Understanding Deep Endometriosis: From Molecular to Neuropsychiatry Dimension
Abstract
:1. Introduction
2. Methods
3. Endometriosis—Epidemiology and Pathophysiology
4. Molecular Basis of Endometriosis
4.1. Gen Mutation
4.2. Epigenetic Changes
4.3. Hormonal Influences
4.4. Immune Dysregulation
4.5. Invasion and Angiogenesis
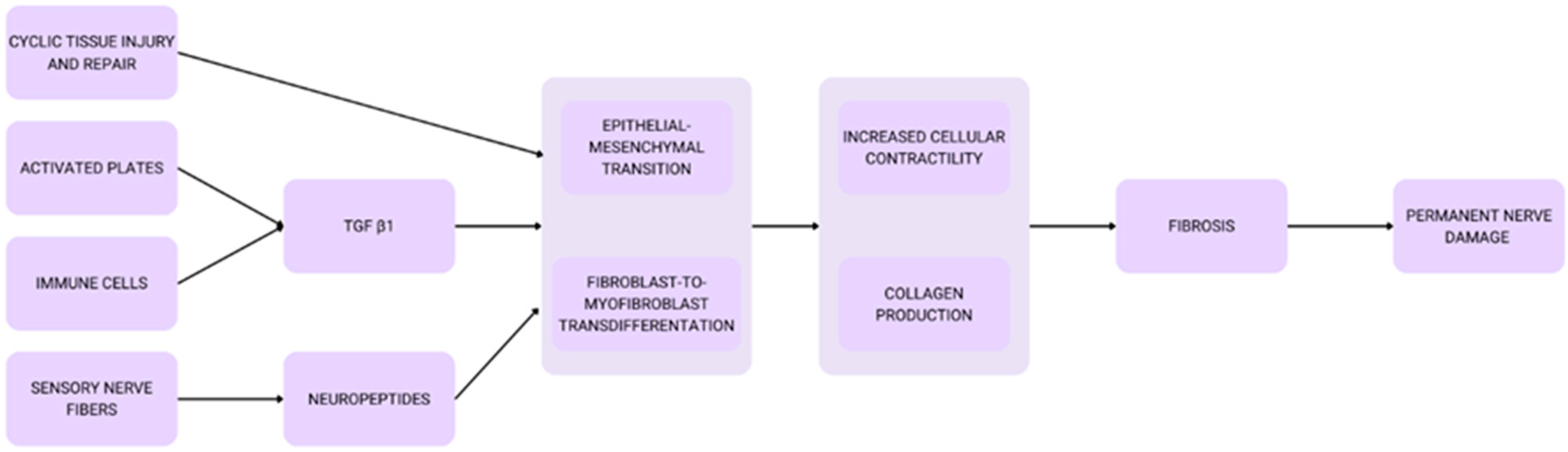
4.6. Fibrosis Formation and Oxidative Stress
5. Endometriosis and the Difficulties in Making a Diagnosis
6. Deep Endometriosis
6.1. Definition, Pathology, and Comparison with Superficial Endometriosis
6.2. Correlation Between Deep Endometriosis and Migraine
6.3. Correlation of Deep Endometriosis, Depression, and Anxiety
6.4. Deep Endometriosis, Neurodegenerative Diseases, and Mental Health
7. Sciatic and Obturator Nerve Endometriosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Frankel, L.R. A 10-Year Journey to Diagnosis With Endometriosis: An Autobiographical Case Report. Cureus 2022, 14, e21329. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem Cells and the Pathogenesis of Endometriosis. Ann. N. Y Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef] [PubMed]
- van Stein, K.; Schubert, K.; Ditzen, B.; Weise, C. Understanding Psychological Symptoms of Endometriosis from a Research Domain Criteria Perspective. J. Clin. Med. 2023, 12, 4056. [Google Scholar] [CrossRef] [PubMed]
- Maulitz, L.; Stickeler, E.; Stickel, S.; Habel, U.; Tchaikovski, S.N.; Chechko, N. Endometriosis, psychiatric comorbidities and neuroimaging: Estimating the odds of an endometriosis brain. Front. Neuroendocrinol. 2022, 65, 100988. [Google Scholar] [CrossRef] [PubMed]
- Middleton, L.; Sitch, A. How to determine if a treatment works for some people but not for others? BJOG 2020, 127, 1015. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.S.; Sepulveda, W.; Etchegaray, A.; Ventura, W.; Corral, E.; Gutierrez-Marin, J.; Espinoza, J. Uterine rupture in subsequent pregnancies following in utero spina bifida closure without stapled hysterotomy. Am. J. Obstet. Gynecol. 2022, 226, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.L.; Albert, A.; Liu, Y.D.; Lum, A.; Hong, J.; Ionescu, C.L.; Senz, J.; Nazeran, T.M.; Lee, A.F.; Noga, H.; et al. KRAS mutations and endometriosis burden of disease. J. Pathol. Clin. Res. 2023, 9, 302–312. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Nyholt, D.R.; Le, L.; Martin, N.G.; James, M.R.; Treloar, S.A.; Montgomery, G.W. KRAS variation and risk of endometriosis. MHR Basic. Sci. Reprod. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Govatati, S.; Kodati, V.L.; Deenadayal, M.; Chakravarty, B.; Shivaji, S.; Bhanoori, M. Mutations in the PTEN tumor gene and risk of endometriosis: A case-control study. Human Reprod. 2014, 29, 324–336. [Google Scholar] [CrossRef]
- Maeda, D.; Shih, I.-M. Pathogenesis and the Role of ARID1A Mutation in Endometriosis-related Ovarian Neoplasms. Adv. Anat. Pathol. 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Yachida, N.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Ueda, H.; Sugino, K.; Yamaguchi, M.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. ARID1A protein expression is retained in ovarian endometriosis with ARID1A loss-of-function mutations: Implication for the two-hit hypothesis. Sci. Rep. 2020, 10, 14260. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.; Noske, A.; Dedes, K.; Fink, D.; Imesch, P. ARID1A Mutations and PI3K/AKT Pathway Alterations in Endometriosis and Endometriosis-Associated Ovarian Carcinomas. Int. J. Mol. Sci. 2013, 14, 18824–18849. [Google Scholar] [CrossRef]
- de Almeida Da Costa, K.; Malvezzi, H.; Dobo, C.; Neme, R.M.; Filippi, R.Z.; Aloia, T.P.A.; Prado, E.R.; Meola, J.; de Azevedo Piccinato, C. Site-Specific Regulation of Sulfatase and Aromatase Pathways for Estrogen Production in Endometriosis. Front. Mol. Biosci. 2022, 9, 854991. [Google Scholar] [CrossRef]
- Mori, T.; Ito, F.; Koshiba, A.; Kataoka, H.; Takaoka, O.; Okimura, H.; Khan, K.N.; Kitawaki, J. Local estrogen formation and its regulation in endometriosis. Reprod. Med. Biol. 2019, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Osiński, M.; Wirstlein, P.; Wender-Ożegowska, E.; Mikołajczyk, M.; Jagodziński, P.P.; Szczepańska, M. HSD3B2, HSD17B1, HSD17B2, ESR1, ESR2 and AR expression in infertile women with endometriosis. Ginekol. Pol. 2018, 89, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ranganathan, P.; Kattal, N.; Pasqualotto, F.; Hallak, J.; Khayal, S.; Mascha, E. Fertility after cancer: A prospective review of assisted reproductive outcome with banked semen specimens. Fertil. Steril. 2004, 81, 342–348. [Google Scholar] [CrossRef]
- Joshi, N.R.; Miyadahira, E.H.; Afshar, Y.; Jeong, J.-W.; Young, S.L.; Lessey, B.A.; Serafini, P.C.; Fazleabas, A.T. Progesterone resistance in endometriosis is modulated by the altered expression of microRNA-29c and FKBP4. J. Clin. Endocrinol. Metab. 2017, 102, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Lessons From the Testosterone Trials. Endocr. Rev. 2018, 39, 369–386. [Google Scholar] [CrossRef]
- Malutan, A.M.; Drugan, T.; Costin, N.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Clinical immunology Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol. 2015, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Sophonsritsuk, A.; Attawattanakul, N.; Sroyraya, M.; Songkoomkrong, S.; Waiyaput, W.; Dittharot, K.; Chansoon, T.; Jinawath, A.; Tingthanatikul, Y. Macrophages and Natural Killer Cells Characteristics in Variously Colored Endometriotic Lesions: A Cross-Sectional Analytic Study. Int. J. Fertil. Steril. 2022, 16, 108–114. [Google Scholar] [CrossRef]
- Evert, J.H.-V.; Paap, R.; Nap, A.; van der Molen, R. The Promises of Natural Killer Cell Therapy in Endometriosis. Int. J. Mol. Sci. 2022, 23, 5539. [Google Scholar] [CrossRef]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef]
- Mazor, R.; Alsaigh, T.; Shaked, H.; Altshuler, A.E.; Pocock, E.S.; Kistler, E.B.; Karin, M.; Schmid-Schönbein, G.W. Matrix Metalloproteinase-1-mediated Up-regulation of Vascular Endothelial Growth Factor-2 in Endothelial Cells. J. Biol. Chem. 2013, 288, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.-K.; Ishii, G.; Saito, S.; Yano, K.; Hoshino, A.; Suzuki, T.; Ochiai, A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009, 113, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-angiogenic Therapy for Endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of Anti-VEGF/VEGFR Agents on Animal Models of Endometriosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Smith, S.K. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Human Reprod. 1996, 11, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano, P.; Benaglia, L.; Busnelli, A.; Vercellini, P.; Fedele, L. Adhesion Prevention in Endometriosis: A Neglected Critical Challenge. J. Minim. Invasive Gynecol. 2012, 19, 415–421. [Google Scholar] [CrossRef]
- Viganò, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.G.; Vannuzzi, V.; Donati, C.; Bernacchioni, C.; Bruni, P.; Petraglia, F. Endometriosis: Cellular and Molecular Mechanisms Leading to Fibrosis. Reprod. Sci. 2023, 30, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- González-Foruria, I.; Santulli, P.; Chouzenoux, S.; Carmona, F.; Chapron, C.; Batteux, F. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: From oxidative stress to fibrosis. MHR Basic Sci. Reprod. Med. 2017, 23, 488–499. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Tu, F.; Soliman, A.M.; Chiuve, S.; Cross, S.; Eichner, S.; Flores, O.A.; Horne, A.; Schneider, B.; As-Sanie, S. Impact of endometriosis on women’s life decisions and goal attainment: A cross-sectional survey of members of an online patient community. BMJ Open 2022, 12, e052765. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.C.M.; de Souza Ferreira, L.P.; Della Giustina, A. It is time to change the definition: Endometriosis is no longer a pelvic disease. Clinics 2024, 79, 100326. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.M.; Missmer, S.; Petrozza, J.; Tomassetti, C.; Zondervan, K.T.; et al. Endometriosis classification, staging and reporting systems: A review on the road to a universally accepted endometriosis classification. Hum. Reprod. Open 2021, 28, 1822–1848. [Google Scholar] [CrossRef]
- Drevet, P.; Lemaire, C.; Gasparini, S.; Zinn-Justin, S.; Lajeunesse, E.; Ducancel, F.; Pinkasfeld, S.; Courçon, M.; Tremeau, O.; Boulain, J.C.; et al. High-level production and isotope labeling of snake neurotoxins, disulfide-rich proteins. Protein Expr. Purif. 1997, 10, 293–300. [Google Scholar] [CrossRef]
- Henthorne, B.H.; Henthorne, T.L.; Alcorn, J.D. Enhancing the provider/patient relationship: The case for patient advocacy programs. J. Health Care Mark. 1994, 14, 52–55. [Google Scholar] [PubMed]
- Yarborough, E.S. The family transition program: A metaphor-based correspondence program for family care-givers. Nurs. Homes 1986, 35, 18–22. [Google Scholar] [PubMed]
- Lemon, H.M.; Rodriguez-Sierra, J.F. Timing of breast cancer surgery during the luteal menstrual phase may improve prognosis. Neb. Med. J. 1996, 81, 110–115. [Google Scholar]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Keckstein, J.; Wattiez, A.; Adamyan, L. Epidemiology of subtle, typical, cystic, and deep endometriosis: A systematic review. Gynecol. Surg. 2016, 13, 457–467. [Google Scholar] [CrossRef]
- Lebovic, D.I.; Mueller, M.D.; Taylor, R.N. Immunobiology of endometriosis. Fertil. Steril. 2001, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ianieri, M.M.; Mautone, D.; Ceccaroni, M. Recurrence in Deep Infiltrating Endometriosis: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2018, 25, 786–793. [Google Scholar] [CrossRef]
- Gordts, S.; Koninckx, P.; Brosens, I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017, 108, 872–885.e1. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Singh, S.S.; Murji, A.; Satkunaratnam, A.; Atri, M.; Reid, S.; Condous, G. Deep Endometriosis: A Diagnostic Dilemma With Significant Surgical Consequences. J. Obstet. Gynaecol. Can. 2018, 40, 1198–1203. [Google Scholar] [CrossRef]
- de Paula Andres, M.; Borrelli, G.M.; Kho, R.M.; Abrão, M.S. The current management of deep endometriosis: A systematic review. Minerva Obstet. Gynecol. 2017, 69, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-Y.; Wang, Y.-P.; Ren, R.; Sun, Y.-R.; Xiao, S.-Q.; Han, L. Clinicopathological correlations of peritoneal endometriosis and deep infiltrating endometriosis. Ann. Med. 2023, 55. [Google Scholar] [CrossRef]
- Somigliana, E. Association rate between deep peritoneal endometriosis and other forms of the disease: Pathogenetic implications. Human Reprod. 2004, 19, 168–171. [Google Scholar] [CrossRef]
- Aguilar-Shea, A.L.; Membrilla Md, J.A.; Diaz-de-Teran, J. Migraine review for general practice. Aten Primaria 2022, 54, 102208. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Migraine. Lancet 2004, 363, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Selntigia, A.; Exacoustos, C.; Ortoleva, C.; Russo, C.; Monaco, G.; Martire, F.G.; Rizzo, G.; Della-Morte, D.; Mercuri, N.B.; Albanese, M. Correlation between endometriosis and migraine features: Results from a prospective case-control study. Cephalalgia 2024, 44, 3331024241235210. [Google Scholar] [CrossRef] [PubMed]
- Maitrot-Mantelet, L.; Hugon-Rodin, J.; Vatel, M.; Marcellin, L.; Santulli, P.; Chapron, C.; Plu-Bureau, G. Migraine in relation with endometriosis phenotypes: Results from a French case-control study. Cephalalgia 2020, 40, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.; Caraballo-Rivera, E.J.; Isola, S.; Oraka, K.; Akter, S.; Verma, S.; Patel, R.S. Demographics and Hospital Outcomes in American Women With Endometriosis and Psychiatric Comorbidities. Cureus 2020, 12, e9935. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.A.A.; de Paiva, A.M.F.; da Costa Porto Taliberti, B.; Gonçalves, A.L.L.; Condes, R.P.; Ribeiro, P.A.G.A. Psychological Problems Experienced by Patients with Bowel Endometriosis Awaiting Surgery. Rev. Bras. De Ginecol. E Obs./RBGO Gynecol. Obstet. 2021, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Siedentopf, F.; Tariverdian, N.; Rücke, M.; Kentenich, H.; Arck, P.C. ORIGINAL ARTICLE: Psychosocial Distress and Reduced Quality of Life in Infertile Patients with Endometriosis. Am. J. Reprod. Immunol. 2008, 60, 449–461. [Google Scholar] [CrossRef]
- González-Mesa, E.; Moya-Bejarano, D.; Butrón-Hinojo, C.A.; Marín-Sánchez, P.; Blasco-Alonso, M.; Jimenez-López, J.S.; Villegas-Muñoz, E.; Lubián-López, D.M. Correlates of Sexual Function in a Sample of Spanish Women with Endometriosis. J. Clin. Med. 2021, 10, 4957. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.J. Deep Dyspareunia in Endometriosis: A Proposed Framework Based on Pain Mechanisms and Genito-Pelvic Pain Penetration Disorder. Sex. Med. Rev. 2017, 5, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- van Barneveld, E.; Manders, J.; van Osch, F.H.M.; van Poll, M.; Visser, L.; van Hanegem, N.; Lim, A.C.; Bongers, M.Y.; Leue, C. Depression, Anxiety, and Correlating Factors in Endometriosis: A Systematic Review and Meta-Analysis. J. Womens Health 2022, 31, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, L.; Di Filippo, C.; Gabrielli, O.; Reppuccia, S.; La Rosa, V.L.; Ragusa, R.; Fichera, M.; Commodari, E.; Bifulco, G.; Giampaolino, P. The Burden of Endometriosis on Women’s Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 4683. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Yang, Y.-C.; Hsu, C.-Y.; Shen, Y.-C. Risk of bipolar disorder in patients with endometriosis: A nationwide population-based cohort study. J. Affect. Disord. 2020, 270, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.J.; Sharma, V.; Sharma, S.; Mazmanian, D. A Systematic Review of the Association Between Psychiatric Disturbances and Endometriosis. J. Obstet. Gynaecol. Can. 2015, 37, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Mahale, S.D. Endometriosis Knowledgebase: A gene-based resource on endometriosis. Database 2019, 2019, baz062. [Google Scholar] [CrossRef] [PubMed]
- Latourelle, J.C.; Dybdahl, M.; Destefano, A.L.; Myers, R.H.; Lash, T.L. Estrogen-related and other disease diagnoses preceding Parkinson’s disease. Clin. Epidemiol. 2010, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Zamurovic, M.; Tomic, A.; Djordjevic, K.; Simanic, S.; Sopta, J.; Rasulic, L.; Simic, L.; Jevtic, J.; Nedeljkovic-Arsenovic, O.; Rovcanin, M. Isolated Deep Infiltrating Endometriosis of the Sciatic Nerve: A Case Report and Overview of the Literature. Medicina 2023, 59, 2161. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Booi, L.; Jenkins, N.; Bridgeman, K.; Muniz-Terrera, G.; Farina, F.R. Hormonal contraception and risk for cognitive impairment or Alzheimer’s disease and related dementias in young women: A scoping review of the evidence. Front. Glob. Womens Health 2023, 4, 1289096. [Google Scholar] [CrossRef]
- Szypłowska, M.; Tarkowski, R.; Kułak, K. The impact of endometriosis on depressive and anxiety symptoms and quality of life: A systematic review. Front. Public Health 2023, 11, 1230303. [Google Scholar] [CrossRef]
- Totev, T.; Tihomirova, T.; Tomov, S.; Gorchev, G. Deep infiltrating endometriosis-diagnosis and principles of surgical treatment. Akush. Ginekol. (Mosk) 2014, 53, 37–41. [Google Scholar]
- Lukac, S.; Schmid, M.; Pfister, K.; Janni, W.; Schäffler, H.; Dayan, D. Extragenital endometriosis in the differential diagnosis of non-gynecological diseases. Dtsch. Arztebl. Int. 2022, 119, 361–367. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Graves, M.J.; Henry, B.M.; Popieluszko, P.; Roy, J.; Pękala, P.A.; Hsieh, W.C.; Vikse, J.; Walocha, J.A. Surgical anatomy of the sciatic nerve: A meta-analysis. J. Orthop. Res. 2016, 34, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Bindra, V.; Nori, M.; Reddy, R.; Reddy, R.; Satpathy, G.; Reddy, C.A. Sciatic nerve endometriosis—The correct approach matters: A case report. Case Rep. Womens Health 2023, 38, e00515. [Google Scholar] [CrossRef]
- Kale, A.; Aboalhasan, Y.; Gündoğdu, E.C.; Usta, T.; Oral, E. Obturator nerve endometriosis: A systematic review of the literature. Facts Views Vis. Obgyn 2022, 14, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Possover, M. Laparoscopic morphological aspects and tentative explanation of the aetiopathogenesis of isolated endometriosis of the sciatic nerve: A review based on 267 patients. Facts Views Vis. Obgyn 2021, 13, 369–375. [Google Scholar] [CrossRef] [PubMed]
- van Poll, M.; van Barneveld, E.; Aerts, L.; Maas, J.W.M.; Lim, A.C.; de Greef, B.T.A.; Bongers, M.Y.; van Hanegem, N. Endometriosis and Sexual Quality of Life. Sex. Med. 2020, 8, 532–544. [Google Scholar] [CrossRef] [PubMed]
| ROS Marker in Endometrial Tissue | Description | Reference |
|---|---|---|
| hydrogen peroxide | Superoxide anion, with higher concentration in endometriotic cells than in endometrial cells | [40] |
| glutathione peroxidase | Antioxidant enzyme, with higher concentration in endometriotic cells than in endometrial cells | [40] |
| catalase | The antioxidant enzyme has a lower concentration in endometriosis cells than endometrial cells | [40] |
| c-Fos and c-Jun | Members of mitogen-activated protein (MAP) kinase/extracellular signal-regulated kinase (ERK) pathway | [40] |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | Marker of oxidative stress damage, with higher concentration in endometriotic cells than in endometrial cells | [40] |
| malondialdehyde (MDA) | A byproduct of lipid peroxidation | [40] |
| Disease | Association with Endometriosis | Pain Perception | |
|---|---|---|---|
| Migraine | Migraine is more frequent in women with endometriosis, with the highest risk in ovarian endometrioma and deep infiltrating endometriosis. | Patients with both migraine and endometriosis reported higher pain intensity | |
| Depression | Overall, 77.1% of the patients suffering from endometriosis had anxiety and depression, from which 77% had anxiety and depression simultaneously. | Endometriosis can have a significant effect on social life as well as on sexual life. Dyspareunia, or painful intercourse, is reported by 32% to 70% of women who have endometriosis. | Anxiety and depression increase pain perception |
| Anxiety | Anxiety in the context of endometriosis encompasses the emotional toll of subfertility, the potential for disease recurrence, and uncertainty regarding future outcomes. | ||
| Bipolar Disorder | Endometriosis may be linked to a higher risk of bipolar disorder. | Chronic pelvic pain and quality of life deterioration | |
| Dementia | Elevated levels of estrogen contribute to the development of endometriosis, whereas reduced estrogen exposure is associated with neurodegenerative diseases such as Alzheimer’s disease. | Alterations in pain processing | |
| Sciatic Nerve Endometriosis | The occurrence of deep infiltrating endometriosis (DIE) involving sacral nerve roots or major pelvic nerves is less than 0.1%. | Sciatic nerve endometriosis is associated with pain in the buttock, radiation to the leg and heel, and mobility disorder. Surgical treatment is the most common. | Pain typically occurs as “catamenial sciatica” which means worsening of sciatica during menstruation |
| Obturator Nerve Endometriosis | Endometriosis of the obturator nerve is only about 1% of peripheral nerves associated with endometriosis. It is associated with thigh pain, weakness, and impaired adduction of the legs. The treatment is radical excision of the nerve using laparoscopy. | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczołowska, M.; Walczak, K.; Kołodziejczyk, W.; Kozłowska, M.; Kozłowski, G.; Gachowska, M.; Leszek, J. Understanding Deep Endometriosis: From Molecular to Neuropsychiatry Dimension. Int. J. Mol. Sci. 2025, 26, 839. https://doi.org/10.3390/ijms26020839
Pszczołowska M, Walczak K, Kołodziejczyk W, Kozłowska M, Kozłowski G, Gachowska M, Leszek J. Understanding Deep Endometriosis: From Molecular to Neuropsychiatry Dimension. International Journal of Molecular Sciences. 2025; 26(2):839. https://doi.org/10.3390/ijms26020839
Chicago/Turabian StylePszczołowska, Magdalena, Kamil Walczak, Weronika Kołodziejczyk, Magdalena Kozłowska, Gracjan Kozłowski, Martyna Gachowska, and Jerzy Leszek. 2025. "Understanding Deep Endometriosis: From Molecular to Neuropsychiatry Dimension" International Journal of Molecular Sciences 26, no. 2: 839. https://doi.org/10.3390/ijms26020839
APA StylePszczołowska, M., Walczak, K., Kołodziejczyk, W., Kozłowska, M., Kozłowski, G., Gachowska, M., & Leszek, J. (2025). Understanding Deep Endometriosis: From Molecular to Neuropsychiatry Dimension. International Journal of Molecular Sciences, 26(2), 839. https://doi.org/10.3390/ijms26020839







