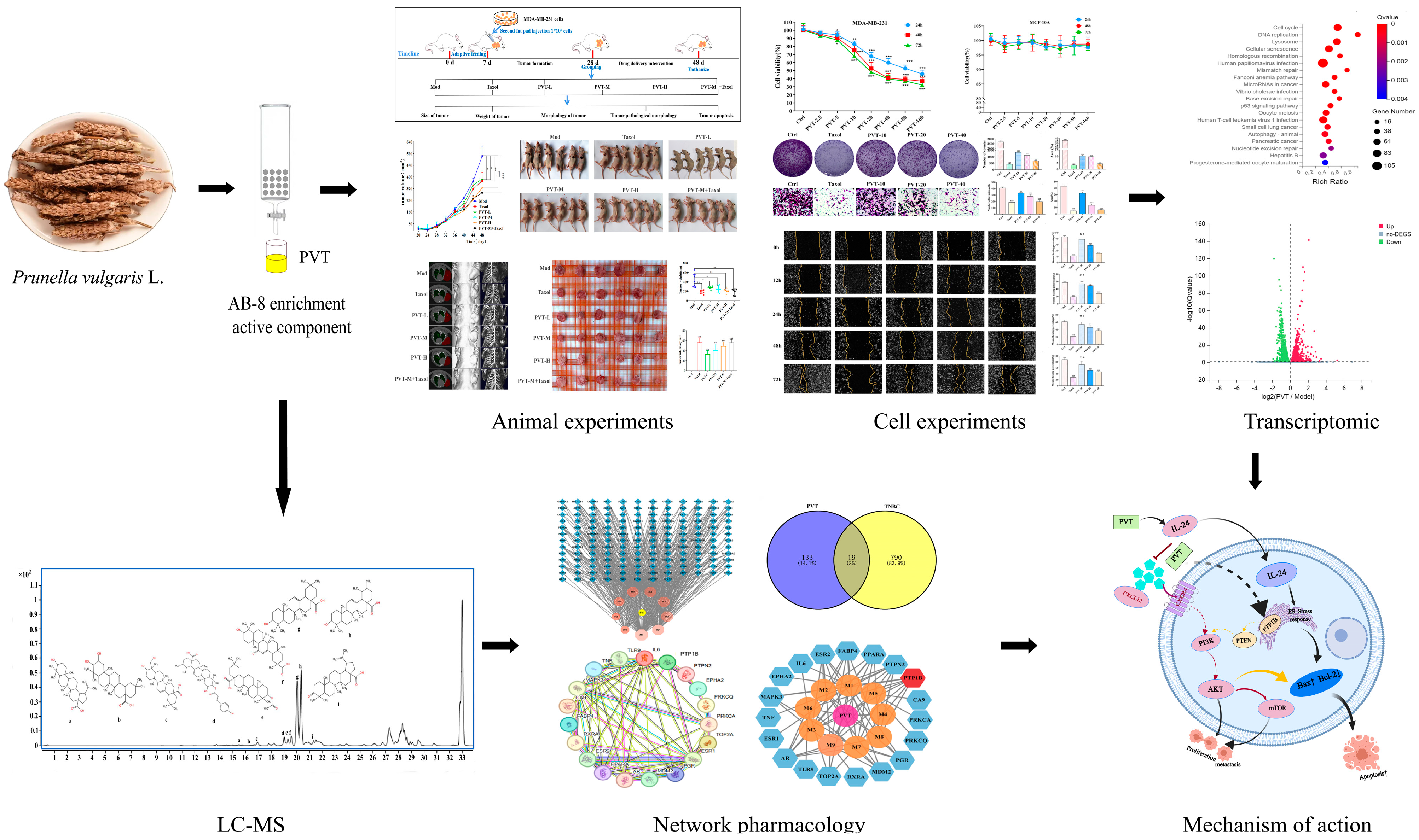
Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways
Abstract
:1. Introduction
2. Results
2.1. Identification of PVT and Determination of Ursolic Acid and Oleanolic Acid Contents
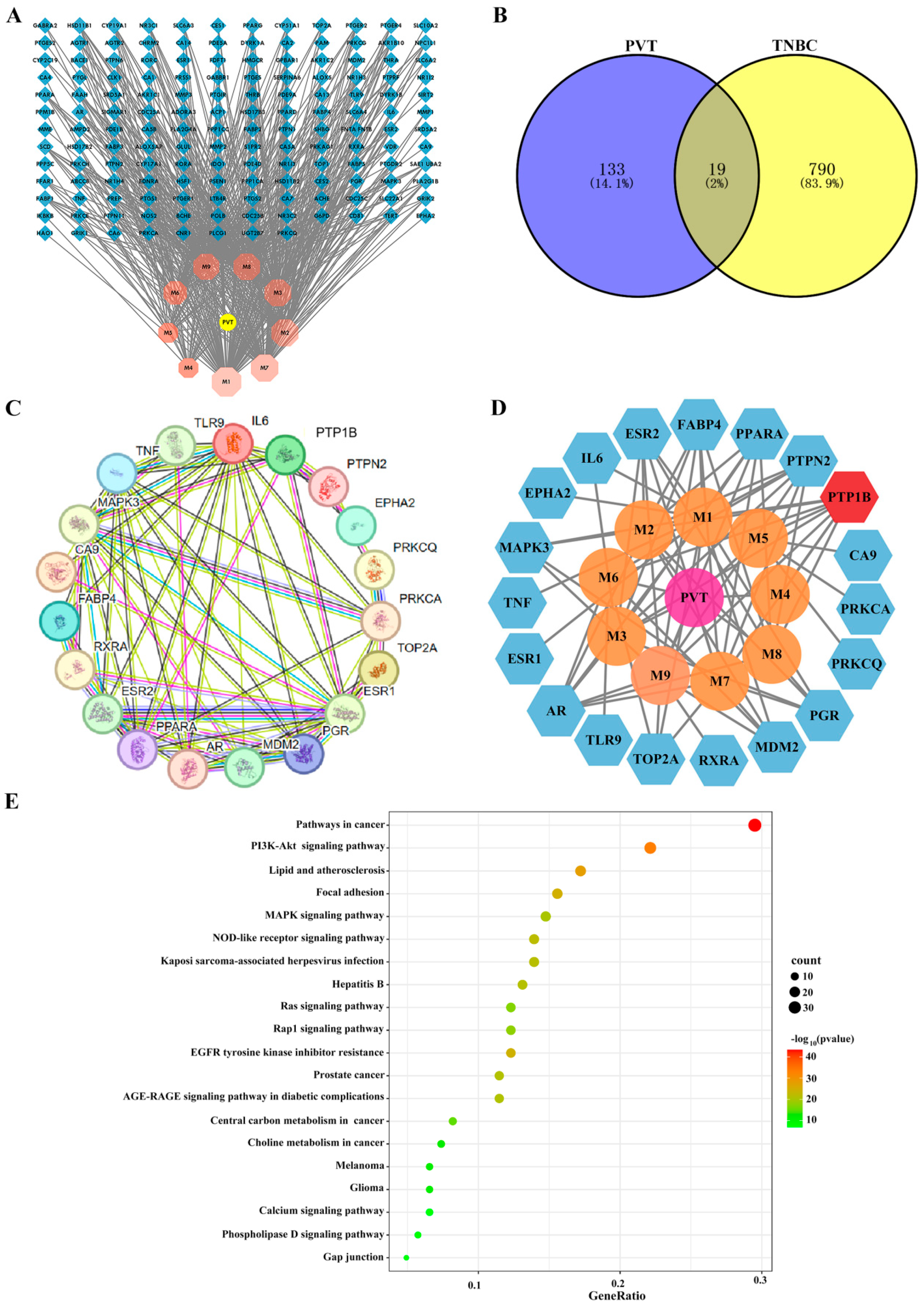
2.2. Network Pharmacology Analysis of Effects of PVT on TNBC
2.3. PVT Inhibited the Proliferation, Migration and Invasion of MDA-MB-231 Cells
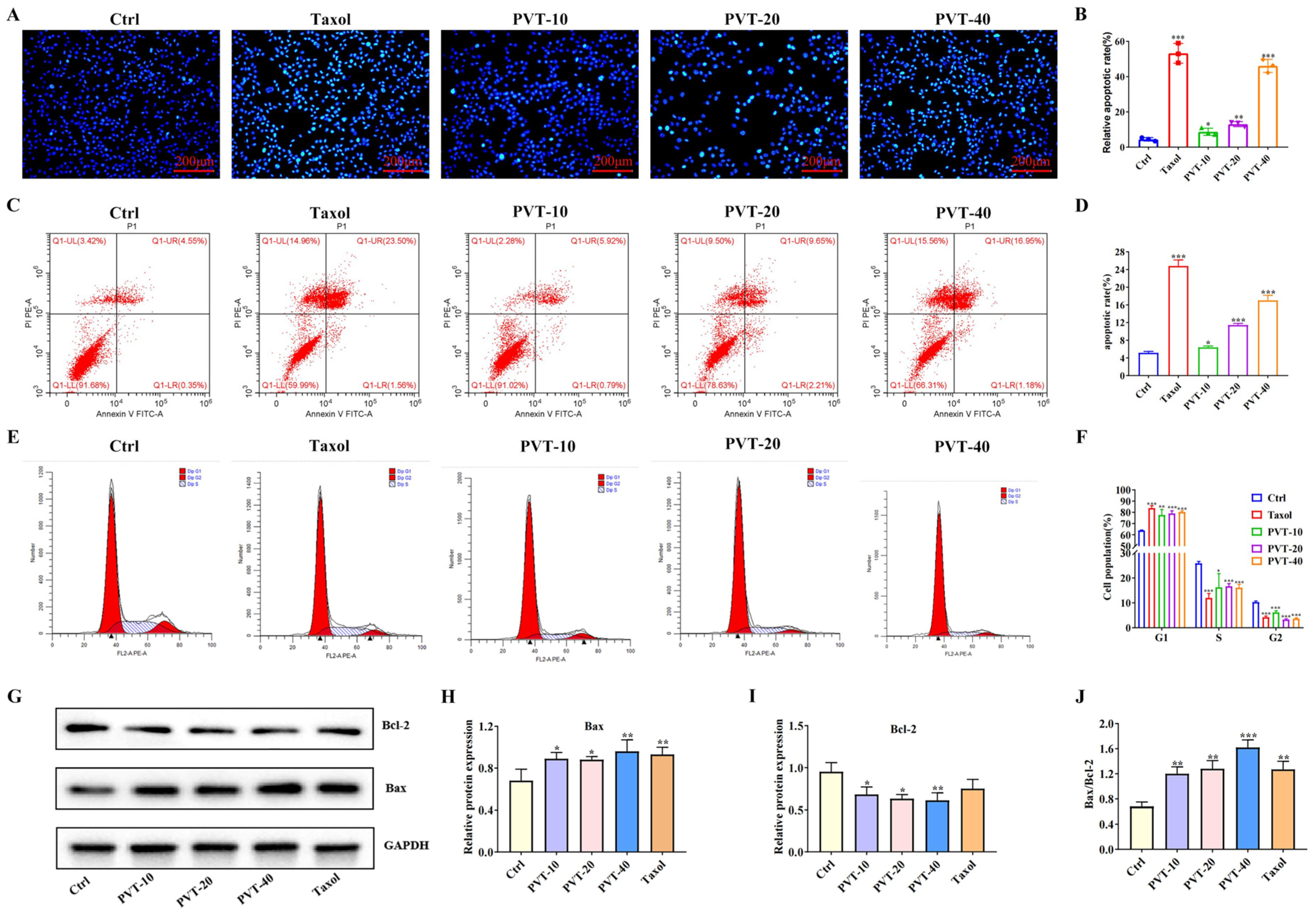
2.4. PVT Effectively Promoted the Apoptosis of MDA-MB-231 Cells
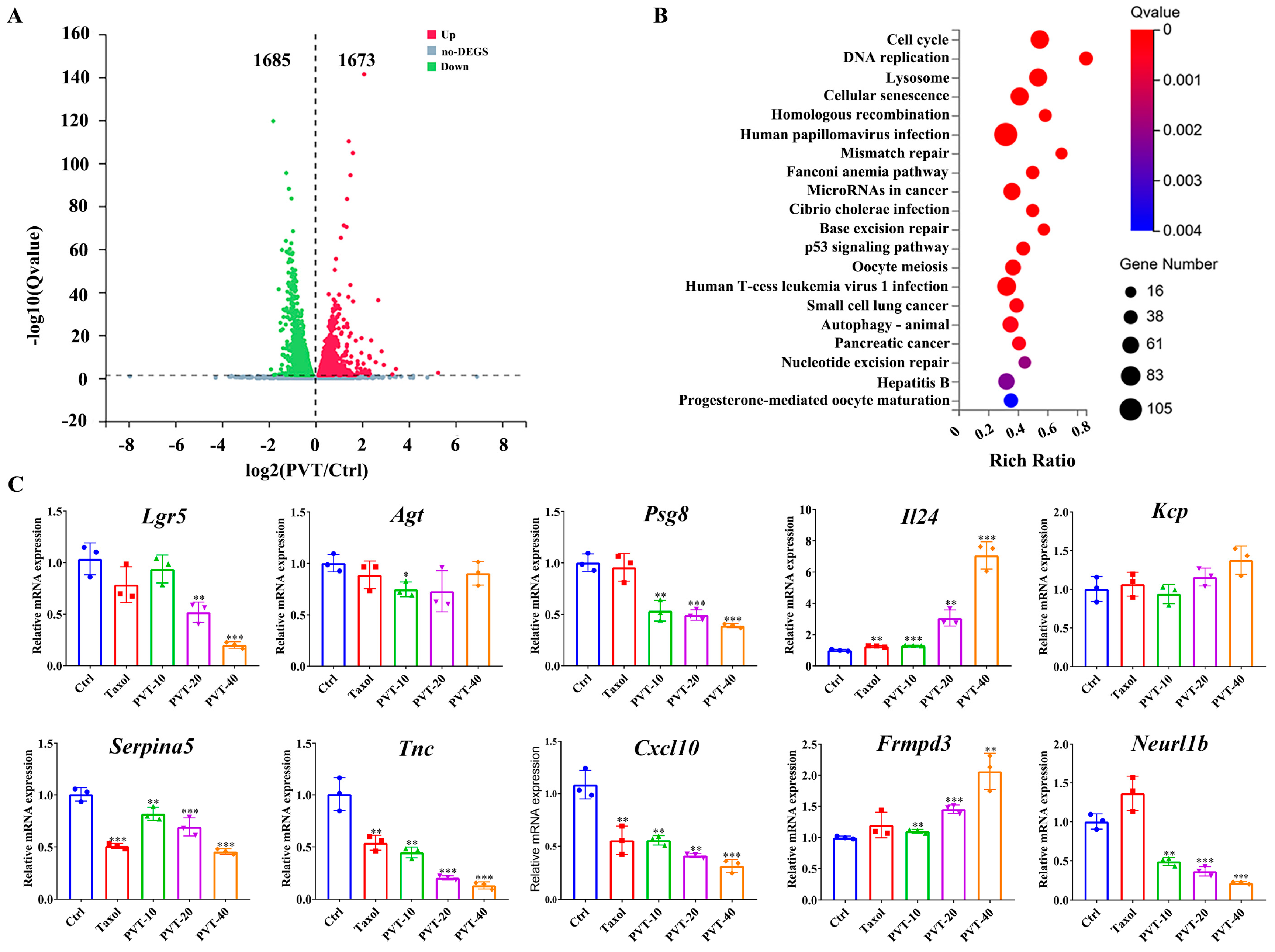
2.5. Transcriptomic Analysis of Effects of PVT on MDA-MB-231 Cells
2.6. PVT Effectively Inhibited the Growth of TNBC Tumors
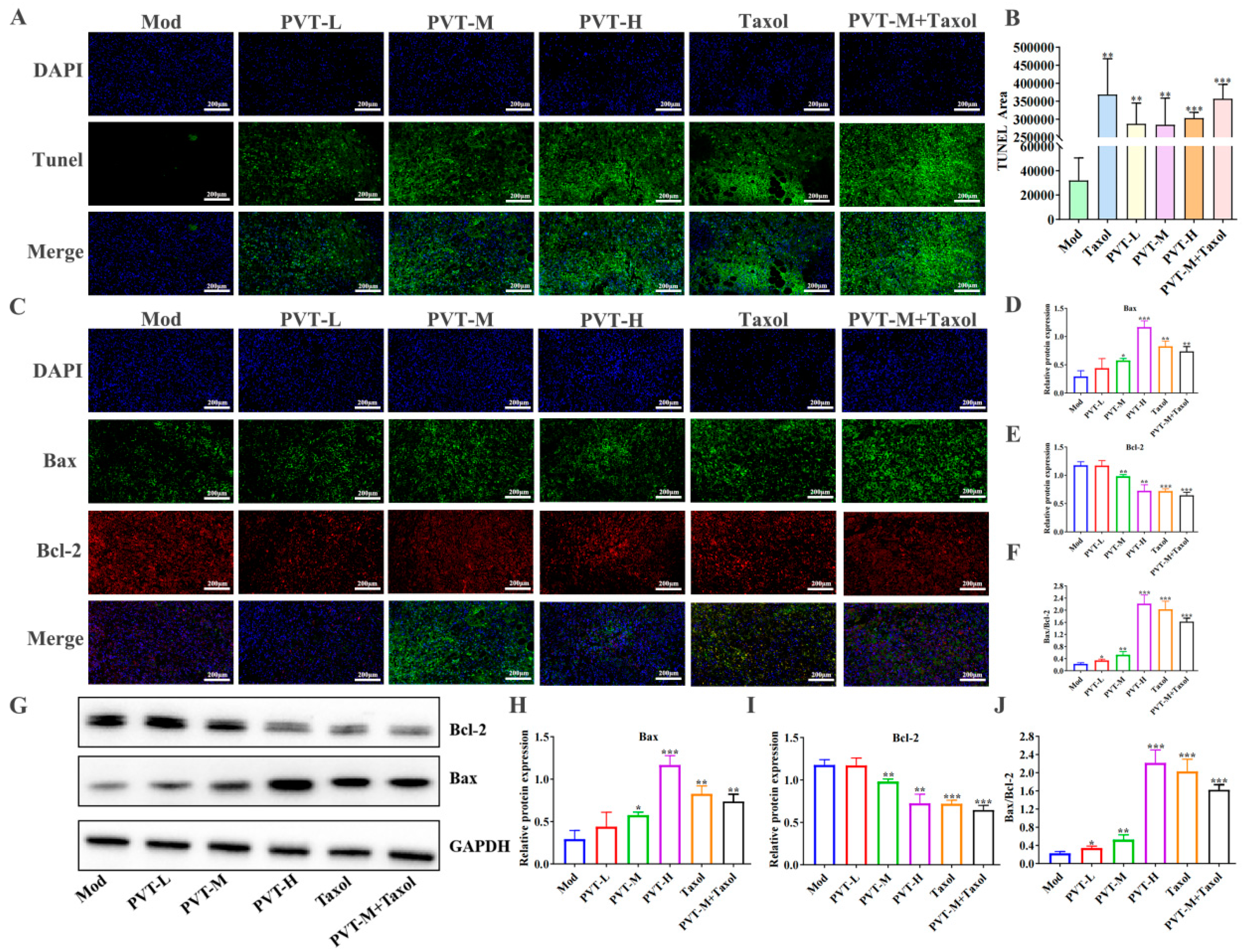
2.7. PVT Promoted Tumor Apoptosis in TNBC Model Mice
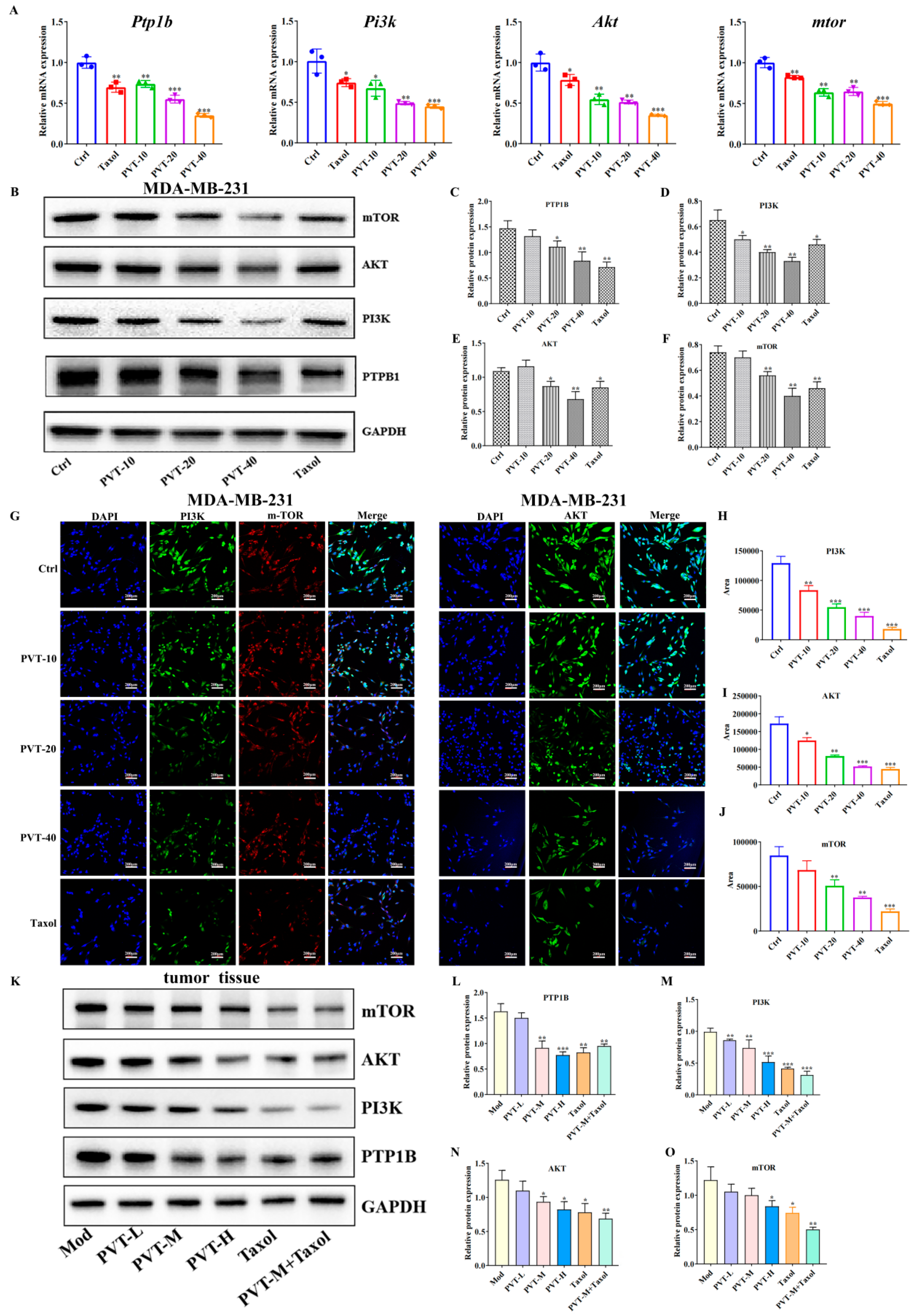
2.8. Effects of PVT on PTP1B and PI3K/AKT/mTOR Signaling Pathways in TNBC Cells and Tumor Tissues
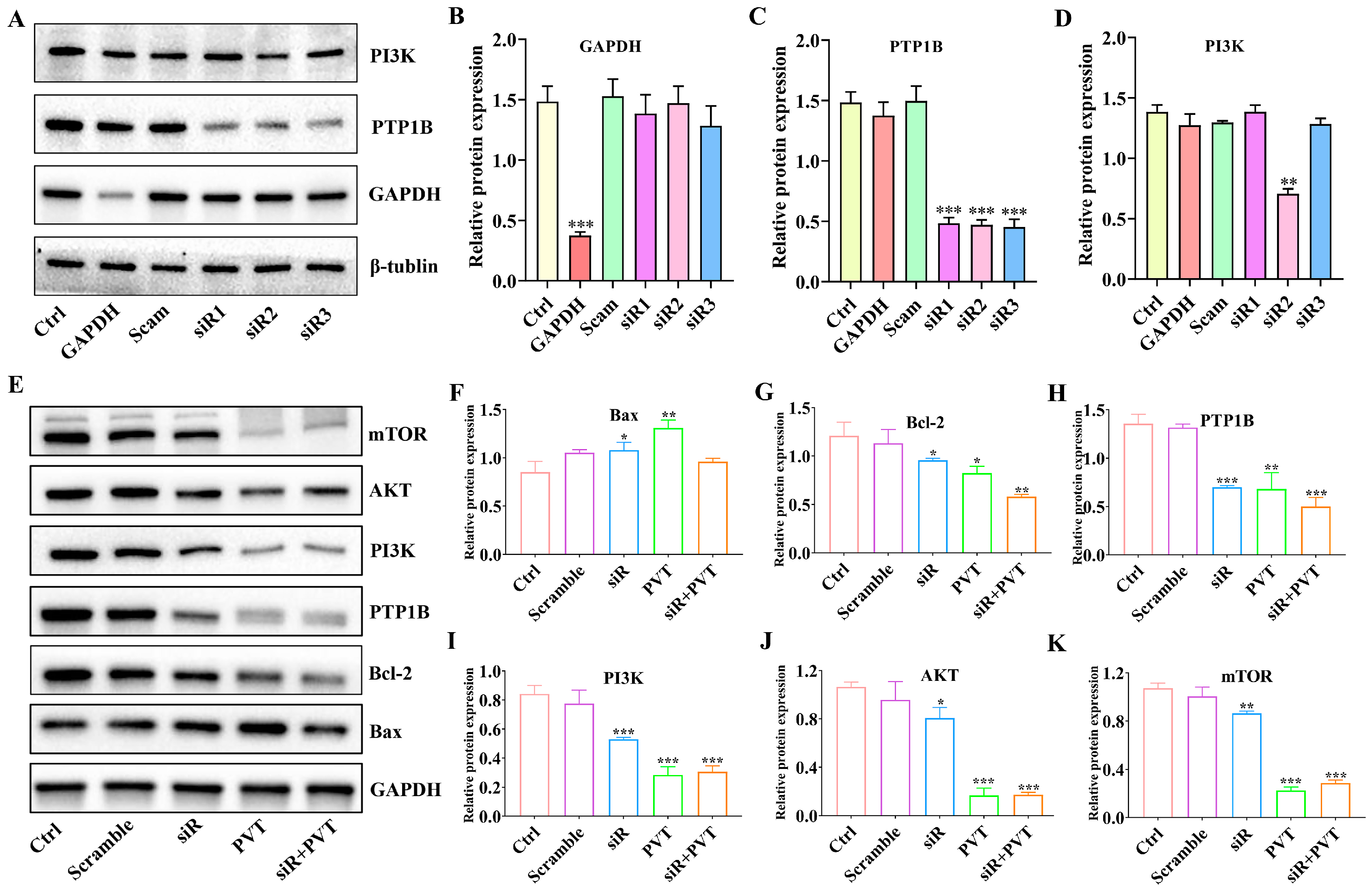
2.9. Silencing PTP1B Can Play an Anti-TNBC Role
2.10. PVT Inhibited the Metastasis of TNBC by Regulating the IL-24/CXCL12/CXCR4 Signaling Axis
3. Discussion
4. Materials and Methods
4.1. Preparation of PVT
4.2. Chemical Profile of PVT
4.3. Network Pharmacology Analysis of PVT in the Treatment of TNBC
4.4. Cell Viability Assay
4.5. Cell Proliferation Assay
4.6. Scratch Assay
4.7. Transwell Migration Assay
4.8. Flow Cytometry
4.9. Cell Transcriptomics
4.9.1. RNA Sequencing
4.9.2. Quantitative Real-Time PCR
4.10. Hoechst 33342 Staining
4.11. Triple-Negative Breast Cancer Mouse Model Construction and Treatment
4.12. Micro-CT Scanning
4.13. Hematoxylin and Eosin (H&E) Staining
4.14. Western Blotting Analysis
4.15. Immunofluorescence Assay
4.16. Silencing the PTP1B Gene Using siRNA Technology
4.17. Statistical Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Committee National Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. Part 1; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Hao, J.; Ding, X.L.; Yang, X.; Wu, X.Z. Prunella vulgaris polysaccharide inhibits growth and migration of breast carcinoma-associated fibroblasts by suppressing expression of basic fibroblast growth factor. Chin. J. Integr. Med. 2020, 26, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Han, B.F.; Zheng, R.S.; Zeng, H.M. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Roberts, K.E.; Adsett, I.T.; Rickett, K.; Conroy, S.M.; Chatfield, M.D.; Woodward, N.E. Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst. Rev. 2022, 1, CD013167. [Google Scholar] [CrossRef] [PubMed]
- Braybrooke, J.; Bradley, R.; Gray, R.; Hills, R.K.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; Aihara, T.; et al. Anthracycline-containing and taxane-containing chemotherapy for early- stage operable breast cancer: A patient-level meta-analysis of 100,000 women from 86 randomised trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Tolaney, S.M. Progress in breast cancer management. Lancet 2024, 404, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Reductions in recurrence in women with early breast cancer entering clinical trials between 1990 and 2009: A pooled analysis of 155 746 women in 151 trials. Lancet 2024, 404, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer-expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.W.; Qin, Y.N.; Sun, C.P.; Chen, J.J.; Ruan, Y.Y.; Chen, L.X.; Liu, S.; Liu, G.Y. Clinical observation on the effect of Chinese medicine-“TCM formula” intervention on recurrence and metastasis of triple negative breast cancer. Complement. Ther. Med. 2020, 52, 102456. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Y.; Zhang, T.; Zheng, C.; Ding, X.; Zhu, C.; Shi, J.; Jing, Y. Traditional Chinese medicine for breast cancer treatment: A bibliometric and visualization analysis. Pharm. Biol. 2024, 62, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhao, L.; Li, Y.; Xia, B.; Lin, Y.; Xie, J.; Wu, P.; Liao, D.; Zhang, Z.; Lin, L. An in vivo and in vitro assessment of the anti-breast cancer activity of crude extract and fractions from Prunella vulgaris L. Heliyon 2022, 8, e11183. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, Q.; Li, Y.; Zhao, L.; Fu, R.; Wu, P.; Zhang, Z.; Gong, Y.; Xia, B.; Liao, D.; et al. Anti breast cancer activity and mechanism study on appropriate components from Prunella vulgaris. Chin. Tradit. Herb. Drugs 2019, 50, 5298–5306. [Google Scholar]
- Zhang, S.; Meng, F.; Pan, X.; Qiu, X.; Li, C.; Lu, S. Chromosome-level genome assembly of Prunella vulgaris L. provides insights into pentacyclic triterpenoid biosynthesis. Plant J. 2024, 118, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, B.; Xie, W.; Zhou, Y.; Xie, J.; Li, H.; Liao, D.; Lin, L.; Li, C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016, 204, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.-A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef] [PubMed]
- Mîrza, C.M.; Mîrza, T.V.; Odagiu, A.C.M.; Uifălean, A.; But, A.E.; Pârvu, A.E.; Bulboacă, A.E. Phytochemical analysis and antioxidant effects of Prunella vulgaris in experimental acute inflammation. Int. J. Mol. Sci. 2024, 25, 4843. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Xie, J.; Xu, C.; Zhang, Z.; Li, M.; Xia, B.; Shi, Z.; Lin, L. Effect and mechanism of Prunella vulgaris L. extract on alleviating lipopolysaccharide-induced acute mastitis in protecting the blood-milk barrier and reducing inflammation. J. Ethnopharmacol. 2024, 28, 117998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Palen, K.; Johnson, B.; Bui, D.; Xiong, D.; Pan, J.; Hu, M.; Wang, Y.; You, M. Cancer chemoprevention with PV-1, a novel Prunella vulgaris-containing herbal mixture that remodels the tumor immune microenvironment in mice. Front. Immunol. 2023, 14, 1196434. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hiang, H.; Li, Y.L.; Liu, Y.Y.; Xu, Y.X. Root extract of Prunella vulgaris inhibits in vitro and in vivo carcinogenesis in MCF-5 human breast carcinoma via suppression of angiogenesis, induction of apoptosis, cell cycle arrest and modulation of PI3K/AKT signalling pathway. J. BUON 2019, 24, 549–554. [Google Scholar] [PubMed]
- Yang, A.P.; Zheng, Z.G.; Liu, F.; Liu, J.; Liu, H. Screening for potential anti-breast cancer components from prunellae spica using MCF-7 cell extraction coupled with HPLC-ESI-MS/MS. Nat. Prod. Commun. 2020, 15, 1934578X2093196. [Google Scholar]
- Bai, Y.; Li, C.; Zhou, Y.; Pi, S.; Xia, B.; Li, Y.; Lin, L.; Liao, D.F. Chemical constituents of triterpenoids from Prunella vulgaris and their anti tumor activities. Chin. Tradit. Herb Drugs 2015, 46, 3623–3629. [Google Scholar]
- Zhou, Y.; Tang, J.; Xiong, S.; Li, Y.; Lin, Y.; Xia, B.; Lin, L.; Liao, D.F. Polar chemical constituents from Prunella vulgaris L. and their anti-human breast tumor activities. Chin. Pharm. J. 2017, 52, 362–366. [Google Scholar]
- Chen, P.J.; Zhang, Y.T. Protein Tyrosine Phosphatase 1B (PTP1B): Insights into its New Implications in Tumorigenesis. Curr. Cancer Drug Targets 2022, 22, 181–194. [Google Scholar] [CrossRef]
- Tonks, N.K.; Muthuswamy, S.K. A brake becomes an accelerator: PTP1B—A new therapeutic target for breast cancer. Cancer Cell 2007, 11, 214–216. [Google Scholar] [CrossRef]
- Hu, C.; Li, G.; Mu, Y.; Wu, W.; Cao, B.; Wang, Z.; Yu, H.; Guan, P.; Han, L.; Li, L.; et al. Discovery of anti-TNBC agents targeting PTP1B: Total synthesis, structure-activity relationship, in vitro and in vivo investigations of Jamunones. J. Med. Chem. 2021, 64, 6008–6020. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; He, Q. Ursolic acid inhibits proliferation, migration and invasion of human papillary thyroid carcinoma cells via CXCL12/CXCR4/CXCR7 axis through cancer-associated fibroblasts. Hum. Exp. Toxicol. 2022, 41, 9603271221111333. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, J.; Jin, J.; Shanker, M.; Lauderdale, J.; Bates, J.; Wang, Q.; Zhao, Y.D.; Archibald, S.J.; Hubin, T.J.; Ramesh, R. IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. PLoS ONE 2015, 10, e0122439. [Google Scholar] [CrossRef]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 alleviates desmoplasia, increases lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Seshadri, M.; Komorowski, M.P.; Abrams, S.I.; Kozbor, D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc. Natl. Acad. Sci. USA 2013, 110, E1291–E1300. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Qiu, Y.; Su, X. Targeting CXCL12-CXCR4 Signaling enhances immune checkpoint blockade therapy against triple negative breast cancer. Eur. J. Pharm. Sci. 2021, 157, 105606. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yan, H.; Kang, Z. A Review of Traditional Chinese Medicine for Triple Negative Breast Cancer and the Pharmacological Mechanisms. Am. J. Chin. Med. 2024, 52, 987–1011. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch. Pharm. Res. 2011, 34, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Zheng, M.; Tiriveedhi, V. Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells. Cell Biochem. Biophys. 2016, 74, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Song, C.K.; Viernstein, H.; Unger, F.; Liang, Z.S. Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chem. 2013, 138, 1998–2007. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, X.; Tao, H.; Zou, H.; Cao, J.; Chen, Y.; Zhang, Z.; Zhu, Y.; Li, Q.; Li, M. Liquidambaric acid inhibits the proliferation of hepatocellular carcinoma cells by targeting PPARα-RXRα to down-regulate fatty acid metabolism. Toxicol. App.l Pharmacol. 2024, 490, 117042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Tsai, C.W.; Mong, M.C.; Yin, M.C. Maslinic Acid Protected PC12 Cells Differentiated by Nerve Growth Factor against β-Amyloid-Induced Apoptosis. J. Agric. Food Chem. 2015, 63, 10243–10249. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Pachón-Peña, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Zhou, J.Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Feng, L.; Jia, X.B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412. [Google Scholar] [CrossRef]
- Su, F.; Sui, X.; Xu, J.; Liu, Q.; Li, J.; Liu, W.; Xu, Y.; Zhang, Z.; Tao, F. Hederagenin suppresses ovarian cancer via targeting mitochondrial fission through dynamin-related protein 1. Eur. J. Pharmacol. 2024, 963, 176188. [Google Scholar] [CrossRef] [PubMed]
- Notte, A.; Ninane, N.; Arnould, T.; Michiels, C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: Role of autophagy and JNK activation. Cell Death Dis. 2013, 4, e638. [Google Scholar] [CrossRef]
- Škubník, J.; Svobodová Pavlíčková, V.; Ruml, T.; Rimpelová, S. Autophagy in cancer resistance to paclitaxel: Development of combination strategies. Biomed. Pharmacother. 2023, 161, 114458. [Google Scholar] [CrossRef]
- Johnson, T.O.; Ermolieff, J.; Jirousek, M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Hu, X.G.; Zhang, X.C.; Fu, T.W.; Liu, Q.; Liang, Y.; Zhao, X.L.; Zhang, X.; Ping, Y.F.; et al. PTP1B promotes aggressiveness of breast cancer cells by regulating PTEN but not EMT. Tumour. Biol. 2016, 37, 13479–13487. [Google Scholar] [CrossRef] [PubMed]
- Wiede, F.; Lu, K.H.; Du, X.; Zeissig, M.N.; Xu, R.; Goh, P.K.; Xirouchaki, C.E.; Hogarth, S.J.; Greatorex, S.; Sek, K.; et al. PTP1B is an intracellular checkpoint that limits T-cell and CAR T-cell antitumor immunity. Cancer Discov. 2022, 12, 752–773. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: Evidence from a phase 1 trial of mTOR inhibition in combination with liposomal Doxorubicin and Bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Everaert, C.; Luypaert, M.; Maag, J.L.V.; Cheng, Q.X.; Dinger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci Rep. 2017, 7, 1559. [Google Scholar] [CrossRef] [PubMed]
- Emdad, L.; Bhoopathi, P.; Talukdar, S.; Pradhan, A.K.; Sarkar, D.; Wang, X.Y.; Das, S.K.; Fisher, P.B. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin. Cancer Biol. 2020, 66, 140–154. [Google Scholar] [CrossRef]
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Guo, C.; Yuan, F.; Li, Y.J.; Archer, M.C.; Zacksenhaus, E.; Windle, J.J.; et al. MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer. Oncotarget 2015, 6, 36928–36942. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mohan, C.D.; Shanmugam, M.K.; Jung, Y.Y.; Chinnathambi, A.; Alharbi, S.A.; Ashrafizadeh, M.; Mahale, M.; Bender, A.; Kumar, A.P.; et al. CXCR4 expression is elevated in TNBC patient derived samples and Z-guggulsterone abrogates tumor progression by targeting CXCL12/CXCR4 signaling axis in preclinical breast cancer model. Environ. Res. 2023, 232, 116335. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Z.; Zhu, P.; Wang, Y.; Ren, Q.; Chen, H.; Xu, J. CXCL12/CXCR4 promotes proliferation, migration, and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway. J. Cell Biochem. 2019, 120, 9724–9736. [Google Scholar] [CrossRef]
- Dheeraj, A.; Garcia Marques, F.J.; Tailor, D.; Bermudez, A.; Resendez, A.; Pandrala, M.; Grau, B.; Kumar, P.; Haley, C.B.; Honkala, A.; et al. Inhibition of protein translational machinery in triple-negative breast cancer as a promising therapeutic strategy. Cell Rep Med. 2024, 5, 101552. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Coyle, K.; Marcato, P.; Vasantha Rupasinghe, H.P.; Hoskin, D.W. Phloridzin docosahexaenoate, a novel fatty acid ester of a plant polyphenol, inhibits mammary carcinoma cell metastasis. Cancer Lett. 2019, 465, 68–81. [Google Scholar] [CrossRef]
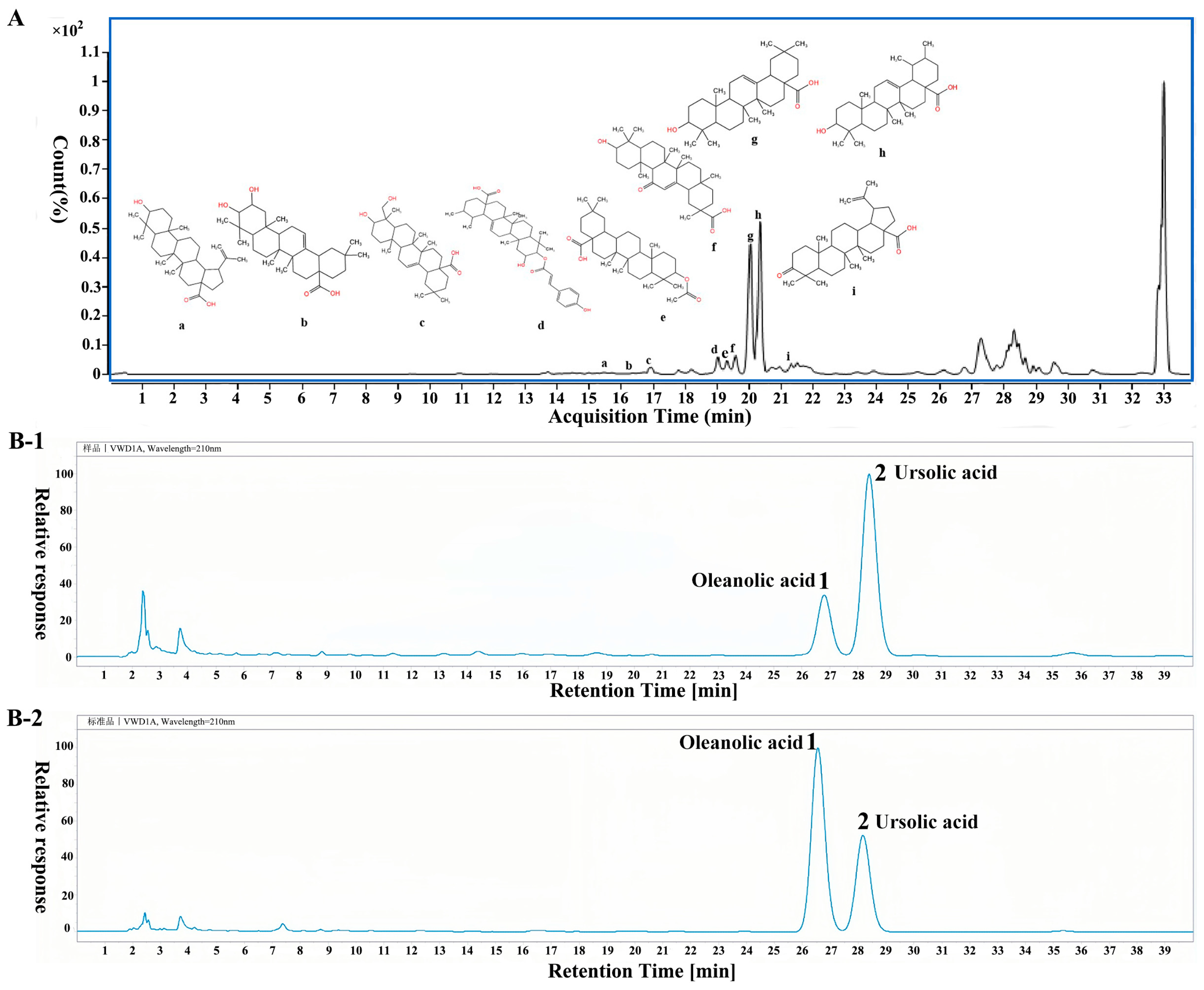




| Serial Number | tR/min | Measured Value [M-H]− | Theoretical Value | Molecular Formula | Compound | Characteristic Ion |
|---|---|---|---|---|---|---|
| 1 | 14.066 | 487.3436 | 487.3430 | C30 H48O5 | Ananasic acid | 487.3436 |
| 2 | 14.498 | 311.1689 | 311.1798 | C20H24O3 | Triptophenolide | 311.1689, 271.1335, 225.1273 |
| 3 | 14.950 | 491.2732 | 491.2657 | C28H36N4O4 | Mucronine B | 491.2659 |
| 4 | 15.021 | 285.0409 | 285.0408 | C15H10O6 | Kaempferol | 285.0408 |
| 5(a) | 15.755 | 469.3328 | 469.3333 | C30H46O4 | 18α-Glycyrrhetinic acid | 469.3328, 373.1238, 175.6028 |
| 6(b) | 16.415 | 471.3476 | 471.3483 | C30H48O4 | Maslinic acid | 471.3476, 409.3477 |
| 7(c) | 16.773 | 471.3479 | 471.3486 | C30H48O4 | Hederagenin | 471.3485 |
| 8(d) | 18.843 | 617.3851 | 617.3849 | C39H54O6 | cis-p-Coumaroylcorosolic acid | 617.3851 |
| 9(e) | 19.152 | 499.3799 | 499.3804 | C32H52O4 | Oleananoic acid acetate | 499.3799, 409.364 |
| 10(f) | 19.527 | 455.3544 | 455.3546 | C30H48O3 | Betulinic acid | 455.3544 |
| 11(g) | 20.213 | 455.3534 | 455.3539 | C30H48O3 | Oleanolic acid | 455.3534 |
| 12(h) | 20.551 | 455.3531 | 455.3536 | C30H48O3 | Ursolic acid | 455.3531 |
| 13 | 20.745 | 592.2687 | 592.7000 | C34H40O9 | Isomoreollic acid | 592.2687, 515.2454 |
| 14(i) | 21.650 | 453.3368 | 453.3382 | C30H46O3 | Liquidambaric acid | 453.3366 |
| 15 | 26.171 | 381.3372 | 381.3378 | C24H46O3 | 3-oxo-tetracosanoic acid | 381.3372 |
| 16 | 27.313 | 714.5094 | 714.5074 | C39H74NO8P | 1-Palmitoyl-2-linoleoyl PE | 714.5094 |
| 17 | 28.275 | 633.4378 | 633.4237 | C35H58O6 | Stigmasteryl glucoside | 633.4378 |
| 18 | 33.054 | 293.1765 | 293.1837 | C17H26O4 | Embelin | 293.1765 |
| 19 | 33.081 | 809.5176 | 809.5159 | C41H79O13P | PI(16:0/16:0) | 809.5159 |
| Serial Number | Molecular Formula | Compound | Canonical SMILES |
|---|---|---|---|
| M1 | C30H48O4 | Maslinic acid | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)C)O)O)C)C)C2C1)C)C(=O)O)C |
| M2 | C30H48O4 | Hederagenin | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)CO)O)C)C)C2C1)C)C(=O)O)C |
| M3 | C39H54O6 | cis-p-Coumaroylcorosolic acid | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)C)OC(=O)/C=C/C6=CC=C(C=C6)O)O)C)C)C2C1C)C)C(=O)O |
| M4 | C32H52O4 | Oleananoic acid acetate | CC(=O)OC1CCC2(C3CCC4C5CC(CCC5(CCC4(C3(CCC2C1(C)C)C)C)C(=O)O)(C)C)C |
| M5 | C30H46O4 | 18α-Glycyrrhetinic acid | CC1(C2CCC3(C(C2(CCC1O)C)C(=O)C=C4C3(CCC5(C4CC(CC5)(C)C(=O)O)C)C)C)C |
| M6 | C30H48O3 | Betulinic acid | CC(=C)C1CCC2(C1C3CCC4C5(CCC(C(C5CCC4(C3(CC2)C)C)(C)C)O)C)C(=O)O |
| M7 | C30H48O3 | Oleanolic acid | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1)C)C(=O)O)C |
| M8 | C30H48O3 | Ursolic acid | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1C)C)C(=O)O |
| M9 | C30H46O3 | Liquidambaric acid | CC(=C)C1CCC2(C1C3CCC4C5(CCC(=O)C(C5CCC4(C3(CC2)C)C)(C)C)C)C(=O)O |
| Gene ID | Gene Symbol | log2 (PVT/Ctrl) | Qvalue (PVT/Ctrl) | Gene ID | Gene Symbol | log2 (PVT/Ctrl) | Qvalue (PVT/Ctrl) |
|---|---|---|---|---|---|---|---|
| 8549 | Lgr5 | 5.2587 | 0.0035 | 5104 | Serpina5 | −1.9025 | 8.97 × 10−5 |
| 183 | Agt | 3.4494 | 5.20 × 10−5 | 3371 | Tnc | −1.8073 | 2.69 × 10−120 |
| 440533 | Psg8 | 3.2907 | 0.0159 | 3627 | Cxcl10 | −1.7788 | 0.0335 |
| 11009 | Il24 | 2.9291 | 7.06 × 10−7 | 84443 | Frmpd3 | −1.7137 | 0.0217 |
| 375616 | Kcp | 2.8352 | 3.48 × 10−13 | 54492 | Neurl1b | −1.5728 | 4.50 × 10−42 |
| Gene | Primer Sequences (5′−3′) FORWARD | Primer Sequences (5′−3′) REVERSE |
|---|---|---|
| Gapdh | TGACATCAAGAAGGTGGTGAAGCAG | GTGTCGCTGTTGAAGTCAGAGGAG |
| Lgr5 | TAATCAGCTAAGACACGTACCC | GGGATTTCTGTTAACGCATTGT |
| Agt | GAGAAGATTGACAGGTTCATGC | GAAGTGGACGTAGGTGTTGAAA |
| Psg8 | TGTGAAATACGGAACCCAGTGAGTG | TTGTTGATGGTGATGTAGGGCTTGG |
| Il24 | TTTGTTCTCATCGTGTCACAAC | GTTTGAATGCTCTCCGGAATAG |
| Kcp | ACTGTGACATGCTCCTTGGTTGAC | GCCGTCCACAAACACCTCTTCC |
| Serpina5 | CCAGAAAAGCTCAGAGAAGGAG | CAGAGTCCCTAAAGTTGGTAGG |
| Tnc | TAGTGAAAAACAATACCCGGGG | TGACATCTTTCACCTCGATCTG |
| Cxcl10 | CTCTCTCTAGAACTGTACGCTG | ATTCAGACATCTCTTCTCACCC |
| Frmpd3 | CACAATCACTCCTGAATCATCG | CGACATCATCTCTGACATGTCT |
| Neurl1b | TATGACCTTCAGTGTCAACCAG | GTGTAGATGACCGTGTCCAC |
| Ptp1b | CCAGCCAAAGGGGAGCCGTC | CTATGTGTTGCTGTTGAACA |
| Pi3k | GGAAGCAGCAACCGAAACAAAG | TCCACCACTACAGAGCAGGCATAG |
| Akt | GCAGGATGTGGACCAACGTGAG | GCAGGCAGCGGATGATGAAGG |
| mtor | CAACCAGCCAATCATTCGCATTCAG | ATGTCCGTTGCTGCCCATAAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Luo, H.; Lin, X.; Hua, L.; Wang, J.; Xie, J.; Zhang, Z.; Shi, Z.; Li, M.; Peng, Q.; et al. Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways. Int. J. Mol. Sci. 2025, 26, 1959. https://doi.org/10.3390/ijms26051959
Li Y, Luo H, Lin X, Hua L, Wang J, Xie J, Zhang Z, Shi Z, Li M, Peng Q, et al. Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways. International Journal of Molecular Sciences. 2025; 26(5):1959. https://doi.org/10.3390/ijms26051959
Chicago/Turabian StyleLi, Yamei, Hongshan Luo, Xiulian Lin, Linye Hua, Jiayao Wang, Jingchen Xie, Zhimin Zhang, Zhe Shi, Minjie Li, Qiuxian Peng, and et al. 2025. "Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways" International Journal of Molecular Sciences 26, no. 5: 1959. https://doi.org/10.3390/ijms26051959
APA StyleLi, Y., Luo, H., Lin, X., Hua, L., Wang, J., Xie, J., Zhang, Z., Shi, Z., Li, M., Peng, Q., Lin, L., Liao, D., & Xia, B. (2025). Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways. International Journal of Molecular Sciences, 26(5), 1959. https://doi.org/10.3390/ijms26051959





