Energy Metabolism and Stemness and the Role of Lauric Acid in Reversing 5-Fluorouracil Resistance in Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
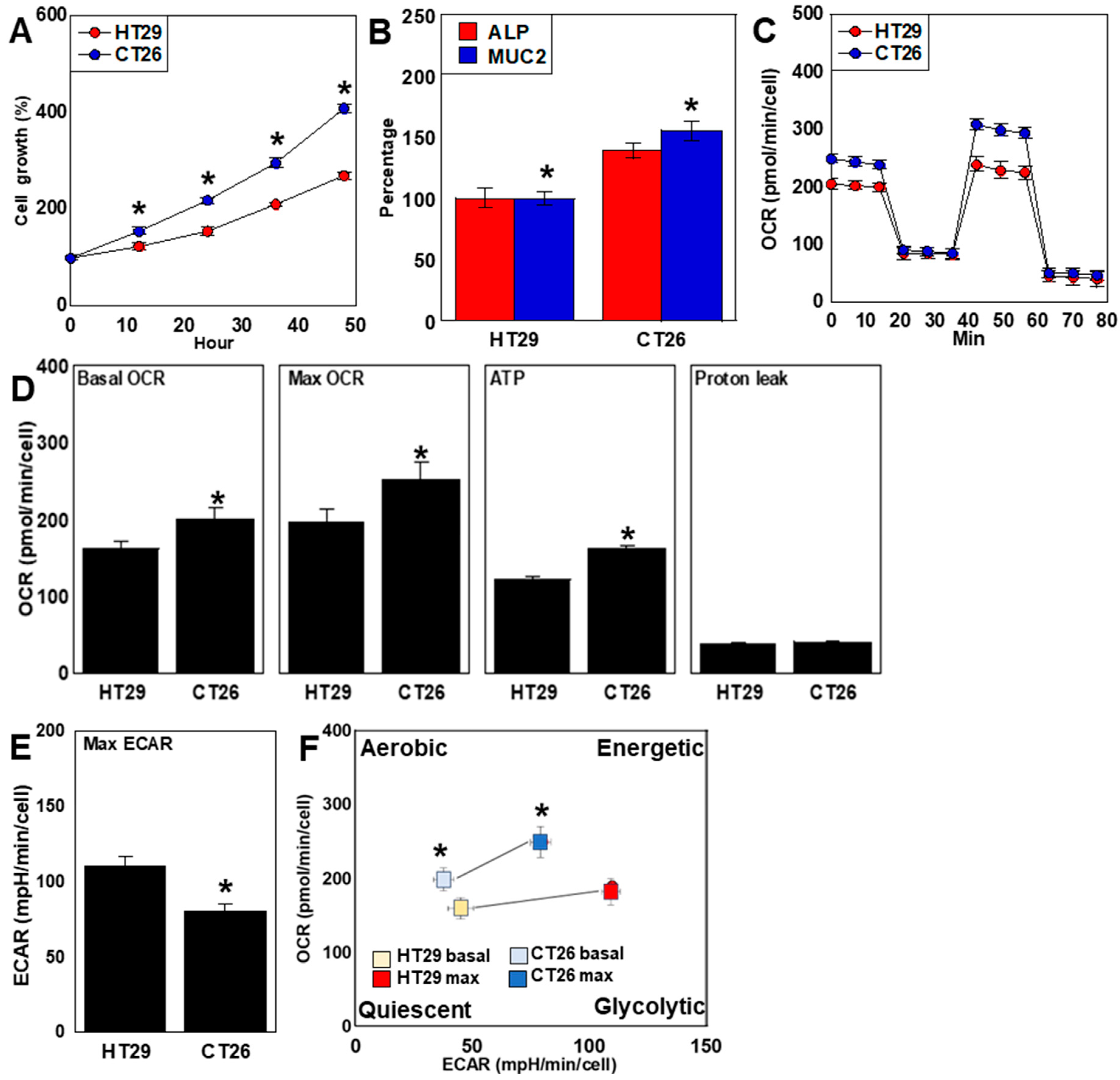
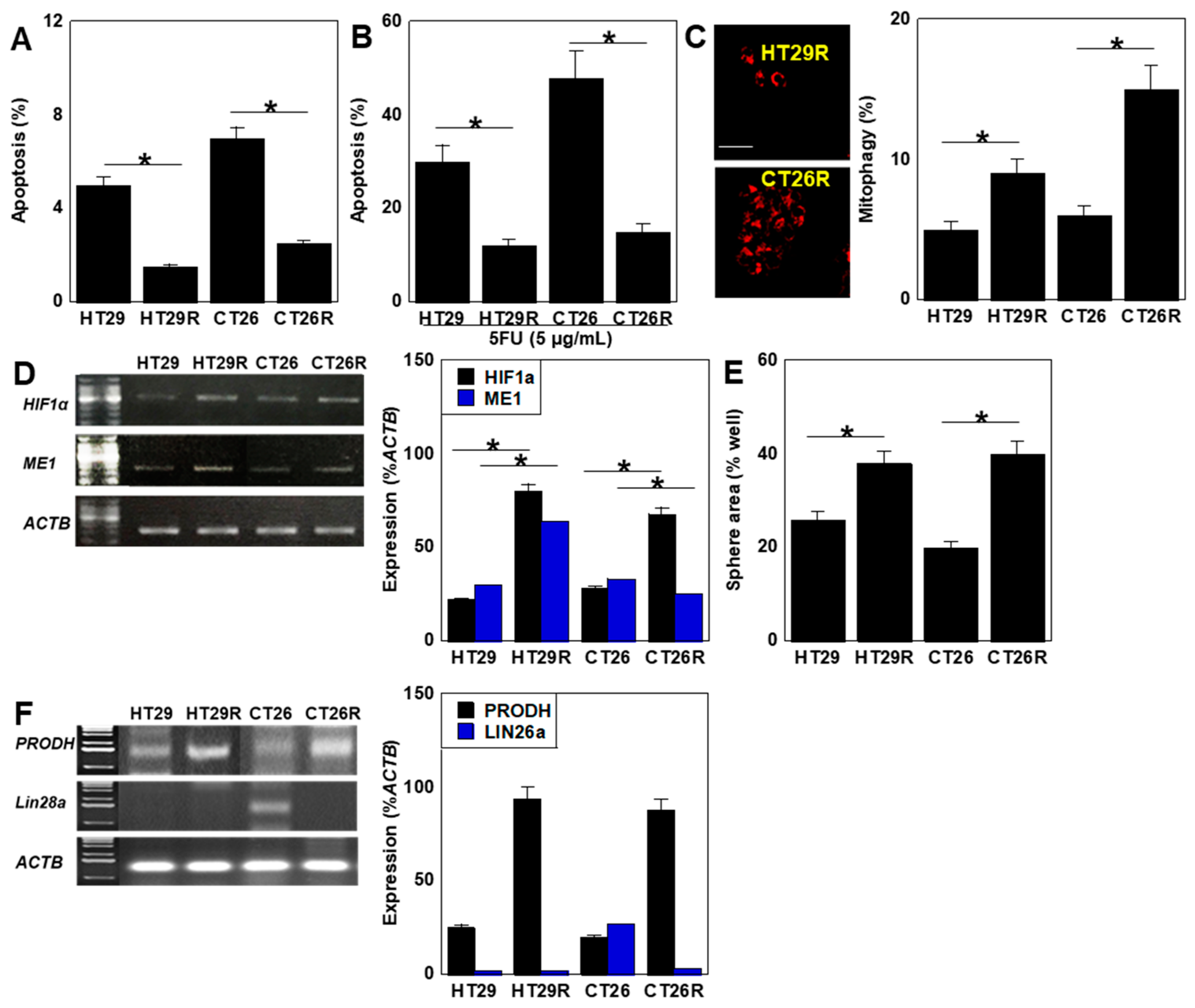
2.1. Differences in Proliferation, Differentiation, and Energy Metabolism
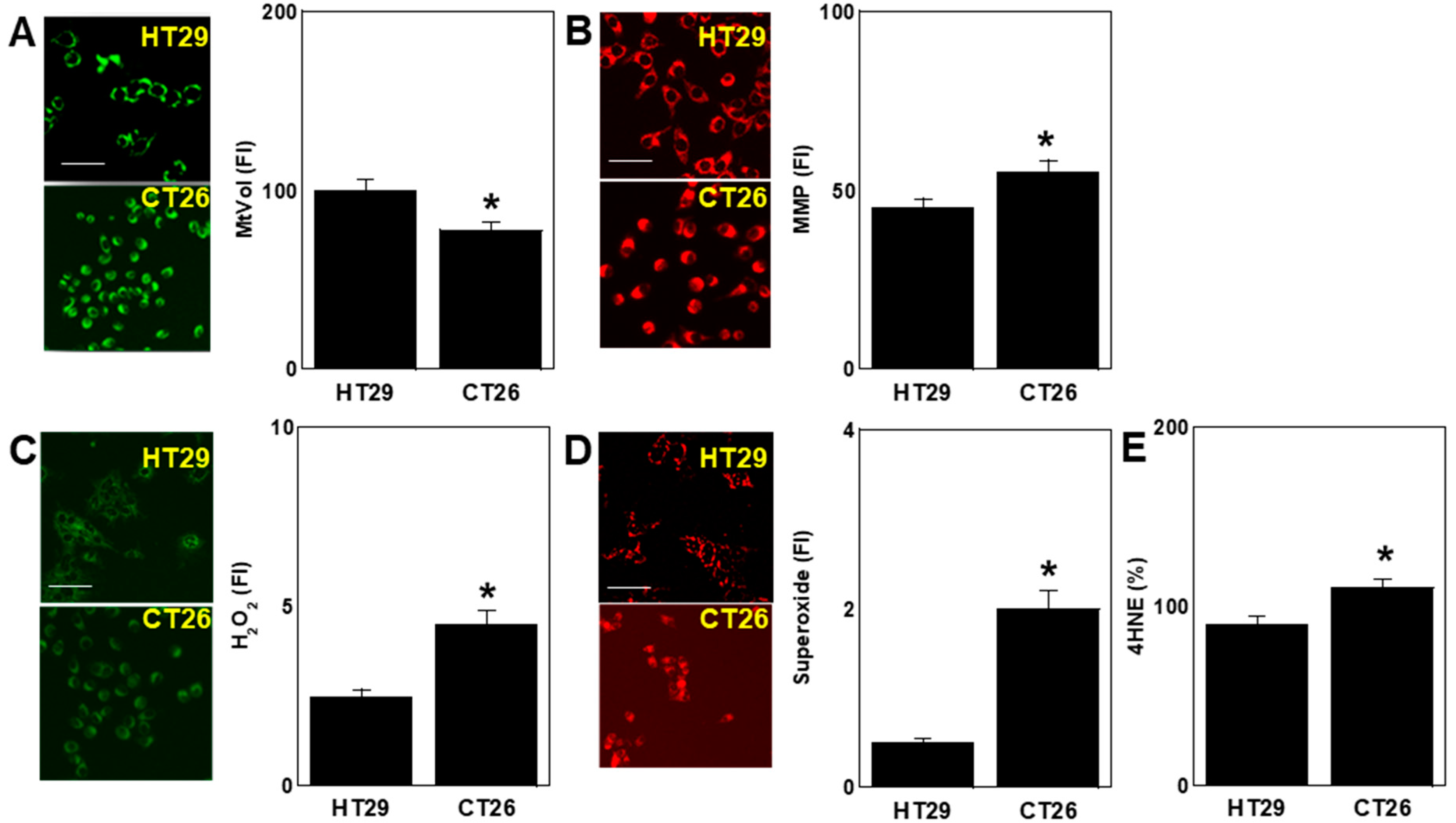
2.2. Differences in Oxidative Stress
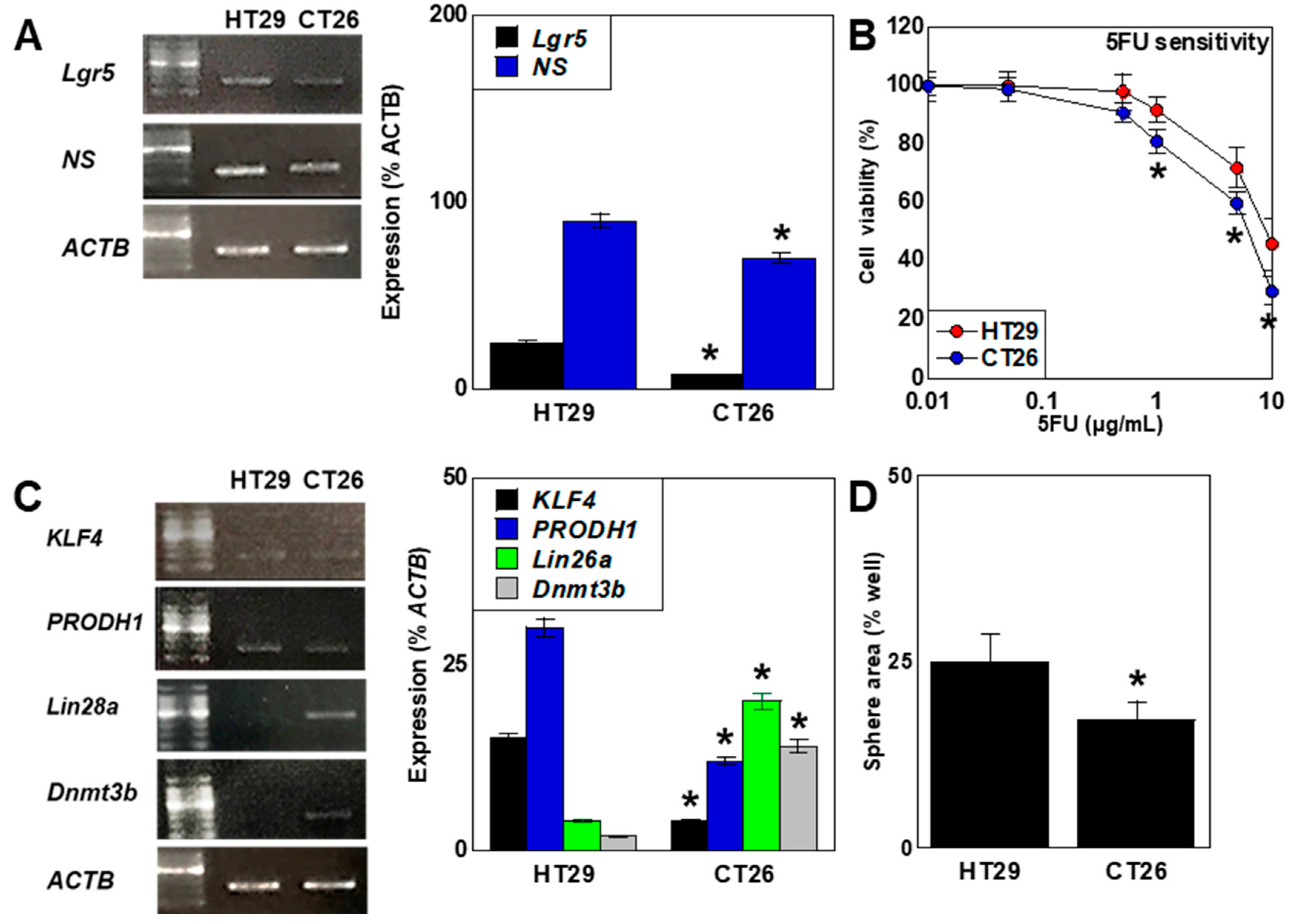
2.3. Differences in Stemness
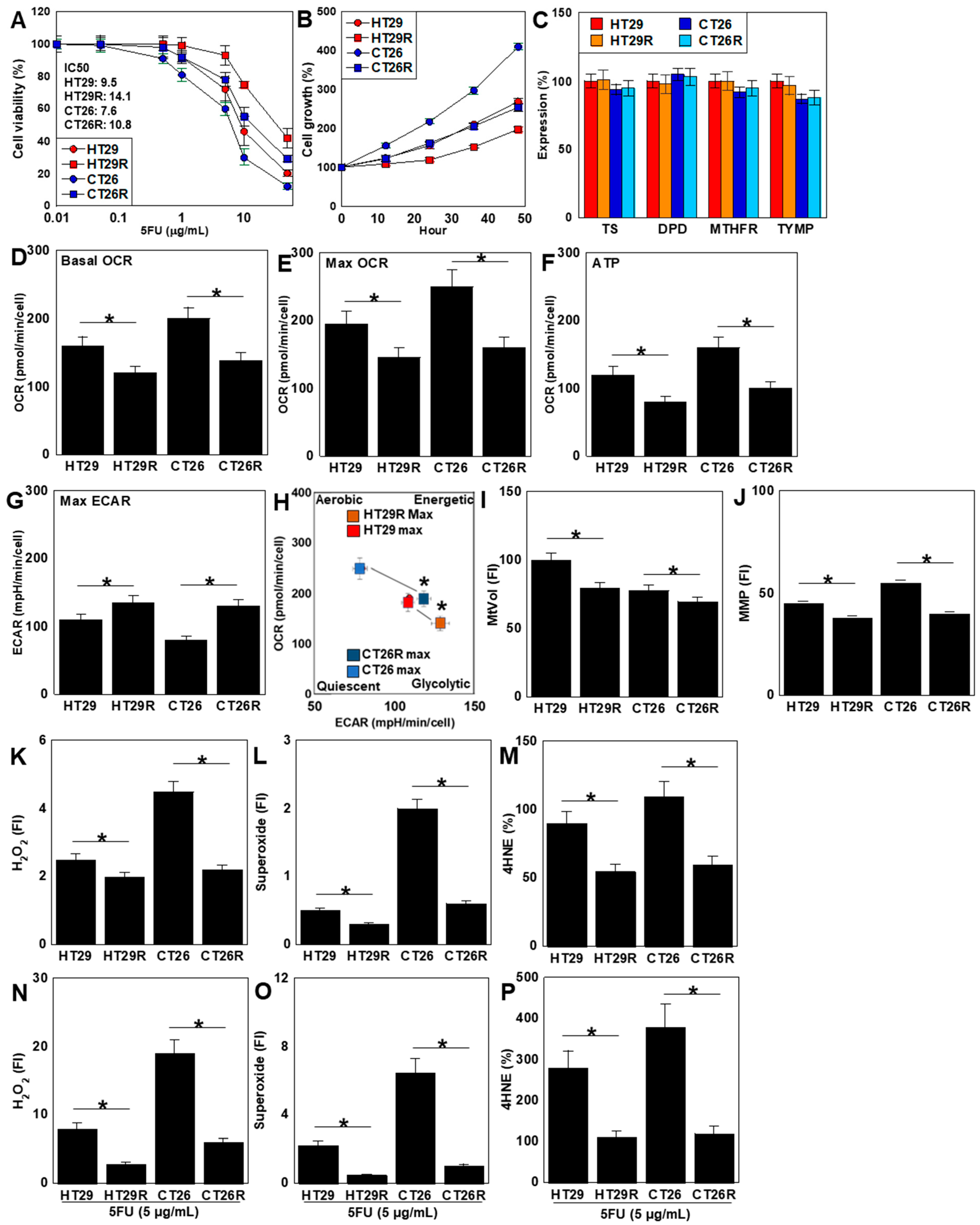
2.4. Characterization of 5FU-Resistant Cell Lines
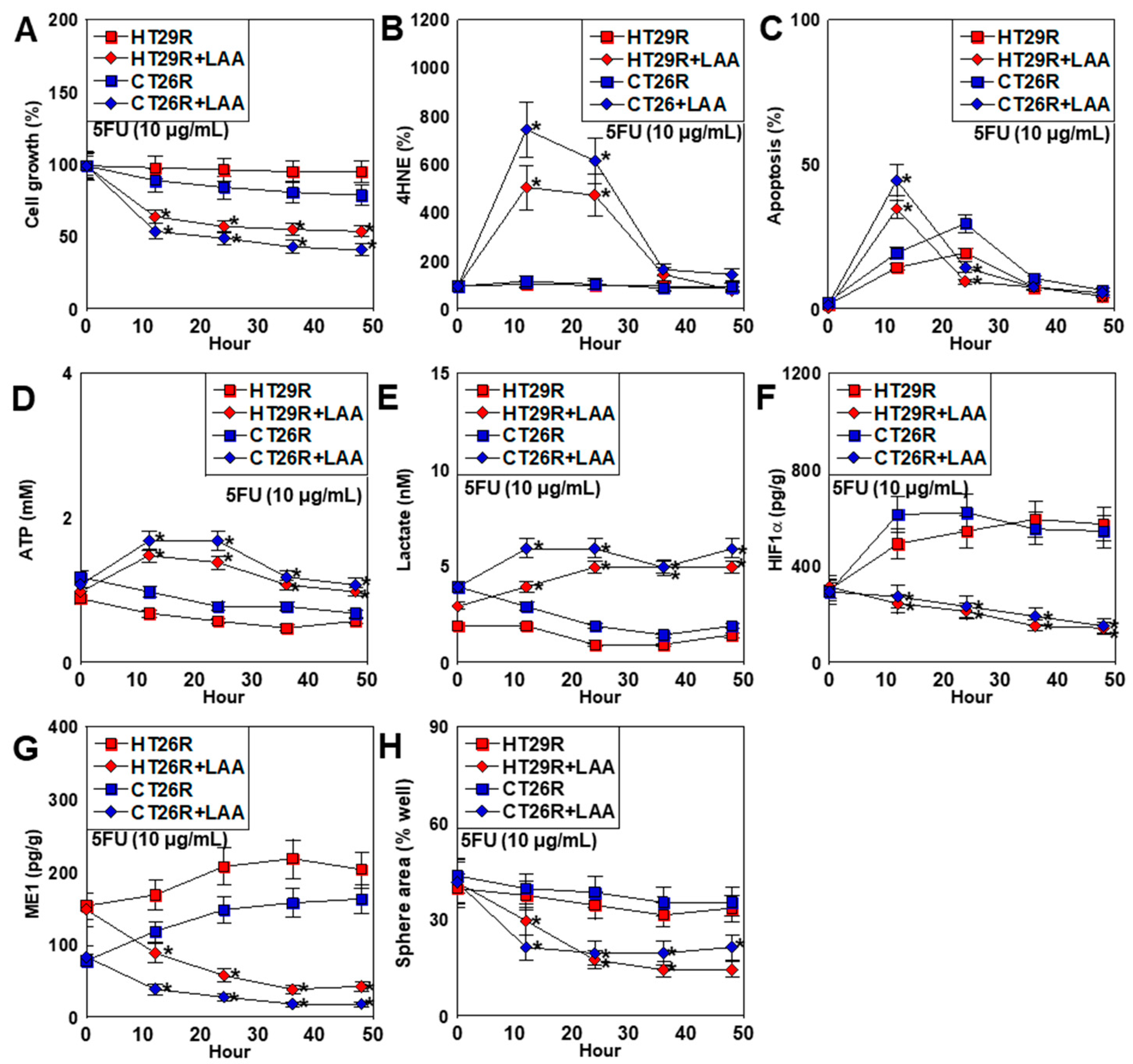
2.5. Effect of LAA on 5FU-Resistant CRC Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. [3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (MTS) Assay
4.3. Mitochondrial Imaging
4.4. Protein Extraction
4.5. Enzyme-Linked Immunosorbent Assay (ELISA) and Fluorometric Assay
4.6. Sphere Assay
4.7. Mitochondrial Stress Test
4.8. Glycolytic Stress Test
4.9. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5FU | 5-fluorouracil |
| CRC | colorectal cancer |
| OXPHOS | oxidative phosphorylation |
| ROS | reactive oxygen species |
| CSC | cancer stem cell |
| TS | thymidylate synthase |
| DPD | dihydropyrimidine dehydrogenase |
| MTHFR | methylenetetrahydrofolate reductase |
| TYMP | thymidine phosphorylase |
| HIF1α | hypoxia-inducible factor-1α |
| LAA | lauric acid |
| MCFA | medium-chain fatty acid |
| ALP | alkaline phosphatase |
| MUC2 | mucin 2 |
| MMP | mitochondrial membrane potential |
| 4HNE | 4-hydroxynonenal |
| LGR5 | leucine-rich repeat-containing G-protein coupled receptor 5 |
| NS | Nucleostemin |
| KLF4 | Krüppel-like factor 4 |
| PRODH | proline dehydrogenase |
| Dnmt3b | DNA methyltransferase-3b |
| ME1 | cytosolic NADPH dehydrogenase 1 (malic enzyme 1) |
| PSC | pluripotent stem cell |
References
- Li, J.; Yang, B.; Teng, Z.; Liu, Y.; Li, D.; Qu, X. Efficacy and safety of first-line treatments for advanced hepatocellular carcinoma patients: A systematic review and network meta-analysis. Front. Immunol. 2024, 15, 1430196. [Google Scholar] [CrossRef]
- Orillard, E.; Adhikari, A.; Malouf, R.S.; Calais, F.; Marchal, C.; Westeel, V. Immune checkpoint inhibitors plus platinum-based chemotherapy compared to platinum-based chemotherapy with or without bevacizumab for first-line treatment of older people with advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2024, 8, CD015495. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yashiro, M. Molecular Mechanism for Malignant Progression of Gastric Cancer Within the Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 11735. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Rashed, M.; Winum, J.Y. Novel Anticancer Drug Discovery Strategies Targeting Hypoxia-Inducible factors. Expert Opin. Drug Discov. 2024, 20, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Rastegar-Pouyani, N.; Abdolvahab, M.H.; Farzin, M.A.; Zare, H.; Kesharwani, P.; Sahebkar, A. Targeting cancer-associated fibroblasts with pirfenidone: A novel approach for cancer therapy. Tissue Cell 2024, 91, 102624. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The Microenvironment of Lung Cancer and Therapeutic Implications. Adv. Exp. Med. Biol. 2016, 890, 75–110. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Kalscheuer, S.M.; Panyam, J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Parveen, N.; Rehman, U.; Aziz, A.; Sheikh, A.; Abourehab, M.A.S.; Guo, W.; Huang, J.; Wang, Z.; Kesharwani, P. Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer. Mol. Cancer 2023, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Niharika; Garg, M. Understanding the autophagic functions in cancer stem cell maintenance and therapy resistance. Expert Rev. Mol. Med. 2024, 26, e23. [Google Scholar] [CrossRef] [PubMed]
- Jamroze, A.; Liu, X.; Tang, D.G. Treatment-induced stemness and lineage plasticity in driving prostate cancer therapy resistance. Cancer Heterog. Plast. 2024, 1, 0005. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Shang, C.; Hong, Y. The Role and Applied Value of Mitochondria in Glioma-Related Research. CNS Neurosci. Ther. 2024, 30, e70121. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Fang, W.; Dong, Y.; Zhang, H.; Luo, Q. Tumor energy metabolism: Implications for therapeutic targets. Mol. Biomed. 2024, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Chen, L.; Wang, X.; Liu, J.; Huang, Y.; Qi, H.; Wang, T.; Li, Q. Targeting extracellular matrix interaction in gastrointestinal cancer: Immune modulation, metabolic reprogramming, and therapeutic strategies. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189225. [Google Scholar] [CrossRef]
- Monzer, A.; Ghamlouche, F.; Wakimian, K.; Ballout, F.; Al Bitar, S.; Yehya, A.; Kanso, M.; Saheb, N.; Tawil, A.; Doughan, S.; et al. ONC206, an imipridone derivative, demonstrates anti-colorectal cancer activity against stem/progenitor cells in 3D cell cultures and in patient-derived organoids. Pharmacol. Rep. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Zhao, Y. Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer (Review). Exp. Ther. Med. 2015, 9, 289–293. [Google Scholar] [CrossRef]
- Saha, T.; Lukong, K.E. Breast Cancer Stem-Like Cells in Drug Resistance: A Review of Mechanisms and Novel Therapeutic Strategies to Overcome Drug Resistance. Front. Oncol. 2022, 12, 856974. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Cordani, M.; Michetti, F.; Zarrabi, A.; Zarepour, A.; Rumio, C.; Strippoli, R.; Marcucci, F. The role of glycolysis in tumorigenesis: From biological aspects to therapeutic opportunities. Neoplasia 2024, 58, 101076. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef]
- Elhinnawi, M.A.; Boushra, M.I.; Hussien, D.M.; Hussein, F.H.; Abdelmawgood, I.A. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma. Stem Cell Rev. Rep. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Uslu, C.; Kapan, E.; Lyakhovich, A. Cancer resistance and metastasis are maintained through oxidative phosphorylation. Cancer Lett. 2024, 587, 216705. [Google Scholar] [CrossRef] [PubMed]
- Friess, D.; Brauer, S.; Pöysti, A.; Choudhury, C.; Harris, L. Tools to study neural and glioma stem cell quiescence. Trends Neurosci. 2024, 47, 736–748. [Google Scholar] [CrossRef]
- Du, Y.; Gupta, P.; Qin, S.; Sieber, M. The role of metabolism in cellular quiescence. J. Cell. Sci. 2023, 136, 325880. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Okechukwu, C.C. Review of 5-FU resistance mechanisms in colorectal cancer: Clinical significance of attenuated on-target effects. Cancer Drug Resist. 2023, 6, 257–272. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Singh, H.; Thareja, S.; Kumar, P. Regulation of thymidylate synthase: An approach to overcome 5-FU resistance in colorectal cancer. Med. Oncol. 2022, 40, 3. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Luo, D.D.; Wan, S.B.; Qu, X.J. S1PR2 inhibitors potently reverse 5-FU resistance by downregulating DPD expression in colorectal cancer. Pharmacol. Res. 2020, 155, 104717. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Mo, J.L.; Liu, J.H.; Li, X.; Tan, L.M.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Pharmacogenomics of 5-fluorouracil in colorectal cancer: Review and update. Cell. Oncol. 2020, 43, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Li, G.; Hu, F.; Hou, Z.; Chen, Q.; Luo, X.; Hu, J.; Feng, Y. PIK3R3 promotes chemotherapeutic sensitivity of colorectal cancer through PIK3R3/NF-kB/TP pathway. Cancer Biol. Ther. 2018, 19, 222–229. [Google Scholar] [CrossRef]
- Baba, H.; Watanabe, M.; Okabe, H.; Miyamoto, Y.; Sakamoto, Y.; Baba, Y.; Iwatsuki, M.; Chikamoto, A.; Beppu, T. Upregulation of ERCC1 and DPD expressions after oxaliplatin-based first-line chemotherapy for metastatic colorectal cancer. Br. J. Cancer 2012, 107, 1950–1955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sreekumar, R.; Al-Saihati, H.; Emaduddin, M.; Moutasim, K.; Mellone, M.; Patel, A.; Kilic, S.; Cetin, M.; Erdemir, S.; Navio, M.S.; et al. The ZEB2-dependent EMT transcriptional programme drives therapy resistance by activating nucleotide excision repair genes ERCC1 and ERCC4 in colorectal cancer. Mol. Oncol. 2021, 15, 2065–2083. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara-Tani, R.; Sasaki, T.; Bhawal, U.K.; Mori, S.; Ogata, R.; Sasaki, R.; Ikemoto, A.; Kishi, S.; Fujii, K.; Ohmori, H.; et al. Nuclear MAST4 Suppresses FOXO3 through Interaction with AKT3 and Induces Chemoresistance in Pancreatic Ductal Carcinoma. Int. J. Mol. Sci. 2024, 25, 4056. [Google Scholar] [CrossRef]
- Takagi, T.; Fujiwara-Tani, R.; Mori, S.; Kishi, S.; Nishiguchi, Y.; Sasaki, T.; Ogata, R.; Ikemoto, A.; Sasaki, R.; Ohmori, H.; et al. Lauric Acid Overcomes Hypoxia-Induced Gemcitabine Chemoresistance in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7506. [Google Scholar] [CrossRef] [PubMed]
- Scheig, R. Absoption of dietary fat: Use of medium-chain triglycerides in malabsorption. Am. J. Clin. Nutr. 1968, 21, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeldt, L.; Wahren, J.; Pernow, B.; Räf, L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J. Clin. Investig. 1972, 51, 2324–2330. [Google Scholar] [CrossRef]
- Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sci 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 2013, 59, 214–224. [Google Scholar] [CrossRef]
- Metges, C.C.; Wolfram, G. Medium- and long-chain triglycerides labeled with 13C: A comparison of oxidation after oral or parenteral administration in humans. J. Nutr. 1991, 121, 31–36. [Google Scholar] [CrossRef]
- Kadochi, Y.; Mori, S.; Fujiwara-Tani, R.; Luo, Y.; Nishiguchi, Y.; Kishi, S.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Remodeling of energy metabolism by a ketone body and medium-chain fatty acid suppressed the proliferation of CT26 mouse colon cancer cells. Oncol. Lett. 2017, 14, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ogata, R.; Mori, S.; Ohmori, H.; Kishi, S.; Fujiwara-Tani, R.; Sasaki, T.; Nishiguchi, Y.; Nakashima, C.; Goto, K.; Kawahara, I.; et al. Suppressive GLI2 fragment enhances liver metastasis in colorectal cancer. Oncotarget 2022, 13, 122–135. [Google Scholar] [CrossRef]
- Kita, M.; Fujiwara-Tani, R.; Kishi, S.; Mori, S.; Ohmori, H.; Nakashima, C.; Goto, K.; Sasaki, T.; Fujii, K.; Kawahara, I.; et al. Role of creatine shuttle in colorectal cancer cells. Oncotarget 2023, 14, 485–501. [Google Scholar] [CrossRef]
- Du, P.; Wu, J. Hallmarks of totipotent and pluripotent stem cell states. Cell Stem Cell 2024, 31, 312–333. [Google Scholar] [CrossRef]
- Varzideh, F.; Gambardella, J.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Molecular Mechanisms Underlying Pluripotency and Self-Renewal of Embryonic Stem Cells. Int. J. Mol. Sci. 2023, 24, 8386. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, Y.; Zhang, R.R.; Kimura, M.; Cai, Y.; Adam, M.; Parameswaran, S.; Masaki, H.; Mizuno, N.; Bhadury, J.; Maezawa, S.; et al. Inter-cellular mRNA Transfer Alters Human Pluripotent Stem Cell State. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Q.; Chen, W.; Gong, Z.; Kang, B.; Sui, M.; Huang, L.; Wang, Y.J. PRODH safeguards human naive pluripotency by limiting mitochondrial oxidative phosphorylation and reactive oxygen species production. EMBO Rep. 2024, 25, 2015–2044. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jia, M.; Zhao, G.; Liu, Y.; Song, Y.; Sun, M.; Chi, W.; Wang, X.; Jiang, Q.; Xin, G.; et al. STAG2 promotes naive-primed transition via activating Lin28a transcription in mouse embryonic stem cells. J. Biol. Chem. 2024, 300, 107958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yu, X.; Shi, J.; Ma, S.; Li, S.; Shi, H.; Xia, S.; Ye, Y.; Zhang, Y.; Du, Y.; et al. A stepwise mode of TGFβ-SMAD signaling and DNA methylation regulates naïve-to-primed pluripotency and differentiation. Nat. Commun. 2024, 15, 10123. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Yamamoto, K.; Fujiwara-Tani, R.; Luo, Y.; Matsushima, S.; Fujii, K.; Ohmori, H.; Sasahira, T.; Sasaki, T.; Kitadai, Y.; et al. Expression of cytosolic malic enzyme (ME1) is associated with disease progression in human oral squamous cell carcinoma. Cancer Sci. 2018, 109, 2036–2045. [Google Scholar] [CrossRef]
- Nakashima, C.; Kirita, T.; Yamamoto, K.; Mori, S.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kawahara, I.; Mori, T.; et al. Malic Enzyme 1 Is Associated with Tumor Budding in Oral Squamous Cell Carcinomas. Int. J. Mol. Sci. 2020, 21, 7149. [Google Scholar] [CrossRef]
- Ishihara, N.; Nomura, M.; Jofuku, A.; Kato, H.; Suzuki, S.O.; Masuda, K.; Otera, H.; Nakanishi, Y.; Nonaka, I.; Goto, Y.; et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, J.; Zhang, Z.; Wakabayashi, N.; Tamura, Y.; Fukaya, M.; Kensler, T.W.; Iijima, M.; Sesaki, H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009, 186, 805–816. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.; Lewis, N.E.; Jamshidi, N.; Mineta, K.; Gao, X.; Gojobori, T. The Emerging Facets of Non-Cancerous Warburg Effect. Front. Endocrinol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Folmes, C.D.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Kawano, I.; Bazila, B.; Ježek, P.; Dlasková, A. Mitochondrial Dynamics and Cristae Shape Changes During Metabolic Reprogramming. Antioxid. Redox Signal 2023, 39, 684–707. [Google Scholar] [CrossRef]
- Kondadi, A.K.; Anand, R.; Reichert, A.S. Cristae Membrane Dynamics—A Paradigm Change. Trends Cell Biol. 2020, 30, 923–936. [Google Scholar] [CrossRef]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Perez-Terzic, C.; Behfar, A.; Terzic, A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4 (Suppl. S1), S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Varum, S.; Rodrigues, A.S.; Moura, M.B.; Momcilovic, O.; Easley, C.A.t.; Ramalho-Santos, J.; Van Houten, B.; Schatten, G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 2011, 6, e20914. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, D.G.; Guo, F.F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zuo, M.; Zeng, L.; Cui, K.; Liu, B.; Yan, C.; Chen, L.; Dong, J.; Shangguan, F.; Hu, W.; et al. OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 2021, 22, e50827. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Mauro, C.; Leow, S.C.; Anso, E.; Rocha, S.; Thotakura, A.K.; Tornatore, L.; Moretti, M.; De Smaele, E.; Beg, A.A.; Tergaonkar, V.; et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011, 13, 1272–1279. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Rouleau, M.; Gounon, P.; Brahimi-Horn, M.C.; Pouysségur, J.; Mazure, N.M. Hypoxic enlarged mitochondria protect cancer cells from apoptotic stimuli. J. Cell. Physiol. 2010, 222, 648–657. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Engstová, H.; Alán, L.; Špaček, T.; Dlasková, A.; Smolková, K.; Špačková, J.; Tauber, J.; Strádalová, V.; Malínský, J.; et al. Hypoxic HepG2 cell adaptation decreases ATP synthase dimers and ATP production in inflated cristae by mitofilin down-regulation concomitant to MICOS clustering. FASEB J. 2016, 30, 1941–1957. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef]
- Kung-Chun Chiu, D.; Pui-Wah Tse, A.; Law, C.T.; Ming-Jing Xu, I.; Lee, D.; Chen, M.; Kit-Ho Lai, R.; Wai-Hin Yuen, V.; Wing-Sum Cheu, J.; Wai-Hung Ho, D.; et al. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019, 10, 934. [Google Scholar] [CrossRef]
- Gdynia, G.; Sauer, S.W.; Kopitz, J.; Fuchs, D.; Duglova, K.; Ruppert, T.; Miller, M.; Pahl, J.; Cerwenka, A.; Enders, M.; et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat. Commun. 2016, 7, 10764. [Google Scholar] [CrossRef]
- Wong, C.C.; Xu, J.; Bian, X.; Wu, J.L.; Kang, W.; Qian, Y.; Li, W.; Chen, H.; Gou, H.; Liu, D.; et al. In Colorectal Cancer Cells with Mutant KRAS, SLC25A22-Mediated Glutaminolysis Reduces DNA Demethylation to Increase WNT Signaling, Stemness, and Drug Resistance. Gastroenterology 2020, 159, 2163–2180.e2166. [Google Scholar] [CrossRef]
- Deshmukh, A.; Deshpande, K.; Arfuso, F.; Newsholme, P.; Dharmarajan, A. Cancer stem cell metabolism: A potential target for cancer therapy. Mol. Cancer 2016, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.W.; Chuang, Y.C.; Lee, H.L.; Kuo, C.C.; Shen, Y.A. Therapeutic Targeting of Glutaminolysis as a Novel Strategy to Combat Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 15296. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ohmori, H.; Luo, Y.; Mori, S.; Miyagawa, Y.; Nukaga, S.; Goto, K.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; et al. Giving combined medium-chain fatty acids and glucose protects against cancer-associated skeletal muscle atrophy. Cancer Sci. 2019, 110, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Nukaga, S.; Mori, T.; Miyagawa, Y.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Nakashima, C.; et al. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci. 2020, 111, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.T.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Smith, A.L.M.; Whitehall, J.C.; Greaves, L.C. Mitochondrial DNA mutations in ageing and cancer. Mol. Oncol. 2022, 16, 3276–3294. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Kukwa, W.; Krawczyk, T.; Scinska, A.; Kukwa, A.; Cappello, F. Mitochondrial DNA mutations in cancer--from bench to bedside. Front. Biosci. 2010, 15, 437–460. [Google Scholar] [CrossRef][Green Version]
- Kopinski, P.K.; Singh, L.N.; Zhang, S.; Lott, M.T.; Wallace, D.C. Mitochondrial DNA variation and cancer. Nat. Rev. Cancer 2021, 21, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mahmood, M.; Reznik, E.; Gammage, P.A. Mitochondrial DNA is a major source of driver mutations in cancer. Trends Cancer 2022, 8, 1046–1059. [Google Scholar] [CrossRef]
- Artusi, M.; Nicoli, S.; Colombo, P.; Bettini, R.; Sacchi, A.; Santi, P. Effect of chemical enhancers and iontophoresis on thiocolchicoside permeation across rabbit and human skin in vitro. J. Pharm. Sci. 2004, 93, 2431–2438. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Qaed, E.; Zhang, J.; Song, Y.; Wu, R.; Bu, X.; Wang, Q.; Tang, Z. Salinomycin, as an autophagy modulator—A new avenue to anticancer: A review. J. Exp. Clin. Cancer Res. 2018, 37, 26. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Li, J.; Luo, R.; Wang, R.; Yin, L.; Liu, M.; Zeng, Y.; Zeng, Z.; Xie, T. Recent Advances in Understanding the Mechanisms of Elemene in Reversing Drug Resistance in Tumor Cells: A Review. Molecules 2021, 26, 5792. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Xu, W.W.; Huan, X.K.; Wu, G.N.; Li, G.; Zhou, Y.H.; Najafi, M. Mechanisms of cancer cell killing by metformin: A review on different cell death pathways. Mol. Cell. Biochem. 2023, 478, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Castiglione, S.; Pavanello, C. Metformin: From diabetes to cancer to prolongation of life. Pharmacol. Res. 2024, 208, 107367. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Fujiwara-Tani, R.; Gyoten, M.; Nukaga, S.; Sasaki, R.; Ikemoto, A.; Ogata, R.; Kishi, S.; Fujii, K.; Kuniyasu, H. Berberine Induces Combined Cell Death in Gastrointestinal Cell Lines. Int. J. Mol. Sci. 2023, 24, 6588. [Google Scholar] [CrossRef]
- Hojo, Y.; Kishi, S.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Nishiguchi, Y.; Nakashima, C.; Luo, Y.; Shinohara, H.; et al. Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8. Int. J. Mol. Sci. 2022, 23, 4002. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kishi, S.; Honoki, K.; Fujiwara-Tani, R.; Moriguchi, T.; Sasaki, T.; Fujii, K.; Tsukamoto, S.; Fujii, H.; Kido, A.; et al. Anti-Stem Cell Property of Pterostilbene in Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9347. [Google Scholar] [CrossRef] [PubMed]
- Nishiguch, Y.; Fujiwara-Tani, R.; Nukaga, S.; Nishida, R.; Ikemoto, A.; Sasaki, R.; Mori, S.; Ogata, R.; Kishi, S.; Hojo, Y.; et al. Pterostilbene Induces Apoptosis from Endoplasmic Reticulum Stress Synergistically with Anticancer Drugs That Deposit Iron in Mitochondria. Int. J. Mol. Sci. 2024, 25, 2611. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Oue, N.; Wakikawa, A.; Shigeishi, H.; Matsutani, N.; Kuraoka, K.; Ito, R.; Yokozaki, H.; Yasui, W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2002, 196, 163–170. [Google Scholar] [CrossRef] [PubMed]
| RT-PCR Primers | ||||
|---|---|---|---|---|
| Gene | Species | ID | Left | Right |
| Actb | Mouse | NM_007393.5 | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
| ACTB | Human | NM_001101.3 | GGACTTCGAGCAAGAGATGG | AGCACTGTGTTGGCGTACAG |
| Lgr5 | Mouse | NM_010195.2 | CATTCACTTTTGGCCGTTTT | AGGGCCAACAGGACACATAG |
| LGR5 | Human | AF061444.1 | CTCTTCCTCAAACCGTCTGC | GATCGGAGGCTAAGCAACTG |
| Klf4 | Mouse | NM_010637.3 | CTGAACAGCAGGGACTGTCA | GTGTGGGTGGCTGTTCTTTT |
| KLF4 | Human | KJ901962.1 | CCCACACAGGTGAGAAACCT | CCCACACAGGTGAGAAACCT |
| Ns | Mouse | BC037996.1 | ATGTGGGGAAAAGCAGTGTC | TGGGGGAGTTACAAGGTGAG |
| NS | Human | BC001024.2 | ATTGCCAACAGTGGTGTTCA | AATGGCTTTGCTGCAAGTTT |
| Lin28a | Mouse | NM_145833.1 | GTGTCCAACCAGCAGTTTGC | CTCTTCCTCTTCCTCCCGGA |
| LIN28A | Human | NM_024674.6 | CGTGTCCAACCAGCAGTTTG | TGGCTTTCCCTGTGCACTAG |
| Prodh | Mouse | AF120279.1 | AAGCAGTATCAGGTGCACCC | CCTCCTCAGTGAACCGTGAC |
| PRODH | Human | AF120278.1 | GGTAGAGTCAGCGATGACGG | TGTGTTGAAGATGAGCGGCT |
| Dnmt3b | Mouse | BC105677.1 | TGTGGGGAAAGATCAAGGGC | CGTTCTCGGCTCTCCTCATC |
| DNMT3B | Human | AF331857.1 | TTCTCCGAGGTCTCTGCAGA | CTGCCACAAGACAAACAGCC |
| Hif1a | Mouse | AF003695.1 | TGCTTGCCAAAAGAGGTGGA | CAGAAGGACTTGCTGGCTGA |
| HIF1A | Human | AF208487.1 | GAAAGCGCAAGTCCTCAAAG | TGGGTAGGAGATGGAGATGC |
| Me1 | Mouse | NM_008615.2 | GGAGTTGCTGCAATTGGTGG | TGCAGGCCACGGATAACAAT |
| ME1 | Human | NM_002395.5 | GGATTGCACACCTGATTGTG | TCTTCATGTTCATGGGCAAA |
| Ts | Mouse | NM_021288.4 | TTCAAGAAGGAGGACCGCAC | CACGCCCAGACCCATATCTC |
| TS | Human | NM_001071.4 | CTGGGGCAGATCCAACACAT | CTGGCGATGTTGAAAGGCAC |
| Dpd | Mouse | NM_170778.3 | GTATGGCCCTGGACAAAGCT | GCAGTTCCTGACACTCCTCC |
| DPD | Human | NM_000110.4 | GTATGGCCCTGGACAAAGCT | GCAATGGAGGTCACAGCTCT |
| Mthfr | Mouse | NM_001161798.1 | CAGCTGGGCACTGTTATCCA | GCTTCCCAGTGGTCACCTAC |
| MTHFR | Human | NM_001330358.2 | TCTACCGTACCCAGGAGTGG | GTGGGCTGGATGATCTCTCG |
| Tymp | Mouse | NM_138302.3 | CTGGAGGTGGAAGAAGCGTT | GGGAGGACAAGTTCAGCGAA |
| TYMP | Human | BC018160.1 | CAAGGTGCCAATGATCAGCG | CAGGTCCCTTAAGTCTGGCG |
| ELISA | ||||
| Target | Species | Cat# | Company | |
| Alp | Mouse | ab285274 | Abcam, Waltham, MA, USA | |
| ALP | Human | ab285149 | Abcam, Waltham, MA, USA | |
| Muc2 | Mouse | M0EB0548 | AssayGenie, Dublin, Ireland | |
| MUC2 | Human | ab282871 | Abcam, Waltham, MA, USA | |
| 4HNE | - | ab287803 | Abcam, Waltham, MA, USA | |
| Lactate | - | ab65331 | Abcam, Waltham, MA, USA | |
| ATP | - | ab83355 | Abcam, Waltham, MA, USA | |
| Hif1α | Mouse | #88-8022-88 | Thermo Fisher, Tokyo, Japan | |
| HIF1α | Human | #A43658 | Thermo Fisher, Tokyo, Japan | |
| Me1 | Mouse | E1556Mo | Bioassay Technology Laboratory, Shanghai, China | |
| ME1 | Human | ABIN6231269 | Antibodies-online.com, Limerick, PA, USA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara-Tani, R.; Luo, Y.; Ogata, R.; Fujii, K.; Sasaki, T.; Sasaki, R.; Nishiguchi, Y.; Mori, S.; Ohmori, H.; Kuniyasu, H. Energy Metabolism and Stemness and the Role of Lauric Acid in Reversing 5-Fluorouracil Resistance in Colorectal Cancer Cells. Int. J. Mol. Sci. 2025, 26, 664. https://doi.org/10.3390/ijms26020664
Fujiwara-Tani R, Luo Y, Ogata R, Fujii K, Sasaki T, Sasaki R, Nishiguchi Y, Mori S, Ohmori H, Kuniyasu H. Energy Metabolism and Stemness and the Role of Lauric Acid in Reversing 5-Fluorouracil Resistance in Colorectal Cancer Cells. International Journal of Molecular Sciences. 2025; 26(2):664. https://doi.org/10.3390/ijms26020664
Chicago/Turabian StyleFujiwara-Tani, Rina, Yi Luo, Ruiko Ogata, Kiyomu Fujii, Takamitsu Sasaki, Rika Sasaki, Yukiko Nishiguchi, Shiori Mori, Hitoshi Ohmori, and Hiroki Kuniyasu. 2025. "Energy Metabolism and Stemness and the Role of Lauric Acid in Reversing 5-Fluorouracil Resistance in Colorectal Cancer Cells" International Journal of Molecular Sciences 26, no. 2: 664. https://doi.org/10.3390/ijms26020664
APA StyleFujiwara-Tani, R., Luo, Y., Ogata, R., Fujii, K., Sasaki, T., Sasaki, R., Nishiguchi, Y., Mori, S., Ohmori, H., & Kuniyasu, H. (2025). Energy Metabolism and Stemness and the Role of Lauric Acid in Reversing 5-Fluorouracil Resistance in Colorectal Cancer Cells. International Journal of Molecular Sciences, 26(2), 664. https://doi.org/10.3390/ijms26020664






