Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Clinical Status
3.2. Vitamin D
3.3. Calcium
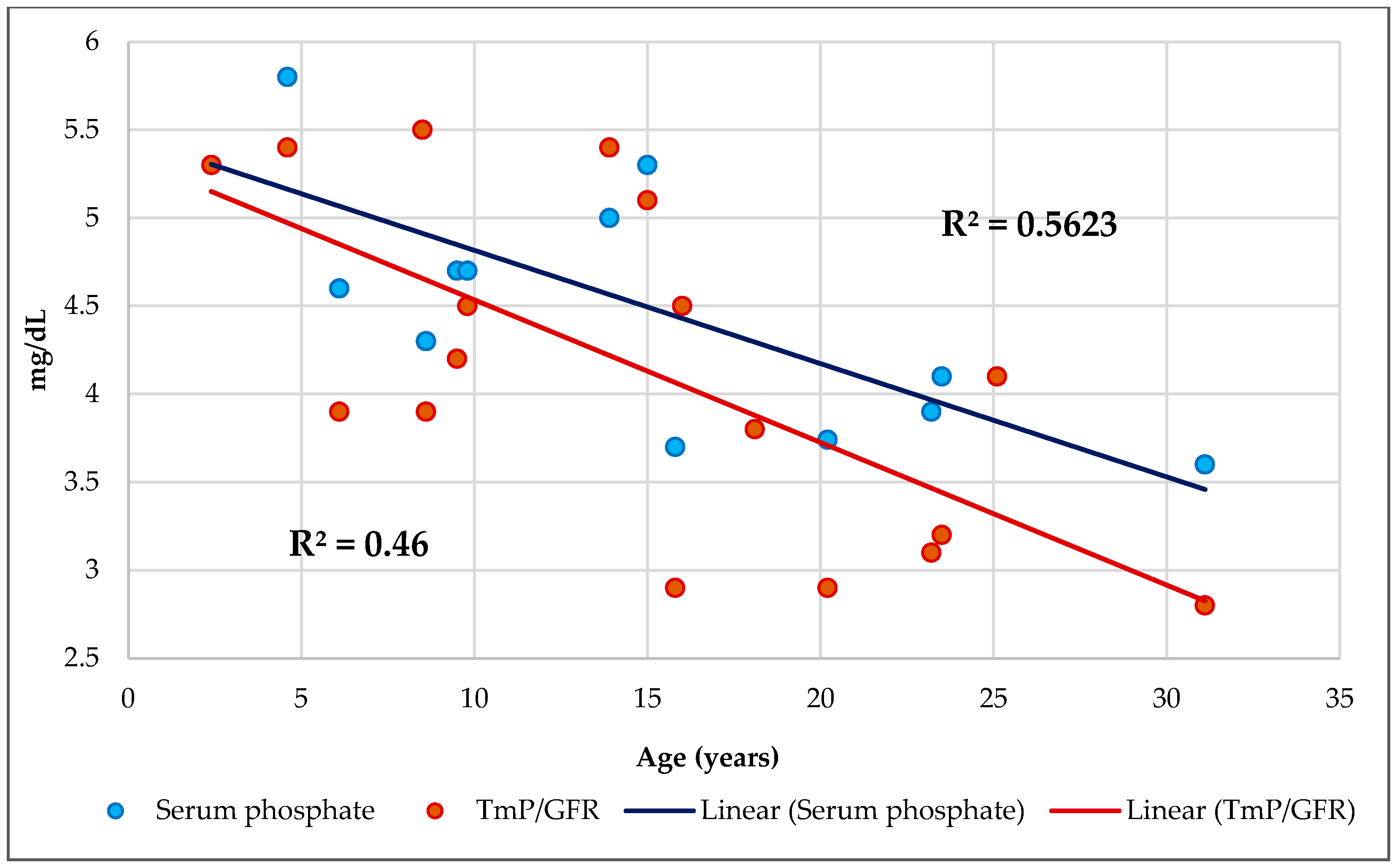
3.4. Phosphorus
3.5. Calcium/Phosphorus Ratios
4. Materials and Methods
4.1. Study Site, Design, and Participants
4.2. Ethical Consideration
4.3. Clinical Evaluation
4.4. Assessment of Phenotypical Characteristics
4.5. Dietary Assessment
4.6. Laboratory Exploration
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| Ca | Calcium |
| P | Phosphorus or phosphate |
| Vit | Vitamin |
| PA | Physical Activity |
| RI | Respiratory insufficiency |
| RS | Respiratory sufficiency |
| PI | Pancreatic insufficiency |
| PS | Pancreatic sufficiency |
| BMI | Body mass index |
| %DRI | % Dietary Reference Intake |
| ALP | Alkaline phosphatase |
| Cr | Creatinine |
| TRP | Fractional tubular reabsorption of phosphate |
| TmP/GFR | Tubular maximum phosphate reabsorption per glomerular filtration rate |
| EE | Energy expenditure |
| ECFS | European Cystic Fibrosis Society |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| WHO | World Health Organization |
| NB | Nitrogen balance |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| NHANES | National Health and Nutrition Examination Survey |
| PERT | Pancreatic enzyme replacement therapy |
| FAC | Fat absorption coefficient |
| FFM | Fat free mass |
| FM | Fat mass |
| BIA | Bioelectrical impedance analysis |
References
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Personalized or Precision Medicine? The Example of Cystic Fibrosis. Front. Pharmacol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Marcos-Temprano, M.; Marugán-Miguelsanz, J.M. Magnesium Status and Calcium/Magnesium Ratios in a Series of Cystic Fibrosis Patients. Nutrients 2022, 14, 1793. [Google Scholar] [CrossRef]
- Kelly, A.; Schall, J.; Stallings, V.A.; Zemel, B.S. Trabecular and cortical bone deficits are present in children and adolescents with cystic fibrosis. Bone 2016, 90, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Marach-Mocarska, A.; Sands, D. Uncommon clinical presentation of cystic fibrosis in a patient homozygous for a rare CFTR mutation: A case report. BMC Pediatr. 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Molecular Genetic Epidemiology of Cystic Fibrosis. Available online: https://www.who.int/genomics/publications/en/HGN_WB_04.02_report.pdf?ua=1 (accessed on 11 March 2023).
- Foundation, Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2020 Annual Data Report. Cystic Fibrosis y 2021. Available online: https://www.cff.org/medical-professionals/patient-registry (accessed on 4 August 2022).
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 3, 557–577. [Google Scholar] [CrossRef]
- Moheet, A.; Moran, A. CF-related diabetes: Containing the metabolic miscreant of cystic fibrosis. Pediatr. Pulmonol. 2017, 52, S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.H.; Ryom, L.; Faurholt-Jepsen, D.; Pressler, T.; Katzenstein, T.L. Prevalence and characteristics of chronic kidney disease among Danish adults with cystic fibrosis. J. Cyst. Fibros. 2017, 17, 478–483. [Google Scholar] [CrossRef]
- Polgreen, P.M.; Comellas, A.P. Clinical Phenotypes of Cystic Fibrosis Carriers. Annu. Rev. Med. 2022, 73, 563–574. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision medicine: Concept and tools. Med. J. Armed. Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef]
- de Lamas, C.; de Castro, M.J.; Gil-Campos, M.; Gil, Á.; Couce, M.L.; Leis, R. Effects of Dairy Product Consumption on Height and Bone Mineral Content in Children: A Systematic Review of Controlled Trials. Adv. Nutr. 2019, 10, S88–S96. [Google Scholar] [CrossRef]
- Civitelli, R.; Ziambaras, K. Calcium and phosphate homeostasis: Concerted interplay of new regulators. J. Endocrinol. Investig. 2011, 34 (Suppl. S7), 3–7. [Google Scholar]
- Sadiq, N.M.; Naganathan, S.; Badireddy, M. Hypercalcemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kiela, P.R.; Radhakrishnan, V.M.; Ghishan, F.K. Phosphorus: Basic Nutritional Aspects. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 34; pp. 413–427. [Google Scholar]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Charoenngam, N.; Ayoub, D.; Holick, M.F. Nutritional rickets and vitamin D deficiency: Consequences and strategies for treatment and prevention. Expert Rev. Endocrinol. Metab. 2022, 17, 351–364. [Google Scholar] [CrossRef]
- Wood, C.; Hasan, S.; Darukhanavala, A.; Tangpricha, V. A Clinician’s guide to vitamin D supplementation for patients with cystic fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100273. [Google Scholar] [CrossRef]
- Carlberg, C. A Pleiotropic Nuclear Hormone Labelled Hundred Years Ago Vitamin D. Nutrients 2022, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Goyal, A.; Givler, A. Vitamin D Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532266/ (accessed on 31 May 2023).
- Escobedo Monge, M.F.; Barrado, E.; Alonso Vicente, C.; Redondo del Río, M.P.; Marugán de Miguelsanz, J.M. Zinc Nutritional Status in Patients with Cystic Fibrosis. Nutrients 2019, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Alonso Vicente, C.; Escobedo-Monge, M.A.; TorreserumHinojal, M.C.; Marugán-Miguelsanz, J.M.; Redondo del Río, M.P. Copper and Copper/Zinc Ratio in a Series of Cystic Fibrosis Patients. Nutrients 2020, 12, 3344. [Google Scholar] [CrossRef] [PubMed]
- CFTR2. Clinical Functional Translation of CFTR. Available online: https://www.cftr2.org (accessed on 1 September 2023).
- Mędza, A.; Kaźmierska, K.; Wielgomas, B.; Konieczna, L.; Olędzka, I.; SzlagatyserumSidorkiewicz, A.; Sznurkowska, K. DeltaF508 CFTR Hetero- and Homozygous Paediatric Patients with Cystic Fibrosis Do Not Differ with Regard to Nutritional Status. Nutrients 2021, 13, 1402. [Google Scholar] [CrossRef]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Alicandro, G.; Bisogno, A.; Battezzati, A.; Bianchi, M.L.; Corti, F.; Colombo, C. Recurrent pulmonary exacerbations are associated with low fat free mass and low bone mineral density in young adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 328–334. [Google Scholar] [CrossRef]
- Kaminski, B.A.; Goldsweig, B.K.; Sidhaye, A.; Blackman, S.M.; Schindler, T.; Moran, A. Cystic fibrosis related diabetes: Nutrition and growth considerations. J Cyst Fibros. 2019, 18 (Suppl. S2), S32–S37. [Google Scholar] [CrossRef]
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H.; MS Clinical Practice Guidelines on Growth and Nutrition Subcommittee & Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J. Am. Diet. Assoc. 2008, 108, 832–839. [Google Scholar] [CrossRef]
- Kastner-Cole, D.; Palmer, C.N.; Ogston, S.A.; Mehta, A.; Mukhopadhyay, S. Overweight and obesity in deltaF508 homozygous cystic fibrosis. J. Pediatr. 2005, 147, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Langer, D.; Vrijsen, B.; Segers, J.; Wouters, K.; Janssens, W.; Gosselink, R.; Decramer, M.; Dupont, L. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur Respir. J. 2009, 33, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mukherjee, A.; Lodha, R.; Kabra, M.; Deepak, K.K.; Khadgawat, R.; Talwar, A.; Kabra, S.K. Effects of Exercise Intervention Program on Bone Mineral Accretion in Children and Adolescents with Cystic Fibrosis: A Randomized Controlled Trial. Indian J. Pediatr. 2019, 86, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Murata, E.M.; Yu, C.; Danik, J.; Kotler, G.; Cook, N.R.; Bubes, V.; Mora, S.; Chandler, P.D.; Tobias, D.K.; et al. Effects of Vitamin D3 Supplementation on Body Composition in the VITamin D and OmegA-3 TriaL (VITAL). J. Clin. Endocrinol. Metab. 2021, 106, 1377–1388. [Google Scholar] [CrossRef]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Wagner, C.L.; Shab-Bidar, S. Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef]
- Çığrı, E.; İnan, F.Ç. The Relationship between Anthropometric Measurements and Vitamin D Levels and Insulin Resistance in Obese Children and Adolescents. Children 2022, 9, 1837. [Google Scholar] [CrossRef]
- Keast, D.R.; Hill Gallant, K.M.; Albertson, A.M.; Gugger, C.K.; Holschuh, N.M. Associations between yogurt, dairy, calcium, and vitamin D intake and obesity among U.S. children aged 8–18 years: NHANES, 2005–2008. Nutrients 2015, 7, 1577–1593. [Google Scholar] [CrossRef]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D deficiency and its treatment in cystic fibrosis. J. Cyst. Fibros. 2019, 18, S66–S73. [Google Scholar] [CrossRef]
- Kirwan, R. Differential effects of vitamin D on upper and lower body fat-free mass: Potential mechanisms. Mol. Biol. Rep. 2023, 50, 883–888. [Google Scholar] [CrossRef]
- Hassan-Smith, Z.K.; Jenkinson, C.; Smith, D.J.; Hernandez, I.; Morgan, S.A.; Crabtree, N.J.; Gittoes, N.J.; Keevil, B.G.; Stewart, P.M.; Hewison, M. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 2017, 12, e0170665. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Ganji, V.; Martineau, B.; Van Fleit, W.E. Association of serum vitamin D concentrations with dietary patterns in children and adolescents. Nutr. J. 2018, 17, 58. [Google Scholar] [CrossRef]
- Timmers, N.K.L.M.; Stellato, R.K.; van der Ent, C.K.; Houwen, R.H.J.; Woestenenk, J.W. Vitamin D intake, serum 25-hydroxy vitamin D and pulmonary function in paediatric patients with cystic fibrosis: A longitudinal approach. Br. J. Nutr. 2019, 121, 195–201. [Google Scholar] [CrossRef]
- Abu-Fraiha, Y.; Elyashar-Earon, H.; Shoseyov, D.; Cohen-Cymberknoh, M.; Armoni, S.; Kerem, E.; Wilschanski, M. Increasing Vitamin D Serum Levels Is Associated With Reduced Pulmonary Exacerbations in Patients With Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sánchez, C.; Garriga-García, M.; Serrano-Nieto, M.J.; García-Romero, R.; Álvarez-Beltrán, M.; Crehuá-Gaudiza, E.; Muñoz-Codoceo, R.; Suárez-Cortina, L.; Vicente-Santamaría, S.; Martínez-Costa, C.; et al. Vitamin D Status in Pediatric and Young Adult Cystic Fibrosis Patients. Are the New Recommendations Effective? Nutrients 2021, 13, 4413. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mukherjee, A.; Khadgawat, R.; Kabra, M.; Lodha, R.; Kabra, S.K. Bone Mineral Density of Indian Children and Adolescents with Cystic Fibrosis. Indian Pediatr. 2017, 54, 545–549. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Vitamin, D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 15 May 2023).
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment & Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [PubMed]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.A.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef]
- Holick, M.F. The One-Hundred-Year Anniversary of the Discovery of the Sunshine Vitamin D3: Historical, Personal Experience and Evidence-Based Perspectives. Nutrients 2023, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Planté-Bordeneuve, T.; Berardis, S.; Bastin, P.; Gruson, D.; Henri, L.; Gohy, S. Vitamin D intoxication in patients with cystic fibrosis: Report of a single-center cohort. Sci. Rep. 2021, 11, 7719. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Melamed, M.L.; Kumar, J.; Roy, C.N.; Miller, E.R., 3rd; Furth, S.L.; Fadrowski, J.J. Vitamin D, race, and risk for anemia in children. J. Pediatr. 2014, 164, 153–158. [Google Scholar] [CrossRef]
- Gupta, V.; Mishra, S.; Gazala, M.P.; Vandana, K.L.; Ratre, M.S. Serum Vitamin D level and its association with red blood cell indices in patients with periodontitis. J. Indian Soc. Periodontol. 2022, 26, 446–450. [Google Scholar]
- Doudin, A.; Becker, A.; Rothenberger, A.; Meyer, T. Relationship between serum 25-hydroxyvitamin D and red blood cell indices in German adolescents. Eur. J. Pediatr. 2018, 177, 583–591. [Google Scholar] [CrossRef]
- Bruscia, E.M.; Bonfield, T.L. Update on Innate and Adaptive Immunity in Cystic Fibrosis. Clin. Chest Med. 2022, 43, 603–615. [Google Scholar] [CrossRef]
- Chesdachai, S.; Tangpricha, V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem. Mol. Biol. 2016, 164, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; TorreserumHinojal, M.C.; Marugán-Miguelsanz, J.M. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; TorreserumHinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A CrosserumSectional Study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.; Plesa, M.; Mogas, A.; Jalaleddine, N.; Hamid, Q.; Al Heialy, S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir Res. 2022, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Loukou, I.; Moustaki, M.; Sardeli, O.; Plyta, M.; Douros, K. Association of vitamin D status with lung function measurements in children and adolescents with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Hu, B.; Zhou, Z.; Xing, X.; Wu, Y.; Gao, J.; He, Y.; Hu, Y.; Cheng, Q.; Gong, Q. Vitamin D levels correlate with lymphocyte subsets in elderly patients with age-related diseases. Sci. Rep. 2018, 8, 7708. [Google Scholar] [CrossRef]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium deficiency worldwide: Prevalence of inadequate intakes and associated health outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Mora Vallellano, J.; Delgado Pecellín, C.; Delgado Pecellín, I.; Quintana Gallego, E.; López-Campos, J.L. Evaluation of bone metabolism in children with cystic fibrosis. Bone 2021, 147, 115929. [Google Scholar] [CrossRef]
- Lee, M.J.; Alvarez, J.A.; Smith, E.M.; Killilea, D.W.; Chmiel, J.F.; Joseph, P.M.; Grossmann, R.E.; Gaggar, A.; Ziegler, T.R.; Tangpricha, V. Vitamin D for Enhancing the Immune System in Cystic Fibrosis Investigators. Changes in Mineral Micronutrient Status During and After. Nutr. Clin. Pract. 2015, 30, 838–843. [Google Scholar] [CrossRef]
- Tham, A.; Katz, T.E.; Sutherland, R.E.; Garg, M.; Liu, V.; Tong, C.W.; Brunner, R.; Quintano, J.; Collins, C.; Ooi, C.Y. Micronutrient intake in children with cystic fibrosis in Sydney, Australia. J. Cyst. Fibros. 2020, 19, 146–152. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar] [PubMed]
- Putman, M.S.; Anabtawi, A.; Le, T.; Tangpricha, V.; Sermet-Gaudelus, I. Cystic fibrosis bone disease treatment: Current knowledge and future directions. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.K.; Garg, M.K.; Dang, N.; Mithal, A.; Narang, A.; Chadha, A.; Gupta, N.; Kumar, M.R. Reference range of random urinary calcium creatinine ratio in North Indian children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Taneja, A. Hypercalciuria. In Stat Pearls [Internet]; Stat Pearls: Treasure Island, FL, USA, 2017; [Updated 14 April 2023]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448183/ (accessed on 11 October 2023).
- Galindo-Zavala, R.; Bourine Torrent, R.; MagallareserumLópez, B.; Mir-Perelló, C.; Palmourine Fontana, N.; Sevilla-Pérez, B.; Medrano-San Ildefonso, M.; González-Fernández, M.I.; Román-Pascual, A.; Alcañiz-Rodríguez, P.; et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr. Rheumatol. Online J. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef]
- Moryousef, J.; Kwong, J.; Kishibe, T.; Ordon, M. Systematic Review of the Prevalence of Kidney Stones in Cystic Fibrosis. J. Endourol. 2021, 3, 1693–1700. [Google Scholar] [CrossRef]
- Bihuniak, J.D.; Insogna, K.L. The effects of dietary protein and amino acids on skeletal metabolism. Mol. Cell Endocrinol. 2015, 410, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hanseree, P.; Staples, A.C.; Cryns, V.L.; Hansen, K.E. Hypocalciuria as a Predictor of Reduced Intestinal Calcium Absorption. J. Endocr. Soc. 2017, 1, 1179–1187. [Google Scholar] [CrossRef]
- Bogdanova, A.; Makhro, A.; Wang, J.; Lipp, P.; Kaestner, L. Calcium in red blood cells-a perilous balance. Int. J. Mol. Sci. 2013, 14, 9848–9872. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Bozoky, Z.; Ahmadi, S.; Milman, T.; Kim, T.H.; Du, K.; Di Paola, M.; Pasyk, S.; Pekhletski, R.; Keller, J.P.; Bear, C.E.; et al. Synergy of cAMP and calcium signaling pathways in CFTR regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E2086–E2095. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Mueller, C.; Braag, S.A.; Keeler, A.; Hodges, C.; Drumm, M.; Flotte, T.R. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am. J. Respir. Cell Mol. Biol. 2011, 44, 922–929. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Sequí-Dominguez, I.; Reina-Gutiérrez, S.; Núñez de Arenas-Arroyo, S.; Garrido-Miguel, M. Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength. Nutrients 2021, 13, 4498. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef] [PubMed]
- Nappo, A.; Sparano, S.; Intemann, T.; Kourides, Y.A.; Lissner, L.; Molnar, D.; Moreno, L.A.; Pala, V.; Sioen, I.; Veidebaum, T.; et al. Dietary calcium intake and adiposity in children and adolescents: Cross-sectional and longitudinal results from IDEFICS/I. Family cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 440–449. [Google Scholar] [CrossRef]
- Li, P.; Fan, C.; Lu, Y.; Qi, K. Effects of calcium supplementation on body weight: A meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflugers. Arch. 2019, 471, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Verploegen, M.F.A.; VargaserumPoussou, R.; Walsh, S.B.; Alpay, H.; Amouzegar, A.; Ariceta, G.; Atmis, B.; Bacchetta, J.; Bárány, P.; Baron, S.; et al. Parathyroid hormone and phosphate homeostasis in patients with Bartter and Gitelman syndrome: An international crosserumsectional study. Nephrol. Dial. Transplant. 2022, 37, 2474–2486. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B. Renal tubular reabsorption of phosphate (TmP/GFR): Indications and interpretation. Ann. Clin. Biochem. 1998, 35, 201–206. [Google Scholar] [CrossRef]
- Bacchetta, J.; Bernardor, J.; Garnier, C.; Naud, C.; Ranchin, B. Hyperphosphatemia and Chronic Kidney Disease: A Major Daily Concern Both in Adults and in Children. Calcif. Tissue Int. 2021, 108, 116–127. [Google Scholar] [CrossRef]
- Naffaa, M.E.; Mustafa, M.; Azzam, M.; Nasser, R.; Andria, N.; Azzam, Z.S.; Braun, E. Serum inorganic phosphorus levels predict 30-day mortality in patients with community acquired pneumonia. BMC Infect Dis. 2015, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Gil Gómez, R.; Milano Manso, G. Electrolitos urinarios. An. Pediatría Contin. 2014, 12, 133–136. [Google Scholar] [CrossRef]
- Sands, D.; Mielus, M.; Umławska, W.; Lipowicz, A.; Oralewska, B.; Walkowiak, J. Evaluation of factors related to bone disease in Polish children and adolescents with cystic fibrosis. Adv. Med. Sci. 2015, 60, 315–320. [Google Scholar] [CrossRef]
- Harrison, C.M.; Johnson, K.; McKechnie, E. Osteopenia of prematurity: A national survey and review of practice. Acta Pediatr. 2008, 97, 407–413. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Rachel, M.; Galiniak, S.; Biesiadecki, M.; Gala-Błądzińska, A. Renal Function in Patients with Cystic Fibrosis: A Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 5454. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Mazzaferro, S.; Mitterhofer, A.P.; Bonci, E.; Marotta, P.G.; Pelligra, F.; Murciano, M.; Celani, C.; Troiani, P.; Cimino, G.; et al. Renal involvement and metabolic alterations in adults patients affected by cystic fibrosis. J. Transl. Med. 2019, 17, 388. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Bhadauria, D. Renal phosphate handling: Physiology. Indian J. Endocrinol. Metab. 2013, 17, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Maeda, Y.; Matsuki, H.; Matsumoto, Y.; Akazawa, M.; Kuyama, T. Urinary phosphorus excretion per creatinine clearance as a prognostic marker for progression of chronic kidney disease: A retrospective cohort study. BMC Nephrol. 2015, 16, 116. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M. Urinary Phosphorus Excretion: Not What We Have Believed It to Be? Clin. J. Am. Soc. Nephrol. 2018, 13, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Derain Dubourg, L.; Aurelle, M.; Chardon, L.; Flammier, S.; Lemoine, S.; Bacchetta, J. Tubular phosphate handling: References from child to adulthood in the era of standardized serum creatinine. Nephrol. Dial. Transplant. 2022, 37, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Bernardor, J.; Lemoine, S.; Bertholet-Thomas, A.; Bacchetta, J. Rare diseases of phosphate and calcium metabolism: Crossing glances between nephrology and endocrinology. Ann. Endocrinol. 2021, 82, 30–35. [Google Scholar] [CrossRef]
- Imel, E.A. Congenital Conditions of Hypophosphatemia in Children. Calcif. Tissue Int. 2021, 108, 74–90. [Google Scholar] [CrossRef]
- Tabara, Y.; Kohara, K.; Okada, Y.; Ohyagi, Y.; Igase, M. Creatinine-to-cystatin C ratio as a marker of skeletal muscle mass in older adults: J-SHIPP study. Clin. Nutr. 2020, 39, 1857–1862. [Google Scholar] [CrossRef]
- Gordon, S.; Sethna, C.; Frank, R.; Vento, S.; Goilav, B.; Tau, M.; Trachtman, H. BUN: Creatinine ratio—definition of the normal range in children. Nephrol. Rev. 2010, 2, 49–52. [Google Scholar] [CrossRef]
- Shen, S.; Yan, X.; Xu, B. The blood urea nitrogen/creatinine (BUN/cre) ratio was U-shaped associated with all-cause mortality in general population. Ren Fail. 2022, 44, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yan, W.; Zhao, Q.; Ji, B.; Ban, B.; Zhang, M. Association between Serum Calcium and Phosphorus Levels and Insulin-Like Growth Factor-1 in Chinese Children and Adolescents with Short Stature. Int. J. Gen. Med. 2020, 13, 1167–1173. [Google Scholar] [CrossRef]
- Robinson, C.A.; Hofer, M.; Benden, C.; Schmid, C. Evaluation of bone disease in patients with cystic fibrosis and end-stage lung disease. J. Bras. Pneumol. 2019, 45, e20170280. [Google Scholar] [CrossRef]
- Ferrari, V.; Terlizzi, V.; Stagi, S. Endocrinological Features in Children and Adolescents with Cystic Fibrosis. J. Clin. Med. 2022, 11, 4041. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Shanmugalingam, T.; Bosco, C.; Wulaningsih, W.; Rohrmann, S. The association between circulating IGF1, IGFBP3, and calcium: Results from NHANES III. Endocr. Connect. 2015, 4, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Madeo, B.; Kara, E.; Cioni, K.; Vezzani, S.; Trenti, T.; Santi, D.; Simoni, M.; Rochira, V. Serum Calcium to Phosphorous (Ca/P) Ratio Is a Simple, Inexpensive, and Accurate Tool in the Diagnosis of Primary Hyperparathyroidism. JBMR Plus 2017, 2, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Madeo, B.; De Vincentis, S.; Repaci, A.; Altieri, P.; Vicennati, V.; Kara, E.; Vescini, F.; Amadori, P.; Balestrieri, A.; Pagotto, U.; et al. The calcium-to-phosphorous (Ca/P) ratio in the diagnosis of primary hyperparathyroidism and hypoparathyroidism: A multicentric study. Endocrine 2020, 68, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Grez-Capdeville, M.; Crenshaw, T.D. Evaluation of calcium to phosphorus ratio in spot urine samples as a practical method to monitor phosphorus intake adequacy in sows. J. Anim. Sci. 2021, 99, skab335. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.; Campos-Obando, N.; Medina-Gomez, C.; Voortman, T.; Uitterlinden, A.G.; Zillikens, M.C. Serum Phosphate, BMI, and Body Composition of Middle-Aged and Older Adults: A Cross-Sectional Association Analysis and Bidirectional Mendelian Randomization Study. J. Nutr. 2022, 152, 276–285. [Google Scholar] [CrossRef]
- Lind, L.; Lithell, H.; Hvarfner, A.; Pollare, T.; Ljunghall, S. On the relationships between mineral metabolism, obesity and fat distribution. Eur. J. Clin. Investig. 1993, 23, 307–310. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B.; Gaziano, J.M., Sr.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef]
- Park, W.; Kim, B.S.; Lee, J.E.; Huh, J.K.; Kim, B.J.; Sung, K.C.; Kang, J.H.; Lee, M.H.; Park, J.R.; Rhee, E.J.; et al. Serum phosphate levels and the risk of cardiovascular disease and metabolic syndrome: A double-edged sword. Diabetes Res. Clin. Pract. 2009, 83, 119–125. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Ji, L.; Cang, J.; Zhao, H. Association between aspartate aminotransferase to alanine aminotransferase ratio and the risk of diabetes in Chinese prediabetic population: A retrospective cohort study. Front Public Health 2023, 10, 1045141. [Google Scholar] [CrossRef]
- Adeli, K.; Higgins, V.; Trajcevski, K.; White-Al Habeeb, N. The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Crit. Rev. Clin. Lab. Sci. 2017, 54, 358–413. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. [Updated 5 October 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 10 December 2023).
- Cannalire, G.; Pilloni, S.; Esposito, S.; Biasucci, G.; Di Franco, A.; Street, M.E. Alkaline phosphatase in clinical practice in childhood: Focus on rickets. Front. Endocrinol. 2023, 14, 1111445. [Google Scholar] [CrossRef] [PubMed]
- Zierk, J.; Arzideh, F.; Haeckel, R.; Cario, H.; Frühwald, M.C.; Groß, H.J.; Gscheidmeier, T.; Hoffmann, R.; Krebs, A.; Lichtinghagen, R.; et al. Pediatric reference intervals for alkaline phosphatase. Clin. Chem. Lab. Med. 2017, 55, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Banjar, H.; AbdulAziz, N.; Khader, J.; Ghomraoui, F.; Alansari, A.; Al-Hoshan, A.; AlKaf, S.; Aldakheel, W. Liver disease in cystic fibrosis patients in a tertiary care center in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2022, 9, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Gholami Bahnemiri, M.; Mirabedini, S.; Mohammadi, P.; Barmaki, H.; Qaffaripour, Z.; Rezapour, M.; Alijanpour, M. Determination of serum alkaline phosphatase reference in healthy children aged 1-18 years. Caspian J. Intern. Med. 2022, 13, 749–756. [Google Scholar]
- Zhang, H.; Jia, Q.; Piao, M.; Chang, Y.; Zhang, J.; Tong, X.; Han, T. Screening of Serum Alkaline Phosphatase and Phosphate Helps Early Detection of Metabolic Bone Disease in Extremely Low Birth Weight Infants. Front Pediatr. 2021, 9, 642158. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Colangelo, L.; Pepe, J.; Diacinti, D.; Cipriani, C.; Rao, S.D. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR Plus 2020, 5, e10447. [Google Scholar] [CrossRef]
- Woestenenk, J.W.; Schulkes, D.A.; Schipper, H.S.; van der Ent, C.K.; Houwen, R.H.J. Dietary intake and lipid profile in children and adolescents with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Szczepanik, M.; Wojsyk-Banaszak, I.; Mądry, E.; Wykrętowicz, A.; Krzyżanowska-Jankowska, P.; Drzymała-Czyż, S.; Nowicka, A.; Pogorzelski, A.; Sapiejka, E.; et al. Cystic fibrosis dyslipidaemia: A cross-sectional study. J. Cyst. Fibros. 2019, 18, 566–571. [Google Scholar] [CrossRef]
- González Jiménez, D.; Muñoz-Codoceo, R.; Garriga-García, M.; Molina-Arias, M.; Álvarez-Beltrán, M.; García-Romero, R.; Martínez-Costa, C.; Meavilla-Olivas, S.M.; Peña-Quintana, L.R.; Gallego Gutiérrez, S.; et al. Excess weight in patients with cystic fibrosis: Is it always beneficial? Nutr. Hosp. 2017, 34, 578–583. [Google Scholar] [CrossRef][Green Version]
- Borowitz, D.; Aronoff, N.; Cummings, L.C.; Maqbool, A.; Mulberg, A.E. Coefficient of Fat Absorption to Measure the Efficacy of Pancreatic Enzyme Replacement Therapy in People With Cystic Fibrosis: Gold Standard or Coal Standard? Pancreas 2022, 51, 310–318. [Google Scholar] [CrossRef]
- World Health Organization. Physical Activity Surveillance. Noncommunicable Disease Surveillance, Monitoring and Reporting. Available online: www.who.int/teams/noncommunicable-diseases/surveillance/systemserumtools/physical-activity-surveillance (accessed on 11 May 2023).
- Frisancho, A.R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Sobradillo, B.; Aguirre, A.; Aresti, U.; Bilbao, A.; Fernández-Ramos, C.; Lizárraga, A.; Lorenzo, H.; Madariaga, L.; Rica, I. Curvas y Tablas de Crecimiento (Estudios Longitudinal y Transversal); Fundación Faustino Orbegozo: Bilbao, Spain, 1985. [Google Scholar]
- Mataix Verdú, J.; García Diaz, J. NUTRIBER. V. 1.0; Fundación Universitaria Iberoamericana: Barcelona, Spain, 2005. [Google Scholar]
- Cuervo, M.; Corbalán, M.; Baladía, E.; Cabrerizo, L.; Formiguera, X.; Iglesias, C.; Lorenzo, H.; Polanco, I.; Quiles, J.; De Avila, M.D.R.; et al. Comparison of dietary reference intakes (DRI) between different countries of the European Union, The United States and the World Health Organization. Nutr. Hosp. 2009, 24, 384–414. [Google Scholar] [PubMed]
- Shroff, R.; Wan, M.; Nagler, E.V.; Bakkaloglu, S.; Fischer, D.C.; Bishop, N.; Cozzolino, M.; Bacchetta, J.; Edefonti, A.; Stefanidis, C.J.; et al. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol. Dial. Transplant. 2017, 32, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846. [Google Scholar] [PubMed]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic & Laboratory Test Reference, 14th ed.; Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Çullas İlarslan, N.E.; Şıklar, Z.; Berberoğlu, M. Childhood Sustained Hypercalcemia: A Diagnostic Challenge. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Approach to Hypercalcemia. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Díaz Romero, C.; Henríquez Sánchec, P.; López Blanco, F.; Rodríguez Rodríguez, E.; Serra Majem, L. Concentrations of Na, K, Ca, and P in serum from a representative sample of the Canary Islands population. Nutr. Hosp. 2002, 17, 204–212. [Google Scholar] [PubMed]
- García Nieto, V.M.; Luis Yanes, M.I.; Tejera Carreño, P.; Perez Suarez, G.; Moraleda Mesa, T. The idiopathic hypercalciuria reviewed. Metabolic abnormality or disease? Nefrologia (Engl. Ed.). 2019, 39, 592–602. [Google Scholar] [CrossRef]
- Alon, U.; Hellerstein, S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr. Nephrol. 1994, 8, 250–251. [Google Scholar] [CrossRef]
- Brodehl, J. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr. Nephrol. 1994, 8, 645. [Google Scholar] [CrossRef] [PubMed]


| Characteristics (Mean ± SD) | Total | Homozygous ΔF508 | Compound Heterozygous ΔF508 | p-Value |
|---|---|---|---|---|
| Age (years) | 14. 8 ± 8 | 18.7 ± 10 | 12.7 ± 6.2 | 0.147 |
| Triceps skinfold (mm) | 8.5 ± 4.6 | 11.6 ± 5.7 | 6.8 ± 2.9 | 0.034 * |
| Polyunsaturated fats (%DRI) | 17.1 ± 5.2 | 20.4 ± 5.6 | 15.1 ± 3.9 | 0.043 * |
| Vitamin C (%DRI) | 170 ± 140 | 270 ± 159 | 110 ± 90 | 0.021 * |
| Vitamin D intake (%DRI) | 623 ± 966 | 1259 ± 1281 | 243 ± 241 | 0.036 * |
| Iodine (%DRI) | 53 ± 21 | 67 ± 16 | 45 ± 20 | 0.043 * |
| Transferrin (mg/dL) | 258 ± 39 | 231 ± 26 | 274 ± 38 | 0.027 * |
| Blood urea nitrogen (mg/dL) | 32.7 ± 11.3 | 41.5 ± 12.2 | 28.0 ± 7.8 | 0.013 * |
| Hemoglobin (g/dL) | 14.3 ± 1.3 | 13.5 ± 1.0 | 14.9 ± 1.3 | 0.037 * |
| Leucocytes (cell/mm3) | 7870 ± 1360 | 6877 ± 616 | 8466 ± 1349 | 0.017 * |
| Platelets (cell/mm3) | 299 ± 103 | 224 ± 45 | 344 ± 103 | 0.018 * |
| IgG3 (mg/dL) | 34.6 ± 22.2 | 18.4 ± 9.1 | 42.6 ± 22.6 | 0.043 * |
| Age (Years) | Type of Mutations |
|---|---|
| 2 | 1898 + 1G->A |
| 6 | 1341 + 1G->A |
| 8 | 711 + 1G->T |
| 8 | 1717-1G->A |
| 9 | 2183AA->G |
| 13 | G542X and Q890X |
| 15 | 1777-1G |
| 15 | L997F |
| 16 | 2183 AA->G |
| 20 | 1341 + 1G->A and G673X |
| 23 | S549N |
| Age (Years) | Sex | BMI | Dietary Intake (%DRI) | Blood Analytical | Urine Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vit-D | Ca | Vit-D | Ca | P | Ca/P ratio | ALP | Calcium | Ca/Cr Ratio | Phosphorus | TRP % | TmP/ GFR | Ca/P Ratio | |||||
| mg/kg/d | 24 hU | mg/kg/d | 24 hU | ||||||||||||||
| Homozygous | |||||||||||||||||
| 4 | M | −0.59 | 50 | 109 | 15.0 | 9.9 | 5.80 | 1.69 | 775 | 1.20 | 22 | 0.02 | 33.93 | 621 | 88.7 | 5.4 | 0.61 |
| 9 | F | −0.09 | 69 | 137 | 28.0 | 10.4 | 4.70 | 2.12 | 555 | 3.55 | 94 | 0.02 | 11.96 | 317 | 87.9 | 4.2 | 0.88 |
| 18 | F | −2.30 | 2293 | 95 | 15.0 | 8.9 | 3.80 | 2.26 | 128 | 2.86 | 105 | 0.07 | 29.32 | 1076 | 91.0 | 3.8 | 1.17 |
| 23 | F | −0.02 | 159 | 114 | 11.0 | 9.4 | 4.10 | 2.27 | 129 | 3.89 | 235 | 0.53 | 13.70 | 828 | 78.8 | 3.2 | 1.38 |
| 25 | F | 0.64 | 2574 | 147 | 26.0 | 9.4 | 4.10 | 2.27 | 130 | 1.59 | 92 | 0.08 | 13.41 | 775 | 90.8 | 4.1 | 1.86 |
| 31 | F | −1.85 | 2406 | 96 | 75.0 | 9.7 | 3.60 | 2.69 | 73 | 4.50 | 223 | 0.62 | 12.85 | 636 | 79.1 | 2.8 | 1.46 |
| Heterozygous | |||||||||||||||||
| 2 | M | −0.79 | 35 | 147 | 23.0 | 10.0 | 5.30 | 1.88 | 550 | 3.83 | 41 | 0.02 | 22.71 | 24 | 90.9 | 5.3 | 0.63 |
| 6 | F | −1.16 | 3 | 123 | 14.0 | 9.9 | 4.60 | 2.13 | 442 | 1.24 | 22 | 0.03 | 17.68 | 313 | 85.5 | 3.9 | 0.75 |
| 8 | M | −0.15 | 85 | 267 | 23.0 | 10.2 | 5.50 | 1.80 | 595 | 3.92 | 127 | 0.07 | 26.57 | 861 | 91.1 | 5.5 | 0.82 |
| 8 | M | −0.62 | 39 | 154 | 22.0 | 9.6 | 4.30 | 2.23 | 948 | 1.64 | 51 | 0.03 | 22.79 | 709 | 89.4 | 3.9 | 1.32 |
| 9 | F | −0.12 | 672 | 85 | 17.0 | 10.1 | 4.70 | 2.12 | 428 | 6.35 | 169 | 0.05 | 32.39 | 861 | 89.5 | 4.5 | 0.81 |
| 13 | M | −0.52 | 19 | 109 | 24.0 | 9.7 | 5.00 | 1.94 | 1071 | 4.74 | 207 | 0.10 | 19.61 | 857 | 93.4 | 5.4 | 1.51 |
| 15 | M | −1.66 | 99 | 155 | 11.0 | 9.9 | 5.30 | 1.87 | 516 | 3.40 | 69 | 0.05 | 30.86 | 1293 | 89.7 | 5.1 | 0.91 |
| 15 | F | −1.72 | 12 | 103 | 20.7 | 10.6 | 3.70 | 2.97 | 420 | 1.79 | 72 | 0.06 | 17.54 | 705 | 80.7 | 2.9 | 0.98 |
| 16 | F | −3.81 | 1425 | 87 | 17.0 | 10.2 | 4.50 | 2.22 | 275 | 4.65 | 165 | 0.06 | 16.24 | 575 | 90.5 | 4.5 | 1.66 |
| 20 | F | −0.04 | 623 | 127 | 7.0 | 10.0 | 3.74 | 2.70 | 187 | 2.45 | 145 | 0.05 | 12.28 | 726 | 92.1 | 2.9 | 3.37 |
| 23 | M | −1.35 | 38 | 96 | 3.4 | 9.5 | 3.90 | 2.44 | 113 | 3.22 | 115 | 0.12 | 20.74 | 712 | 79.7 | 3.1 | 1.26 |
| Serum Calcium (mg/dL) | Serum Phosphorus (mg/dL) | Ca/Cr Ratio | Urine Ca (mg/dL) | Urine Phosphate (mg/dL) | Urine Ca/P Ratio | Calcium Excretion Rate | TRP (%) | TmP/GFR (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Linear | regression | analysis | ||||||
| FM (kg) BIA R2 = 0.746 p = 0.006 | Age (years) R2 = 0.562 p = 0.001 | Indirect calorimetry R2 = 0.604 p = 0.001 | Serum Ca/P ratio R2 = 0.839 p = 0.000 | |||||
| Serum Ca/P ratio R2 = 0.838 p = 0.000 | Basophiles R2 = 0.537 p = 0.001 | MCHC R2 = 0.712 p = 0.000 | Serum phosphorus R2 = 0.875 p = 0.000 | |||||
| Multilinear | regression | analysis | ||||||
| Creatinine R2 = 0.732 p = 0.000 | FFM kg A R2 = 0.537 p = 0.009 | Hip C R2 = 0.510 p = 0.002 | Creatinine R2 = 0.722 p = 0.000 | Creatinine R2 = 0.588 p = 0.000 | ||||
| CER R2 = 0.999 p = 0.000 | Vitamin B12 (%DRI) R2 = 0.555 p = 0.001 | FFM kg A R2 = 0.569 p = 0.001 | ||||||
| CD3 T L R2 = 0.631 p = 0.000 | CD19 T L R2 = 0.638 p = 0.000 | CD3 T L R2 = 0.621 p = 0.000 | IgM R2 = 0.616 p = 0.000 | |||||
| BUN and GGT R2 = 0.586 p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Monge, M.F.; Marcos-Temprano, M.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-Vicente, C.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 1900. https://doi.org/10.3390/ijms25031900
Escobedo-Monge MF, Marcos-Temprano M, Parodi-Román J, Escobedo-Monge MA, Alonso-Vicente C, Torres-Hinojal MC, Marugán-Miguelsanz JM. Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. International Journal of Molecular Sciences. 2024; 25(3):1900. https://doi.org/10.3390/ijms25031900
Chicago/Turabian StyleEscobedo-Monge, Marlene Fabiola, Marianela Marcos-Temprano, Joaquín Parodi-Román, María Antonieta Escobedo-Monge, Carmen Alonso-Vicente, María Carmen Torres-Hinojal, and José Manuel Marugán-Miguelsanz. 2024. "Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study" International Journal of Molecular Sciences 25, no. 3: 1900. https://doi.org/10.3390/ijms25031900
APA StyleEscobedo-Monge, M. F., Marcos-Temprano, M., Parodi-Román, J., Escobedo-Monge, M. A., Alonso-Vicente, C., Torres-Hinojal, M. C., & Marugán-Miguelsanz, J. M. (2024). Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. International Journal of Molecular Sciences, 25(3), 1900. https://doi.org/10.3390/ijms25031900








