Role of Regulatory T Cells and Their Potential Therapeutic Applications in Celiac Disease
Abstract
:1. Introduction
2. Anti-Inflammatory Cytokines in CeD
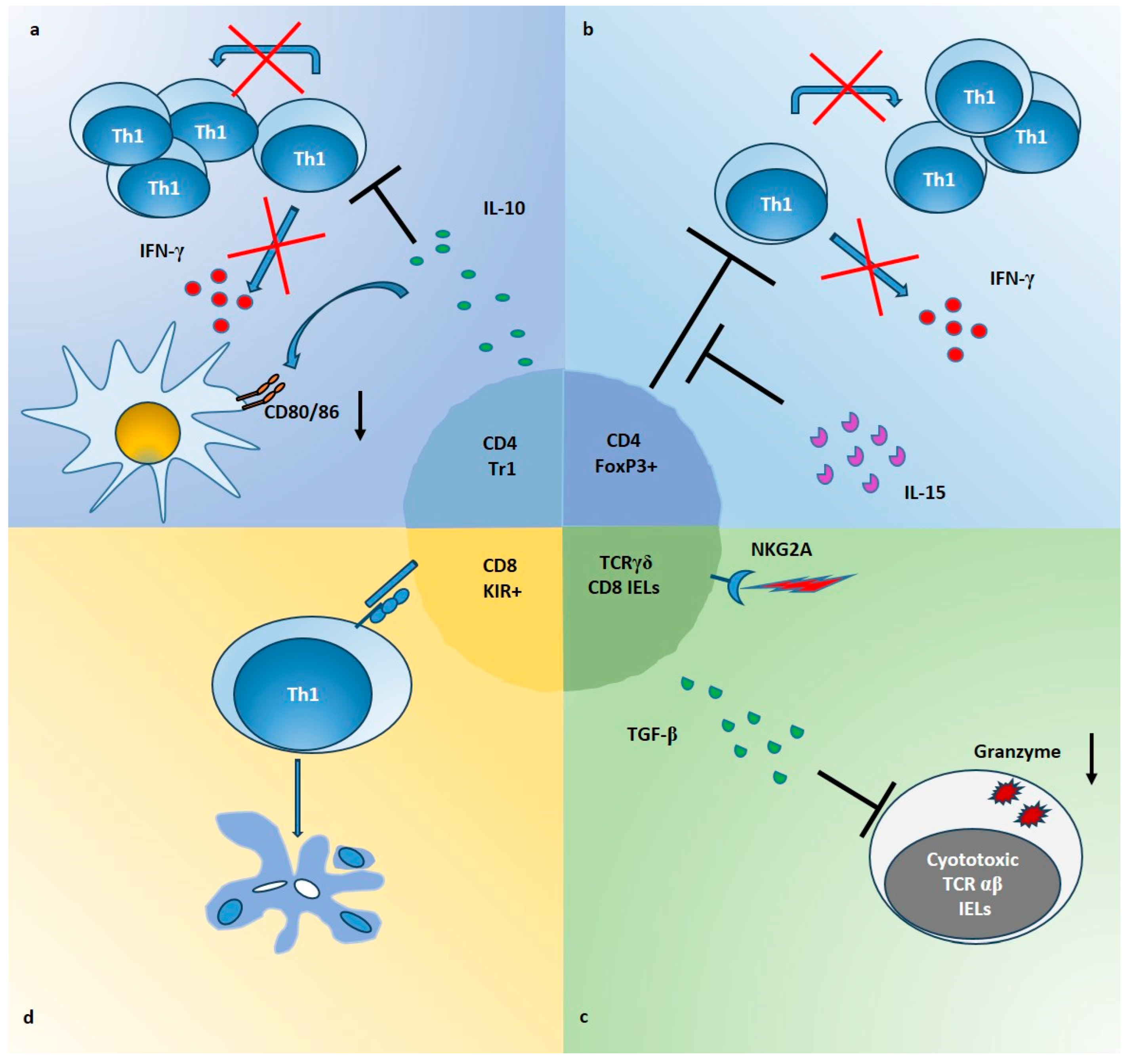
3. Regulatory T-Cell Populations in CeD
- (1)
- Production of inhibitory cytokines such as IL-10, TGF-β and IL-35;
- (2)
- Direct cytotoxic activity via granzyme A/B and perforin;
- (3)
- Inhibition by cell–cell contact through co-inhibitory receptors including CTLA4, PDL1, LAG3, TIGIT, TIM3, NKG2A;
- (4)
- Metabolic perturbation of T effector cells by subtraction of IL-2, production of adenosine via CD73 and CD39 ATP-ectoenzymes, induction of IDO in dendritic cells (DCs) and others.
3.1. Type 1 Regulatory T Cells
3.2. Foxp3+ Treg Cells
3.3. CD8 T Lymphocytes with Regulatory Activity
4. Regulatory T Cells in the Context of CeD Pathogenesis
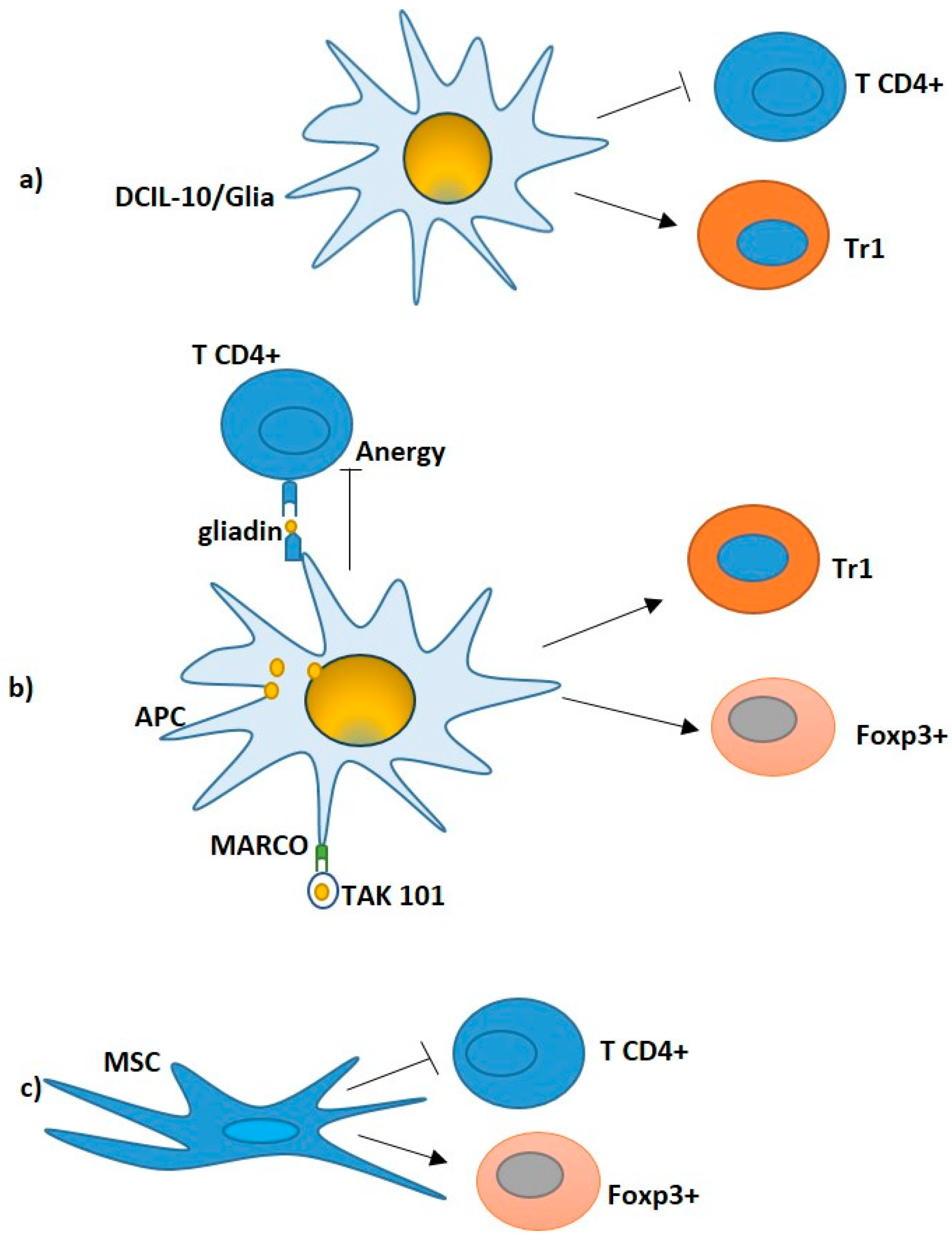
5. Therapeutic Applications of Treg Cells in CeD
- -
- Approaches based on in vivo administration of drugs such as rapamycin, or biologicals, such as IL-10 or low-dose IL-2, to suppress Teff cells and promote Tregs.
- -
- Approaches resting on the administration of the autoantigen by using lentiviral vectors or antigen-specific nanoparticles, promoting tolerogenic cells and the expansion of Treg cells.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iversen, R.; Sollid, L.M. The Immunobiology and Pathogenesis of Celiac Disease. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Voisine, J.; Abadie, V. Interplay Between Gluten, HLA, Innate and Adaptive Immunity Orchestrates the Development of Coeliac Disease. Front. Immunol. 2021, 12, 674313. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Muratori, P.; Cassani, F.; Pappas, G.; Muratori, L.; Agostinelli, D.; Veronesi, L.; Bortolotti, R.; Petrolini, N.; Bianchi, F.B.; et al. Anti-actin IgA antibodies in severe coeliac disease. Clin. Exp. Immunol. 2004, 137, 386–392. [Google Scholar] [CrossRef]
- Cervio, E.; Volta, U.; Verri, M.; Boschi, F.; Pastoris, O.; Granito, A.; Barbara, G.; Parisi, C.; Felicani, C.; Tonini, M.; et al. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 2007, 133, 195–206. [Google Scholar] [CrossRef]
- Schuppan, D.; Ciccocioppo, R. Coeliac disease and secondary autoimmunity. Dig. Liver Dis. 2002, 34, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Zauli, D.; Muratori, P.; Muratori, L.; Grassi, A.; Bortolotti, R.; Petrolini, N.; Veronesi, L.; Gionchetti, P.; Bianchi, F.B.; et al. Anti-Saccharomyces cerevisiae and perinuclear anti-neutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment. Pharmacol. Ther. 2005, 21, 881–887. [Google Scholar] [CrossRef]
- Mazzarella, G. Effector and Suppressor T cells in Celiac Disease. World J. Gastroenterol. 2015, 21, 7349–7356. [Google Scholar] [CrossRef]
- Castellanos-Rubio, A.; Santin, I.; Irastorza, I.; Castaño, L.; Vitoria, J.C.; Bilbao, J.R. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity 2009, 42, 69–73. [Google Scholar] [CrossRef]
- Monteleone, I.; Sarra, M.; Blanco, G.D.V.; Paoluzi, O.A.; Franzè, E.; Fina, D.; Fabrizi, A.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Characterization of IL-17A–producing cells in celiac disease mucosa. J. Immunol. (Baltim. Md. 1950) 2010, 184, 2211–2218. [Google Scholar] [CrossRef]
- Lahdenperä, A.I.; Fälth-Magnusson, K.; Högberg, L.; Ludvigsson, J.; Vaarala, O. Expression pattern of T-helper 17 cell signaling pathway and mucosal inflammation in celiac disease. Scand. J. Gastroenterol. 2013, 49, 145–156. [Google Scholar] [CrossRef]
- Fernández, S.; Molina, I.J.; Romero, P.; González, R.; Peña, J.; Sánchez, F.; Reynoso, F.R.; Pérez-Navero, J.L.; Estevez, O.; Ortega, C.; et al. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am. J. Gastroenterol. 2011, 106, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Lahat, N.; Shapiro, S.; Karban, A.; Gerstein, R.; Kinarty, A.; Lerner, A. Cytokine profile in coeliac disease. Scand. J. Immunol. 1999, 49, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T.; Ulfgren, A.K.; Lindroos, E.; DannAEus, A.; Dahlbom, I.; Klareskog, L. Transforming growth factor-β (TGF-β) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand. J. Immunol. 2002, 56, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Salvati, V.M.; Mazzarella, G.; Gianfrani, C.; Levings, M.K.; Stefanile, R.; De Giulio, B.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; Roncarolo, M.G.; et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut 2005, 54, 46–53. [Google Scholar] [CrossRef]
- Borrelli, M.; Salvati, V.M.; Maglio, M.; Zanzi, D.; Ferrara, K.; Santagata, S.; Ponticelli, D.; Aitoro, R.; Mazzarella, G.; Lania, G.; et al. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. Am. J. Gastroenterol. 2013, 108, 1775–1784. [Google Scholar] [CrossRef]
- Forsberg, G.; Hernell, O.; Hammarström, S.; Hammarström, M.-L. Concomitant increase of IL-10 and pro-inflammatory cytokines in intraepithelial lymphocyte subsets in celiac disease. Int. Immunol. 2007, 19, 993–1001. [Google Scholar] [CrossRef]
- Forsberg, G.; Hernell, O.; Melgar, S.; Israelsson, A.; Hammarström, S.; Hammarström, M. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 2002, 123, 667–678. [Google Scholar] [CrossRef]
- Hmida, N.B.; Ben Ahmed, M.; Moussa, A.; Rejeb, M.B.; Said, Y.; Kourda, N.; Meresse, B.; Abdeladhim, M.; Louzir, H.; Cerf-Bensussan, N. Impaired control of effector T cells by regulatory t cells: A clue to loss of oral tolerance and autoimmunity in celiac disease? Am. J. Gastroenterol. 2012, 107, 604–611. [Google Scholar] [CrossRef]
- Faghih, M.; Barartabar, Z.; Nasiri, Z.; Rostami-Nejad, M. The role of Th1 and Th17 in the pathogenesis of celiac disease. Gastroenterol. Hepatol. Open Access 2018, 9, 83–87. [Google Scholar]
- Meresse, B.; Ripoche, J.; Heyman, M.; Cerf-Bensussan, N. Celiac disease: From oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009, 2, 8–23. [Google Scholar] [CrossRef]
- Brazowski, E.; Cohen, S.; Yaron, A.; Filip, I.; Eisenthal, A. FOXP3 expression in duodenal mucosa in pediatric patients with celiac disease. Pathobiology 2010, 77, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, C.; Levings, M.K.; Sartirana, C.; Mazzarella, G.; Barba, G.; Zanzi, D.; Camarca, A.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; et al. Gliadin-specific type 1 regulatory T cells from the intestinal mucosa of treated celiac patients inhibit pathogenic T cells. J. Immunol. 2006, 177, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Zanzi, D.; Stefanile, R.; Santagata, S.; Iaffaldano, L.; Iaquinto, G.; Giardullo, N.; Lania, G.; Vigliano, I.; Vera, A.R.; Ferrara, K.; et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am. J. Gastroenterol. 2011, 106, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, M.; Meresse, B.; Arnulf, B.; Barbe, U.; Mention, J.; Verkarre, V.; Allez, M.; Cellier, C.; Hermine, O.; Cerf–Bensussan, N. Inhibition of TGF-β signaling by IL-15: A new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology 2007, 132, 994–1008. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. (Baltim. Md. 1950) 1991, 146, 3444–3451. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef]
- Buelens, C.; Willems, F.; Delvaux, A.; Piérard, G.; Delville, J.P.; Velu, T.; Goldman, M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur. J. Immunol. 1995, 25, 2668–2672. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Yssel, H.; de Vries, J.E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 1993, 150, 4754–4765. [Google Scholar] [CrossRef]
- Taga, K.; Mostowski, H.; Tosato, G. Human interleukin-10 can directly inhibit T-cell growth. Blood 1993, 81, 2964–2971. [Google Scholar] [CrossRef]
- Groux, H.; Bigler, M.; de Vries, J.E.; Roncarolo, M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996, 184, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Pender, S.L.; Breese, E.J.; Günther, U.; Howie, D.; Wathen, N.C.; Schuppan, D.; MacDonald, T.T. Suppression of T cell–mediated injury in human gut by interleukin 10: Role of matrix metalloproteinases. Gastroenterology 1998, 115, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.M.; Jahnsen, F.L.; Lundin, K.E.; Johansen, F.E.; Fausa, O.; Sollid, L.M.; Jahnsen, J.; Scott, H.; Brandtzaeg, P. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 1998, 115, 551–563. [Google Scholar] [CrossRef]
- Beckett, C.G.; Dell'Olio, D.; Kontakou, M.; Przemioslo, R.T.; Rosen-Bronson, S.; Ciclitira, P.J. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut 1996, 39, 818–823. [Google Scholar] [CrossRef] [PubMed]
- van de Wal, Y.; Kooy, Y.; van Veelen, P.; Peña, S.; Mearin, L.; Papadopoulos, G.; Koning, F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. (Baltim. Md. 1950) 1998, 161, 1585–1588. [Google Scholar] [CrossRef]
- Camarca, A.; Del Mastro, A.; Gianfrani, C. Repertoire of gluten peptides active in celiac disease patients: Perspectives for translational therapeutic applications. Endocrine Metab. Immune Disord. Drug Targets 2012, 12, 207–219. [Google Scholar] [CrossRef]
- Ráki, M.; Dahal-Koirala, S.; Yu, H.; Korponay-Szabó, I.R.; Gyimesi, J.; Castillejo, G.; Jahnsen, J.; Qiao, S.W.; Sollid, L.M. Similar Responses of Intestinal T Cells from Untreated Children and Adults with Celiac Disease to Deamidated Gluten Epitopes. Gastroenterology 2017, 153, 787–798. [Google Scholar] [CrossRef]
- Gregori, S.; Tomasoni, D.; Pacciani, V.; Scirpoli, M.; Battaglia, M.; Magnani, C.F.; Hauben, E.; Roncarolo, M.G. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10–dependent ILT4/HLA-G pathway. Blood 2010, 116, 935–944. [Google Scholar] [CrossRef]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-β-mediated negative regulation of gut inflammation. Trends Immunol. 2004, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Feagins, L.A. Role of transforming growth factor-b in inflammatory bowel disease and colitis-associated colon cancer. Inflamm. Bowel Dis. 2010, 16, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Rotondi Aufiero, V.; Marena, P.; Venezia, A.; Troncone, R.; Auricchio, S.; Mazzarella, G. Laser Capture Microdissection as a Tool to Study the Mucosal Immune Response in Celiac Disease. Methods Mol. Biol. 2018, 1723, 139–154. [Google Scholar] [CrossRef]
- Bhagat, G.; Naiyer, A.J.; Shah, J.G.; Harper, J.; Jabri, B.; Wang, T.C.; Green, P.H.; Manavalan, J.S. Small intestinal CD8+TCRγδ+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J. Clin. Investig. 2008, 118, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fang, Q.; Zheng, S.-G. Regulatory T Cells: Concept, Classification, Phenotype, and Biological Characteristics. Adv. Exp. Med. Biol. 2021, 1278, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Sartirana, C.; Levings, M.; Bordignon, C.; Narula, S.; Roncarolo, M.-G. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur. J. Immunol. 2002, 32, 2237–2245. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef]
- Freeborn, R.A.; Strubbe, S.; Roncarolo, M.G. Type 1 regulatory T cell-mediated tolerance in health and disease. Front. Immunol. 2022, 13, 1032575. [Google Scholar] [CrossRef]
- Cook, L.; Stahl, M.; Han, X.; Nazli, A.; MacDonald, K.N.; Wong, M.Q.; Tsai, K.; Dizzell, S.; Jacobson, K.; Bressler, B.; et al. Suppressive and Gut-Reparative Functions of Human Type 1 T Regulatory Cells. Gastroenterology 2019, 157, 1584–1598. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Du Pré, M.F.; Kozijn, A.E.; van Berkel, L.A.; ter Borg, M.N.; Lindenbergh–Kortleve, D.; Jensen, L.T.; Kooy-Winkelaar, Y.; Koning, F.; Boon, L.; Nieuwenhuis, E.E.; et al. Tolerance to ingested deamidated gliadin in mice is maintained by splenic, type 1 regulatory T cells. Gastroenterology 2011, 141, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Tiittanen, M.; Westerholm-Ormio, M.; Verkasalo, M.; Savilahti, E.; Vaarala, O. Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clin. Exp. Immunol. 2008, 152, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Vorobjova, T.; Uibo, O.; Heilman, K.; Rägo, T.; Honkanen, J.; Vaarala, O.; Tillmann, V.; Ojakivi, I.; Uibo, R. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand. J. Gastroenterol. 2009, 44, 422–430. [Google Scholar] [CrossRef]
- Granzotto, M.; dal Bo, S.; Quaglia, S.; Tommasini, A.; Piscianz, E.; Valencic, E.; Ferrara, F.; Martelossi, S.; Ventura, A.; Not, T. Regulatory T-cell function is impaired in celiac disease. Dig. Dis. Sci. 2008, 54, 1513–1519. [Google Scholar] [CrossRef]
- Frisullo, G.; Nociti, V.; Iorio, R.; Patanella, A.K.; Marti, A.; Assunta, B.; Plantone, D.; Cammarota, G.; Tonali, P.A.; Batocchi, A.P. Increased CD4+CD25+Foxp3+ T cells in peripheral blood of celiac disease patients: Correlation with dietary treatment. Hum. Immunol. 2009, 70, 430–435. [Google Scholar] [CrossRef]
- van Leeuwen, M.A.; du Pré, M.F.; van Wanrooij, R.L.; de Ruiter, L.F.; Raatgeep, H.R.; Lindenbergh-Kortleve, D.J.; Mulder, C.J.; de Ridder, L.; Escher, J.C.; Samsom, J.N. Changes in natural Foxp3(+)Treg but not mucosally-imprinted CD62L(neg)CD38(+)Foxp3(+)Treg in the circulation of celiac disease patients. PLoS ONE 2013, 8, e68432. [Google Scholar] [CrossRef]
- Åkesson, K.; Tompa, A.; Rydén, A.; Faresjö, M. Low expression of CD39[+] /CD45RA[+] on regulatory T cells [Treg ] cells in type 1 diabetic children in contrast to high expression of CD101[+] /CD129[+] on Treg cells in children with coeliac disease. Clin. Exp. Immunol. 2015, 180, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, A.; Risnes, L.F.; Bergseng, E.; Lundin, K.E.; Sollid, L.M.; Qiao, S.-W. Healthy HLA-DQ2.5+ Subjects Lack Regulatory and Memory T Cells Specific for Immunodominant Gluten Epitopes of Celiac Disease. J. Immunol. (Baltim. Md. 1950) 2016, 196, 2819–2826. [Google Scholar] [CrossRef]
- Cook, L.; Munier, C.M.L.; Seddiki, N.; van Bockel, D.; Ontiveros, N.; Hardy, M.Y.; Gillies, J.K.; Levings, M.K.; Reid, H.H.; Petersen, J.; et al. Circulating gluten-specific FOXP3 + CD39 + regulatory T cells have impaired suppressive function in patients with celiac disease. J. Allergy Clin. Immunol. 2017, 140, 1592–1603. [Google Scholar] [CrossRef]
- Kumar, S.; Lal, S.; Bhatnagar, A. Regulatory T cell subsets in peripheral blood of celiac disease patients and TLR2 expression: Correlation with oxidative stress. APMIS 2017, 125, 888–901. [Google Scholar] [CrossRef]
- Asri, N.; Rostami-Nejad, M.; Nikzamir, A.; Aghamohamadi, E.; Asadzadeh-Aghdaei, H.; Zali, M.R. Reduced frequency of circulating regulatory T cells and their related immunosuppressive mediators in treated celiac patients. Mol. Biol. Rep. 2022, 49, 8527–8535. [Google Scholar] [CrossRef]
- Sanchez-Solares, J.; Sanchez, L.; Pablo-Torres, C.; Diaz-Fernandez, C.; Sørensen, P.; Barber, D.; Gomez-Casado, C. Celiac Disease Causes Epithelial Disruption and Regulatory T Cell Recruitment in the Oral Mucosa. Front. Immunol. 2021, 12, 623805. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, M.A.; Costes, L.M.M.; van Berkel, L.A.; Simons-Oosterhuis, Y.; du Pré, M.F.; Kozijn, A.E.; Raatgeep, H.C.; Lindenbergh-Kortleve, D.J.; van Rooijen, N.; Koning, F.; et al. Macrophage-mediated gliadin degradation and concomitant IL-27 production drive IL-10- and IFN-γ-secreting Tr1-like-cell differentiation in a murine model for gluten tolerance. Mucosal Immunol. 2017, 10, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Scheinecker, C.; Göschl, L.; Bonelli, M. Treg cells in health and autoimmune diseases: New insights from single cell analysis. J. Autoimmun. 2019, 110, 102376. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M.; Gallimore, A.; Godkin, A.; Gouttefangeas, C.; de Gruijl, T.D.; Koenen, H.J.P.M.; et al. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immunother. 2015, 64, 1271–1286. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2016, 27, 109–118. [Google Scholar] [CrossRef]
- Szymczak-Workman, A.L.; Workman, C.J.; Vignali, D.A. Cutting edge: Regulatory T cells do not require stimulation through their TCR to suppress. J. Immunol. 2009, 182, 5188–5192. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Lu, W.; Sindhava, V.J.; Huang, Y.; Burkhardt, J.K.; Yang, E.; Riese, M.J.; Maltzman, J.S.; Jordan, M.S.; Kambayashi, T. Regulatory T cells require TCR signaling for their suppressive function. J. Immunol. 2015, 194, 4362–4370. [Google Scholar] [CrossRef]
- Vorobjova, T.; Uibo, O.; Heilman, K.; Uibo, R. Increased density of tolerogenic dendritic cells in the small bowel mucosa of celiac patients. World J. Gastroenterol. 2015, 21, 439–452. [Google Scholar] [CrossRef]
- Kivling, A.; Nilsson, L.; Fälth-Magnusson, K.; Söllvander, S.; Johanson, C.; Faresjö, M. Diverse Foxp3 expression in children with type 1 diabetes and CeD. Ann. N. Y. Acad. Sci. 2008, 1150, 273–727. [Google Scholar] [CrossRef] [PubMed]
- Serena, G.; Yan, S.; Camhi, S.; Patel, S.; Lima, R.S.; Sapone, A.; Leonard, M.M.; Mukherjee, R.; Nath, B.J.; Lammers, K.M.; et al. Proinflammatory cytokine interferon-γ and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin. Exp. Immunol. 2017, 187, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, M.; Belhadj Hmida, N.; Moes, N.; Buyse, S.; Abdeladhim, M.; Louzir, H.; Cerf-Bensussan, N. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J. Immunol. (Baltim. Md. 1950) 2009, 182, 6763–6770. [Google Scholar] [CrossRef]
- Halstensen, T.S.; Scott, H.; Brandtzaeg, P. Intraepithelial Tcells of theTcRγ/δ+ CD8− andVδ1/Jδ1+ phenotypes are increased in coeliac disease. Scand. J. Immunol. 1989, 30, 665–672. [Google Scholar] [CrossRef]
- Li, G.-Q.; Xia, J.; Zeng, W.; Luo, W.; Liu, L.; Zeng, X.; Cao, D. The intestinal γδ T cells: Functions in the gut and in the distant organs. Front. Immunol. 2023, 14, 1206299. [Google Scholar] [CrossRef]
- Hüe, S.; Mention, J.J.; Monteiro, R.C.; Zhang, S.; Cellier, C.; Schmitz, J.; Verkarre, V.; Fodil, N.; Bahram, S.; Cerf-Bensussan, N.; et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nanno, M.; Shiohara, T.; Yamamoto, H.; Kawakami, K.; Ishikawa, H. γδ T cells: Firefighters or fire boosters in the front lines of inflammatory responses. Immunol. Rev. 2007, 215, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Inagaki-Ohara, K.; Chinen, T.; Matsuzaki, G.; Sasaki, A.; Sakamoto, Y.; Hiromatsu, K.; Nakamura-Uchiyama, F.; Nawa, Y.; Yoshimura, A. mucosal T cells bearing TCRγδ play a protective role in intestinal inflammation. J. Immunol. (Baltim. Md. 1950) 2004, 173, 1390–1398. [Google Scholar] [CrossRef]
- Mishra, S.; Srinivasan, S.; Ma, C.; Zhang, N. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 708874. [Google Scholar] [CrossRef]
- Shytikov, D.; Rohila, D.; Li, D.; Wang, P.; Jiang, M.; Zhang, M.; Xu, Q.; Lu, L. Functional Characterization of Ly49+CD8 T-Cells in Both Normal Condition and during Anti-Viral Response. Front. Immunol. 2021, 11, 602783. [Google Scholar] [CrossRef]
- Saligrama, N.; Zhao, F.; Sikora, M.J.; Serratelli, W.S.; Fernandes, R.A.; Louis, D.M.; Yao, W.; Ji, X.; Idoyaga, J.; Mahajan, V.B.; et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 2019, 572, 481–487. [Google Scholar] [CrossRef]
- Li, J.; Zaslavsky, M.; Su, Y.; Guo, J.; Sikora, M.J.; van Unen, V.; Christophersen, A.; Chiou, S.H.; Chen, L.; Li, J.; et al. KIR+ CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 2022, 376, eabi9591. [Google Scholar] [CrossRef]
- Levescot, A.; Cerf-Bensussan, N. Regulatory CD8+ T cells suppress disease. Science 2022, 376, 243–244. [Google Scholar] [CrossRef]
- Mazzarella, G.; Stefanile, R.; Camarca, A.; Giliberti, P.; Cosentini, E.; Marano, C.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; Sette, A.; et al. Gliadin activates HLA class I-restricted CD8+ T cells in celiac disease intestinal mucosa and induces the enterocyte apoptosis. Gastroenterology 2008, 134, 1017–1027. [Google Scholar] [CrossRef]
- Arellano, B.; Graber, D.J.; Sentman, C.L. Regulatory T cell-based therapies for autoimmunity. Discov. Med. 2016, 22, 73–80. [Google Scholar] [PubMed]
- Wang, S.; Zou, X.; Zhang, Y.; Wang, X.; Yang, W.; Li, Y. The generation and regulation of tissue-resident tregs and their role in autoimmune diseases. J. Immunol. Res. 2020, 2020, 8815280. [Google Scholar] [CrossRef]
- Esensten, J.H.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J. Allergy Clin. Immunol. 2018, 142, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef]
- Harris, D.T.; Kranz, D.M. Adoptive T Cell Therapies: A Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol. Sci. 2016, 37, 220–230. [Google Scholar] [CrossRef]
- Dawson, N.A.J.; Levings, M.K. Antigen-specific regulatory T cells: Are police CARs the answer? Transl. Res. 2017, 187, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415. [Google Scholar] [CrossRef] [PubMed]
- Giannoukakis, N.; Phillips, B.; Finegold, D.; Harnaha, J.; Trucco, M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011, 34, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2020, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Inaba, K.; Tarbell, K.V.; Steinman, R.M. Dendritic cells expand antigen-specific Foxp3(+)CD25(+)CD4(+) regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 2006, 212, 314–329. [Google Scholar] [CrossRef]
- Passeri, L.; Andolfi, G.; Bassi, V.; Russo, F.; Giacomini, G.; Laudisa, C.; Marrocco, I.; Cesana, L.; Di Stefano, M.; Fanti, L.; et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J. Autoimmun. 2023, 138, 103051. [Google Scholar] [CrossRef]
- Getts, D.R.; Martin, A.J.; McCarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J.; et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224. [Google Scholar] [CrossRef]
- Prasad, S.; Neef, T.; Xu, D.; Podojil, J.R.; Getts, D.R.; Shea, L.D.; Miller, S.D. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J. Autoimmun. 2018, 89, 112–124. [Google Scholar] [CrossRef]
- Hunter, Z.; McCarthy, D.P.; Yap, W.T.; Harp, C.T.; Getts, D.R.; Shea, L.D.; Miller, S.D. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 2014, 8, 2148–2160. [Google Scholar] [CrossRef]
- McCarthy, D.P.; Yap, J.W.; Harp, C.T.; Song, W.K.; Chen, J.; Pearson, R.M.; Miller, S.D.; Shea, L.D. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 191–200. [Google Scholar] [CrossRef]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Deffrasnes, C.; Müller, M.; van Vreden, C.; Ashhurst, T.M.; Chami, B.; McCarthy, D.; Wu, H.; et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014, 6, 219ra7. [Google Scholar] [CrossRef] [PubMed]
- Jamison, B.L.; Neef, T.; Goodspeed, A.; Bradley, B.; Baker, R.L.; Miller, S.D.; Haskins, K. Nanoparticles Containing an Insulin–ChgA Hybrid Peptide Protect from Transfer of Autoimmune Diabetes by Shifting the Balance between Effector T Cells and Regulatory T Cells. J. Immunol. (Baltim. Md. 1950) 2019, 203, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Freitag, T.L.; Podojil, J.R.; Pearson, R.M.; Fokta, F.J.; Sahl, C.; Messing, M.; Andersson, L.C.; Leskinen, K.; Saavalainen, P.; Hoover, L.I.; et al. Gliadin Nanoparticles Induce Immune Tolerance to Gliadin in Mouse Models of Celiac Disease. Gastroenterology 2020, 158, 1667–1681. [Google Scholar] [CrossRef]
- Freitag, T.L.; Rietdijk, S.; Junker, Y.; Popov, Y.; Bhan, A.K.; Kelly, C.P.; Terhorst, C.; Schuppan, D. Gliadin-primed CD4+CD45RBlowCD25- T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut 2009, 58, 1597–1605. [Google Scholar] [CrossRef]
- Kelly, C.P.; Murray, J.A.; Leffler, D.A.; Getts, D.R.; Bledsoe, A.C.; Smithson, G.; First, M.R.; Morris, A.; Boyne, M.; Elhofy, A.; et al. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021, 161, 66–80. [Google Scholar] [CrossRef]
- Drucker, N.A.; McCulloh, C.J.; Li, B.; Pierro, A.; Besner, G.E.; Markel, T.A. Stem cell therapy in necrotizing enterocolitis: Current state and future directions. Semin. Pediatr. Surg. 2018, 27, 57–64. [Google Scholar] [CrossRef]
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z.; et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013, 65, 1181–1193. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef]
- Si, Y.; Zhao, Y.; Hao, H.; Liu, J.; Guo, Y.; Mu, Y.; Shen, J.; Cheng, Y.; Fu, X.; Han, W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes 2012, 61, 1616–1625. [Google Scholar] [CrossRef]
- Jones, J.; Estirado, A.; Redondo, C.; Pacheco-Torres, J.; Sirerol-Piquer, M.-S.; Garcia-Verdugo, J.M.; Martinez, S. Mesenchymal stem cells improve motor functions and decrease neurodegeneration in ataxic mice. Mol. Ther. 2015, 23, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Huang, H.; Sun, P.; Ma, S.Z.; Liu, A.H.; Xue, J.; Fu, J.H.; Liang, Y.Q.; Liu, B.; Wu, D.Y.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016, 5, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Rehorova, M.; Vargova, I.; Forostyak, S.; Vackova, I.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodička, P.; Kubinová, Š.; Syková, E.; Jendelová, P. A combination of intrathecal and intramuscular application of human mesenchymal stem cells partly reduces the activation of necroptosis in the spinal cord of SOD1(G93A) rats. Stem Cells Transl. Med. 2019, 8, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Soria, B.; Martin-Montalvo, A.; Aguilera, Y.; Mellado-Damas, N.; López-Beas, J.; Herrera-Herrera, I.; López, E.; Barcia, J.A.; Alvarez-Dolado, M.; Hmadcha, A.; et al. Human Mesenchymal Stem Cells Prevent Neurological Complications of Radiotherapy. Front. Cell. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef]
- Azevedo, R.I.; Minskaia, E.; Fernandes-Platzgummer, A.; Vieira, A.I.S.; da Silva, C.L.; Cabral, J.M.S.; Lacerda, J.F. Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells 2020, 38, 1007–1019. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Calliada, F.; et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Camarca, A.; Cangemi, G.C.; Radano, G.; Vitale, S.; Betti, E.; Ferrari, D.; Visai, L.; Strada, E.; Badulli, C.; et al. Tolerogenic effect of mesenchymal stromal cells on gliadin-specific T lymphocytes in celiac disease. Cytotherapy 2014, 16, 1080–1091. [Google Scholar] [CrossRef]
| Treg Cell Phenotype | Patients/ Specimens | Main Findings | Ref. |
|---|---|---|---|
| Tr1 cells (IL10hi, IFN-γpos, IL4low/neg, IL-2low/neg ) | Untreated and treated adults/ Duodenal mucosa | Suppression of gliadin-specific Th0/Th1 response by Tr1 cells. | [22] |
| CD25+Foxp3+ | Children with Overt or potential CeD/ Duodenal mucosa | Increase in Foxp3 mRNA, CD25+ cells, and FoxP3+ cells. | [52] |
| CD25+Foxp3+ | CeD children with or without T1D/ Duodenal mucosa | Increase in Foxp3 mRNA, CD25+ cells, and Foxp3+ cells in partial or subtotal villus atrophy. | [53] |
| CD4+CD25+Foxp3+ | Children/ Peripheral Blood | Impaired suppression activity of Treg. | [54] |
| CD4+CD25+Foxp3+ | Untreated and treated adults/ Peripheral blood | Increase in CD4+CD25+Foxp3+ frequency in untreated. | [55] |
| CD4+CD25+Foxp3+ | Untreated and treated Adults/ Duodenal mucosa and peripheral blood | Increase in CD4+CD25+Foxp3+ in untreated. Gliadin-dependent expansion of Foxp3 Treg. Inhibition of Treg suppression activity by IL-15. | [23] |
| CD4+CD25+Foxp3+ | Untreated and treated adults/ Duodenal mucosa and peripheral blood | Increase in FoxP3 mRNA and CD4+CD25+Foxp3+ LPLs in biopsies of untreated. Resistance of LPLs and IELs to the suppressive activity of peripheral blood Tregs. | [18] |
| CD4+CD25+Foxp3+ | Children with overt or potential CeD/ Duodenal mucosa and peripheral blood | Increase in mucosal Foxp3+CD25+CD4+ cells. No influence of IL-15 on intestinal Tregs from potCeD. | [15] |
| CD62L+Foxp3+ nTreg and CD62-CD38+Foxp3+ iTreg | CeD children and treated or refractory adults/ Duodenal mucosa and peripheral blood | Increase in circulating nTreg in adult treated CeD and RCeD. Increase in Foxp3 + cells in LPLs of children and adult CeD. | [56] |
| CD4+CD25+Foxp3highCD127low | Children with or without T1D/ Peripheral blood | No differences. | [57] |
| CD4+Foxp3+CD25+ T cells and IL-10highCD4 Tr1 cells | HLA-DQ2.5+ healthy and CeD subjects/ Duodenal mucosa and peripheral blood | No detection of Foxp3+ and of Tr1 cells within gluten-tetramer binding CD4+ T cells. | [58] |
| CD39+Foxp3+ CD4+ T cells | Treated adults undergone to SGC/ Peripheral blood | Increase in circulating CD39+Foxp3+ CD4+ Treg cells after gluten challenge. Impaired suppressive function of gluten-specific Treg cells. | [59] |
| CD4+Foxp3+CD25+ nTreg and CD4+Foxp3+ iTreg | Untreated and treated Children/ Peripheral blood | Decrease in nTreg and iTreg frequency in both untreated and treated. | [60] |
| CD4+CD25+Foxp3+ | Treated adults/ Duodenal mucosa and peripheral blood | Increase in CD25 and Foxp3 mRNA expression in duodenal mucosa. | [61] |
| CD4+Foxp3+ | Untreated and treated Adults/ Oral mucosa | Increase in Foxp3+ cells. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarca, A.; Rotondi Aufiero, V.; Mazzarella, G. Role of Regulatory T Cells and Their Potential Therapeutic Applications in Celiac Disease. Int. J. Mol. Sci. 2023, 24, 14434. https://doi.org/10.3390/ijms241914434
Camarca A, Rotondi Aufiero V, Mazzarella G. Role of Regulatory T Cells and Their Potential Therapeutic Applications in Celiac Disease. International Journal of Molecular Sciences. 2023; 24(19):14434. https://doi.org/10.3390/ijms241914434
Chicago/Turabian StyleCamarca, Alessandra, Vera Rotondi Aufiero, and Giuseppe Mazzarella. 2023. "Role of Regulatory T Cells and Their Potential Therapeutic Applications in Celiac Disease" International Journal of Molecular Sciences 24, no. 19: 14434. https://doi.org/10.3390/ijms241914434





