TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep
Abstract
1. Introduction
2. Results
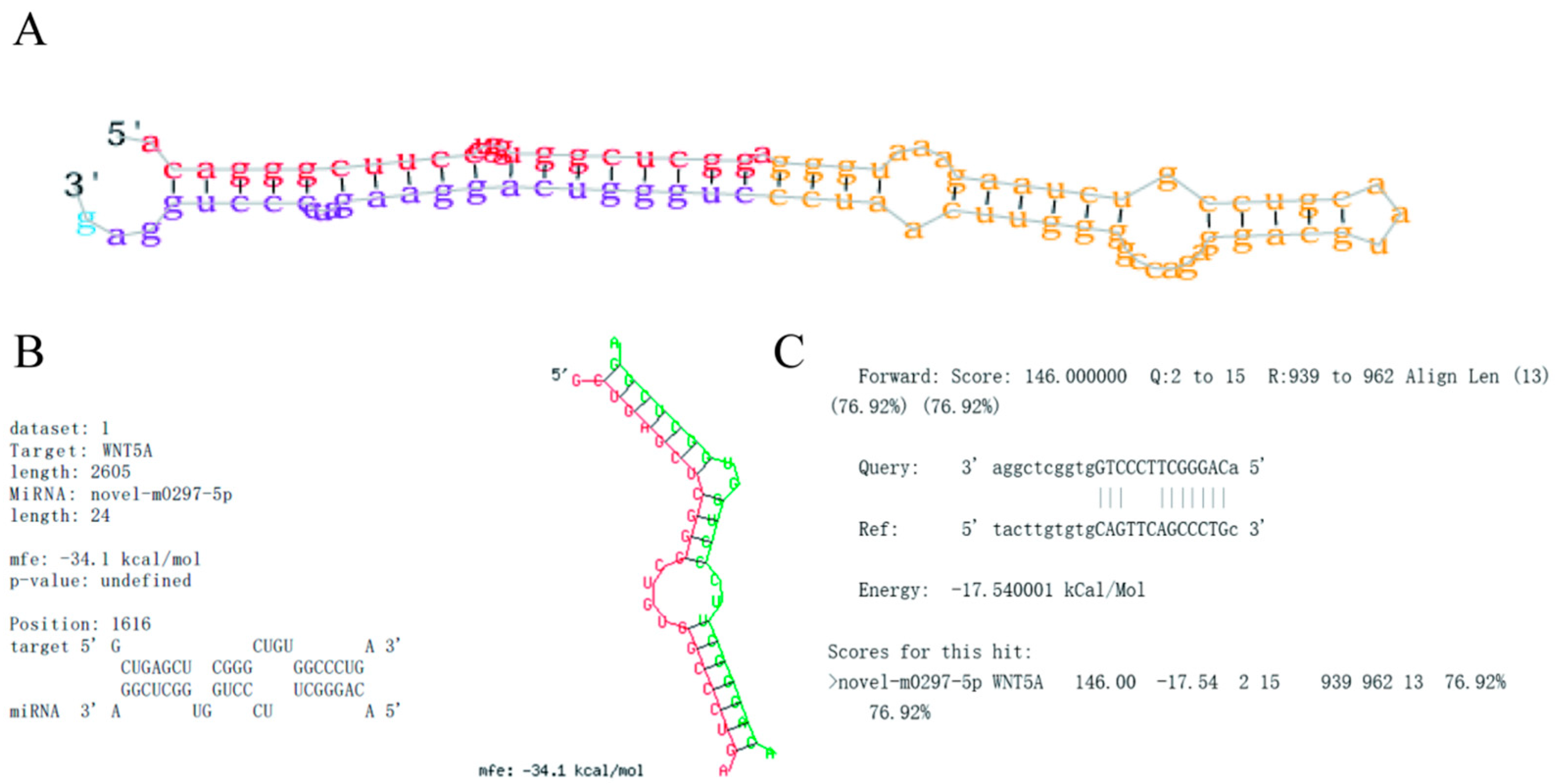
2.1. Novel-m0297-5p Secondary Structure and Target Gene Binding Site Prediction
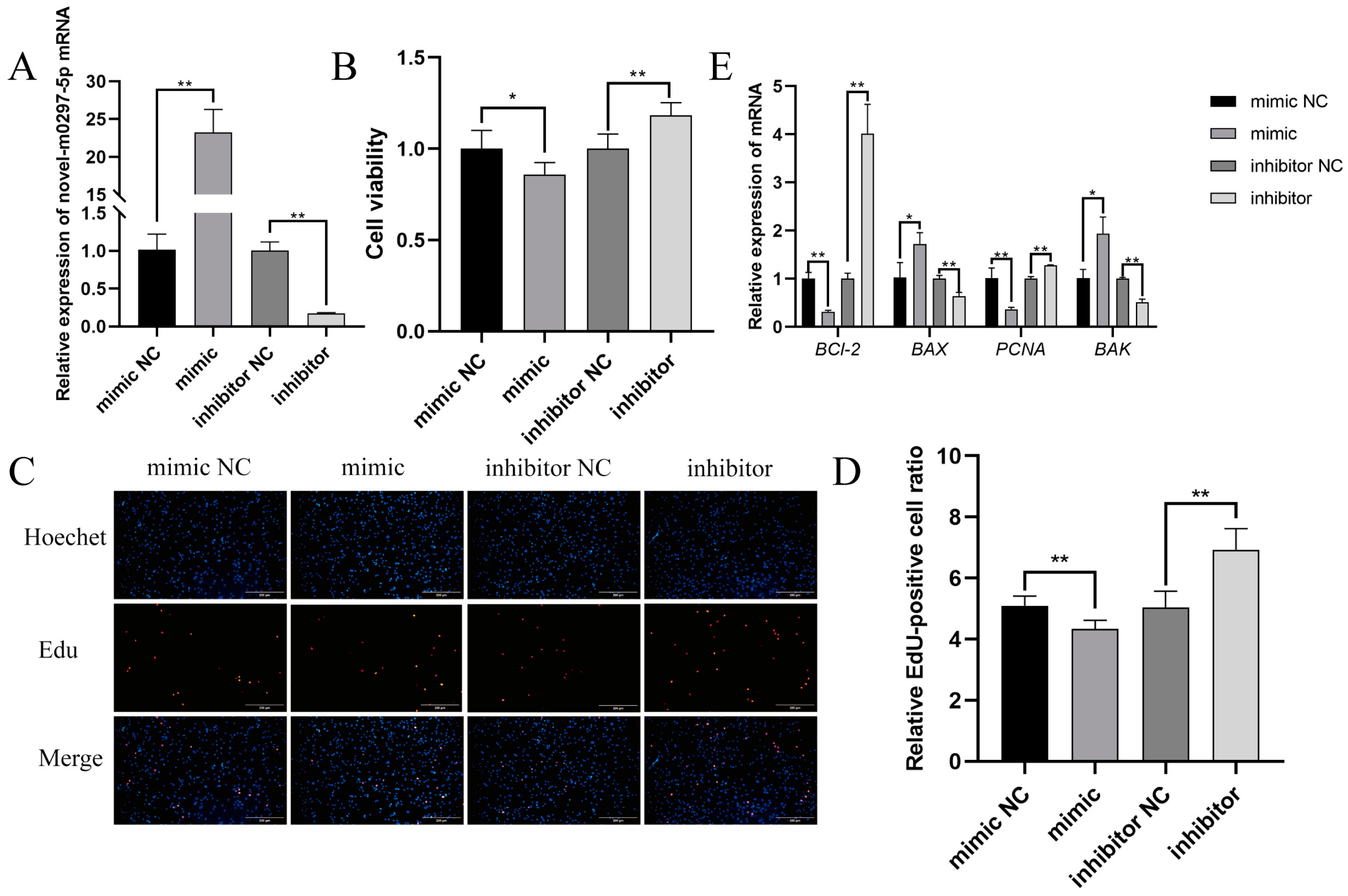
2.2. Effects of Novel-m0297-5p on GC Proliferation
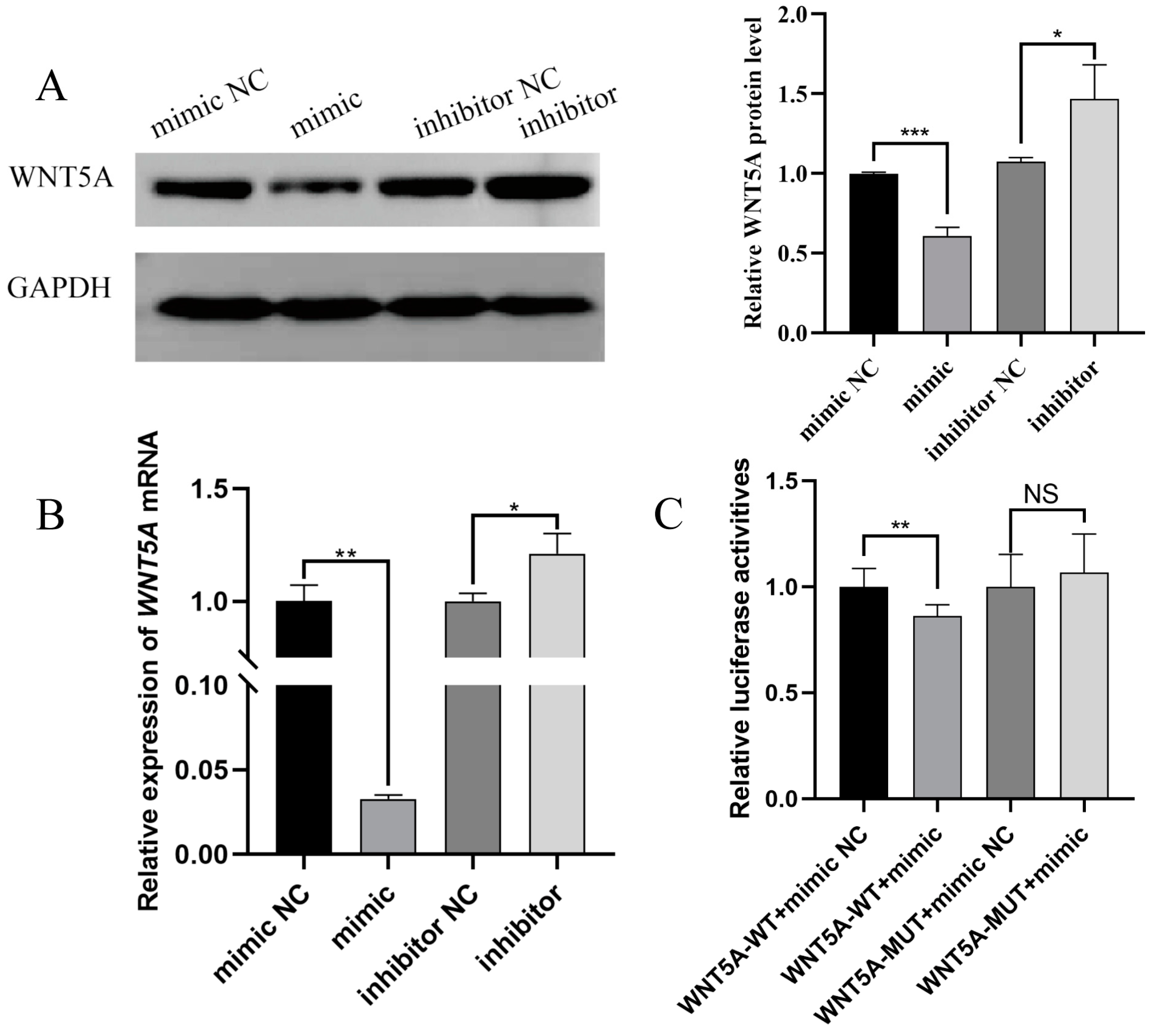
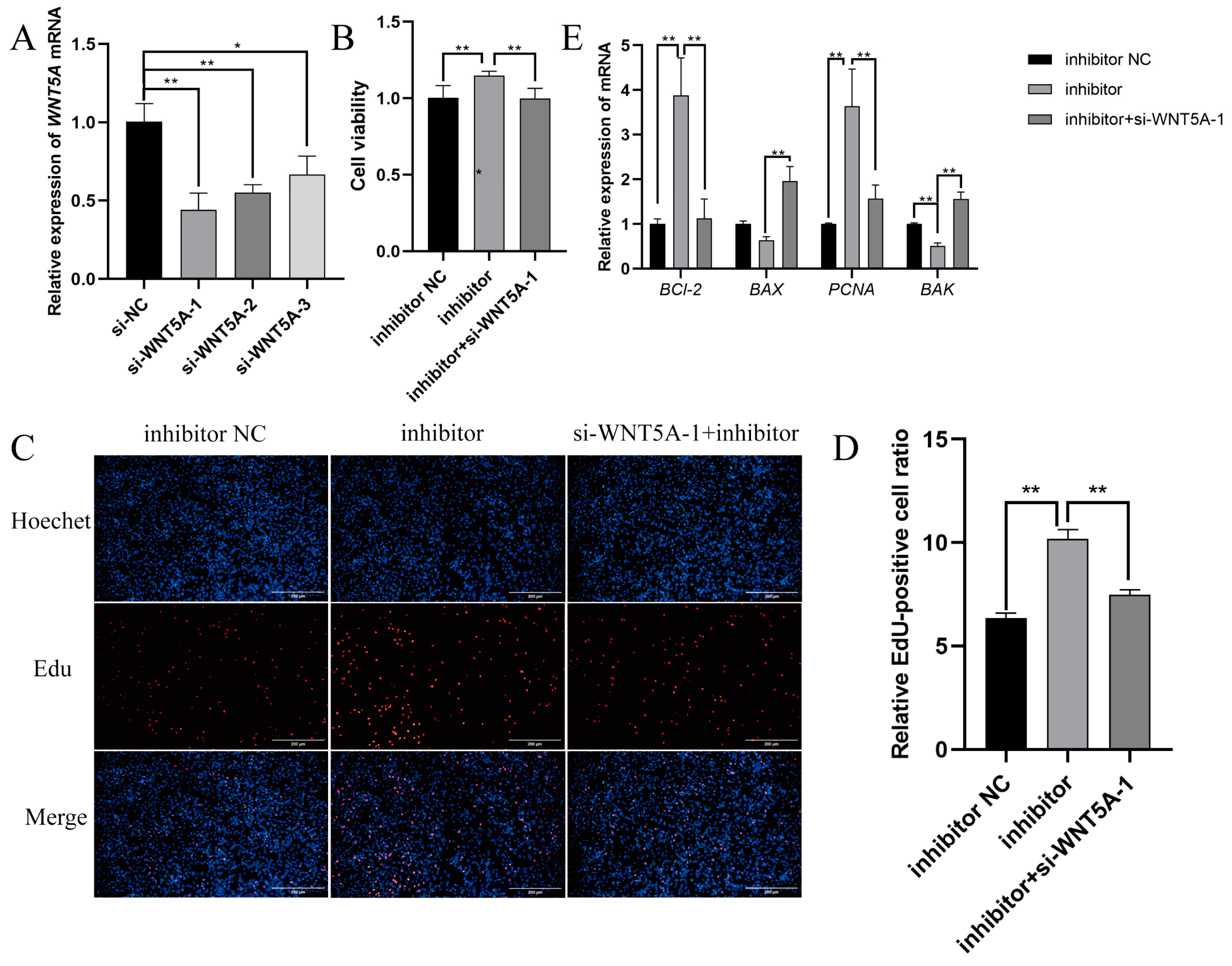
2.3. Targeting Validation Between Novel-m0297-5p and WNT5A
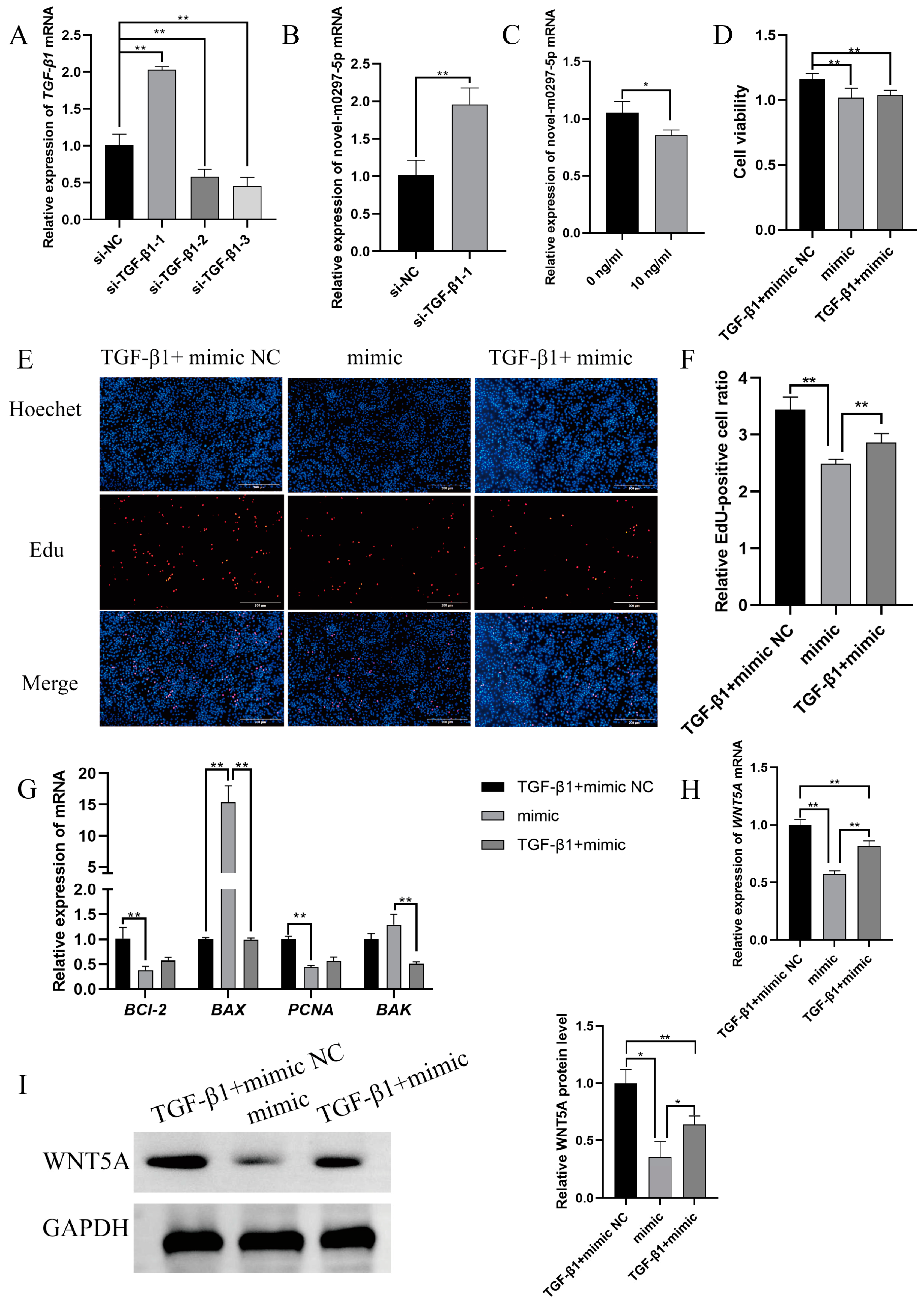
2.4. TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in Cell Proliferation
2.5. Novel-m0297-5p Targeting WNT5A to Participate in Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Culture, Passage, and Identification of Ovarian GCs
4.3. TGF-β1 Treatment and Cell Transfection
4.4. Dual-Luciferase Assay
4.5. Total RNA Extraction, Reverse Transcription, and Real-Time Fluorescence Quantitative PCR (qRT-PCR) Test
4.6. Western Blot Test
4.7. Cell Viability and Proliferation Detection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, M.X.; Yang, J.; Feng, T.; Cao, G.L.; Fang, L.; Di, R.; Huang, D.W.; Tang, Q.Q.; Ma, Y.H.; Li, K.; et al. GDF9 as a candidate gene for prolificacy of Small Tail Han sheep. Mol. Biol. Rep. 2011, 38, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, J.; Xu, B.; Chrusciel, M.; Gao, J.; Bazert, M.; Stelmaszewska, J.; Xu, Y.; Zhang, H.; Pawelczyk, L.; et al. Functional Characterization of MicroRNA-27a-3p Expression in Human Polycystic Ovary Syndrome. Endocrinology 2018, 159, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Dhori, X.; Gioiosa, S.; Gonfloni, S. An integrated analysis of multiple datasets reveals novel gene signatures in human granulosa cells. Sci. Data 2024, 11, 972. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Du, Z.; Yao, Z.; Guo, T.; Cheng, Y.; Wang, K.; Ma, X.; Chen, C.; Kebreab, E.; et al. Effects of BAMBI on luteinized follicular granulosa cell proliferation and steroid hormone production in sheep. Mol. Reprod. Dev. 2023, 90, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Zhao, X.; Du, F.; Liu, X.; Ruan, Q.; Wu, Z.; Lei, C.; Deng, Y.; Luo, C.; Jiang, J.; Shi, D.; et al. Brain-derived neurotrophic factor (BDNF) is expressed in buffalo (Bubalus bubalis) ovarian follicles and promotes oocyte maturation and early embryonic development. Theriogenology 2019, 130, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol. Cell 2018, 72, 1021–1034.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Z.; Wang, P.; He, X.; Liu, Y.; Chu, M. The SLC19A1-AS/miR-1343/WNT11 axis is a novel positive regulatory ceRNA network governing goat granulosa cell proliferation. Int. J. Biol. Macromol. 2024, 264, 130658. [Google Scholar] [CrossRef] [PubMed]
- Dai, A.; Sun, H.; Fang, T.; Zhang, Q.; Wu, S.; Jiang, Y.; Ding, L.; Yan, G.; Hu, Y. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett. 2013, 587, 2474–2482. [Google Scholar] [CrossRef]
- Tao, H.; Yang, J.; Xu, M.; Liu, Z.; Liu, Y.; Xiong, Q. MicroRNA-27a-3p targeting Vangl1 and Vangl2 inhibits cell proliferation in mouse granulosa cells. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194885. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, X.; Zhang, Y.; Zhu, Q.; Yin, H.; Han, S. MiR-223 inhibits proliferation and steroid hormone synthesis of ovarian granulosa cell via the AKT signaling pathway by targeting CRIM1 in chicken. Poult. Sci. 2024, 103, 103910. [Google Scholar] [CrossRef]
- Peng, J.Y.; An, X.P.; Fang, F.; Gao, K.X.; Xin, H.Y.; Han, P.; Bao, L.; Ma, H.; Cao, B. MicroRNA-10b suppresses goat granulosa cell proliferation by targeting brain-derived neurotropic factor. Domest. Anim. Endocrinol. 2016, 54, 60–67. [Google Scholar] [CrossRef]
- Gui, H.; Li, F.; Chen, C.; Zhu, Q.; Zhang, C.; Zhang, J.; Meng, C.; Qian, Y.; Cao, S.; Li, Y. miR-27a-3p targets NR5A2 to regulate CYP19A1 expression and 17-β estradiol synthesis in ovine granulosa cells. Anim. Reprod. Sci. 2023, 248, 107160. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bao, Y.; Yang, F.; Li, X.; Wang, F.; Zhang, C. miR-134-3p Regulates Cell Proliferation and Apoptosis by Targeting INHBA via Inhibiting the TGF-β/PI3K/AKT Pathway in Sheep Granulosa Cells. Biology 2024, 14, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Duan, C.; Li, J.; Ji, S.; Yan, H.; Liu, Y.; Zhang, Y. LncGSAR Controls Ovarian Granulosa Cell Steroidogenesis via Sponging MiR-125b to Activate SCAP/SREBP Pathway. Int. J. Mol. Sci. 2022, 23, 12132. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Hill, C.S. TGF-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Prud’homme, G.J. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Liu, X.M.; Wang, Y.; Chen, Z.Y. Activating transcription factor 3 (ATF3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (TGF-beta)/SMAD signaling pathway. Bioengineered 2021, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M.; Gorospe, W.C. Biochemical identification of apoptosis(programmed cell death)in granulosa cells: Evidence for a potential mechanism underlying follicular atresia. Endocrinol. Endocrinol. 1991, 129, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Doraiswamy, V.; Skinner, M.K. Transforming growth factor-beta isoform expression during bovine ovarian antral follicle development. Mol. Reprod. Dev. 2010, 66, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gao, X.; Bao, Y.; El-Samahy, M.A.; Yang, J.; Wang, Z.; Li, X.; Zhang, G.; Zhang, Y.; Liu, W.; et al. lncRNA FDNCR promotes apoptosis of granulosa cells by targeting the miR-543-3p/DCN/TGF-β signaling pathway in Hu sheep. Mol. Ther. Nucleic Acids. 2021, 24, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Pan, Z.; Du, X.; Li, X.; Ma, B.; Yao, W.; Li, Q.; Liu, H. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol. Cell Endocrinol. 2015, 409, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, W.; Luo, Y.; Wang, J.; Liu, X.; Li, S.; Hao, Z. Transforming growth factor-β1 mediates the SMAD4/BMF pathway to regulate ovarian granulosa cell apoptosis in small tail Han sheep. Theriogenology 2024, 214, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Yasuda, S.Y.; Kahn, M. Wnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. Rep. 2011, 7, 836–846. [Google Scholar] [CrossRef]
- Abedini, A.; Zamberlam, G.; Boerboom, D.; Price, C.A. Non-canonical WNT5A is a potential regulator of granulosa cell function in cattle. Mol. Cell Endocrinol. 2015, 403, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Zamberlam, G.; Lapointe, E.; Tourigny, C.; Boyer, A.; Paquet, M.; Hayashi, K.; Honda, H.; Kikuchi, A.; Price, C.; et al. WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 2016, 30, 1534–1547. [Google Scholar] [CrossRef]
- Liu, N.; Wang, S.Y.; Yao, Q.C.; Li, Y.; Hu, H.; Tang, X.; Ran, H.; Price, C.A.; Jiang, Z. Activin A attenuates apoptosis of granulosa cells in atretic follicles through ERβ-induced autophagy. Reprod. Domest. Anim. 2022, 57, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Li, Q.; Wu, W.; Shang, P.; Chamba, Y.; Pan, Z.; Li, Q. miR-130a/TGF-β1 axis is involved in sow fertility by controlling granulosa cell apoptosis. Theriogenology 2020, 157, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Boerboom, D.; Carrière, P.D. Transforming growth factor-beta1 inhibits luteinization and promotes apoptosis in bovine granulosa cells. Reproduction 2009, 137, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, H.; Jiang, Y.; Ding, L.; Wu, S.; Fang, T.; Yan, G.; Hu, Y. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS ONE 2013, 8, e59667. [Google Scholar] [CrossRef]
- Liu, J.; Yao, W.; Yao, Y.; Du, X.; Zhou, J.; Ma, B.; Liu, H.; Li, Q.; Pan, Z. MiR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting Smad7 gene. FEBS Lett. 2014, 588, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yin, M.; Lian, J.; Tian, H.; Liu, L.; Li, X.; Sun, F. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol. Endocrinol. 2010, 24, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Pande, H.; Tesfaye, D.; Hoelker, M.; Gebremedhn, S.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Wondim, D. MicroRNA-424/503 cluster members regulate bovine granulosa cell proliferation and cell cycle progression by targeting SMAD7 gene through activin signalling pathway. J. Ovarian Res. 2018, 11, 34. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, B.W.; Guo, H.X.; Ren, Q.L.; Wang, X.W.; Chen, J.F.; Wang, J.; Zhang, Z.J.; Ma, Q.; Xing, B.S. miR-181a promotes porcine granulosa cell apoptosis by targeting TGFBR1 via the activin signaling pathway. Mol. Cell Endocrinol. 2020, 499, 110603. [Google Scholar] [CrossRef]
- Hu, H.; Fu, Y.; Zhou, B.; Li, Z.; Liu, Z.; Jia, Q. Long non-coding RNA TCONS_00814106 regulates porcine granulosa cell proliferation and apoptosis by sponging miR-1343. Mol. Cell Endocrinol. 2021, 520, 111064. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, X.; Wang, L.; Shi, K.; Li, Q. TGF-β1 controls porcine granulosa cell states: A miRNA-mRNA network view. Theriogenology 2021, 160, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chang, H.M.; Yi, Y.; Yao, Y.; Leung, P.C.K. TGF-β1 increases GDNF production by upregulating the expression of GDNF and Furin in human granulosa-lutein cells. Cells 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y.; Wang, F.; Meng, C.; Niu, J.; Guo, M.; Sizhu, S.; Xu, Y. BMP15 modulates the H19/miR-26b/SMAD1 axis influences yak granulosa cell proliferation, autophagy, and apoptosis. Reprod. Sci. 2023, 30, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Pan, Z.X.; Yao, Y.; Liu, H.L.; Liu, S.R.; Xie, Z.; Li, Q. miR-34a targets the inhibin beta B gene, promoting granulosa cell apoptosis in the porcine ovary. Genet. Mol. Res. 2014, 13, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hao, L.; Zeng, X.; Yang, R.; Qiao, S.; Wang, C.; Yu, H.; Wang, S.; Jiao, Y.; Jia, H.; et al. A novel miRNA Y-56 targeting IGF-1R mediates the proliferation of porcine skeletal muscle satellite cells through AKT and ERK pathways. Front. Vet. Sci. 2022, 9, 754435. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, P.; Wang, X.; Chu, M.; Wang, P. Mutation of the ETS1 3′UTR interacts with miR-216a-3p to regulate granulosa cell apoptosis in sheep. Theriogenology 2023, 210, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; El-Samahy, M.A.; Li, X.; Bao, Y.; Guo, J.; Yang, F.; Wang, Z.; Li, K.; Zhang, Y.; Wang, F. LncRNA-412.25 activates the LIF/STAT3 signaling pathway in ovarian granulosa cells of Hu sheep by sponging miR-346. FASEB J. 2022, 36, e22467. [Google Scholar] [CrossRef]
- Zhou, R.; Miao, Y.; Li, Y.; Li, X.; Xi, J.; Zhang, Z. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene. Theriogenology 2019, 127, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.; Yue, S.; Liu, Y.; Zhang, Y.; Duan, C. Identification of Novel 58-5p and SREBF1 Interaction and Effects on Apoptosis of Ovine Ovarian Granulosa Cell. Int. J. Mol. Sci. 2025, 26, 576. [Google Scholar] [CrossRef]
- Gupta, P.S.P.; Kaushik, K.; Krishna, K.; Nikhil KuTej, J.; Nandi, S.; Mondal, S.; Johnson, P. Regulatory role of Wnt signal in the oestradiol synthesis of different size categories of ovarian follicles in buffalo (Bubalus bubalis). Reprod. Domest. Anim. 2022, 57, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Taweechaipaisankul, A.; Ridlo, M.R.; Kim, G.A.; Lee, B.C. Effect of Klotho protein during porcine oocyte maturation via Wnt signaling. Aging 2020, 12, 23808–23821. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Q.; Yang, L.; Liu, L.; Cao, Q.; Li, Q. SMAD4 activates Wnt signaling pathway to inhibit granulosa cell apoptosis. Cell Death Dis. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, Y.; Li, Z.; Zhou, J.; Zhu, H.; Bu, G.; Liu, Z.; Hou, X.; Zhang, X.; Miao, Y.-L. Maternal cytokines CXCL12, VEGFA, and WNT5A promote porcine oocyte maturation via MAPK activation and canonical WNT inhibition. Front. Cell Dev. Biol. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Shi, J.; Gao, Q.; Fu, J. WNT5A enhances LH-mediated expression of HAS2 in granulosa cells. Reprod. Sci. 2022, 29, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Haseeb, A.; Sun, P.; Zhang, H.; Zhong, J.; Yin, W.; Fan, K.; Yang, H.; Zhang, Z.; Sun, Y.; et al. Scutellarin targets Wnt5a against zearalenone-induced apoptosis in mouse granulosa cells in vitro and in vivo. J. Hazard. Mater. 2024, 464, 132917. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Wu, L.L.; Yan, J.; Pang, X.Y.; Liu, S.J. miR-26a-5p inhibit gastric cancer cell proliferation and invasion through mediated Wnt5a. Onco. Targets Ther. 2020, 13, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, S.D. Identification of novel and known miRNAs in deep-sequencing data with miRDeep2. Curr. Protoc. Bioinform. 2011, 36, 12.10.1–12.10.15. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, Y.; Li, H.; Wei, C.; Niu, L.; Guan, S.; Li, S.; Du, L. Identification of microsatellites in cattle unigenes. J. Genet. Genom. 2008, 35, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Grishagin, I.V. Automatic cell counting with ImageJ. Anal. Biochem. 2015, 473, 63–65. [Google Scholar] [CrossRef] [PubMed]
- One-Way ANOVA Followed by Dunnett’s Multiple Comparisons Test Was Performed Using GraphPad Prism, version 8.0.2 (2019) for Windows; GraphPad Software: San Diego, CA, USA; Available online: https://www.graphpad.com (accessed on 20 May 2024).
| Genes | Primer Sequence (5′→3′) |
|---|---|
| si-TGF-β1-1 | Sense: GCGUGCUAAUGGUGGAAUACGTTAntisense: CGUAUUCCACCAUUAGCACGCTT |
| si-TGF-β1-2 | Sense: GAGCUGUACCAGAAAUAUAGCTTAntisense: GCUAUAUUUCUGGUACAGCUCTT |
| si-TGF-β1-3 | Sense: CCUGUGACAGUAAGGAUAACATTAntisense: UGUUAUCCUUACUGUCACAGGTT |
| si-WNT5A-1 | Sense: AGGUUGUAAUAGAAGCUAAUUTTAntisense: AAUUAGCUUCUAUUACAACCUTT |
| si-WNT5A-2 | Sense: GCGGCGACAACAUCGACUACGTTAntisense: CGUAGUCGAUGUUGUCGCCGCTT |
| si-WNT5A-3 | Sense: CCGACUACUGCGUGCGCAACGTTAntisense: CGUUGCGCACGCAGUAGUCGGTT |
| si-NC | Sense: UUCUCCGAACGUGUCACGUdTdTAntisense: ACGUGACACGUUCGGAGAAdTdT |
| Genes | Sequence Information (5′→3′) |
|---|---|
| mimic | ACAGGGCUUCCCUGGUGGCUCGGAUCCGAGCCACCAGGGAAGCCCUGU |
| mimic NC | UCACAACCUCCUAGAAAGAGUAGAUCUACUCUUUCUAGGAGGUUGUGA |
| inhibitor | UCCGAGCCACCAGGGAAGCCCUGU |
| inhibitor NC | UCUACUCUUUCUAGGAGGUUGUGA |
| Genes | GenBank ID | Primer Sequence (5′–3′) | Product Length/bp |
|---|---|---|---|
| Bax | XM_027978592.2 | F:TCTCCCCGAGAGGTCTTTTT | 177 |
| R:TCGAAGGAAGTCCAATGTCC | |||
| Bcl-2 | XM_027960877.2 | F:TCTTTGAGTTCGGAGGGGTC | 162 |
| R:GGCCATACAGCTCCACAAAG | |||
| BAK | XM_015102660.4 | F:GTCTTCCGCAGCTACGTCTT R:CGGTTGATGTCATCCCCGAT | 161 |
| PCNA | XM_004014340.5 | F:CTTGGTGCAGCTAACCCTTC | 161 |
| R:CCAAGGTGTCCGCATTATCT | |||
| TGF-β1 | NM_001009400.2 | F:GAGCCCTGGACACCAACTAC | 161 |
| R:GCTCCAGATGTAGGGACAGG | |||
| R:ACTTCTCCTTCAGGGCATCAC | |||
| WNT5A | XM_004018630.5 | F:AGGGCAATGTCTTCCAAGTTCTTC R:GTTATTCATACCTAGCGACCACCAAG | 105 |
| GAPDH | NM_001190390.1 | F:GGTCGGAGTGAACGGATTT | 175 |
| R:CTCTGCCTTGACTGTGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Liu, Y.; Guo, Y.; Zhao, Z.; Cui, J.; Li, M.; Wang, J. TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep. Int. J. Mol. Sci. 2025, 26, 1961. https://doi.org/10.3390/ijms26051961
Ren S, Liu Y, Guo Y, Zhao Z, Cui J, Li M, Wang J. TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep. International Journal of Molecular Sciences. 2025; 26(5):1961. https://doi.org/10.3390/ijms26051961
Chicago/Turabian StyleRen, Siyu, Yuan Liu, Yajing Guo, Zhihui Zhao, Jingjing Cui, Mingna Li, and Jiqing Wang. 2025. "TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep" International Journal of Molecular Sciences 26, no. 5: 1961. https://doi.org/10.3390/ijms26051961
APA StyleRen, S., Liu, Y., Guo, Y., Zhao, Z., Cui, J., Li, M., & Wang, J. (2025). TGF-β1 Mediates Novel-m0297-5p Targeting WNT5A to Participate in the Proliferation of Ovarian Granulosa Cells in Small-Tailed Han Sheep. International Journal of Molecular Sciences, 26(5), 1961. https://doi.org/10.3390/ijms26051961






