Abstract
Glycosyltransferases (GTs) play an important role in plant growth and development, as well as responses to biotic and abiotic stresses. However, the function of the GT family in cotton resistance to Verticillium wilt is limited. In the present study, transcriptome analysis revealed eight GTs upregulated in susceptible cotton varieties and downregulated in resistant cotton varieties during early Verticillium dahliae inoculation, indicating they were involved in regulating the infection of V. dahliae in cotton. Promoter analysis revealed a high prevalence of MeJA (methyl jasmonate) and ABA (abscisic acid)-related cis-acting elements among these GTs. Genome-wide and location analysis of the homologous genes showed that these GTs were relatively conserved in evolution. Furthermore, a Virus-Induced Gene Silencing (VIGS) experimental results demonstrated a reduction in disease resistance after GhGT61 silencing. These insights not only deepen our understanding of the GT family’s role in cotton, but also provide a foundation for future research on the disease resistance mechanisms of these genes.
1. Introduction
Cotton is a pivotal economic crop and the primary source of natural fiber for the textile industry [1]. However, it is severely affected by Verticillium wilt caused by V. dahliae. In China, this disease affects over 50% of cotton fields annually, resulting in substantial economic losses [2]. V. dahliae is particularly difficult to control due to its persistence in soil as dormant microsclerotia [3]. Currently, there is no effective chemical control for cotton Verticillium wilt [4]. The most cost-effective and efficient strategy involves the breeding and deployment of disease-resistant cotton varieties [5]. The scarcity of available resistance genes for genetic engineering has significantly constrained cotton molecular breeding.
Some natural glycosides or sugar esters with a more stable structure are usually formed by glycosylation modification [6]. Glycosylation is crucial for promoting plant storage, intracellular transport, and regulating the balance of growth agents within plants [7]. GTs can link sugar molecules to specific receptors and produce glycosidic bonds, which are present in most organisms and can be particularly important in plants [8]. One important role of GTs is as catalyzed plant glycosylation [9]. GTs transfer activated sugars to various plant molecules, including monosaccharides, oligosaccharides, polysaccharides, and non-carbohydrate substrates such as proteins, antibiotics, and lipids, leading to the glycosylation of these compounds [10]. GTs are essential for plant growth, development, and responses to biotic and abiotic stresses.
GTs play crucial roles in plant defense responses by regulating resistance pathways and glycosylation. For instance, AtUGT73C7 is a glycosyltransferase that regulates Arabidopsis thaliana resistance to Pseudomonas syringae by redirecting the phenylpropanoid pathway and activating the Toll-interleukin-1 receptor (TIR)-type nucleotide-binding leucine-rich repeat (NLR) protein SNC1 [11]. AtUGT76B1 could regulate the basic defense and systemic acquired resistance (SAR) by coordinating the glycosylation of three defense activators, such as salicylic acid (SA), isoleucic acid, and N-hydroxypipecolic acid [12]. Additionally, TaUGT6 is a novel UDP-glycosyltransferase gene that enhances wheat resistance to Fusarium head blight by converting deoxynivalenol (DON) into DON-3-glucoside [13]. A recent study also showed that CsUGT87E7 encodes a SA carboxyltransferase that enhances the disease resistance of tea plants to Pseudopestalotiopsis camelliae-sinensis by regulating SA homeostasis [14]. These studies demonstrate that GTs have significant roles in disease resistance, whereas there have been few reports on the role of GTs in cotton against V. dahliae.
In our study, we found that GTs are involved in the regulation of cotton’s defense response to V. dahliae. In order to clarify the molecular function of GTs in cotton, the GTs were analyzed in G. arboreum, G. raimondii, G. hirsutum, and G. barbadense genomes, respectively. The characteristics of GTs were also comprehensively analyzed in G. hirsutum, including their chromosomal locations, motif distributions, gene structures, and the cis-elements in the promoter regions. Additionally, the impact of GhGT61 on V. dahliae was verified by VIGS. This study will preliminarily clarify the function of GTs and provide gene resources for molecular breeding of upland cotton resistant to Verticillium wilt.
2. Results
2.1. GTs Are Involved in the Response of Cotton to V. dahliae Infection
In order to screen the key resistance-associated genes of upland cotton responding to V. dahliae infection, we analyzed the differentially expressed genes (DEGs) in both resistant and susceptible upland cotton varieties after inoculation with V. dahliae. Gene Ontology (GO) enrichment analysis revealed that the transferase activity and transferring glycosyl group pathway were significantly enriched in both cotton varieties at 12 h post-inoculation. However, the expression patterns of these DEGs were completely opposite because the related DEGs were up-regulated and down-regulated in susceptible and resistant upland cotton, respectively (Figure 1A,B).
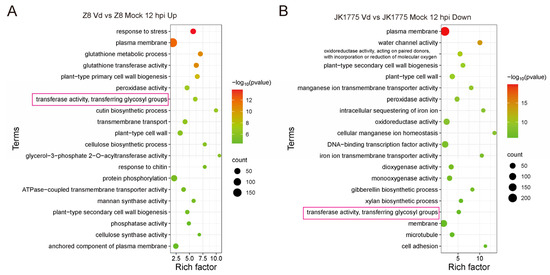
Figure 1.
GO analysis of DEGs in response to V. dahliae infection at 12 hpi in resistant and susceptible upland cotton. (A) GO analysis of up-regulating DEGs in response to V. dahliae infection at 12 hpi in susceptible upland cotton Z8. (B) GO analysis of down-regulating DEGs in response to V. dahliae infection at 12 hpi in resistant upland cotton JK1775. The transferase activity and transferring glycosyl group pathway were marked with red boxes.
There were eight identical GTs involved in the transferase activity and transferring glycosyl group pathway in both the resistant and susceptible upland cotton (Figure 2A,B). These GTs may play a crucial role in the immune response of upland cotton to V. dahlia. In order to verify the reliability of the selected GT data, the differential expression levels of GTs were analyzed by qRT-PCR in the early stage of V. dahliae infection in both resistant and susceptible upland cotton. The results indicated that the expression level of the GTs were basically consistent with the results of the transcriptome data (Figure 2C). Notably, GH_A04G1083 exhibited a high expression in both the inoculated and uninoculated upland cotton, with expression levels higher than those of other GTs (Figure 2). These results suggested that GH_A04G1083 may play a role not only in the immune response to V. dahliae, but also in other vital activities of upland cotton.
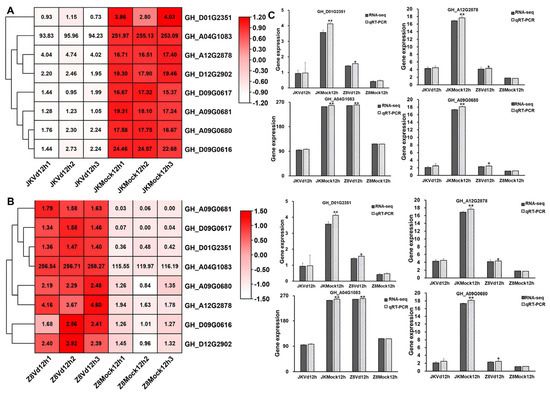
Figure 2.
Expression analysis of eight GTs in response to V. dahliae infection in resistant and susceptible upland cotton at 12 hpi. (A) Heatmap of eight GTs in resistant upland cotton JK1775 in response to V. dahliae infection at 12 hpi. (B) Heatmap of eight GTs in susceptible upland cotton Z8 in response to V. dahliae infection at 12 hpi. (C) qRT-PCR verification of GTs transcriptome data. The data are mean ± SD (n = 3). Significance is determined using Duncan’s multiple range test, indicated by * p ≤ 0.05 and ** p ≤ 0.01.
2.2. Domain Structure and Genetic Relationship of the Eight GTs in Cotton
To elucidate the biological function of the eight GTs further, we analyzed their domain structure and genetic relationship. The results revealed that although the distribution of CDS sequences on the eight GTs showed certain differences (Figure 3C), GH_A09G0681 and GH_D09G0617, GH_A12G2878 and GH_D12G2902, and GH_A09G0680 and GH_D09G0616 were clustered together, respectively, indicating a relatively close relationship (Figure 3A). These six GTs were identified as members of glycosyltransferase family 61 based on the domain analysis (Figure 3B). Additionally, the GH_D01G2351 and GH_AO4G1083 were classified under glycosyltransferase family 1 and glycosyltransferase family 2, respectively (Figure 3A).
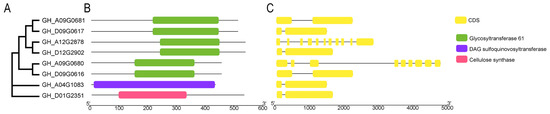
Figure 3.
Phylogenetic tree, gene structure, and conservative motif analysis of eight GTs. (A) Neighbor-joining phylogenetic tree of eight GTs. (B) Prediction of conserved motifs in the GTs, with different colors representing distinct motifs. (C) Analysis of the intron-exon structure of the GTs, where exons and introns are depicted by yellow boxes and gray lines, respectively.
To investigate the evolutionary relationship of the eight GTs in cotton, we obtained their homologous genes in G. arboreum, G. raimondii, G. hirsutum, G. barbadense, and A. thaliana using the Basic Local Alignment Search Tool (BLAST, version 2.16) in cotton and A. thaliana genomes. The phylogenetic analysis revealed that their homologous GTs were divided into three distinct groups. Specifically, seven GTs were classified into Group Ι, while one GT (GH_D01G2351) fell into Group II (Figure 4). Notably, the number of homologous GTs in G. barbadense and G. hirsutum was approximately twice than that of G. arboreum and G. raimondii (Figure 4). These results suggest that these GTs may have undergone different differentiation paths and selection pressures during evolution, resulting in the formation of gene families with diverse functions and characteristics.
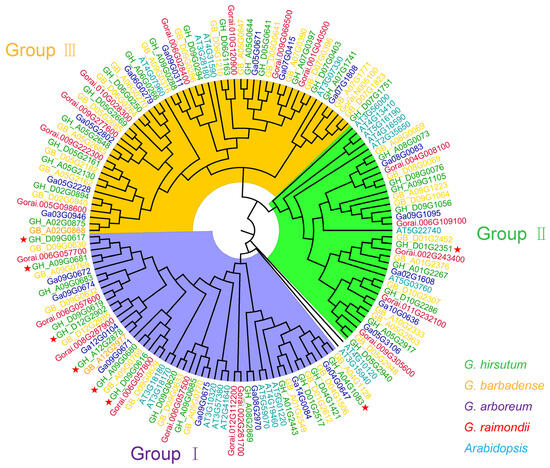
Figure 4.
Phylogenetic analysis of GTs proteins from G. hirsutum, G. barbadense, G. arboreum, G. raimondii, and A. thaliana. The GTs proteins from the five species are indicated by different colors. The phylogenetic tree is constructed using MEGA X with the maximum likelihood method and 1000 bootstrap replications. Eight GTs were marked with red stars.
2.3. Analysis of Promoter Region of the Eight GTs
Promoter regions are crucial for determining the gene expression patterns and functions. In order to predict the roles of GTs, we analyzed the upstream cis-acting elements within their promoter regions. Interestingly, many of these elements were associated with plant hormones, including ABA, gibberellin (GA), SA, and MeJA, as well as abiotic stress factors such as anaerobic conditions, defense and stress responses, light responses, and low-temperature responses (Figure 5A). Among these elements, hormone-related elements (SA, MeJA, auxin, ABA, and gibberellin) account for a significant proportion, representing 50.93% of the total elements (Figure 5B). More importantly, defense and stress response elements accounted for 5.66% of the total (Figure 5B). Notably, each GT contains at least one hormone-related cis-acting element, particularly those related to ABA. Among the eight GTs, GH_A09G0680, GH_D09G0616, and GH_D12G2902 lack MeJA-related elements. Collectively, these results indicate that GTs are crucial for conferring resistance to upland cotton against adverse environmental conditions.
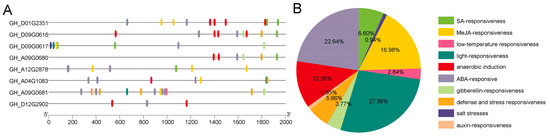
Figure 5.
Cis-acting elements of GTs in upland cotton. (A) Predicted cis-elements in the promoter regions of GTs. (B) Proportion of different types of cis-elements. Ten groups of cis-acting elements are identified and represented by color-coded boxes.
2.4. Chromosomal Localization of Cotton GTs
To investigate the genomic distribution of cotton GTs, a chromosome map was constructed for 41 GTs in upland cotton. A total of 20 GTs were mapped to 9 chromosomes of the At subgenome, while 21 GTs were mapped to 10 chromosomes of the Dt subgenome (Figure 6). The distribution of GTs across At and Dt chromosomes is uneven, ranging from 1 to 6. Specifically, there are 6 GTs located on chromosomes A09 and D09, while 6 GTs are located on chromosomes A02, A04, A06, A12, D02, D04, D06, D10, and D12. Notably, the number and chromosomal location of certain GTs on the At subgenome are the same as those on the Dt subgenome, as evidenced by pairs such as A01-D01, A02-D02, A04-D04, A05-D05, A07-D07, A09-D09, and A12-D12.
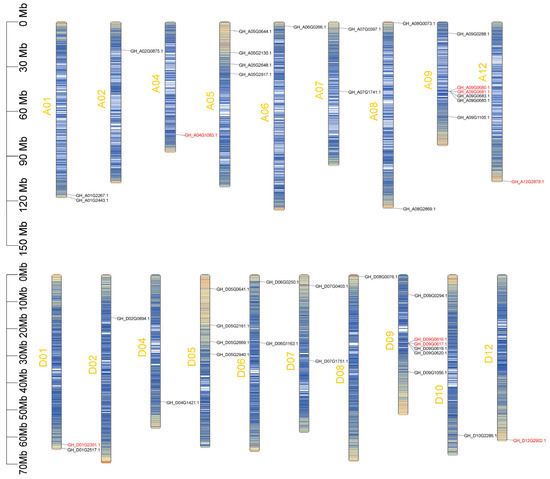
Figure 6.
Chromosome distribution of GTs in G. hirsutum. The heatmap visualizes the chromosome density, with a blue-to-red color scale representing low to high-density values. The chromosome number is annotated along the left side in orange, while eight GTs (red) and their homologous genes (black) are labeled on the right flanking axis, respectively.
2.5. Synteny Analysis of GTs in Cotton
In order to investigate the fragment replication and tandem replication of GTs in upland cotton, the amplification mechanism of cotton GTs were analyzed using BLAST, version 2.16 and MCScanX, https://github.com/wyp1125/MCScanX, accessed on 13 August 2024. The results suggested that six of the eight GTs formed gene clusters, including GH_A04G1083-GH_D01G2517, GH_D01G2351-GH_A09G0685, GH_A12G2878-GH_A09G0680, and GH_D09G0616-GH_D12G2902, which constituted four tandem clusters on chromosomes A12, A09, D09, and D12, respectively (Figure 7A). In contrast, GH_D09G0617 and GH_A09G0681 did not form gene clusters (Figure 7A). These four tandem duplications are inferred to have originated from polyploid ancestors, while GH_D09G0617 and GH_A09G0681 are believed to have arisen in G. hirsutum following its divergence from G. barbadense, as they are absent in G. barbadense (Figure 7B).
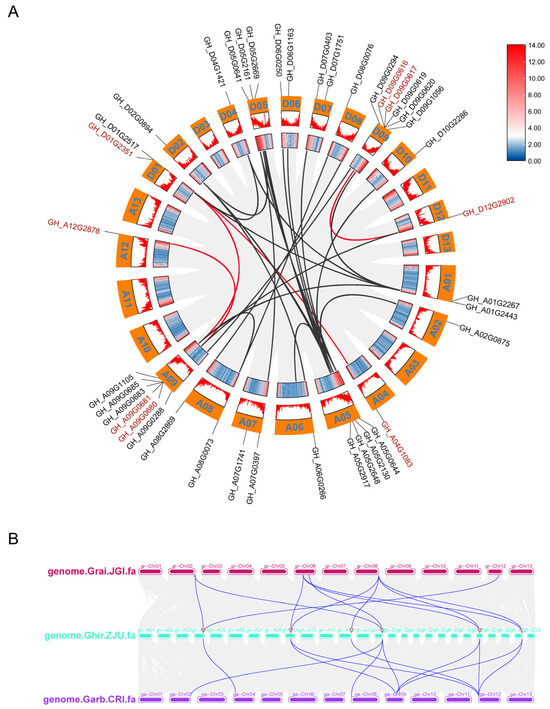
Figure 7.
Intraspecific and interspecific collinearity analysis of eight GTs. (A) Synteny analysis between At and Dt subgenomes in G. hirsutum. Duplicated gene pairs are denoted by red connecting lines, whereas gray lines indicate identical gene pairs genome-wide. Eight GTs are labeled in red, with homologous genes distinguished by black labeling. (B) Synteny of repetitive gene pairs in three cotton species (G. hirsutum, G. arboretum, and G. raimondii).
2.6. Silence of GhGT61 GhGTs Affected the Disease Resistance of Cotton
To elucidate the role of GhGTs on the resistance of cotton to Verticillium wilt, Virus-Induced Gene Silencing (VIGS) and disease resistance assays were carried out. The results showed that an albino phenotype was observed at 14 dpi (Figure 8A). Following inoculation with V. dahliae 13 days after silencing, plants with suppressed GhGT61 exhibited markedly reduced resistance to the V. dahliae (Figure 8B,F), with a significant reduction in the expression level of GhGT61 (Figure 8E). A histological analysis of longitudinal sections of cotton cotyledonary nodes revealed pronounced browning in TRV2:GhGT61 plants compared to TRV2:00 controls (Figure 8C). The fungal biomass quantification via recovery culture showed significantly higher V. dahliae accumulation in stems of TRV2:GhGT61 plants than in TRV2:00 controls (Figure 8D,G). These results collectively demonstrate that silencing GhGT61 enhances cotton susceptibility to V. dahliae, underscoring its critical role in mediating resistance to Verticillium wilt.
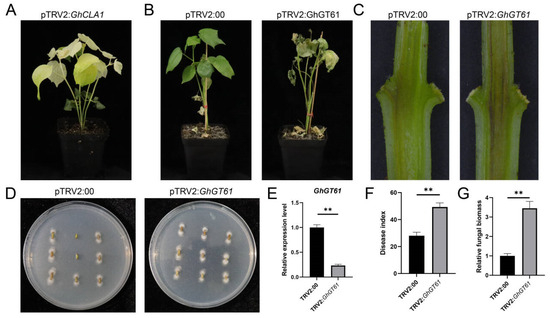
Figure 8.
Function of GhGT61 on the resistance of cotton to V. dahliae. (A) VIGS-mediated silencing of GhCLA1 results in an albino phenotype in cotton. (B) Phenotype of silenced and normal plants inoculated with V. dahliae at 13 dpi. (C) Vascular bundle browning observed in longitudinal stem sections of silenced and control plants. (D) Fungal biomass recovery experiment in stems of silenced and control plants. (E) The expression level of GhGT61 in silenced and normal plants. (F) Disease index of silenced and normal plants at 13 dpi. (G). qRT-PCR quantification of fungal biomass in stems in silenced and control plants. The data are presented as mean ± SD (n = 3). Significance is determined using Duncan’s multiple range test, indicated by ** p ≤ 0.01.
3. Discussion
In recent years, continuous cropping has resulted in a significant increase in Verticillium wilt incidence in the cotton industry, severely impacting cotton yields. The application of modern molecular techniques for identifying resistance-related genes has accelerated the breeding of disease-resistant varieties [4]. Exploring key genes associated with cotton’s resistance to Verticillium wilt and elucidating their mechanism is crucial for developing new cotton germplasm resistant to Verticillium wilt [15]. Recent advancements in sequencing technology and decreasing costs have significantly improved and updated cotton genome sequencing efforts, providing a foundation for studying gene functions at the genomic level [16]. Transcriptome analysis can identify potential genes and molecular mechanisms involved in various biotic stress responses, playing a vital role in elucidating gene function [17]. A more profound comprehension of cotton genomics and genetics opens new pathways for investigating the GT family.
Glycosylation is a common modification of plant secondary metabolites and plays various functions, including the regulation of hormone homeostasis, detoxification of exogenous substances, and the biosynthesis and storage of secondary compounds. These processes are facilitated by specific subclasses of the GT family [18]. However, there are few studies on the biotic stress of cotton GTs. In the present study, we identified that there were eight GTs involved in cotton regulation of the defense response to V. dahliae in the early infection period. The GT family has remained conserved throughout the long-term evolution of cotton, consistent with its evolutionary relationships. Notably, the number of GTs in tetraploid cotton is approximately double that of diploid cotton species, further supporting the existence of two diploid ancestors for tetraploid cotton [19].
Gene function is closely linked to the differentiation of the promoter region, which are the combination regions of many transcription factors (TFs) [20]. TFs usually regulate various aspects of plant growth, development, and stress resistance by regulating gene expression in responses to biotic and abiotic stress factors [21]. An analysis of the cis-elements in the promoter regions of these eight GTs revealed that the cis-elements with the strongest hormone response were ABA- and MEJA-response cis-elements. This suggests that these GTs may be involved in the response of cotton to V. dahliae through the ABA or MeJA pathway. Additionally, light-responsive cis-regulating elements account for a large proportion in the eight GTs, accounting for 27.336% of the total, indicating that GTs may participate in the regulation of light responses in cotton.
At present, the main goal of cotton breeding is to select new high-yield and high-quality varieties resistant to Verticillium wilt [22]. In this study, we analyzed the RNA-Seq data set (PRJNA1121084) of cotton inoculated with V. dahliae and found that eight GTs were differentially expressed in resistant and susceptible cotton after inoculation with V. dahliae, indicating that GTs may be involved in the defense process of cotton against V. dahliae. Significantly, silencing GhGT61 compromised plant resistance, establishing its critical role in disease response.
Studies have shown the multifaceted role of GTs in plant−pathogen interactions. In Brachypodium, a UDP-glycosyltransferase enhances Fusarium resistance through DON detoxification [23], while rice OsTGAL1 modulates bacterial leaf streak susceptibility via SA glycosyltransferase regulation [24]. Arabidopsis UGT73C3/UGT74C4 catalyze immunostimulatory pinoresinol glycosylation [25], in contrast with the immunosuppressive effect of OsGMT1 overexpression in rice [26]. These functional divergences underscore substantial interspecies variation in GT-mediated defense mechanisms. Future investigations should employ transgenic approaches and CRISPR/Cas9-mediated knockout strategies to elucidate functional redundancy and synergistic effects within GT families, particularly in Verticillium wilt resistance.
4. Materials and Methods
4.1. RNA-Seq and qRT-PCR Analysis
The transcriptome data were downloaded from the NCBI database (Project ID: PRJNA1121084) [27]. Data were filtered and quality-controlled using Fastp software, version 0.23.4 [28]. Clean data were aligned to the G. hirsutum TM-1 genome (ZJU-improved_v2.1_a1) using HISAT2, and StringTie was used for the read quantification. The gene expression was evaluated using the RPKM method [29]. For qRT-PCR, primers were designed using Primer 5.3 software based on the cDNA sequences of eight candidate genes (Table S1). Expression levels were analyzed using root tissue cDNA as a template. Each sample was replicated seven times, and GhUBQ7 was used as an internal reference gene. qRT-PCR was performed as described previously [30].
4.2. Identification of GTs in Cotton
Genomic data of G. arboretum (Ga), G. raimondii (Gr), G. hirsutum (Gh), and G. barbadense (Gb) were obtained from the CottonGen database http://www.cottongen.org/ (accessed on 13 August 2024). Homologous sequences were identified using BLAST with an e-value threshold of 1e-5. A total of 21, 21, 41, and 40 GTs were retrieved in Ga, Gr, Gh, and Gb, respectively [31].
4.3. Analysis of Gene Structure and Conserved Motifs
Gene structure analysis was performed using TBtools version 2.149 to identify the exons, introns, and untranslated regions of GTs in cotton. Domain analysis was performed using the NCBI conserved domain database (CDD) to determine the domain types and positions. Subsequently, the gene exons, introns, and conserved motifs were visualized using TBtools version 2.149.
4.4. Phylogenetic Analysis of GTs in Cotton
Multiple sequence alignments were performed using Clustal W in MEGA X version 11.0.13 with default settings (gap opening penalty = 10, gap separation distance = 4). A phylogenetic tree with 1000 bootstrap replicates was constructed using the neighbor-joining (NJ) method. The tree was visualized and edited using FigTree (http://tree.bio.ed.ac.uk/software/Figtree/ (accessed on 16 August 2024)) [32].
4.5. Analysis of Cotton Glycosyltransferase Activity Gene Promoter Region
The 2000 bp upstream DNA sequences of the GTs were extracted from the CottonGen database (http://www.cottongen.org/ (accessed on 11 August 2024)). Cis-acting elements were predicted using the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 11 August 2024)) [33], and their positions were visualized using TBtools version 2.149.
4.6. VIGS of GhGT61
RNA was isolated from cotton roots and reverse-transcribed into cDNA. A 300 bp CDS fragment of GhGT61 (GH_D12G2902) was amplified using primers with BamHI and EcoRI restriction sites. The fragment was cloned into the TRV2 vector by homologous recombination and transformed into Agrobacterium GV3101. The transformed TRV2 vector was injected into Z8 with completely flattened cotyledons, and then the transformed seedlings were transferred to a growth chamber at 25 °C with a light/dark photoperiod of 16 h/ 8 h. VIGS efficiency was systematically validated using TRV2: GhCLA-positive controls, with successful gene suppression phenotypically confirmed by characteristic leaf albinism at 15 days post-agroinfiltration (DPI). For molecular validation, second true leaves were collected from silenced plants (n = 30 across three biological replicates) for RNA extraction and subsequent RT-qPCR analysis using GhUBQ7 as the reference gene (2−ΔΔCt method). Following confirmation silencing efficiency, the plants were challenge-inoculated with the defoliating V. dahliae strain V592 (1 × 10⁶ spores/mL, root-dip method) [34]. The highly pathogenic strain V592 of V. dahliae was provided by Professor Jiafeng Huang from Shihezi University [35], cultured in Czapek Dox liquid medium [36]. Disease progression was monitored at 13 dpi, and the disease index was calculated according to standardized 0–4 grading scales [37]. The fungal biomass was detected by qRT-PCR using fungal-specific ITS1-F primer and V. dahliae-specific reverse primer ST-VE1-R, and GhUBQ7 as the internal reference gene [28]. The fungal recovery test was performed according to the previously described method [38]. After 4 days of culture, the fungal colonies were observed.
4.7. Statistical Analysis
All of the experiments were conducted with three independent biological replicates, each containing three technical repetitions. Quantitative data are presented as mean ± standard deviation (SD). Significant differences between wild-type (WT) and treatment groups were determined using Duncan’s multiple range test (SPSS v26.0), with asterisks denoting significance levels: * p < 0.05, ** p < 0.01. Statistical homogeneity within subgroups was indicated by identical lowercase letters (a, b, c) in the corresponding figures.
5. Conclusions
This study identified eight GTs that were differentially expressed in cotton varieties susceptible and resistant to Verticillium wilt through RNA-Seq analysis. The analysis of the gene number, chromosome location, and evolution revealed that the GT family is conserved in cotton. The presence of ABA- and MeJA-responsive cis-acting elements suggests that these GTs may participate in the response of cotton to V. dahliae infection through these hormonal pathways. Additionally, the presence of photoresponsive elements in the cis-acting elements of each gene suggests that GTs may be involved in the regulation of the cotton photoperiod. VIGS experiments further revealed that GhGT61 is involved in the resistance process of cotton to Verticillium wilt. This study provides a foundation for further research on the role of GTs in cotton and offers insights for breeding efforts aimed at developing more resistant cotton varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26073170/s1.
Author Contributions
Conceptualization, Y.Y. and H.X.; methodology, M.Z.; software, M.Z.; validation, M.Z., F.Z. and G.S.; formal analysis, Y.M.; investigation, M.Z. and X.Z.; resources, Y.Y. and H.X.; data curation, M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, M.Z., X.Z., Y.Y. and H.X.; visualization, H.X.; supervision, S.Z. and H.X.; project administration, Y.Y. and H.X.; funding acquisition, X.Z. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shihezi University high-level talents research project (2022ZK015), Bingtuan Science and Technology Program (2022ZD056, 2023CB007-08), and the Earmarked fund for XJARS-Cotton (XJARS-03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study were from the National Center for Biotechnology Information (NCBI), BioProject ID: PRJNA1121084.
Acknowledgments
We thank senior researcher Yu Yu from Xinjiang Academy of Agricultural Reclamation Sciences for his assistance with the materials used for the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (G. hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 3, 531–537. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, W.; Feng, Z.; Zhu, Q.; Sun, Y.; Li, Y.; Sun, J. Transcriptomic analysis of gene expression of V. dahliae upon treatment of the cotton root exudates. BMC Genom. 2020, 21, 155. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Dong, J.; Yang, X.; Sui, A. Interactions between V. dahliae and its host: Vegetative growth, pathogenicity, plant immunity. Appl. Microbiol. Biotechnol. 2014, 98, 6921–6932. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Wang, X.; Zhang, Y.; Zhang, G.; Zhang, J.; Ma, Z. Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to V. dahliae. Plant J. 2015, 83, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Paquette, S.; Møller, B.L.; Bak, S. On the origin of family 1 plant glycosyltransferases. Phytochemistry 2003, 62, 399–413. [Google Scholar] [CrossRef]
- Gilbert, M.K.; Bland, J.M.; Shockey, J.M.; Cao, H.; Hinchliffe, D.J.; Fang, D.D.; Naoumkina, M. A transcript profiling approach reveals an abscisic acid-specific glycosyltransferase (UGT73C14) induced in developing fiber of Ligon lintless-2 mutant of cotton (Gossypium hirsutum L.). PLoS ONE 2013, 8, e75268. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Henrissat, B.; Davies, G.J. Glycoside hydrolases and glycosyltransferases. Families, modules, and implications for genomics. Plant Physiol. 2000, 124, 1515–1519. [Google Scholar] [CrossRef]
- Yu, J.; Hu, F.; Dossa, K.; Wang, Z.; Ke, T. Genome-wide analysis of UDP-glycosyltransferase super family in Brassica rapa and Brassica oleracea reveals its evolutionary history and functional characterization. BMC Genom. 2017, 18, 474. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Lin, J.; Chen, L.; Li, Y.; Liu, Q.; Wang, G.; Xu, F.; Liu, L.; Hou, B. The novel pathogen-responsive glycosyltransferase UGT73C7 mediates the redirection of phenylpropanoid metabolism and promotes SNC1-dependent Arabidopsis immunity. Plant J. 2021, 107, 149–165. [Google Scholar] [CrossRef]
- Bauer, S.; Mekonnen, D.W.; Hartmann, M.; Yildiz, I.; Janowski, R.; Lange, B.; Geist, B.; Zeier, J.; Schäffner, A.R. UGT76B1, a promiscuous hub of small molecule-based immune signaling, glucosylates N-hydroxypipecolic acid, and balances plant immunity. Plant Cell. 2021, 33, 714–734. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Liu, X.; Jiang, P.; Yu, L.; Qiu, J.; Wang, G.; Zhang, X.; Ma, H. TaUGT6, a novel UDP-glycosyltransferase gene enhances the resistance to FHB and DON accumulation in wheat. Front. Plant Sci. 2020, 11, 574775. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, M.; Lu, M.; Wu, Y.; Jing, T.; Zhao, M.; Zhao, Y.; Feng, Y.; Wang, J.; Gao, T.; et al. Salicylic acid carboxyl glucosyltransferase UGT87E7 regulates disease resistance in Camellia sinensis. Plant Physiol. 2022, 188, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, H.; Wang, L.; Li, Z.; Shi, Y.; Zhao, L.; Feng, Z.; Zhu, H. Potential of endophytic fungi isolated from cotton roots for biological control against Verticillium wilt disease. PLoS ONE 2017, 12, e0170557. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. G. barbadense and G. hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef]
- Guo, L.; Han, L.; Yang, L.; Zeng, H.; Fan, D.; Zhu, Y.; Feng, Y.; Wang, G.; Peng, C.; Jiang, X.; et al. Genome and transcriptome analysis of the fungal pathogen Fusarium oxysporum f. sp. cubense causing banana vascular wilt disease. PLoS ONE 2014, 9, e95543. [Google Scholar] [CrossRef]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Senchina, D.S.; Alvarez, I.; Cronn, R.C.; Liu, B.; Rong, J.; Noyes, R.D.; Paterson, A.H.; Wing, R.A.; Wilkins, T.A.; Wendel, J.F. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol. Biol. Evol. 2003, 20, 633–643. [Google Scholar] [CrossRef]
- Amorim, L.L.B.; da Fonseca Dos Santos, R.; Neto, J.P.B.; Guida-Santos, M.; Crovella, S.; Benko-Iseppon, A.M. Transcription factors involved in plant resistance to pathogens. Curr. Protein Pept. Sci. 2017, 18, 335–351. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Zhao, W.; Fu, L.; Han, Y.; Wang, K.; Yan, L.; Li, Y.; Zhang, X.H.; Min, D.H. Genome-wide analysis of the serine carboxypeptidase-like protein family in Triticum aestivum reveals TaSCPL184-6D is involved in abiotic stress response. BMC Genom. 2021, 22, 350. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Deng, X.; Wang, P.; Geng, S.; Gao, W.; Guo, P.; Chen, Q.; Li, C.; Qu, Y. Genome-wide analysis of serine carboxypeptidase-like protein (SCPL) family and functional validation of Gh_SCPL42 unchromosome conferring cotton Verticillium der Verticillium wilt stress in Gossypium hirsutum. BMC Plant Biol. 2022, 22, 421. [Google Scholar] [CrossRef]
- Pasquet, J.C.; Changenet, V.; Macadré, C.; Boex-Fontvieille, E.; Soulhat, C.; Bouchabké-Coussa, O.; Dalmais, M.; Atanasova-Pénichon, V.; Bendahmane, A.; Saindrenan, P.; et al. UDP-Glycosyltransferase confers root tolerance to deoxynivalenol and resistance to Fusarium infection. Plant Physiol. 2016, 172, 559–574. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zheng, C.; Zheng, E.; Liang, W.; Tan, X.; Xu, R.; Yan, C.; Yang, Y.; Yi, K.; et al. OsTGAL1 suppresses the resistance of rice to bacterial blight disease by regulating the expression of salicylic acid glucosyltransferase OsSGT1. Plant Cell Environ. 2022, 45, 1584–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dong, G.; Liu, C.; Ding, Y.; Ma, Y.; Ma, X.; Yang, X.; Liu, L.; Hou, B. Two pathogen-inducible UDP-glycosyltransferases, UGT73C3 and UGT73C4, catalyze the glycosylation of pinoresinol to promote plant immunity in Arabidopsis. Plant Commun. 2025, 23, 101261. [Google Scholar]
- Lin, Y.; Zhu, Y.; Wang, L.; Zheng, Y.; Xie, Y.; Cai, Q.; He, W.; Xie, H.; Liu, H.; Wang, Y.; et al. Overexpression of a GIPC glycosyltransferase gene, OsGMT1, suppresses plant immunity and delays heading time in rice. Plant Sci. 2023, 331, 111674. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Wang, Y.; Gao, H.; Zhao, S.; Yu, Y.; Zhang, X.; Xi, H. MAPK and phenylpropanoid metabolism pathways involved in regulating the resistance of upland cotton plants to Verticillium dahliae. Front. Plant Sci. 2024, 15, 1451985. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, P.; Gao, W.; Long, Y.; Wang, Y.; Geng, S.; Su, X.; Jiao, Y.; Chen, Q.; Qu, Y. Genome-wide identification of the DUF668 gene family in cotton and expression profiling analysis of GhDUF668 in Gossypium hirsutum under adverse stress. BMC Genom. 2021, 22, 395. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, G.; Zhang, X.; Zhang, X.; Qiao, P.; Long, L.; Yuan, Y.; Cai, Y. Genome-wide identification of the TIFY gene family in three cultivated Gossypium species and the expression of JAZ genes. Sci. Rep. 2017, 7, 42418. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, L.; Wang, Y.; Valkenburg, D.J.; Zhang, X.; Zhu, L.; Thomma, B.P.H.J. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 2018, 16, 638–648. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, B.J.; Li, G.Y.; Jia, P.S.; Li, H.; Zhao, Y.L.; Zhao, P.; Xia, G.X.; Guo, H.S. A glutamic acid-rich protein identified in Verticillium dahliae from an insertional mutagenesis affects microsclerotial formation and pathogenicity. PLoS ONE 2010, 5, e15319. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Yu, B.; Lian, Q.; Huang, J. Combined transcriptome and metabolome analysis reveals that carbon catabolite repression coverns growth and pathogenicity in Verticillium dahliae. Int. J. Mol. Sci. 2024, 25, 11575. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Luo, X.; Xu, L.; Liu, L.; Min, L.; Jin, L.; Zhu, L.; Zhang, X. Cotton WRKY1 mediates the plant defense-to-development transition during infection of cotton by Verticillium dahliae by activating JASMONATE ZIM-DOMAIN1 expression. Plant Physiol. 2014, 166, 2179–2194. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, S.; Chi, J.; Zhang, G.; Ma, Z. Cloning and characterization of a Verticillium wilt resistance gene from Gossypium barbadense and functional analysis in Arabidopsis thaliana. Plant Cell Rep. 2011, 30, 2085–2096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).