traits drive the enrichment of Massilia in the rhizosphere to improve soybean oil content. Microbiome 2024, 12, 224. [Google Scholar]
- Ait-El-Mokhtar, M.; Meddich, A.; Baslam, M. Plant-microbiome interactions under drought—Insights from the molecular machinist’s toolbox. Front. Sustain. Food Syst. 2023, 7, 1253735. [Google Scholar] [CrossRef]
- Yang, N.; Nesme, J.; Røder, H.L.; Li, X.; Zuo, Z.; Petersen, M.; Burmølle, M.; Sørensen, S.J. Emergent bacterial community properties induce enhanced drought tolerance in Arabidopsis. NPJ Biofilms Microbiomes 2021, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Hu, C.; Gan, Y.; Zhou, J.; Tian, G.; Gao, L. Role of microbes in alleviating crop drought stress: A review. Plants 2024, 13, 384. [Google Scholar]
- Thingujam, D.; Liu, J.; Majeed, A.; Mukhtar, M.S. Plant–microbiome dynamics through spatial metatranscriptomics and network biology. Trends Plant Sci. 2024, 29, 1176–1180. [Google Scholar]
- Kodadinne Narayana, N.; Wijewardana, C.; Alsajri, F.A.; Reddy, K.R.; Stetina, S.R.; Bheemanahalli, R. Resilience of soybean genotypes to drought stress during the early vegetative stage. Sci. Rep. 2024, 14, 17365. [Google Scholar]
- Poudel, S.; Vennam, R.R.; Sankarapillai, L.V.; Liu, J.; Reddy, K.R.; Wijewardane, N.K.; Mukhtar, M.S.; Bheemanahalli, R. Negative synergistic effects of drought and heat during flowering and seed setting in soybean. Environ. Exp. Bot. 2024, 222, 105769. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: Community Ecology Package. 2010. Available online: http://vegan.r-forge.r-project.org/ (accessed on 24 December 2024).

| Group Name | Soil Sample ID | Soybean Genotype ID | Amount of Soil for DNA Isolation |
|---|---|---|---|
| G1 (High nodulation group) | SY105 | PI 458505 | 200 mg |
| SY107 | PI 603490 | 200 mg | |
| G2 (Low nodulation group) | SY112 | PI 605839 A | 200 mg |
| SY209 | PI 548400 | 200 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thingujam, D.; Majeed, A.; Sivarathri, B.S.; Narayana, N.K.; Bista, M.K.; Cowart, K.E.; Knight, A.J.; Pajerowska-Mukhtar, K.M.; Bheemanahalli, R.; Mukhtar, M.S. The Impact of Soybean Genotypes on Rhizosphere Microbial Dynamics and Nodulation Efficiency. Int. J. Mol. Sci. 2025, 26, 2878. https://doi.org/10.3390/ijms26072878
Thingujam D, Majeed A, Sivarathri BS, Narayana NK, Bista MK, Cowart KE, Knight AJ, Pajerowska-Mukhtar KM, Bheemanahalli R, Mukhtar MS. The Impact of Soybean Genotypes on Rhizosphere Microbial Dynamics and Nodulation Efficiency. International Journal of Molecular Sciences. 2025; 26(7):2878. https://doi.org/10.3390/ijms26072878
Chicago/Turabian StyleThingujam, Doni, Aqsa Majeed, Bala Subramanyam Sivarathri, Nisarga Kodadinne Narayana, Mohan K. Bista, Katie E. Cowart, Adelle J. Knight, Karolina M. Pajerowska-Mukhtar, Raju Bheemanahalli, and M. Shahid Mukhtar. 2025. "The Impact of Soybean Genotypes on Rhizosphere Microbial Dynamics and Nodulation Efficiency" International Journal of Molecular Sciences 26, no. 7: 2878. https://doi.org/10.3390/ijms26072878
APA StyleThingujam, D., Majeed, A., Sivarathri, B. S., Narayana, N. K., Bista, M. K., Cowart, K. E., Knight, A. J., Pajerowska-Mukhtar, K. M., Bheemanahalli, R., & Mukhtar, M. S. (2025). The Impact of Soybean Genotypes on Rhizosphere Microbial Dynamics and Nodulation Efficiency. International Journal of Molecular Sciences, 26(7), 2878. https://doi.org/10.3390/ijms26072878






