Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells
Abstract
1. Introduction
2. Results
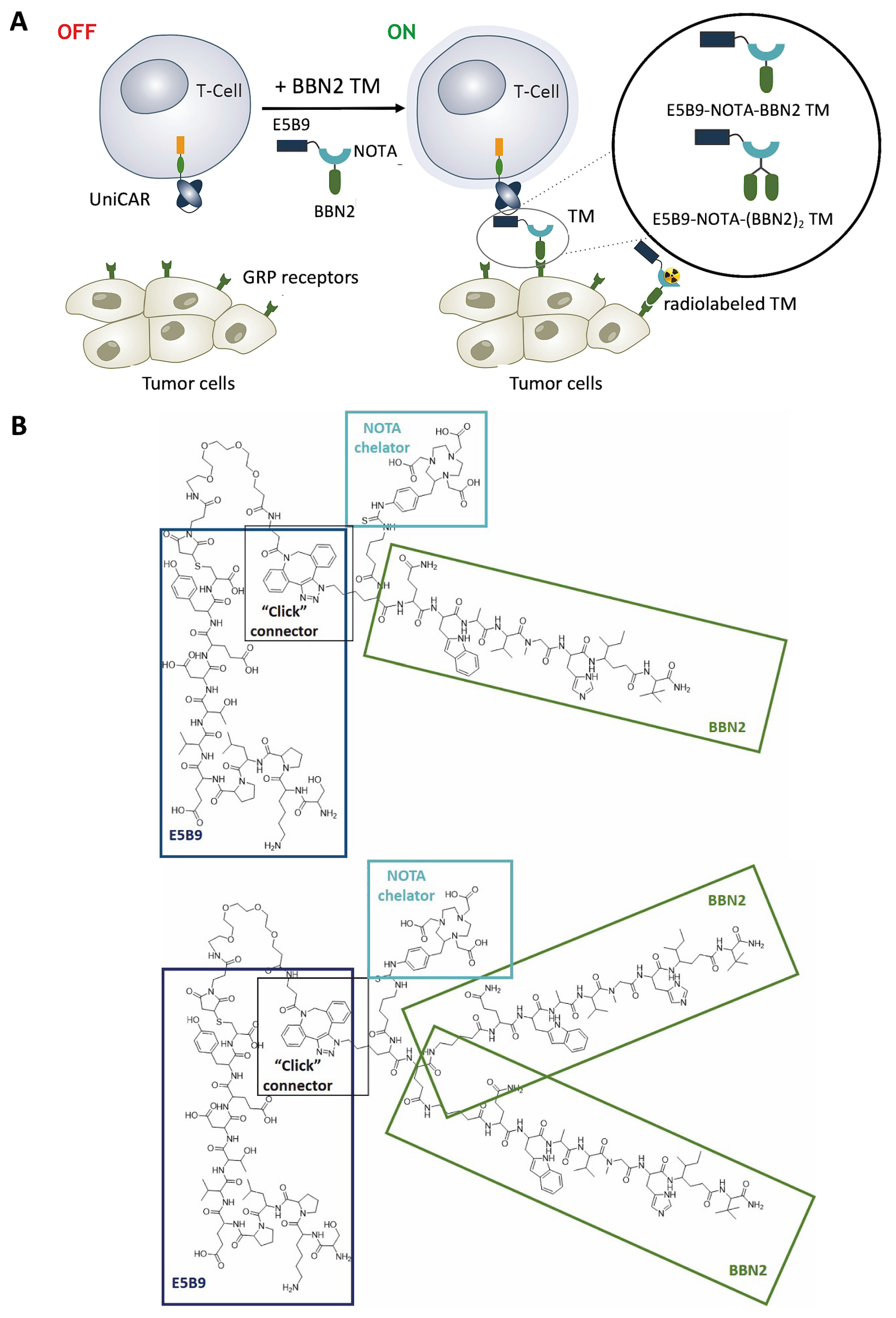
2.1. Targeting GRP Receptor Using UniCAR T-Cells Redirected by BBN2 TMs
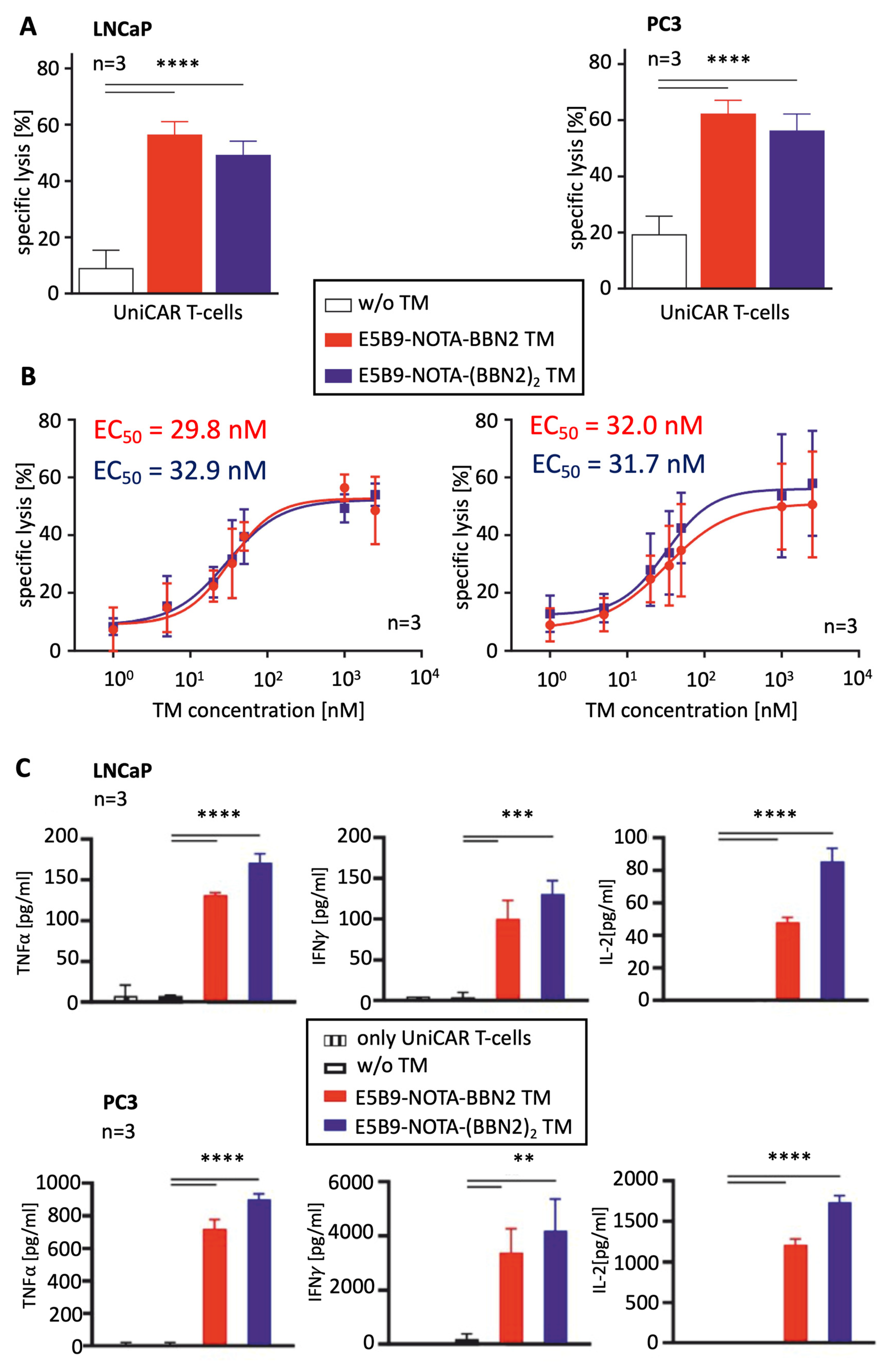
2.2. Therapeutic Evaluation of BBN2 TMs in Combination with UniCAR T-Cells
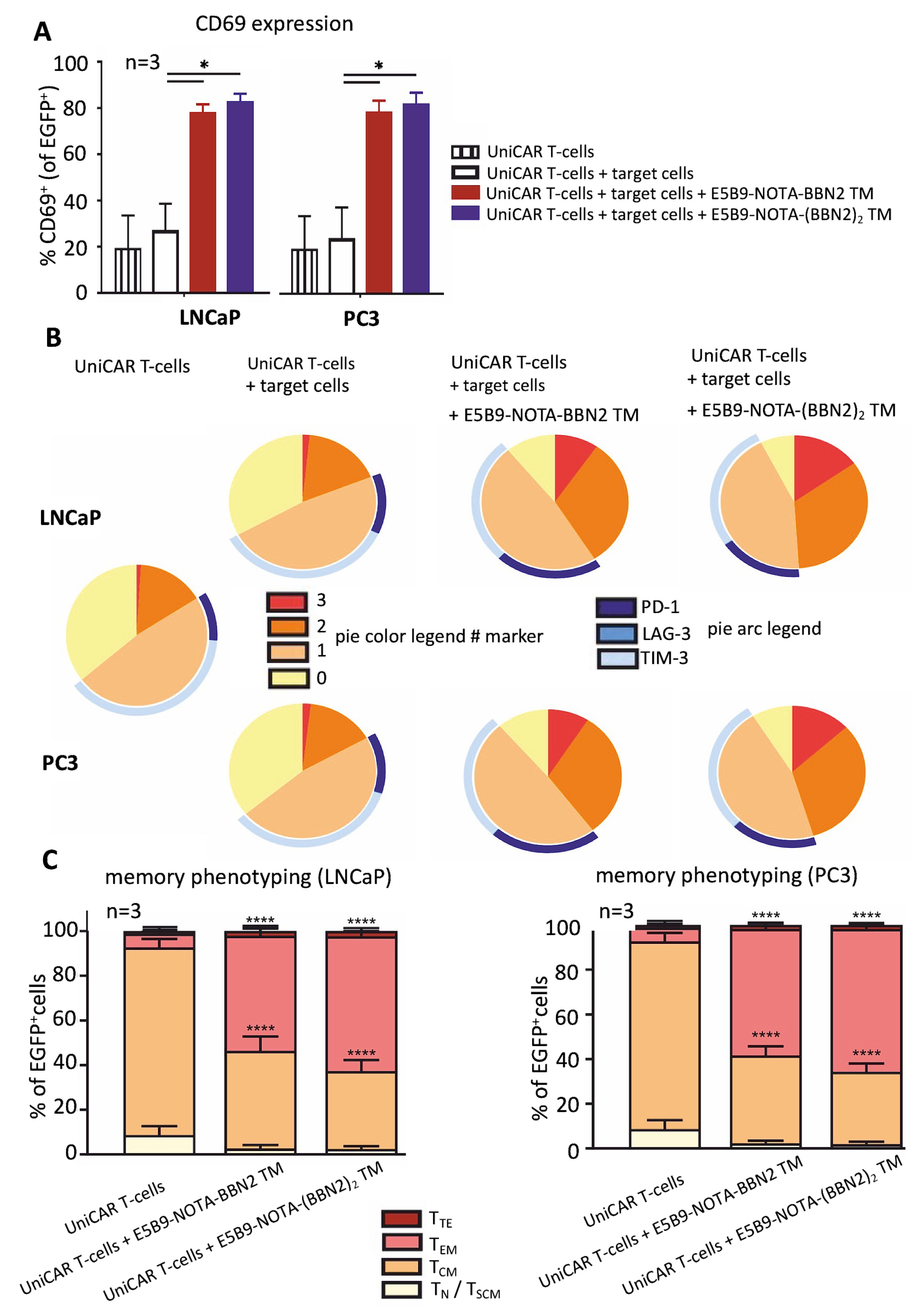
2.3. UniCAR T-Cell Phenotyping Based on Their Activation, Exhaustion, and Memory State
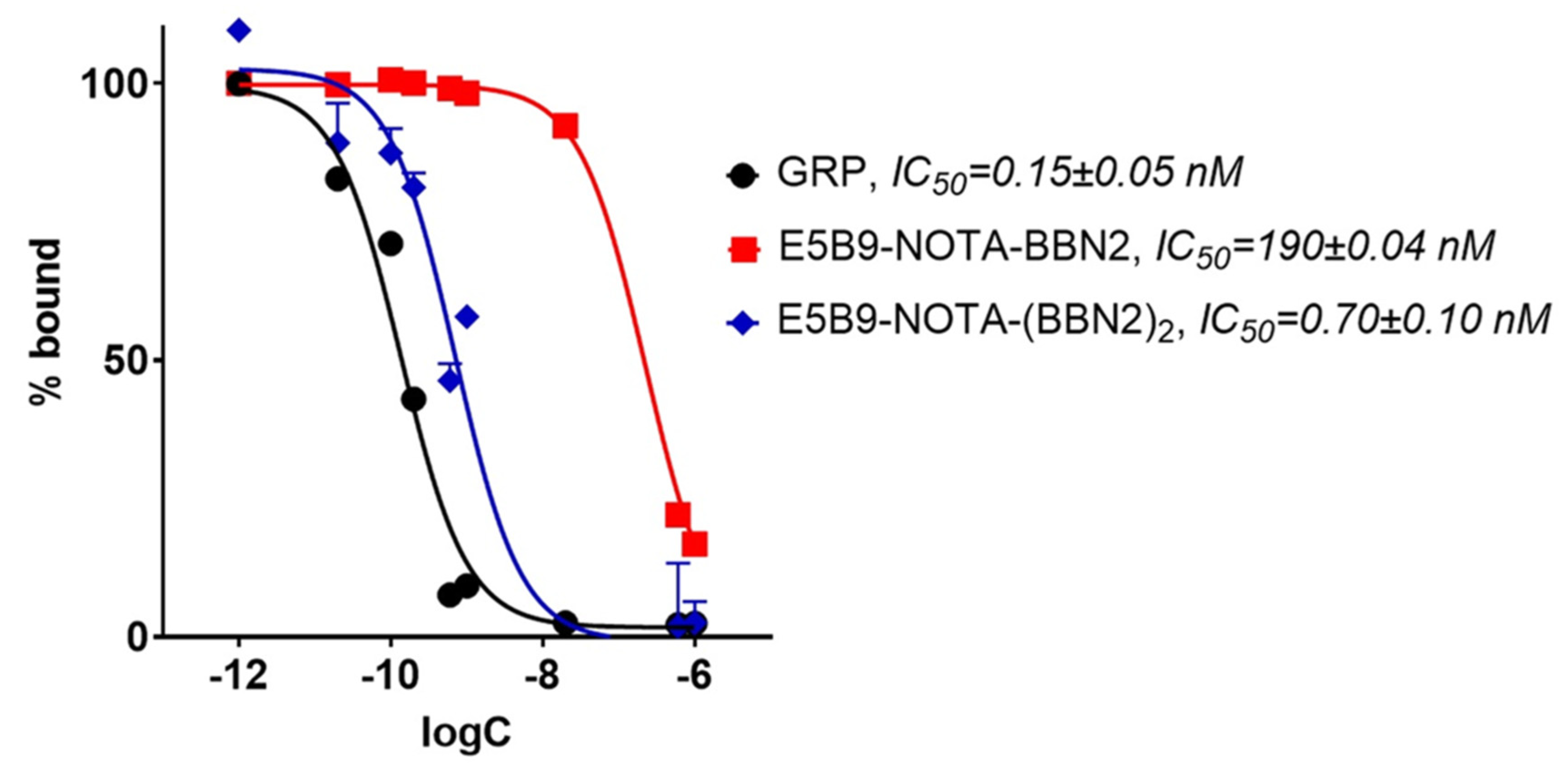
2.4. In Vitro Binding Assessment of BBN2 TMs to GRPR
2.5. 68Ga-Radiolabeling of Monomeric and Dimeric BBN2 TMs
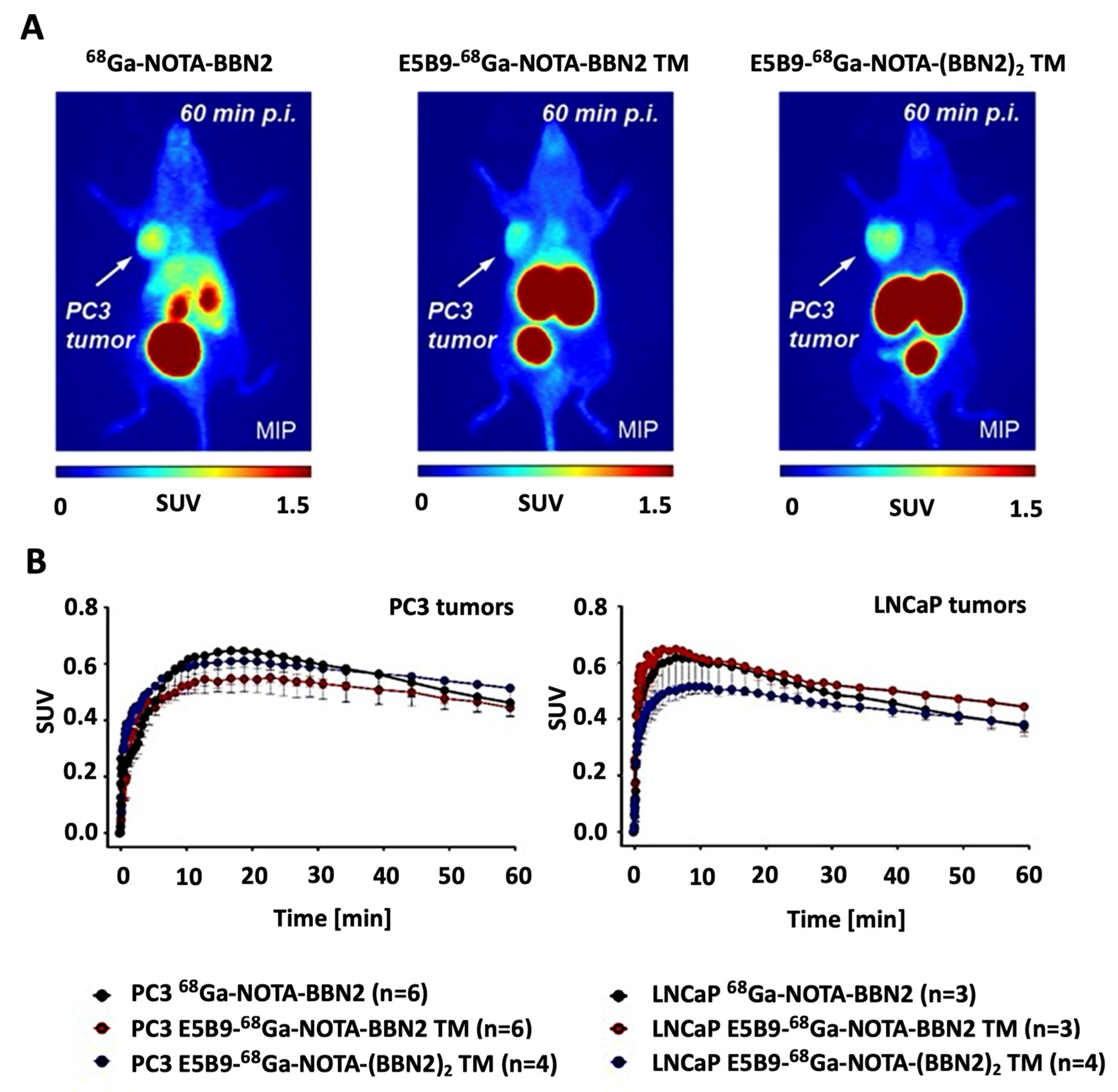
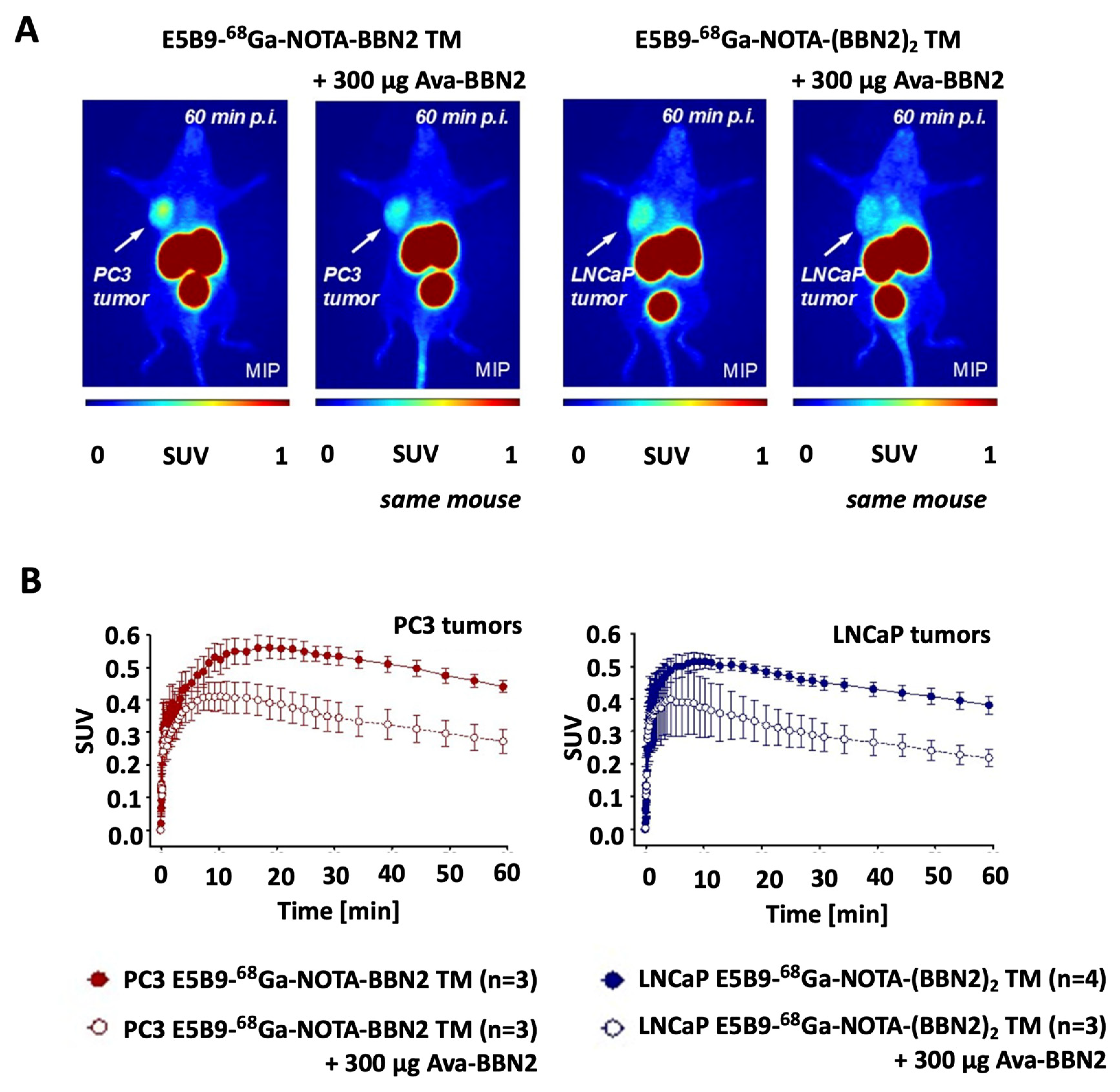
2.6. In Vivo PET Imaging of 68Ga-Labeled Monomeric and Dimeric BBN2 TMs
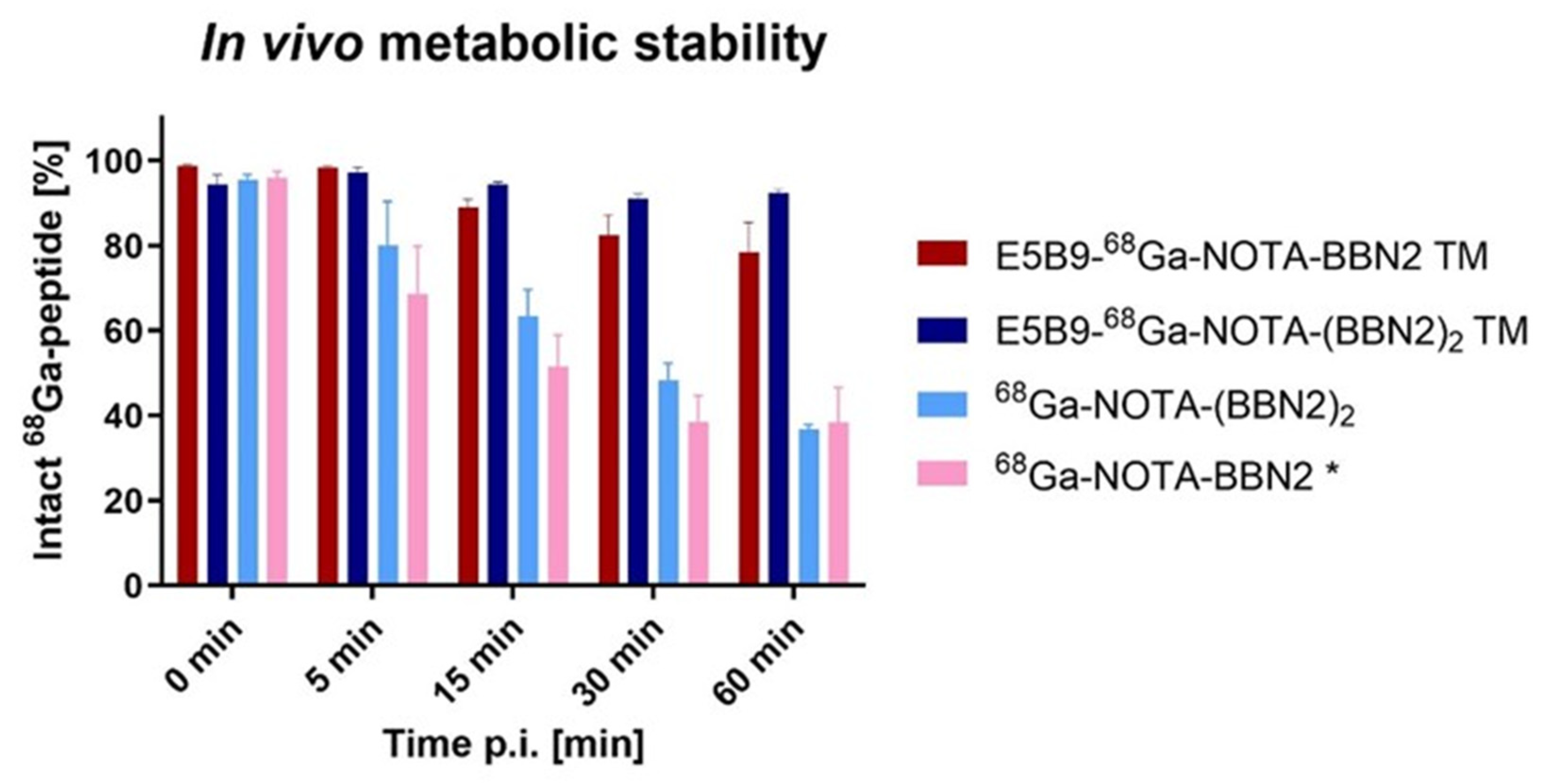
2.7. In Vivo Metabolic Stability of BBN2 TMs
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Lines
4.2. UniCAR T-Cells—T-Cell Isolation and Genetic Modification
4.3. Peptide Synthesis—General Procedure
4.4. NOTA Modification of Ava-Glu-(Ava-BBN2)2
4.5. Competitive Binding Assay
4.6. Binding Assay Using Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Chromium-Release Cytotoxicity Assay
4.8. Cytokine-Release Assay
4.9. T-Cell Activation, Exhaustion, and Memory Phenotype Using Flow Cytometry
4.10. 68Ga-Labeling of E5B9-NOTA-BBN2 Monomeric and Dimeric TMs, and NOTA-Ava-Glu- (Ava-BBN2)2 Dimer
4.11. Metabolic Stability In Vivo
4.12. Dynamic PET Imaging Studies
4.13. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBN2 | Antagonist bombesin peptide |
| CAR | Chimeric Antigen Receptor |
| CT | Computed tomography |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EC50 | Half-maximum effective concentration |
| EDTA | Ethylenediamine tetraacetic acid |
| EGFP | Enhanced green fluorescent protein |
| ELISA | Enzyme-linked immunosorbent assay |
| E:T | Effector-to-target cell |
| FBS | Fetal bovine serum |
| 68Ga | Gallium-68 |
| GRP | Gastrin-releasing peptide |
| GRPR | Gastrin-releasing peptide receptor |
| HPLC | High-performance liquid chromatography |
| IC50 | Half-maximum inhibitory concentration |
| mAb | Monoclonal antibody |
| MW | Molecular weight |
| NOD-SCID | Non-obese diabetic–severe combined immune-deficient |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PET | Positron emission tomography |
| radio-TLC | Radio-thin layer chromatography |
| scFv | Single-chain variable fragment |
| SD | Standard deviation |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SE-HPLC | Size exclusion HPLC |
| SPAAC | Strain-promoted azide–alkyne cycloaddition |
| SPE | Solid-phase extraction |
| SPPS | Solid-phase peptide synthesis |
| SUV | Standard uptake value |
| TAA | Tumor-associated antigen |
| TAC | Time–activity curve |
| TCM | Central memory T-cells |
| TEM | Effector memory T-cells |
| TN/TSCM | Naïve and stem cell-like memory T-cells |
| TTE | Terminal effector T-cells |
| TM | Target module |
| UniCAR | Universal CAR |
References
- Dagar, G.; Gupta, A.; Masoodi, T.; Nisar, S.; Merhi, M.; Hashem, S.; Chauhan, R.; Dagar, M.; Mirza, S.; Bagga, P.; et al. Harnessing the potential of CAR-T cell therapy: Progress, challenges, and future directions in hematological and solid tumor treatments. J. Transl. Med. 2023, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Koristka, S.; Cartellieri, M.; Feldmann, A.; Arndt, C.; Loff, S.; Michalk, I.; Aliperta, R.; von Bonin, M.; Bornhäuser, M.; Ehninger, A.; et al. Flexible Antigen-Specific Redirection of Human Regulatory T Cells Via a Novel Universal Chimeric Antigen Receptor System. Blood 2014, 124, 3494. [Google Scholar] [CrossRef]
- Cartellieri, M.; Feldmann, A.; Koristka, S.; Arndt, C.; Loff, S.; Ehninger, A.; von Bonin, M.; Bejestani, E.P.; Ehninger, G.; Bachmann, M.P. Switching CAR T cells on and off: A novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016, 6, e458. [Google Scholar] [CrossRef]
- Arndt, C.; Bachmann, M.; Bergmann, R.; Berndt, N.; Feldmann, A.; Koristka, S. Theranostic CAR T cell targeting: A brief review. J. Label. Comp. Radiopharm. 2019, 62, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, L.R.; Feldmann, A.; Bergmann, R.; Koristka, S.; Berndt, N.; Arndt, C.; Pietzsch, J.; Novo, C.; Videira, P.; Bachmann, M. Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer J. 2018, 8, 81. [Google Scholar] [CrossRef]
- Mitwasi, N.; Feldmann, A.; Bergmann, R.; Berndt, N.; Arndt, C.; Koristka, S.; Kegler, A.; Jureczek, J.; Hoffmann, A.; Ehninger, A.; et al. Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells. Oncotarget 2017, 8, 108584–108603. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Bergmann, R.; Loff, S.; Cartellieri, M.; Bachmann, D.; Aliperta, R.; Hetzenecker, M.; Ludwig, F.; Albert, S.; et al. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget 2017, 8, 31368–31385. [Google Scholar] [CrossRef]
- Bachmann, M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef]
- Carmo-Fonseca, M.; Pfeifer, K.; Schröder, H.C.; Vaz, M.F.; Fonseca, J.E.; Müller, W.E.; Bachmann, M. Identification of La ribonucleoproteins as a component of interchromatin granules. Exp. Cell Res. 1989, 185, 73–85. [Google Scholar] [CrossRef]
- Arndt, C.; Fasslrinner, F.; Loureiro, L.R.; Koristka, S.; Feldmann, A.; Bachmann, M. Adaptor car platforms—Next generation of T cell-based cancer immunotherapy. Cancers 2020, 12, 1302. [Google Scholar] [CrossRef]
- Loureiro, L.R.; Hoffmann, L.; Neuber, C.; Rupp, L.; Arndt, C.; Kegler, A.; Kubeil, M.; Hagemeyer, C.E.; Stephan, H.; Schmitz, M.; et al. Immunotheranostic target modules for imaging and navigation of UniCAR T-cells to strike FAP-expressing cells and the tumor microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 341. [Google Scholar] [CrossRef]
- Loureiro, L.R.; Feldmann, A.; Bergmann, R.; Koristka, S.; Berndt, N.; Máthé, D.; Hegedüs, N.; Szigeti, K.; Videira, P.A.; Bachmann, M.; et al. Extended half-life target module for sustainable UniCAR T-cell treatment of STn-expressing cancers. J. Exp. Clin. Cancer Res. 2020, 39, 77. [Google Scholar] [CrossRef]
- Albert, S.; Arndt, C.; Feldmann, A.; Bergmann, R.; Bachmann, D.; Koristka, S.; Ludwig, F.; Ziller-Walter, P.; Kegler, A.; Gärtner, S.; et al. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology 2017, 6, e1287246. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Feldmann, A.; Schmitz, M.; Pietzsch, J.; Steinbach, J.; Bachmann, M. From mono- to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo. Oncotarget 2018, 9, 25597. [Google Scholar] [CrossRef]
- Arndt, C.; Feldmann, A.; Koristka, S.; Schäfer, M.; Bergmann, R.; Mitwasi, N.; Berndt, N.; Bachmann, D.; Kegler, A.; Schmitz, M.; et al. A theranostic PSMA ligand for PET imaging and retargeting of T cells expressing the universal chimeric antigen receptor UniCAR. Oncoimmunology 2019, 8, 1659095. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic Prospects of Gastrin-Releasing Peptide Receptor-Radioantagonists in Oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef]
- Maina, T.; Nock, B.A. From Bench to Bed: New Gastrin-Releasing Peptide Receptor-Directed Radioligands and Their Use in Prostate Cancer. PET Clin. 2017, 12, 205–217. [Google Scholar] [CrossRef]
- Mansi, R.; Fleischmann, A.; Mäcke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers--from basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Yuan, L.; Fan, K.; Zhang, Y.; Cai, W.; Lan, X. Radionuclide-Based Imaging of Breast Cancer: State of the Art. Cancers 2021, 13, 5459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Mokotoff, M.; Ball, E.D. Targeting gastrin-releasing peptide receptors for cancer treatment. Anticancer Drugs 2004, 15, 921–927. [Google Scholar] [CrossRef]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled Bombesin Analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2015, 57, 557–562. [Google Scholar] [CrossRef]
- Duan, H.; Ghanouni, P.; Daniel, B.; Rosenberg, J.; Thong, A.; Kunder, C.; Aparici, C.M.; Davidzon, G.A.; Moradi, F.; Sonn, G.A.; et al. A Pilot Study of 68Ga-PSMA11 and 68Ga-RM2 PET/MRI for Biopsy Guidance in Patients with Suspected Prostate Cancer. J. Nucl. Med. 2023, 64, 744–750. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T.; et al. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.; Decristoforo, C.; Uprimny, C.; Hohenberger, P.; Schoenberg, S.O.; Orlandi, F.; Mariani, M.F.; Manzl, C.; Kasseroler, M.T.; Tilg, H.; et al. Imaging Properties and Tumor Targeting of 68Ga-NeoBOMB1, a Gastrin-Releasing Peptide Receptor Antagonist, in GIST Patients. Biomedicines 2022, 10, 2899. [Google Scholar] [CrossRef]
- Wong, K.; Sheehan-Dare, G.; Nguyen, A.; Ho, B.; Liu, V.; Lee, J.; Brown, L.; Dear, R.; Chan, L.; Sharma, S.; et al. 64Cu-SAR-Bombesin PET-CT Imaging in the Staging of Estrogen/Progesterone Receptor Positive, HER2 Negative Metastatic Breast Cancer Patients: Safety, Dosimetry and Feasibility in a Phase I Trial. Pharmaceuticals 2022, 15, 772. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, M.; Krieger, S.S.; Rogers, B.E.; Friebe, M.; Bergmann, R.; Wuest, F. Synthesis and radiopharmacological evaluation of a high-affinity and metabolically stabilized 18F-labeled bombesin analogue for molecular imaging of gastrin-releasing peptide receptor-expressing prostate cancer. Nucl. Med. Biol. 2013, 40, 1025–1034. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, M.; Bergman, C.N.; Krieger, S.; Rogers, B.E.; Wuest, F. Metabolically Stabilized (68)Ga-NOTA-Bombesin for PET Imaging of Prostate Cancer and Influence of Protease Inhibitor Phosphoramidon. Mol. Pharm. 2016, 13, 1347–1357. [Google Scholar] [CrossRef]
- Ferguson, S.; Wuest, M.; Richter, S.; Bergman, C.; Dufour, J.; Krys, D.; Simone, J.; Jans, H.S.; Riauka, T.; Wuest, F. A comparative PET imaging study of 44gSc- and 68Ga-labeled bombesin antagonist BBN2 derivatives in breast and prostate cancer models. Nucl. Med. Biol. 2020, 90, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Gonzalez, B.; Marino, J.; Ayasoufi, K.; Valujskikh, A. Role of Memory T Cells in Allograft Rejection and Tolerance. Front. Immunol. 2017, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, S.; Bove, C.; Mezzanotte, C.; Camisa, B.; Falcone, L.; Manfredi, F.; Bezzecchi, E.; El Khoury, R.; Norata, R.; Sanvito, F.; et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J. Clin. Investig. 2022, 132, e150807. [Google Scholar] [CrossRef] [PubMed]
- López-Cantillo, G.; Urueña, C.; Camacho, B.A.; Ramírez-Segura, C. CAR-T Cell Performance: How to Improve Their Persistence? Front. Immunol. 2022, 13, 878209. [Google Scholar] [CrossRef]
- Watowich, M.B.; Gilbert, M.R.; Larion, M. T cell exhaustion in malignant gliomas. Trends Cancer 2023, 9, 270–292. [Google Scholar] [CrossRef]
- Cytawa, W.; Kircher, S.; Kübler, H.; Werner, R.A.; Weber, S.; Hartrampf, P.; Bandurski, T.; Lass, P.; Połom, W.; Matuszewski, M.; et al. Diverse PSMA expression in primary prostate cancer: Reason for negative [68Ga]Ga-PSMA PET/CT scans? Immunohistochemical validation in 40 surgical specimens. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3938–3949. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discov. 2022, 4, 75. [Google Scholar] [CrossRef]
- Zheng, P.P.; Kros, J.M.; Li, J. Approved CAR T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov. Today 2018, 23, 1175–1182. [Google Scholar] [CrossRef]
- Rangger, C.; Haubner, R. Radiolabelled Peptides for Positron Emission Tomography and Endoradiotherapy in Oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef]
- Jackson, I.M.; Scott, P.J.H.; Thompson, S. Clinical Applications of Radiolabeled Peptides for PET. Semin. Nucl. Med. 2017, 47, 493–523. [Google Scholar] [CrossRef]
- Nock, B.A.; Maina, T. Theranostic Radiopeptides in Nuclear Oncology: Design, Preclinical Screening, and Clinical Translation. In Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P Baum, 1st ed.; Prasad, V., Ed.; Springer: Cham, Switzerland, 2024; pp. 207–224. [Google Scholar]
- Yan, Y.; Chen, X. Peptide heterodimers for molecular imaging. Amino Acids 2011, 41, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, J.; Czerwinski, A.; Schuelke, M.; Płoska, A.; Sowinski, P.; Hood, L.; Mamer, S.B.; Cole, J.A.; Czaplewska, P.; Banach, M.; et al. Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of α V β 3 Integrin Activity. Sci. Rep. 2017, 7, 3185. [Google Scholar] [CrossRef] [PubMed]
- Judmann, B.; Braun, D.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Current State of Radiolabeled Heterobivalent Peptidic Ligands in Tumor Imaging and Therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; De Jong, M. “To Serve and Protect”: Enzyme Inhibitors as Radiopeptide Escorts Promote Tumor Targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef]
- Rethi-Nagy, Z.; Abraham, E.; Udvardy, K.; Klement, E.; Darula, Z.; Pal, M.; Katona, R.L.; Tubak, V.; Pali, T.; Kota, Z.; et al. STABILON, a Novel Sequence Motif That Enhances the Expression and Accumulation of Intracellular and Secreted Proteins. Int. J. Mol. Sci. 2022, 23, 8168. [Google Scholar] [CrossRef]
- Walker, J.R.; Altman, R.K.; Warren, J.W.; Altman, E. Using protein-based motifs to stabilize peptides. J. Pept. Res. 2003, 62, 214–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loureiro, L.R.; Pike, S.; Wuest, M.; Bergman, C.N.; JØrgensen, K.R.; Bergmann, R.; Feldmann, A.; Wuest, F.; Bachmann, M. Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells. Int. J. Mol. Sci. 2025, 26, 2686. https://doi.org/10.3390/ijms26062686
Loureiro LR, Pike S, Wuest M, Bergman CN, JØrgensen KR, Bergmann R, Feldmann A, Wuest F, Bachmann M. Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells. International Journal of Molecular Sciences. 2025; 26(6):2686. https://doi.org/10.3390/ijms26062686
Chicago/Turabian StyleLoureiro, Liliana R., Susan Pike, Melinda Wuest, Cody N. Bergman, Kira R. JØrgensen, Ralf Bergmann, Anja Feldmann, Frank Wuest, and Michael Bachmann. 2025. "Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells" International Journal of Molecular Sciences 26, no. 6: 2686. https://doi.org/10.3390/ijms26062686
APA StyleLoureiro, L. R., Pike, S., Wuest, M., Bergman, C. N., JØrgensen, K. R., Bergmann, R., Feldmann, A., Wuest, F., & Bachmann, M. (2025). Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells. International Journal of Molecular Sciences, 26(6), 2686. https://doi.org/10.3390/ijms26062686










