Coumarin Promotes Hypocotyl Elongation by Increasing the Synthesis of Brassinosteroids in Plants
Abstract
:1. Introduction
2. Results
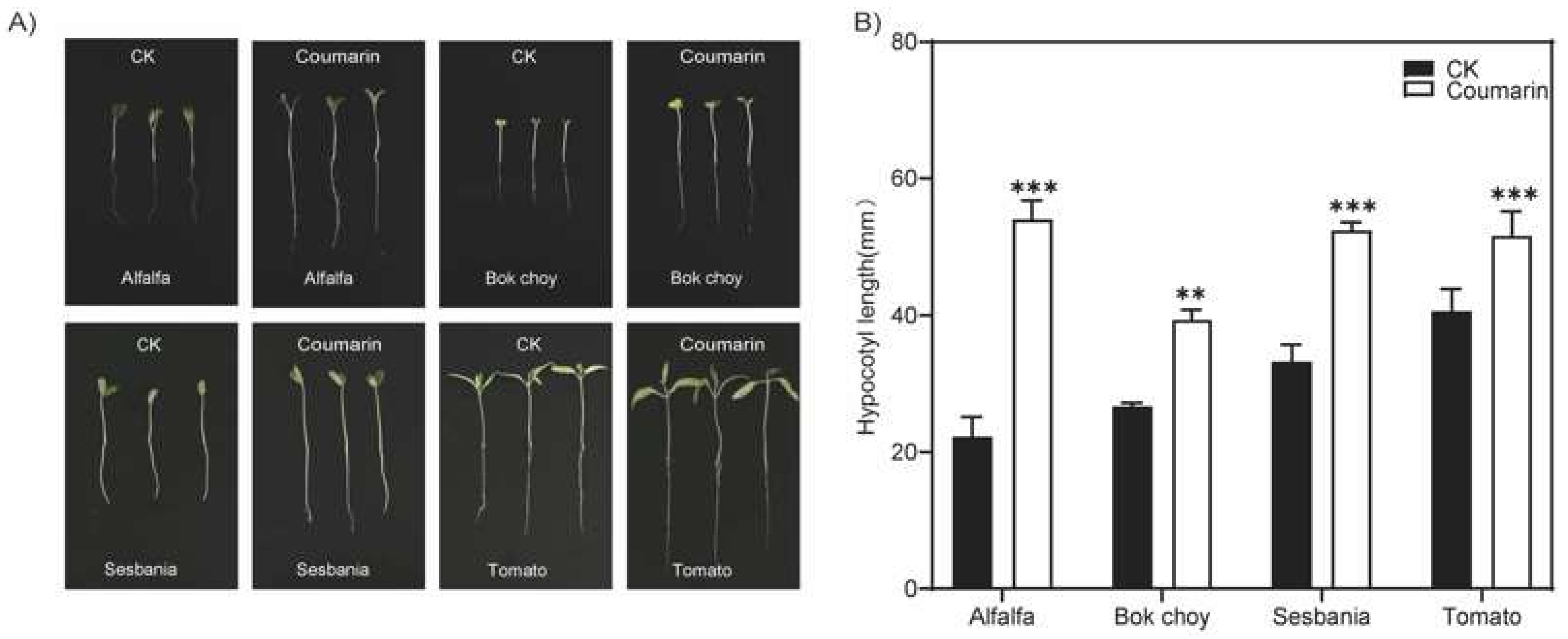
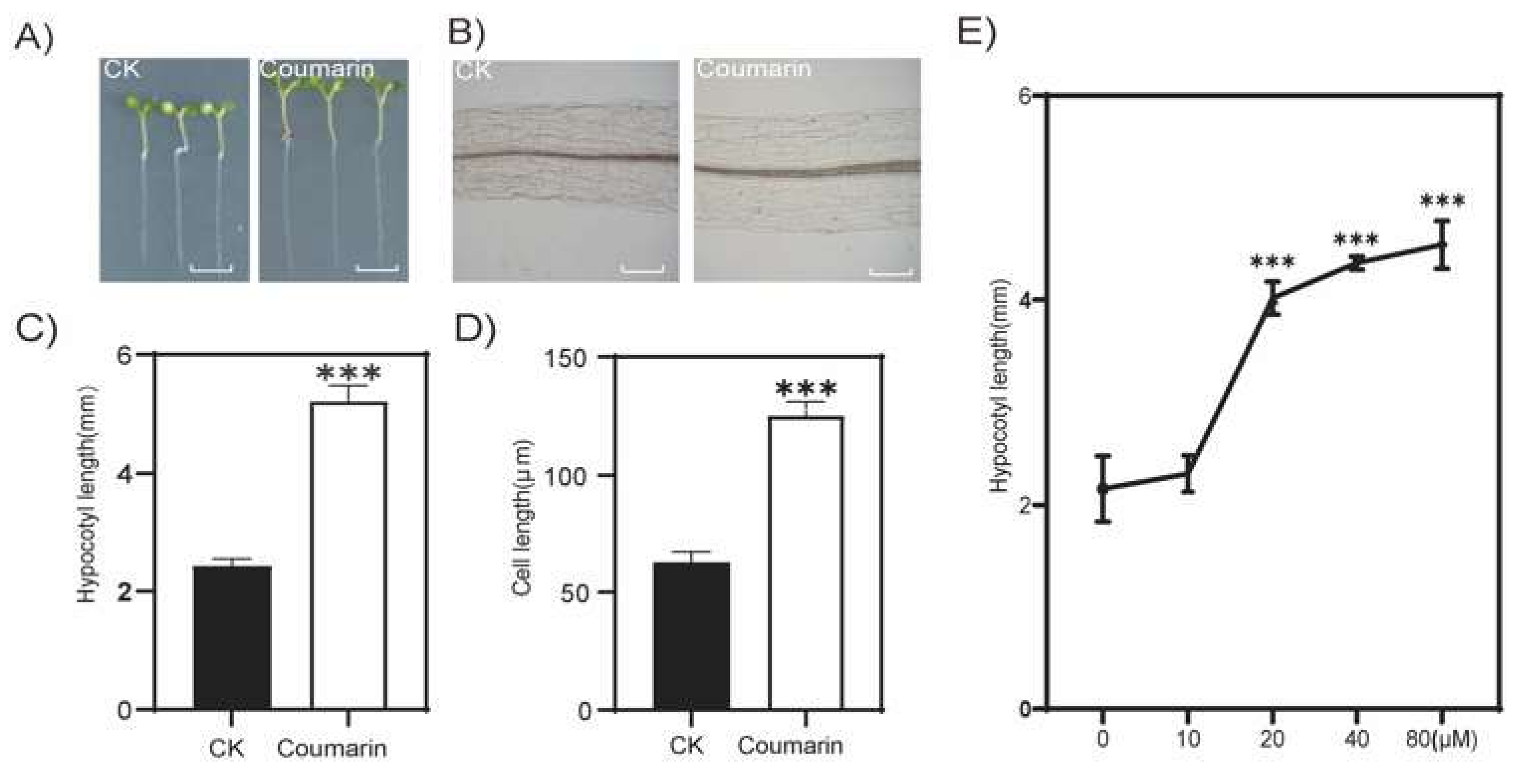
2.1. Coumarin Promotes Elongation of Hypocotyl in Plants
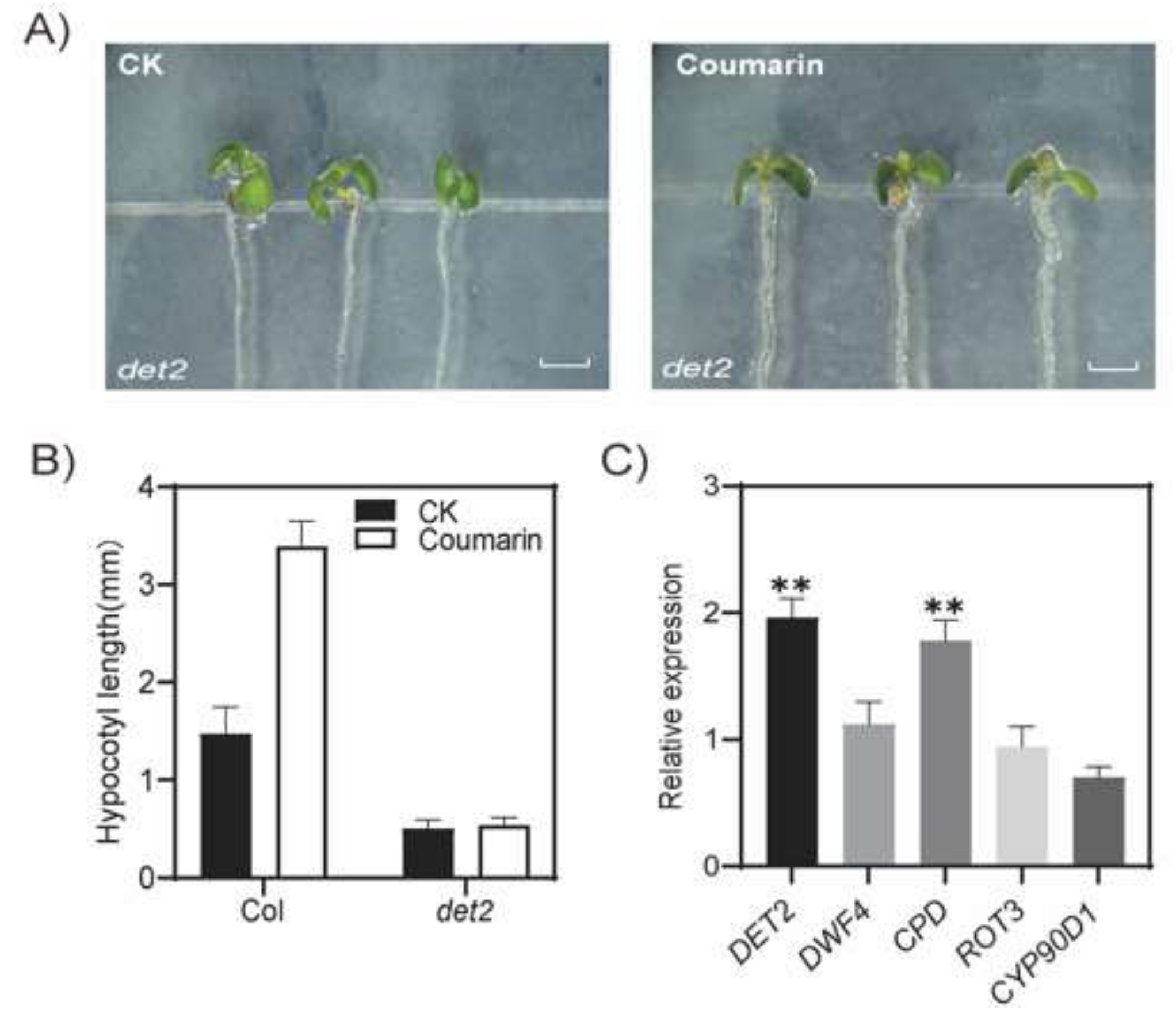
2.2. Mutant DET2 Is Insensitive to Coumarin
2.3. Coumarin Promotes Hypocotyl Elongation Depends on the Synthesis of BRs
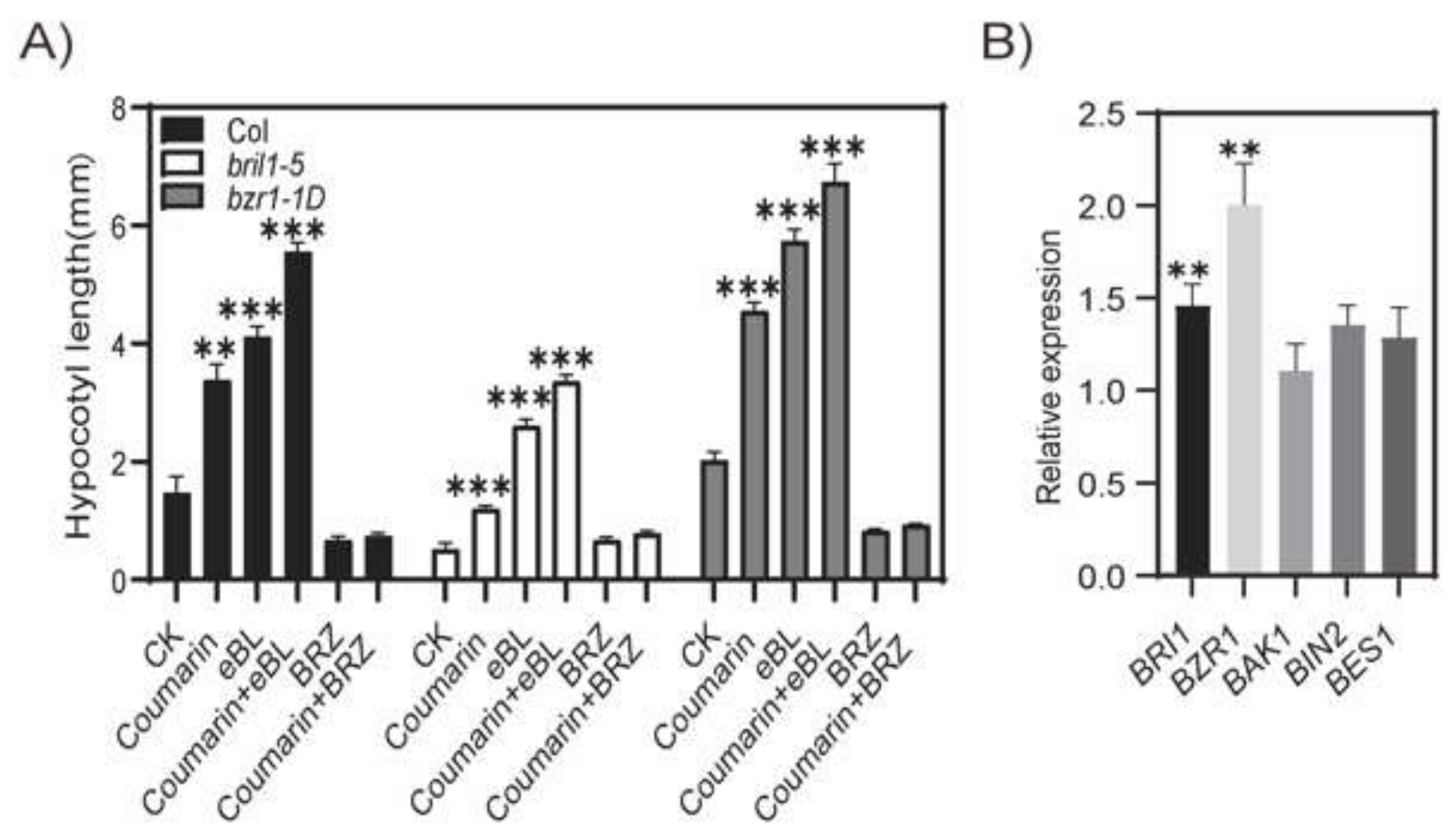
2.4. Coumarin Promotes Hypocotyl Elongation Through BR Signaling
3. Discussion
3.1. Promoting Hypocotyl Elongation Is Another New Effect of Coumarin
3.2. Coumarin Promotes Hypocotyl Elongation by Inducing BR Synthesis
3.3. The Effect of Coumarin on Promoting Hypocotyl Elongation Increase Plant Adaptability
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cytological Observation of Arabidopsis Hypocotyl
4.3. Gene Expression Analysis
4.4. Determination of the Concentration of Brassinolide
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, I.M.; Seigler, D.; Miller, D.A.; Kyung, S.H. Autotoxic compounds from fresh alfalfa leaf extracts: Identification and biological activity. J. Chem. Ecol. 2000, 26, 315–327. [Google Scholar] [CrossRef]
- Lu, C.; Zeng, Z.H.; Zheng, S.Z.; Qi, Z.Q.; Hu, Y.G. The difference of autotoxic compounds content in alfalfa cultivars. Acta Agron. Sin. 2007, 33, 578–582. [Google Scholar]
- Rong, S.C.; Shi, S.L.; Sun, C.C. Determination of coumarins and major phenolic acids in plant and rhizosphere soil of Alfalfa (Medicago sativa L.). Soils 2016, 48, 931–938. [Google Scholar]
- Wu, B.; Shi, S.; Zhang, H.; Lu, B.; Nan, P.; Yun, A. Anabolic metabolism of autotoxic substance coumarins in plants. PeerJ 2023, 11, e16508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Z.; Luo, W.; Chen, S.; Chen, H.L.; Li, G.Y.; Li, L.M. Coumarins from flowers of Stellera chamaejasme and their biological activities. Chin. Tradit. Herb. Drugs 2021, 52, 943–950. [Google Scholar]
- Luo, K. A Study of Genetics Breeding, Seed Multiplication and the Transcriptome of Low Coumarin Sweetclover (Melilotus spp.). Master’s Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Schmid, N.B.; Giehl, R.F.; Döll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef]
- Fourcroy, P.; Tissot, N.; Gaymard, F.; Briat, J.F.; Dubos, C. Facilitated Fe nutrition by phenolic compounds excreted by the Arabidopsis ABCG37/PDR9 transporter requires the IRT1/FRO2 high-affinity root Fe2+ transport system. Mol. Plant 2016, 9, 485–488. [Google Scholar] [CrossRef]
- Fourcroy, P.; Siso-Terraza, P.; Sudre, D.; Saviron, M.; Reyt, G.; Gaymard, F.; Abadia, A.; Abadia, J.; Alvarez-Fernandez, A.; Briat, J.F. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol. 2014, 201, 155–167. [Google Scholar] [CrossRef]
- Rajniak, J.; Giehl, R.F.H.; Chang, E.; Murgia, I.; von Wiren, N.; Sattely, E. Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat. Chem. Biol. 2018, 14, 442–450. [Google Scholar] [CrossRef]
- Robe, K.; Conejero, G.; Gao, F.; Lefebvre-Legendre, L.; Sylvestre-Gonon, E.; Rofidal, V.; Hem, S.; Rouhier, N.; Barberon, M.; Hecker, A.; et al. Coumarin accumulation and trafficking in Arabidopsis thaliana: A complex and dynamic process. New Phytol. 2021, 229, 2062–2079. [Google Scholar] [CrossRef]
- DeLoose, M.; Cho, H.; Bouain, N.; Choi, I.; Prom-U-Thai, C.; Shahzad, Z.; Zheng, L.; Rouached, H. PDR9 allelic variation and MYB63 modulate nutrient-dependent coumarin homeostasis in Arabidopsis. Plant J. 2024, 117, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer: New York, NY, USA, 2013. [Google Scholar]
- Yao, D.D.; Wang, J.Y.; Zhou, Q.; Tang, Q.; Zhao, G.Q.; Wu, C.X. Effect of coumarin on Italian ryegrass seed germination and seedling growth. Acta Prataculturae Sin. 2017, 26, 136–145. [Google Scholar]
- El-Shahawy, T.A.; Abdelhamid, M.T. Potential allelopathic effect of six Phaseolus vulgaris recombinant inbred lines for weed control. Aust. J. Basic Appl. Sci. 2013, 7, 462–467. [Google Scholar]
- Li, X.; Shi, S.; Zhang, X.; Li, C.; Wang, H.; Kang, W.; Yin, G. Potential effect of DIMBOA (2,4-Dihydroxy-7-methoxy-1, 4-benzoxazin-3-one) on alleviating the autotoxic coumarin stress in Alfalfa (Medicago sativa) seedlings. Life 2022, 12, 2140. [Google Scholar] [CrossRef]
- Wang, K.J.; Chen, F.; Fan, J.W.; Li, Q.; Sun, Z.W.; Guo, M.M.; Zhang, G.X. Research progress and prospect of wheat allelopathy. Bull. Agric. Sci. Technol. 2018, 2, 7–11. [Google Scholar]
- Tao, R.; Shi, S.L.; Zhang, C.M.; Chen, J.G.; Wang, X.Y. Effects of exogenous coumarin and caffeic acid on root morphogenesis and anatomical structure of Alfalfa. Acta Agrestia Sin. 2019, 27, 404–412. [Google Scholar]
- Chen, B.X.; Peng, Y.X.; Gao, J.D.; Zhang, Q.; Liu, Q.J.; Fu, H.; Liu, J. Coumarin-induced delay of rice seed germination is mediated by suppression of abscisic acid catabolism and reactive oxygen species production. Front. Plant Sci. 2019, 10, 828. [Google Scholar] [CrossRef]
- Razavi, S.M. Plant coumarins as allelopathic agents. Int. J. Biol. Chem. Sci. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Song, L.; Pan, K.W.; Wang, J.C.; Ma, Y.H. Effects of phenolic acids on seed germination and seedling antioxidant enzyme activity of alfalfa. Acta Ecol. Sin. 2006, 26, 3393–3403. [Google Scholar]
- Dutsadee, C.; Nunta, C. Induction of peroxidase, scopoletin, phenolic compounds and resistance in Hevea brasiliensis by elicitin and a novel protein elicitor purified from Phytophthora palmivora. Physiol. Mol. Plant Pathol. 2008, 72, 179–187. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Zhang, B.; Ma, J.; Hettenhausen, C.; Cao, G.; Wu, J. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signaling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar] [CrossRef]
- Ahrabi, F.; Enteshari, S.H.; Moradshahi, A. Allelopathic potential of para-hydroxybenzoic acid and coumarin on canola: Talaieh cultivar. J. Med. Plants Res. 2011, 5, 5104–5109. [Google Scholar]
- Wang, J.Y.; Wu, C.X.; Zhao, G.Q. Research progress on molecular mechanism of plant allelopathy and differential proteomics. Jiangsu Agric. Sci. 2017, 45, 8–11. [Google Scholar]
- Krahmer, J.; Fankhauser, C. Environmental Control of Hypocotyl elongation. Annu. Rev. Plant Biol. 2024, 75, 489–519. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.; Fan, L.-M.; Wei, N.; Chen, H.; Deng, X.W. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 2016, 7, 11868. [Google Scholar] [CrossRef]
- Yang, C.-J.; Zhang, C.; Lu, Y.-N.; Jin, J.-Q.; Wang, X.-L. The mechanisms of brassinosteroids’ action: From signal transduction to plant development. Mol. Plant 2011, 4, 588–600. [Google Scholar] [CrossRef]
- Takahashi, K.; Hayashi, K.-I.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yuan, M.; Ehrhardt, D.W.; Wang, Z.; Mao, T. Arabidopsis MICROTUBULE DESTABILIZING PROTEIN40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 2012, 24, 4012–4025. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X.-Y.; Cao, D.-M.; Tang, W.; He, K.; Zhu, J.Y.; He, J.X.; Bai, M.Y.; Zhu, S.; Oh, E.; et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Bellow, R.; Lee, K.; Kennaway, R.; Barclay, J.E.; Whibley, A.; Bushell, C.; Spooner, J.; Yu, M.; Brett, P.; Kular, B.; et al. Brassinosteroid coordinates cell layer interactions in plants via cell wall and tissue mechanics. Science 2023, 380, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004, 2, e258. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Vieira, R.F.; Bizzo, H.R.; Silveira, D.; Gimenes, M.A. Secondary Metabolites; Intech Open: London, UK, 2012. [Google Scholar]
- Gouvea, D.R.; Gobbo-Neto, L.; Sakamoto, H.T.; Lopes, N.P.; Lopes, J.L.C.; Meloni, F.; Amaral, J.G. Seasonal variation of the major secondary metabolites present in the extract of Eremanthus mattogrossensis Less (Asteraceae: Vernonieae) leaves. Química Nova 2012, 35, 2139–2145. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Ferrayoli, C.G.; Palacios, S.M. Antifungal synergistic effect of scopoletin: A hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 2005, 53, 2922–2927. [Google Scholar] [CrossRef]
- Graña, E.; Costas-Gil, A.; Longueira, S.; Celeiro, M.; Teijeira, M.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Auxin-like effects of the natural coumarin scopoletin on Arabidopsis cell structure and morphology. J. Plant Physiol. 2017, 218, 45–55. [Google Scholar] [CrossRef]
- Kang, Z.X. Research on the Distribution Rules of 9 Substances Including Coumarin in Lyciun barbarum L. and Their Response to Saline-Alkali Stress. Master’s Thesis, Peking Union Medical College, Beijing, China, 2022. [Google Scholar]
- Döll, S.; Kuhlmann, M.; Rutten, T.; Mette, M.F.; Scharfenberg, S.; Petridis, A.; Mock, H.P. Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J. 2018, 93, 431–444. [Google Scholar] [CrossRef]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.; Guan, R.; Rombolà, A.D.; Schulze-Lefert, P. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837. [Google Scholar] [CrossRef]
- Duan, Z.; Yan, Q.; Wu, F.; Wang, Y.; Wang, S.; Zong, X.; Zhang, J. Genome-wide analysis of the UDP-Glycosyltransferase family reveals its roles in coumarin biosynthesis and abiotic stress in Melilotus albus. Int. J. Mol. Sci. 2021, 22, 10826. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Ma, A.; Li, J.; Du, Z.; Zhu, L.; Feng, G. Coumarin Promotes Hypocotyl Elongation by Increasing the Synthesis of Brassinosteroids in Plants. Int. J. Mol. Sci. 2025, 26, 1092. https://doi.org/10.3390/ijms26031092
Liu S, Ma A, Li J, Du Z, Zhu L, Feng G. Coumarin Promotes Hypocotyl Elongation by Increasing the Synthesis of Brassinosteroids in Plants. International Journal of Molecular Sciences. 2025; 26(3):1092. https://doi.org/10.3390/ijms26031092
Chicago/Turabian StyleLiu, Siqi, Aolin Ma, Jie Li, Zhixuan Du, Longfei Zhu, and Guanping Feng. 2025. "Coumarin Promotes Hypocotyl Elongation by Increasing the Synthesis of Brassinosteroids in Plants" International Journal of Molecular Sciences 26, no. 3: 1092. https://doi.org/10.3390/ijms26031092
APA StyleLiu, S., Ma, A., Li, J., Du, Z., Zhu, L., & Feng, G. (2025). Coumarin Promotes Hypocotyl Elongation by Increasing the Synthesis of Brassinosteroids in Plants. International Journal of Molecular Sciences, 26(3), 1092. https://doi.org/10.3390/ijms26031092




