On the Synergistic Effects of Cold Atmospheric Pressure Plasma Irradiation and Electroporation on Cytotoxicity of HeLa Cells
Abstract
1. Introduction
2. Results
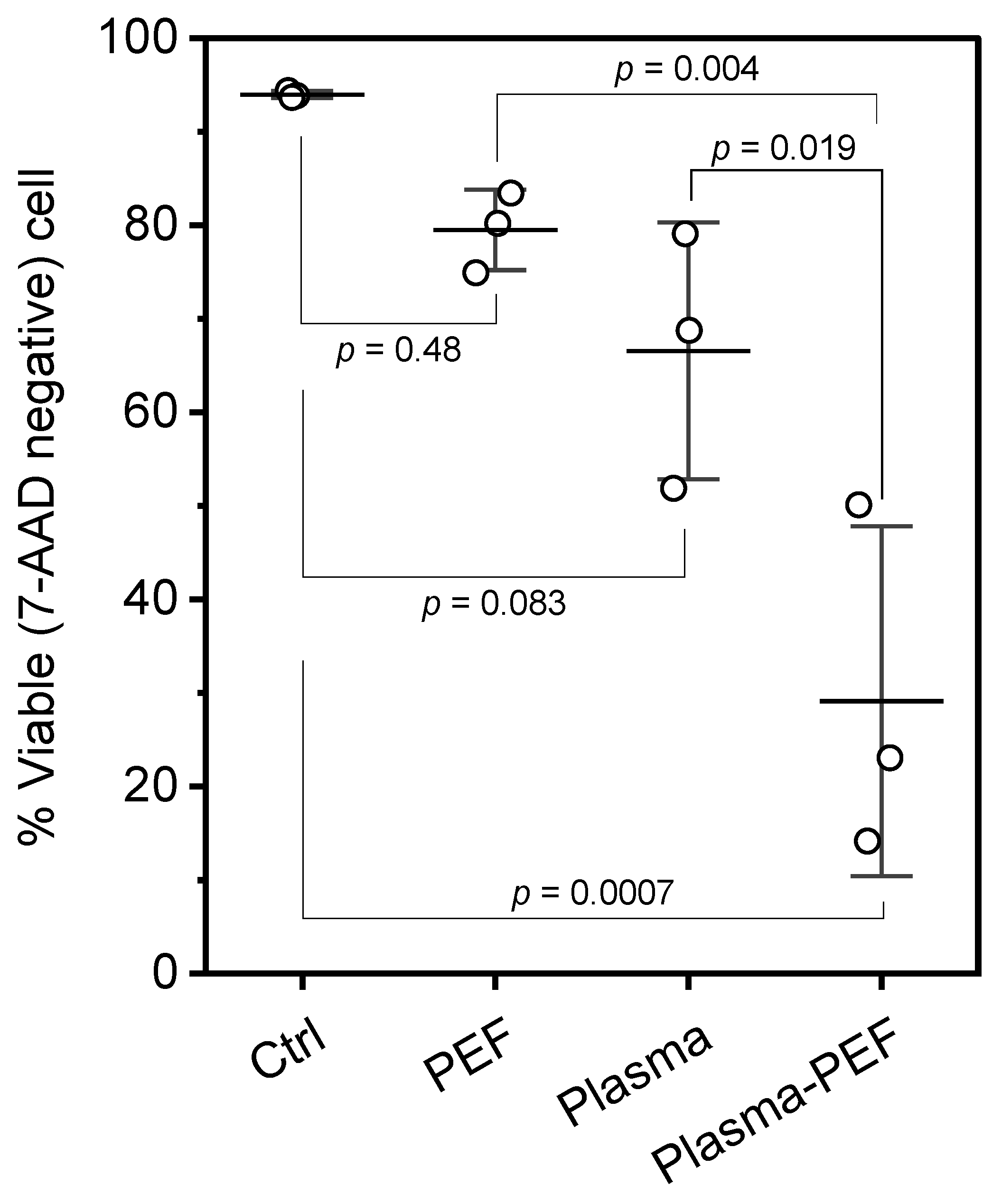
2.1. Cell Viability
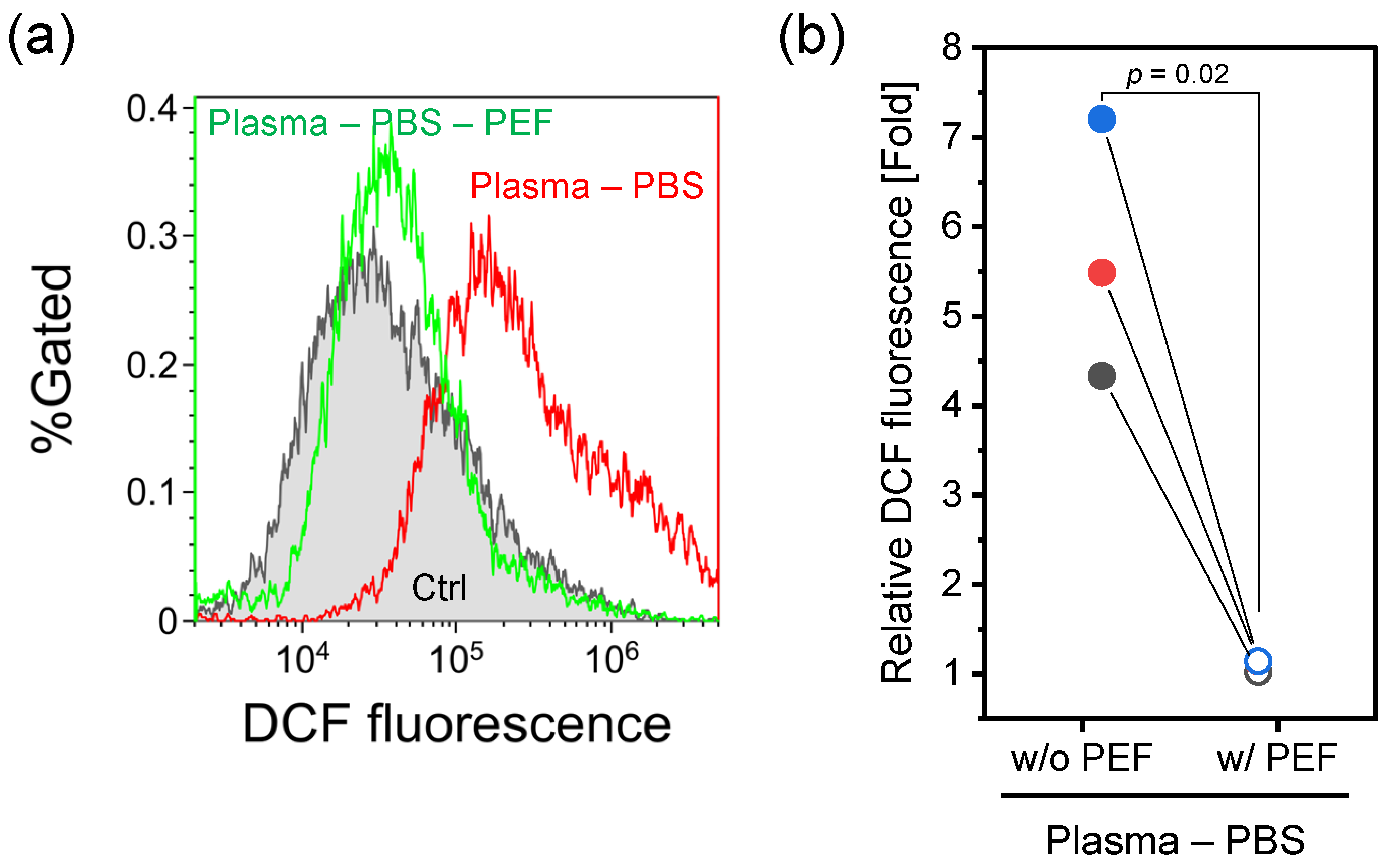
2.2. Intracellular RONS Level
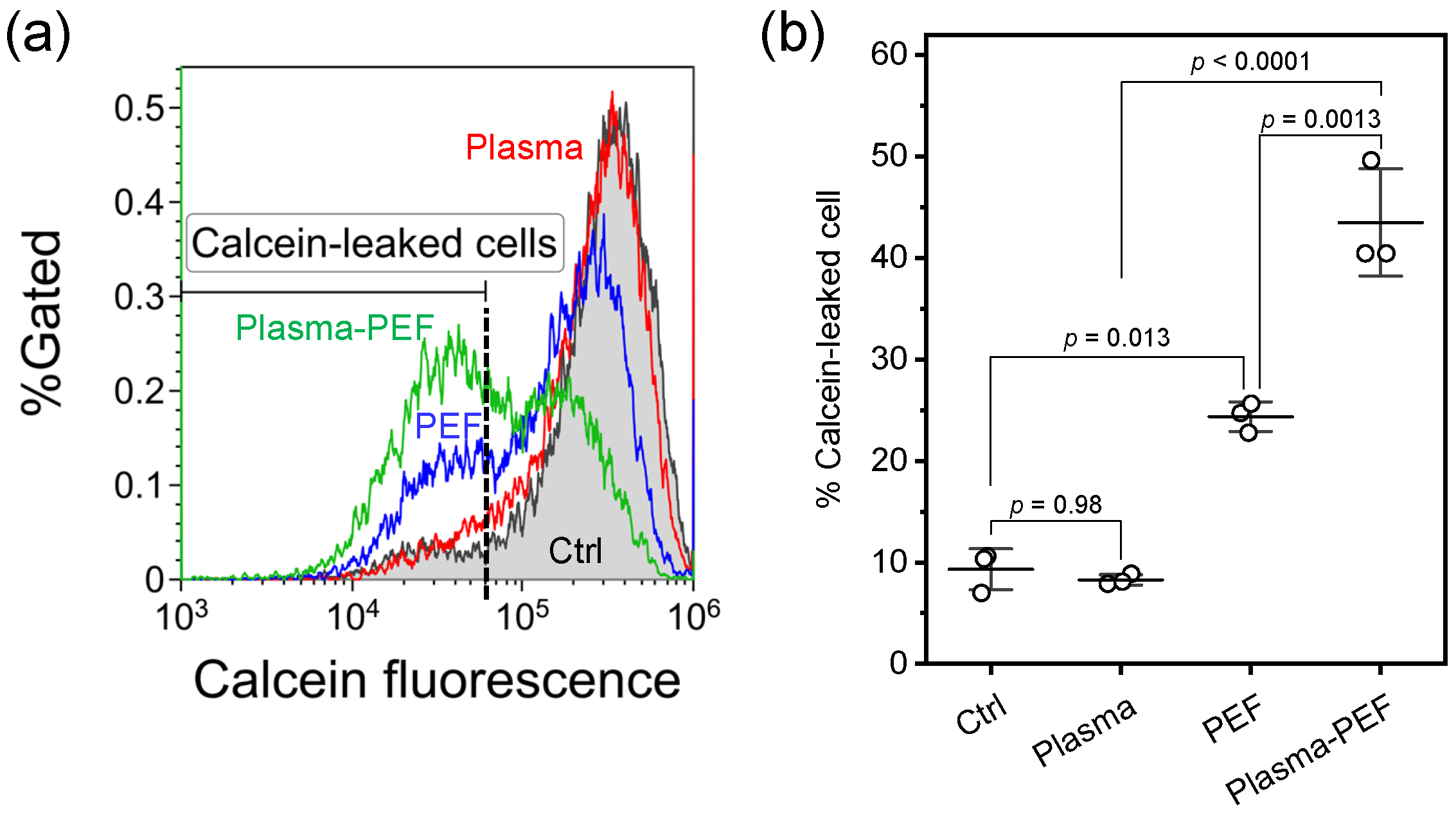
2.3. Membrane Integrity
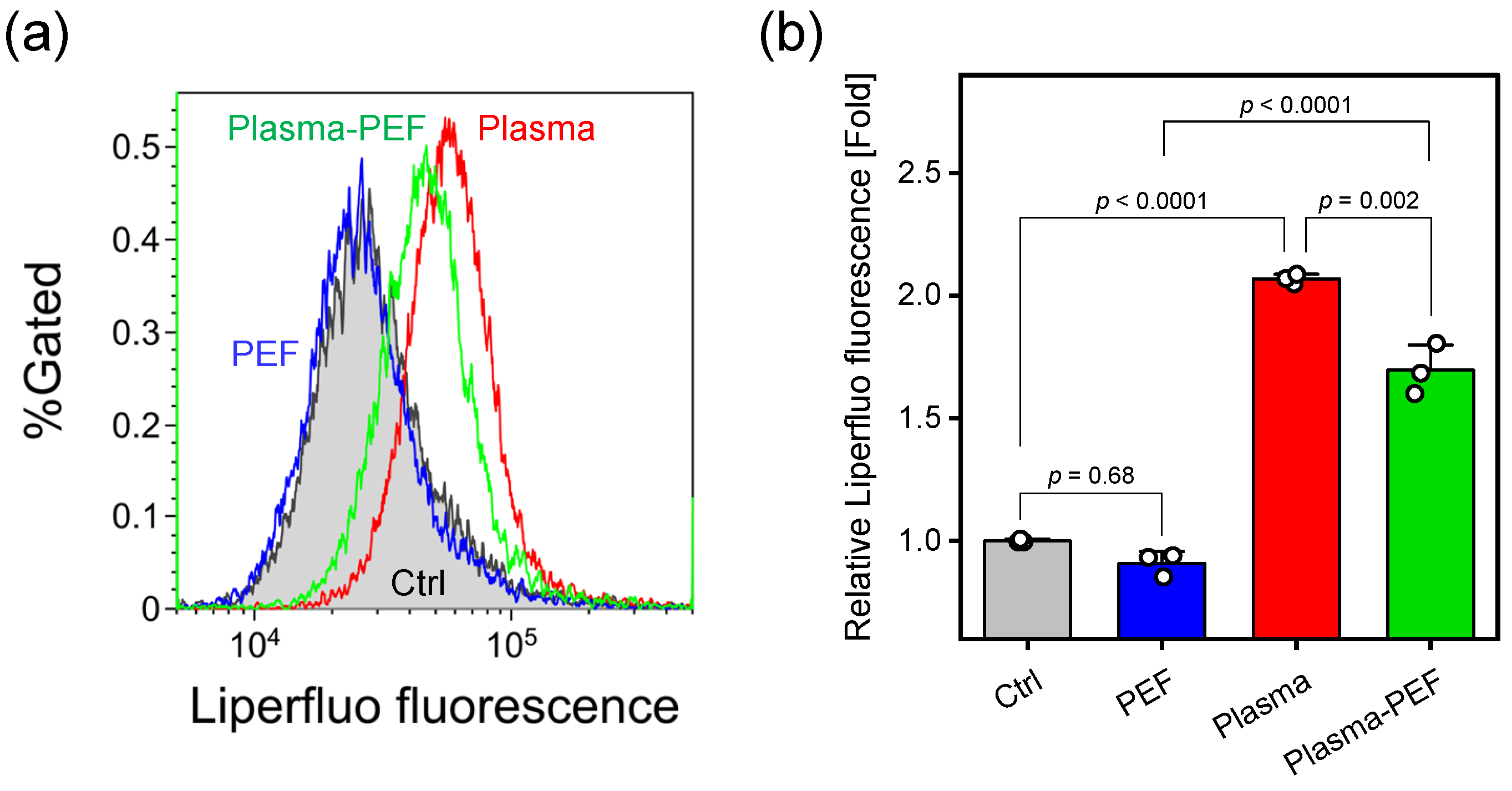
2.4. Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
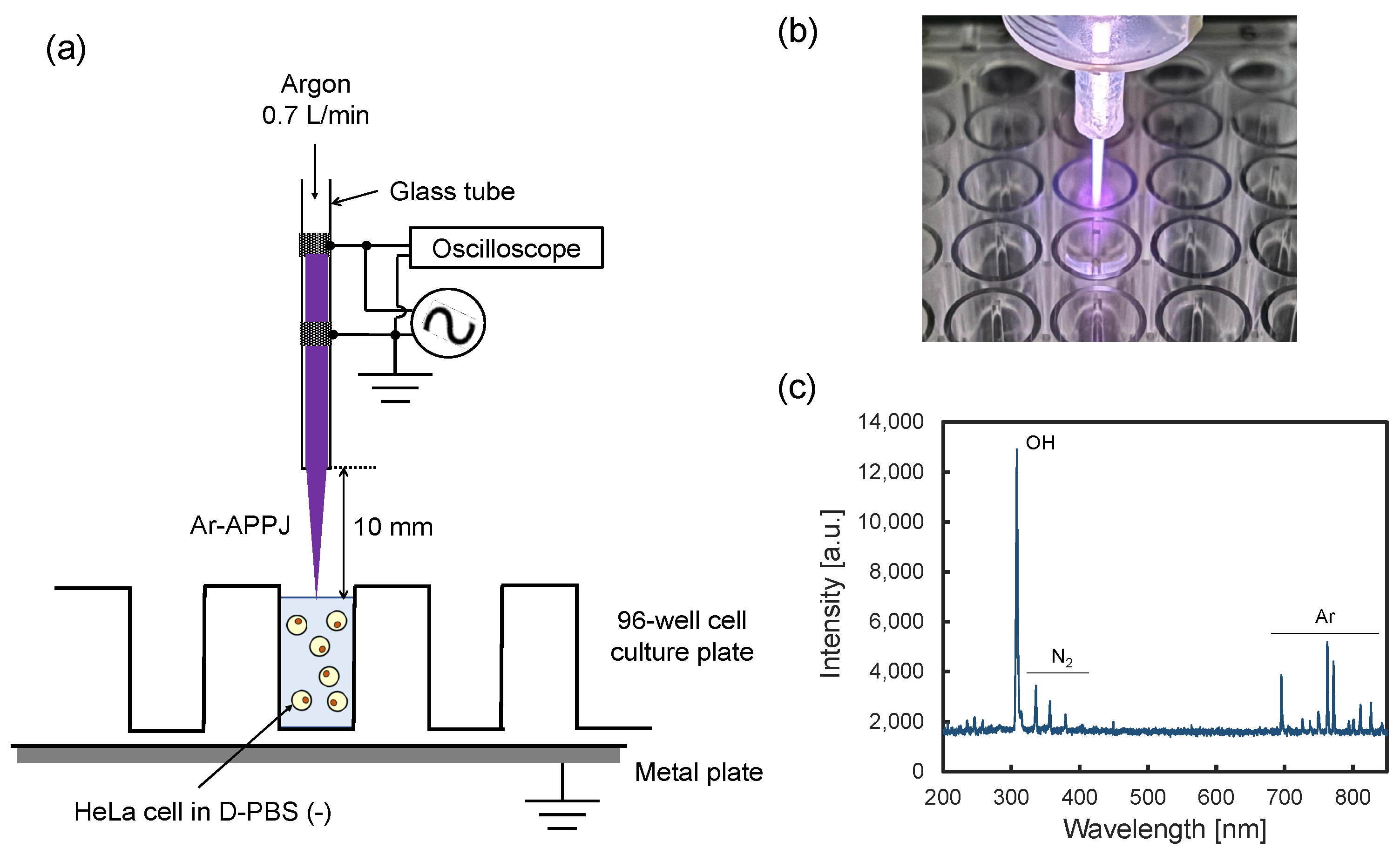
4.2. Ar-APPJ Generator and Plasma Irradiation Setup
4.3. PEF Application
4.4. Cell Viability
4.5. Intracellular RONS Level
4.6. Membrane Integrity
4.7. Lipid Peroxidation
4.8. Flow Cytometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalili, M.; Daniels, L.; Lin, A.; Krebs, F.C.; Snook, A.E.; Bekeschus, S.; Bowne, W.B.; Miller, V. Non-Thermal Plasma-Induced Immunogenic Cell Death in Cancer: A Topical Review. J. Phys. D Appl. Phys. 2019, 52, 423001. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma Cancer Treatment, a Critical Review. Appl. Sci. 2021, 11, 7757. [Google Scholar] [CrossRef]
- Limanowski, R.; Yan, D.; Li, L.; Keidar, M. Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers 2022, 14, 3461. [Google Scholar] [CrossRef]
- Karthik, C.; Sarngadharan, S.C.; Thomas, V. Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview. Int. J. Mol. Sci. 2023, 25, 524. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takeda, K.; Yoshimura, S.; Kondo, T.; Tanaka, H.; Toyokuni, S.; Nakamura, K.; Kajiyama, H.; Mizuno, M.; Hori, M. Generation and measurement of low-temperature plasma for cancer therapy: A historical review. Free Radic. Res. 2023, 57, 239–270. [Google Scholar] [CrossRef]
- Garner, A.L.; Mehlhorn, T.A. A Review of Cold Atmospheric Pressure Plasmas for Trauma and Acute Care. Front. Phys. 2021, 9, 786381. [Google Scholar] [CrossRef]
- Barjasteh, A.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Cold Atmospheric Pressure Plasma: A Growing Paradigm in Diabetic Wound Healing-Mechanism and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 16657. [Google Scholar] [CrossRef]
- Silva, N.; Marques, J.; da Cruz, M.B.; Luís, H.; Sério, S.; Mata, A. The applications of cold atmospheric plasma in dentistry. Plasma Process. Polym. 2023, 20, e2300067. [Google Scholar] [CrossRef]
- Banaszak, A.; Terefinko, D.; Motyka-Pomagruk, A.; Grzebieluch, W.; Wdowiak, J.; Pohl, P.; Sledz, W.; Malicka, B.; Jamroz, P.; Skoskiewicz-Malinowska, K.; et al. Possibilities of Application of Cold Atmospheric Pressure Plasmas in Dentistry—A Narrative Review. Plasma Process. Polym. 2024, e2400246. [Google Scholar] [CrossRef]
- Saito, K.; Toyoda, H.; Okada, M.; Oh, J.S.; Nakazawa, K.; Ban, Y.; Orita, K.; Shimatani, A.; Yao, H.; Shirafuji, T.; et al. Fracture healing on non-union fracture model promoted by non-thermal atmospheric-pressure plasma. PLoS ONE 2024, 19, e0298086. [Google Scholar] [CrossRef]
- Nakazawa, K.; Toyoda, H.; Manaka, T.; Orita, K.; Hirakawa, Y.; Ito, Y.; Saito, K.; Iio, R.; Ban, Y.; Yao, H.; et al. Non-thermal atmospheric pressure gas discharge plasma enhances tendon-to-bone junction repair in a rabbit model. J. Shoulder Elbow Surg. 2024, in press. [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of bacterial inhibition and tolerance around cold atmospheric plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2017, 15, e1700174. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Process. Polym. 2020, 18, e2000162. [Google Scholar] [CrossRef]
- Bauer, G.; Graves, D.B. Mechanisms of Selective Antitumor Action of Cold Atmospheric Plasma-Derived Reactive Oxygen and Nitrogen Species. Plasma Process. Polym. 2016, 13, 1157–1178. [Google Scholar] [CrossRef]
- Chung, W.H. Mechanisms of a novel anticancer therapeutic strategy involving atmospheric pressure plasma-mediated apoptosis and DNA strand break formation. Arch. Pharm. Res. 2016, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Yusupov, M.; Razzokov, J.; Cordeiro, R.M.; Bogaerts, A. Transport of Reactive Oxygen and Nitrogen Species across Aquaporin: A Molecular Level Picture. Oxid. Med. Cell. Longev. 2019, 2019, 2930504. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Sersa, G.; Kotnik, T.; Miklavcic, D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef]
- Campana, L.G.; Edhemovic, I.; Soden, D.; Perrone, A.M.; Scarpa, M.; Campanacci, L.; Cemazar, M.; Valpione, S.; Miklavcic, D.; Mocellin, S.; et al. Electrochemotherapy—Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur. J. Surg. Oncol. 2019, 45, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernandez, A.R.; Campos, L.; Gutierrez-Maldonado, S.E.; Nunez, G.; Villanelo, F.; Perez-Acle, T. Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box. Int. J. Mol. Sci. 2022, 23, 6158. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Dong, Y.; Alhaskawi, A.; Tu, T.; Hasan Abdullah Ezzi, S.; Goutham Kota, V.; Hasan Abdulla Hasan Abdulla, M.; Li, P.; Wu, B.; et al. New advances in treatment of skin malignant tumors with nanosecond pulsed electric field: A literature review. Bioelectrochemistry 2023, 150, 108366. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Oshin, E.A.; Guo, S.; Scott, M.; Li, X.; Mangiamele, C.; Heller, R. Synergistic effects of an atmospheric pressure plasma jet and pulsed electric field on cells and skin. IEEE Trans. Plasma Sci. 2021, 49, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Oshin, E.A.; Minhas, Z.; Biancatelli, R.; Catravas, J.D.; Heller, R.; Guo, S.; Jiang, C. Synergistic effects of nanosecond pulsed plasma and electric field on inactivation of pancreatic cancer cells in vitro. Sci. Rep. 2024, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Kolb, J.F.; Bekeschus, S. Combined In Vitro Toxicity and Immunogenicity of Cold Plasma and Pulsed Electric Fields. Biomedicines 2022, 10, 3084. [Google Scholar] [CrossRef]
- Chung, T.H.; Stancampiano, A.; Sklias, K.; Gazeli, K.; Andre, F.M.; Dozias, S.; Douat, C.; Pouvesle, J.M.; Santos Sousa, J.; Robert, E.; et al. Cell Electropermeabilisation Enhancement by Non-Thermal-Plasma-Treated PBS. Cancers 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Minamijima, Y.; Takashima, K. Characterization of intracellular reactive species production stimulated by cold atmospheric pressure plasma irradiation. Int. J. Plasma Environ. Sci. Technol. 2020, 14, e03003. [Google Scholar] [CrossRef]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.F.; Garcia, F.J.; Onak, C.S.; Carroll, K.S.; Chang, C.J. Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins. Annu. Rev. Biochem. 2015, 84, 765–790. [Google Scholar] [CrossRef] [PubMed]
- Batista Napotnik, T.; Polajzer, T.; Miklavcic, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Sachdev, S.; Potocnik, T.; Rems, L.; Miklavcic, D. Revisiting the role of pulsed electric fields in overcoming the barriers to in vivo gene electrotransfer. Bioelectrochemistry 2022, 144, 107994. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavcic, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, M.; Van der Paal, J.; Neyts, E.C.; Bogaerts, A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 839–847. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, T.; Wang, H.; Wang, X.; Wang, D.; Zhang, Y. Molecular dynamics simulation of the transmembrane transport process of reactive species under the synergistic effect of plasma oxidation and an electric field. Free Radic. Biol. Med. 2023, 208, 372–383. [Google Scholar] [CrossRef]
- Arai, S.; Bidbayasakh, K.; Fukuda, A.; Takashima, K.; Kurita, H. Oxidative modification in nuclear and mitochondrial DNA and its removal in A549 human lung cancer cells exposed to cold atmospheric-pressure plasma. Jpn. J. Appl. Phys. 2022, 61, 096003. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database; ver. 5.12; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. Available online: https://physics.nist.gov/asd (accessed on 23 January 2025).
- Watanabe, Y.; Nihonyanagi, H.; Numano, R.; Shibata, T.; Takashima, K.; Kurita, H. Influence of Electroporation Medium on Delivery of Cell-Impermeable Small Molecules by Electrical Short-Circuiting via an Aqueous Droplet in Dielectric Oil: A Comparison of Different Fluorescent Tracers. Sensors 2022, 22, 2494. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, Y.; Watanabe, Y.; Numano, R.; Shibata, T.; Kurita, H. Influence of DNA characteristics on cell membrane damage stimulated by electrical short-circuiting via a low-conductive aqueous droplet in dielectric oil. PLoS ONE 2023, 18, e0285444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitajima, N.; Makihara, K.; Kurita, H. On the Synergistic Effects of Cold Atmospheric Pressure Plasma Irradiation and Electroporation on Cytotoxicity of HeLa Cells. Int. J. Mol. Sci. 2025, 26, 1093. https://doi.org/10.3390/ijms26031093
Kitajima N, Makihara K, Kurita H. On the Synergistic Effects of Cold Atmospheric Pressure Plasma Irradiation and Electroporation on Cytotoxicity of HeLa Cells. International Journal of Molecular Sciences. 2025; 26(3):1093. https://doi.org/10.3390/ijms26031093
Chicago/Turabian StyleKitajima, Nao, Kosuke Makihara, and Hirofumi Kurita. 2025. "On the Synergistic Effects of Cold Atmospheric Pressure Plasma Irradiation and Electroporation on Cytotoxicity of HeLa Cells" International Journal of Molecular Sciences 26, no. 3: 1093. https://doi.org/10.3390/ijms26031093
APA StyleKitajima, N., Makihara, K., & Kurita, H. (2025). On the Synergistic Effects of Cold Atmospheric Pressure Plasma Irradiation and Electroporation on Cytotoxicity of HeLa Cells. International Journal of Molecular Sciences, 26(3), 1093. https://doi.org/10.3390/ijms26031093






