A Gastroenterologist’s Guide to Care Transitions in Cystic Fibrosis from Pediatrics to Adult Care
Abstract
1. Introduction
1.1. Esophagus and Stomach
1.1.1. GERD and Foregut Dysmotility
Pediatrics
Adults
Transition Take-Home Points
 Reassess the indication for PPI and consider discontinuation trial.
Reassess the indication for PPI and consider discontinuation trial. Screen for alarm features or poor response to therapy and consider upper endoscopy and surgical referral when clinically appropriate due to a higher risk of Barrett’s esophagus and esophageal cancer.
Screen for alarm features or poor response to therapy and consider upper endoscopy and surgical referral when clinically appropriate due to a higher risk of Barrett’s esophagus and esophageal cancer.1.2. Intestine
1.2.1. Small Intestinal Bacterial Overgrowth
Pediatrics
Adults
1.2.2. Intestinal Obstruction Syndromes: Meconium Ileus, DIOS, Constipation
Pediatrics
Adults
Transition Take-Home Points
 Identify patients with nutritional deficiencies with symptoms of SIBO and consider for diagnostic evaluation or empiric therapy.
Identify patients with nutritional deficiencies with symptoms of SIBO and consider for diagnostic evaluation or empiric therapy. Identify patients with a history of meconium ileus requiring surgical intervention during childhood or PI who are at higher risk of DIOS and constipation.
Identify patients with a history of meconium ileus requiring surgical intervention during childhood or PI who are at higher risk of DIOS and constipation. Obtain a thorough history of previously tolerated and unsuccessful treatments for constipation to guide future care which can include optimizing PERT dosing, adjusting bowel regimens, and consideration of combination therapies.
Obtain a thorough history of previously tolerated and unsuccessful treatments for constipation to guide future care which can include optimizing PERT dosing, adjusting bowel regimens, and consideration of combination therapies.2. Pancreas
2.1. Pancreatic Insufficiency
2.1.1. Pediatrics
2.1.2. Adults
2.2. Pancreatitis
2.2.1. Pediatrics
2.2.2. Adults
2.2.3. Transition Take-Home Points
 Identify PwCF and pancreatic sufficiency by checking fecal elastase.
Identify PwCF and pancreatic sufficiency by checking fecal elastase. Acute pancreatitis should be considered as a potential cause of abdominal pain in individuals with pancreatic sufficiency.
Acute pancreatitis should be considered as a potential cause of abdominal pain in individuals with pancreatic sufficiency. Screen for CFRD at time of the transition with oral glucose tolerance test, as the risk increases with age due to progressive pancreatic islet cell destruction.
Screen for CFRD at time of the transition with oral glucose tolerance test, as the risk increases with age due to progressive pancreatic islet cell destruction.3. Hepatobiliary
3.1. Pediatrics
3.2. Adults
3.3. Transition Take-Home Points
 Identify PwCF with previous liver disease or injury and obtain an initial assessment of their liver function and any signs of chronic liver disease.
Identify PwCF with previous liver disease or injury and obtain an initial assessment of their liver function and any signs of chronic liver disease. Screen for and counsel regarding modifiable risk factors for hepatotoxicity such as alcohol use or recreational drug use.
Screen for and counsel regarding modifiable risk factors for hepatotoxicity such as alcohol use or recreational drug use.4. Nutritional Failure
4.1. Pediatrics
4.2. Adults
4.3. Transition Take-Home Points
 Obtain a complete review of previous dietary habits and supplements.
Obtain a complete review of previous dietary habits and supplements. Evaluate for signs of malnutrition, obesity/overweight and disordered eating during the initial transition visit.
Evaluate for signs of malnutrition, obesity/overweight and disordered eating during the initial transition visit.5. GI Cancers
Transition Take-Home Points
 Carefully investigate for any alarm or unexplained GI symptoms during the transition of care. Individualized GI cancer screening strategies depending on the burden of other comorbidities, transplant status, and severity of pulmonary manifestations should be discussed during care transitions.
Carefully investigate for any alarm or unexplained GI symptoms during the transition of care. Individualized GI cancer screening strategies depending on the burden of other comorbidities, transplant status, and severity of pulmonary manifestations should be discussed during care transitions.6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| PwCF | People with Cystic Fibrosis |
| GI | Gastrointestinal |
| CFF | Cystic Fibrosis Foundation |
| LLC | Learning and Leadership Collaboratives |
| DIGEST | Developing Innovative Gastroenterology Specialty Training Program |
| GERD | Gastrointestinal Reflux Disease |
| LES | Lower Esophageal Sphincter |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| PI | Pancreatic Insufficiency |
| PERT | Pancreatic Enzyme Replacement Therapy |
| CwCF | Children with Cystic Fibrosis |
| AwCF | Adults with Cystic Fibrosis |
| MII | Multichannel Intraluminal Impedance |
| PPI | Proton Pump Inhibitor |
| EGD | Esophagogastroduodenoscopy |
| H2-RA | Histamine receptor 2 antagonists |
| SIBO | Small Intestinal Bacterial Overgrowth |
| DIOS | Distal Intestinal Obstruction Syndrome |
| MI | Meconium Ileus |
| PEG | Polyethylene Glycol |
| CFRD | Cystic Fibrosis Related Diabetes |
| PS | Pancreatic Sufficiency |
| CFLD | Cystic Fibrosis Related Liver Disease |
| UDC | Ursodeoxycholic acid |
| CRC | Colorectal Carcinoma |
| RDA | Recommended Daily Allowance |
References
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- McBennett, K.A.; Davis, P.B.; Konstan, M.W. Increasing life expectancy in cystic fibrosis: Advances and challenges. Pediatr. Pulmonol. 2022, 57 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, L.K.; Schwartz, L.A.; Sawicki, G.S.; Britto, M.T. Cystic fibrosis and transition to adult medical care. Pediatrics 2010, 125, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Goralski, J.L.; Nasr, S.Z.; Uluer, A. Overcoming barriers to a successful transition from pediatric to adult care. Pediatr. Pulmonol. 2017, 52, S52–S60. [Google Scholar] [CrossRef]
- Transition, G. Got Transition. The Six Core Elements of Health Care Transition. Available online: http://www.gottransition.org/providers/index.cfm (accessed on 15 September 2022).
- Baker, A.M.; Riekert, K.A.; Sawicki, G.S.; Eakin, M.N. CF RISE: Implementing a Clinic-Based Transition Program. Pediatr. Allergy Immunol. Pulmonol. 2015, 28, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Van Biervliet, S.; de Clercq, C.; Declercq, D.; Van Braeckel, E.; Van Daele, S.; De Baets, F.; De Looze, D. Gastro-intestinal manifestations in cystic fibrosis patients. Acta Gastroenterol. Belg. 2016, 79, 481–486. [Google Scholar] [PubMed]
- Gustafsson, P.M.; Fransson, S.G.; Kjellman, N.I.; Tibbling, L. Gastro-oesophageal reflux and severity of pulmonary disease in cystic fibrosis. Scand. J. Gastroenterol. 1991, 26, 449–456. [Google Scholar] [CrossRef]
- Palm, K.; Sawicki, G.; Rosen, R. The impact of reflux burden on Pseudomonas positivity in children with Cystic Fibrosis. Pediatric. Pulmonol. 2012, 47, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, A.; Blondeau, K.; Dupont, L.J.; Sifrim, D. Mechanisms of increased gastroesophageal reflux in patients with cystic fibrosis. Am. J. Gastroenterol. 2012, 107, 1346–1353. [Google Scholar] [CrossRef]
- Stringer, D.A.; Sprigg, A.; Juodis, E.; Corey, M.; Daneman, A.; Levison, H.J.; Durie, P.R. The association of cystic fibrosis, gastroesophageal reflux, and reduced pulmonary function. Can. Assoc. Radiol. J. 1988, 39, 100–102. [Google Scholar] [PubMed]
- Ng, C.; Prayle, A.P. Gastrointestinal complications of cystic fibrosis. Paediatr. Child Health 2020, 30, 345–349. [Google Scholar] [CrossRef]
- Assis, D.N.; Freedman, S.D. Gastrointestinal Disorders in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Pauwels, A. Cystic Fibrosis and gastroesophageal reflux disease. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Lewindon, P.J.; Robb, T.A.; Moore, D.J.; Davidson, G.P.; Martin, A.J. Bowel dysfunction in cystic fibrosis: Importance of breath testing. J. Paediatr. Child Health 1998, 34, 79–82. [Google Scholar] [CrossRef]
- Mousa, H.M.; Woodley, F.W. Gastroesophageal reflux in cystic fibrosis: Current understandings of mechanisms and management. Curr. Gastroenterol. Rep. 2012, 14, 226–235. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Manti, S.; Parisi, G.F.; Papale, M.; Mulè, E.; Rotolo, N.; Leonardi, S. Focus on gastroesophageal reflux disease in patients with cystic fibrosis. World J. Gastroenterol. 2020, 26, 6322–6334. [Google Scholar] [CrossRef]
- Henen, S.; Denton, C.; Teckman, J.; Borowitz, D.; Patel, D. Review of Gastrointestinal Motility in Cystic Fibrosis. J. Cyst. Fibros. 2021, 20, 578–585. [Google Scholar] [CrossRef]
- Gelfond, D.; Ma, C.; Semler, J.; Borowitz, D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig. Dis. Sci. 2013, 58, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, A.; Decraene, A.; Blondeau, K.; Mertens, V.; Farre, R.; Proesmans, M.; Van Bleyenbergh, P.; Sifrim, D.; Dupont, L.J. Bile acids in sputum and increased airway inflammation in patients with cystic fibrosis. Chest 2012, 141, 1568–1574. [Google Scholar] [CrossRef]
- Hedsund, C.; Gregersen, T.; Joensson, I.M.; Olesen, H.V.; Krogh, K. Gastrointestinal transit times and motility in patients with cystic fibrosis. Scand. J. Gastroenterol. 2012, 47, 920–926. [Google Scholar] [CrossRef]
- Corral, J.E.; Dye, C.W.; Mascarenhas, M.R.; Barkin, J.S.; Salathe, M.; Moshiree, B. Is Gastroparesis Found More Frequently in Patients with Cystic Fibrosis? A Systematic Review. Scientifica 2016, 2016, 2918139. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Rudolph, C.D.; Di Lorenzo, C.; Hassall, E.; Liptak, G.; Mazur, L.; Sondheimer, J.; Staiano, A.; Thomson, M.; Veereman-Wauters, G.; et al. Pediatric gastroesophageal reflux clinical practice guidelines: Joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2009, 49, 498–547. [Google Scholar] [CrossRef] [PubMed]
- Mertens, V.; Blondeau, K.; Pauwels, A.; Farre, R.; Vanaudenaerde, B.; Vos, R.; Verleden, G.; Van Raemdonck, D.E.; Dupont, L.J.; Sifrim, D. Azithromycin reduces gastroesophageal reflux and aspiration in lung transplant recipients. Dig. Dis. Sci. 2009, 54, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Boesch, R.P.; Acton, J.D. Outcomes of fundoplication in children with cystic fibrosis. J. Pediatr. Surg. 2007, 42, 1341–1344. [Google Scholar] [CrossRef]
- Sheikh, S.I.; Ryan-Wenger, N.A.; McCoy, K.S. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr. Pulmonol. 2013, 48, 556–562. [Google Scholar] [CrossRef]
- Blondeau, K.; Dupont, L.J.; Mertens, V.; Verleden, G.; Malfroot, A.; Vandenplas, Y.; Hauser, B.; Sifrim, D. Gastro-oesophageal reflux and aspiration of gastric contents in adult patients with cystic fibrosis. Gut 2008, 57, 1049–1055. [Google Scholar] [CrossRef]
- Lusman, S.S.; Grand, R. Approach to chronic abdominal pain in Cystic Fibrosis. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S24–S31. [Google Scholar] [CrossRef]
- Knotts, R.M.; Solfisburg, Q.S.; Keating, C.; DiMango, E.; Lightdale, C.J.; Abrams, J.A. Cystic fibrosis is associated with an increased risk of Barrett’s esophagus. J. Cyst. Fibros. 2019, 18, 425–429. [Google Scholar] [CrossRef]
- Fridge, J.L.; Conrad, C.; Gerson, L.; Castillo, R.O.; Cox, K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 212–218. [Google Scholar] [CrossRef]
- Lisowska, A.; Wójtowicz, J.; Walkowiak, J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: Combined hydrogen and methane measurements are required for its detection. Acta Biochim. Pol. 2009, 56, 631–634. [Google Scholar] [CrossRef]
- Dorsey, J.; Gonska, T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the Cystic Fibrosis intestine. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.A.; Xie, Y.Y.; Wine, E.; Huynh, H.Q. Diagnosis and pharmacological management of small intestinal bacterial overgrowth in children with intestinal failure. Can. J. Gastroenterol. 2011, 25, 41–45. [Google Scholar] [CrossRef]
- Haller, W.; Ledder, O.; Lewindon, P.J.; Couper, R.; Gaskin, K.J.; Oliver, M. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J. Gastroenterol. Hepatol. 2014, 29, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- De Lisle, R.C.; Roach, E.; Jansson, K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G577–G584. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.; Quera, R. Small intestinal bacterial overgrowth: Roles of antibiotics, prebiotics, and probiotics. Gastroenterology 2006, 130, S78–S90. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Shah, S.C.; Day, L.W.; Somsouk, M.; Sewell, J.L. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2013, 38, 925–934. [Google Scholar] [CrossRef]
- Furnari, M.; De Alessandri, A.; Cresta, F.; Haupt, M.; Bassi, M.; Calvi, A.; Haupt, R.; Bodini, G.; Ahmed, I.; Bagnasco, F.; et al. The role of small intestinal bacterial overgrowth in cystic fibrosis: A randomized case-controlled clinical trial with rifaximin. J. Gastroenterol. 2019, 54, 261–270. [Google Scholar] [CrossRef]
- Bali, A.; Stableforth, D.E.; Asquith, P. Prolonged small-intestinal transit time in cystic fibrosis. Br. Med. J. (Clin. Res. Ed.) 1983, 287, 1011–1013. [Google Scholar] [CrossRef]
- Escobar, H.; Perdomo, M.; Vasconez, F.; Camarero, C.; del Olmo, M.T.; Suárez, L. Intestinal permeability to 51Cr-EDTA and orocecal transit time in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1992, 14, 204–207. [Google Scholar] [CrossRef]
- Report, A.D. Annual Data Report 2020 Cystic Fibrosis Foundation Patient Registry. Available online: https://www.cff.org/sites/default/files/2021-10/2019-Patient-Registry-Annual-Data-Report.pdf (accessed on 15 September 2022).
- Houwen, R.H.; van der Doef, H.P.; Sermet, I.; Munck, A.; Hauser, B.; Walkowiak, J.; Robberecht, E.; Colombo, C.; Sinaasappel, M.; Wilschanski, M. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Alberti, C.; Colombo, C.; Kashirskaya, N.; Ellemunter, H.; Fotoulaki, M.; Houwen, R.; Robberecht, E.; Boizeau, P.; Wilschanski, M. International prospective study of distal intestinal obstruction syndrome in cystic fibrosis: Associated factors and outcome. J. Cyst. Fibros. 2016, 15, 531–539. [Google Scholar] [CrossRef]
- Colombo, C.; Ellemunter, H.; Houwen, R.; Munck, A.; Taylor, C.; Wilschanski, M. Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Declercq, D.; Van Biervliet, S.; Robberecht, E. Nutrition and pancreatic enzyme intake in patients with cystic fibrosis with distal intestinal obstruction syndrome. Nutr. Clin. Pract. 2015, 30, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.O.; Hjelt, K.; Waever, E.; Overgaard, K. The age-related incidence of meconium ileus equivalent in a cystic fibrosis population: The impact of high-energy intake. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 356–360. [Google Scholar] [CrossRef]
- Speck, K.; Charles, A. Distal intestinal obstructive syndrome in adults with cystic fibrosis: A surgical perspective. Arch. Surg. 2008, 143, 601–603. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Anderson, P.J.; Stowe, C.D. Lubiprostone for constipation in adults with cystic fibrosis: A pilot study. Ann. Pharmacother. 2011, 45, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Anderson, P.J.; Stowe, C.D. Use of the chloride channel activator lubiprostone for constipation in adults with cystic fibrosis: A case series. Ann. Pharmacother. 2010, 44, 577–581. [Google Scholar] [CrossRef]
- McHugh, D.R.; Cotton, C.U.; Moss, F.J.; Vitko, M.; Valerio, D.M.; Kelley, T.J.; Hao, S.; Jafri, A.; Drumm, M.L.; Boron, W.F.; et al. Linaclotide improves gastrointestinal transit in cystic fibrosis mice by inhibiting sodium/hydrogen exchanger 3. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G868–G878. [Google Scholar] [CrossRef]
- Singh, V.K.; Schwarzenberg, S.J. Pancreatic insufficiency in Cystic Fibrosis. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S70–S78. [Google Scholar] [CrossRef] [PubMed]
- Augarten, A.; Ben Tov, A.; Madgar, I.; Barak, A.; Akons, H.; Laufer, J.; Efrati, O.; Aviram, M.; Bentur, L.; Blau, H.; et al. The changing face of the exocrine pancreas in cystic fibrosis: The correlation between pancreatic status, pancreatitis and cystic fibrosis genotype. Eur. J. Gastroenterol. Hepatol. 2008, 20, 164–168. [Google Scholar] [CrossRef]
- Brownell, J.N.; Bashaw, H.; Stallings, V.A. Growth and Nutrition in Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 40, 775–791. [Google Scholar] [CrossRef]
- Borowitz, D.; Robinson, K.A.; Rosenfeld, M.; Davis, S.D.; Sabadosa, K.A.; Spear, S.L.; Michel, S.H.; Parad, R.B.; White, T.B.; Farrell, P.M.; et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J. Pediatr. 2009, 155, S73–S93. [Google Scholar] [CrossRef] [PubMed]
- FitzSimmons, S.C.; Burkhart, G.A.; Borowitz, D.; Grand, R.J.; Hammerstrom, T.; Durie, P.R.; Lloyd-Still, J.D.; Lowenfels, A.B. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N. Engl. J. Med. 1997, 336, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Meyerholz, D.K.; Engelhardt, J.F. Pancreatic pathophysiology in cystic fibrosis. J. Pathol. 2016, 238, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Desimone, M.; Kasim, N.; Chan, C.L. Cystic fibrosis-related diabetes: Prevalence, screening, and diagnosis. J. Clin. Transl. Endocrinol. 2022, 27, 100290. [Google Scholar] [CrossRef]
- Witt, H. Chronic pancreatitis and cystic fibrosis. Gut 2003, 52 (Suppl. S2), ii31–ii41. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Dorfman, R.; Cipolli, M.; Gonska, T.; Castellani, C.; Keenan, K.; Freedman, S.D.; Zielenski, J.; Berthiaume, Y.; Corey, M.; et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 2011, 140, 153–161. [Google Scholar] [CrossRef]
- De Boeck, K.; Weren, M.; Proesmans, M.; Kerem, E. Pancreatitis among patients with cystic fibrosis: Correlation with pancreatic status and genotype. Pediatrics 2005, 115, e463–e469. [Google Scholar] [CrossRef]
- Sathe, M.N.; Freeman, A.J. Gastrointestinal, Pancreatic, and Hepatobiliary Manifestations of Cystic Fibrosis. Pediatr. Clin. N. Am. 2016, 63, 679–698. [Google Scholar] [CrossRef]
- Petrocheilou, A.; Kaditis, A.G.; Loukou, I. Pancreatitis in A Patient with Cystic Fibrosis Taking Ivacaftor. Children 2020, 7, 6. [Google Scholar] [CrossRef]
- Megalaa, R.; Gopalareddy, V.; Champion, E.; Goralski, J.L. Time for a gut check: Pancreatic sufficiency resulting from CFTR modulator use. Pediatr. Pulmonol. 2019, 54, E16–E18. [Google Scholar] [CrossRef]
- Howlett, C.; Ronan, N.J.; NiChroinin, M.; Mullane, D.; Plant, B.J. Partial restoration of pancreatic function in a child with cystic fibrosis. Lancet Respir. Med. 2016, 4, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.J.; Ooi, C.Y. Pancreatitis and pancreatic cystosis in Cystic Fibrosis. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S79–S86. [Google Scholar] [CrossRef]
- Debray, D.; Kelly, D.; Houwen, R.; Strandvik, B.; Colombo, C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S29–S36. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Battezzati, P.M. Liver involvement in cystic fibrosis: Primary organ damage or innocent bystander? J. Hepatol. 2004, 41, 1041–1044. [Google Scholar] [CrossRef]
- Lamireau, T.; Monnereau, S.; Martin, S.; Marcotte, J.E.; Winnock, M.; Alvarez, F. Epidemiology of liver disease in cystic fibrosis: A longitudinal study. J. Hepatol. 2004, 41, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shan, A.; Mathews, S.; Sathe, M. Understanding Cystic Fibrosis Comorbidities and Their Impact on Nutritional Management. Nutrients 2022, 14, 1028. [Google Scholar] [CrossRef]
- Debray, D.; Narkewicz, M.R.; Bodewes, F.; Colombo, C.; Housset, C.; de Jonge, H.R.; Jonker, J.W.; Kelly, D.A.; Ling, S.C.; Poynard, T.; et al. Cystic Fibrosis-related Liver Disease: Research Challenges and Future Perspectives. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 443–448. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Friedman, K.J.; Ling, S.C.; Pace, R.G.; Bell, S.C.; Bourke, B.; Castaldo, G.; Castellani, C.; Cipolli, M.; Colombo, C.; et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009, 302, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Wasuwanich, P.; Karnsakul, W. Cystic fibrosis-associated liver disease in children. Minerva Pediatr. 2020, 72, 440–447. [Google Scholar] [CrossRef]
- Cheng, K.; Ashby, D.; Smyth, R.L. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst. Rev. 2017, 9, Cd000222. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.; Sakiani, S.; Surana, P.; Zhao, X.; Eccleston, J.; Kleiner, D.E.; Herion, D.; Liang, T.J.; Hoofnagle, J.H.; Chernick, M.; et al. Adult-onset cystic fibrosis liver disease: Diagnosis and characterization of an underappreciated entity. Hepatology 2017, 66, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C. Liver disease in cystic fibrosis. Curr. Opin. Pulm. Med. 2007, 13, 529–536. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Canlas, K.; Kahi, C.; Temkit, M.; Molleston, J.; Ober, M.; Howenstine, M.; Kwo, P.Y. Hepatobiliary abnormalities and disease in cystic fibrosis: Epidemiology and outcomes through adulthood. J. Clin. Gastroenterol. 2009, 43, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Zemel, B.S.; Kawchak, D.A.; Cnaan, A.; Zhao, H.; Scanlin, T.F.; Stallings, V.A. Prospective evaluation of resting energy expenditure, nutritional status, pulmonary function, and genotype in children with cystic fibrosis. Pediatr. Res. 1996, 40, 578–586. [Google Scholar] [CrossRef]
- Abbott, J.; Morton, A.M.; Musson, H.; Conway, S.P.; Etherington, C.; Gee, L.; Fitzjohn, J.; Webb, A.K. Nutritional status, perceived body image and eating behaviours in adults with cystic fibrosis. Clin. Nutr. 2007, 26, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Feranchak, A.P.; Sontag, M.K.; Wagener, J.S.; Hammond, K.B.; Accurso, F.J.; Sokol, R.J. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J. Pediatr. 1999, 135, 601–610. [Google Scholar] [CrossRef]
- Winklhofer-Roob, B.M.; Tuchschmid, P.E.; Molinari, L.; Shmerling, D.H. Response to a single oral dose of all-rac-alpha-tocopheryl acetate in patients with cystic fibrosis and in healthy individuals. Am. J. Clin. Nutr. 1996, 63, 717–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailey, J.; Rozga, M.; McDonald, C.M.; Bowser, E.K.; Farnham, K.; Mangus, M.; Padula, L.; Porco, K.; Alvarez, J.A. Effect of CFTR Modulators on Anthropometric Parameters in Individuals with Cystic Fibrosis: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2021, 121, 1364–1378.e2. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Laguna, T.; Leschyshyn, J.; Regelmann, W.; Dunitz, J.; Billings, J.; Moran, A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: A small pilot study. Pediatr. Diabetes 2013, 14, 417–421. [Google Scholar] [CrossRef]
- Corey, M.; McLaughlin, F.J.; Williams, M.; Levison, H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J. Clin. Epidemiol. 1988, 41, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Farrell, P.M.; Pencharz, P. Nutritional assessment and management in cystic fibrosis: A consensus report. The Consensus Committee. Am. J. Clin. Nutr. 1992, 55, 108–116. [Google Scholar] [CrossRef]
- Bell, S.C.; Bowerman, A.R.; Davies, C.A.; Campbell, I.A.; Shale, D.J.; Elborn, J.S. Nutrition in adults with cystic fibrosis. Clin. Nutr. 1998, 17, 211–215. [Google Scholar] [CrossRef]
- Boland, M.P.; Stoski, D.S.; MacDonald, N.E.; Soucy, P.; Patrick, J. Chronic jejunostomy feeding with a non-elemental formula in undernourished patients with cystic fibrosis. Lancet 1986, 1, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, G.; von der Hardt, H. Improvement of nutritional status and lung function after long-term nocturnal gastrostomy feedings in cystic fibrosis. J. Pediatr. 1994, 124, 244–249. [Google Scholar] [CrossRef]
- Shepherd, R.W.; Holt, T.L.; Thomas, B.J.; Kay, L.; Isles, A.; Francis, P.J.; Ward, L.C. Nutritional rehabilitation in cystic fibrosis: Controlled studies of effects on nutritional growth retardation, body protein turnover, and course of pulmonary disease. J. Pediatr. 1986, 109, 788–794. [Google Scholar] [CrossRef]
- Harindhanavudhi, T.; Wang, Q.; Dunitz, J.; Moran, A.; Moheet, A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: A single-center analysis. J. Cyst. Fibros. 2020, 19, 139–145. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Blankenheim, Z.; Scott, P.M.; Cormier, R.T. CFTR and Gastrointestinal Cancers: An Update. J. Pers. Med. 2022, 12, 868. [Google Scholar] [CrossRef]
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of gastrointestinal cancers in patients with cystic fibrosis: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Slae, M.; Wilschanski, M. Cystic fibrosis: A gastrointestinal cancer syndrome. Lancet Oncol. 2018, 19, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Hadjiliadis, D.; Khoruts, A.; Zauber, A.G.; Hempstead, S.E.; Maisonneuve, P.; Lowenfels, A.B. Cystic Fibrosis Colorectal Cancer Screening Consensus Recommendations. Gastroenterology 2018, 154, 736–745.e14. [Google Scholar] [CrossRef] [PubMed]

| Vitamin A | Increased Infection susceptibility Growth Failure Corneal Opacities Xerophthalmia Bitot Spots Night Blindness |
| Vitamin D | Bone Pain Motor Delays and Poor Growth Delayed Fontanelle Closure Craniotabes, Frontal Bossing Widening of Ankles and Wrists Bowlegs or Knock Knees Widening of Growth Plate |
| Vitamin E | Decreased Reflexes and Sensation Ataxia Myopathy Retinopathy Hemolytic Anemia |
| Vitamin K | Easy Bruising Mucosal Bleeding Splinter Hemorrhages GI and GU Bleeding |
| Essential Fatty Acids | Scaly Dermatitis Alopecia Thrombocytopenia Poor Growth Poor Cognitive Function Visual Impairment |
| Zinc | Depressed Immunity Impaired Taste and Smell Acrodermatitis |
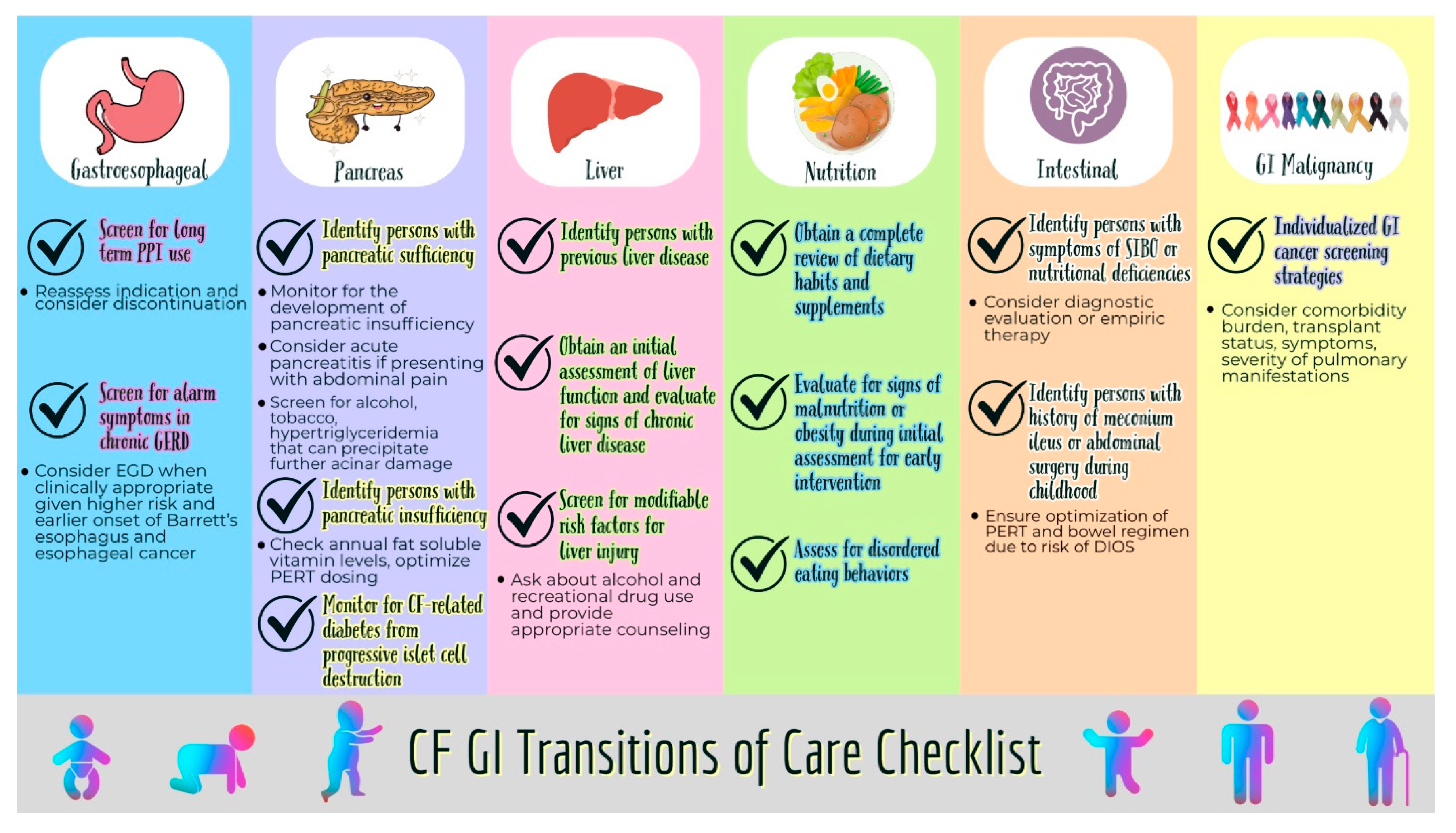
| Esophagus |
|
| Pancreas |
|
| Liver |
|
| Nutrition |
|
| Intestines |
|
| GI malignancy |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.; Baliss, M.; Saikumar, P.; Numan, L.; Teckman, J.; Hachem, C. A Gastroenterologist’s Guide to Care Transitions in Cystic Fibrosis from Pediatrics to Adult Care. Int. J. Mol. Sci. 2023, 24, 15766. https://doi.org/10.3390/ijms242115766
Patel D, Baliss M, Saikumar P, Numan L, Teckman J, Hachem C. A Gastroenterologist’s Guide to Care Transitions in Cystic Fibrosis from Pediatrics to Adult Care. International Journal of Molecular Sciences. 2023; 24(21):15766. https://doi.org/10.3390/ijms242115766
Chicago/Turabian StylePatel, Dhiren, Michelle Baliss, Pavithra Saikumar, Laith Numan, Jeffrey Teckman, and Christine Hachem. 2023. "A Gastroenterologist’s Guide to Care Transitions in Cystic Fibrosis from Pediatrics to Adult Care" International Journal of Molecular Sciences 24, no. 21: 15766. https://doi.org/10.3390/ijms242115766
APA StylePatel, D., Baliss, M., Saikumar, P., Numan, L., Teckman, J., & Hachem, C. (2023). A Gastroenterologist’s Guide to Care Transitions in Cystic Fibrosis from Pediatrics to Adult Care. International Journal of Molecular Sciences, 24(21), 15766. https://doi.org/10.3390/ijms242115766





