Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients?
Abstract
:1. Introduction
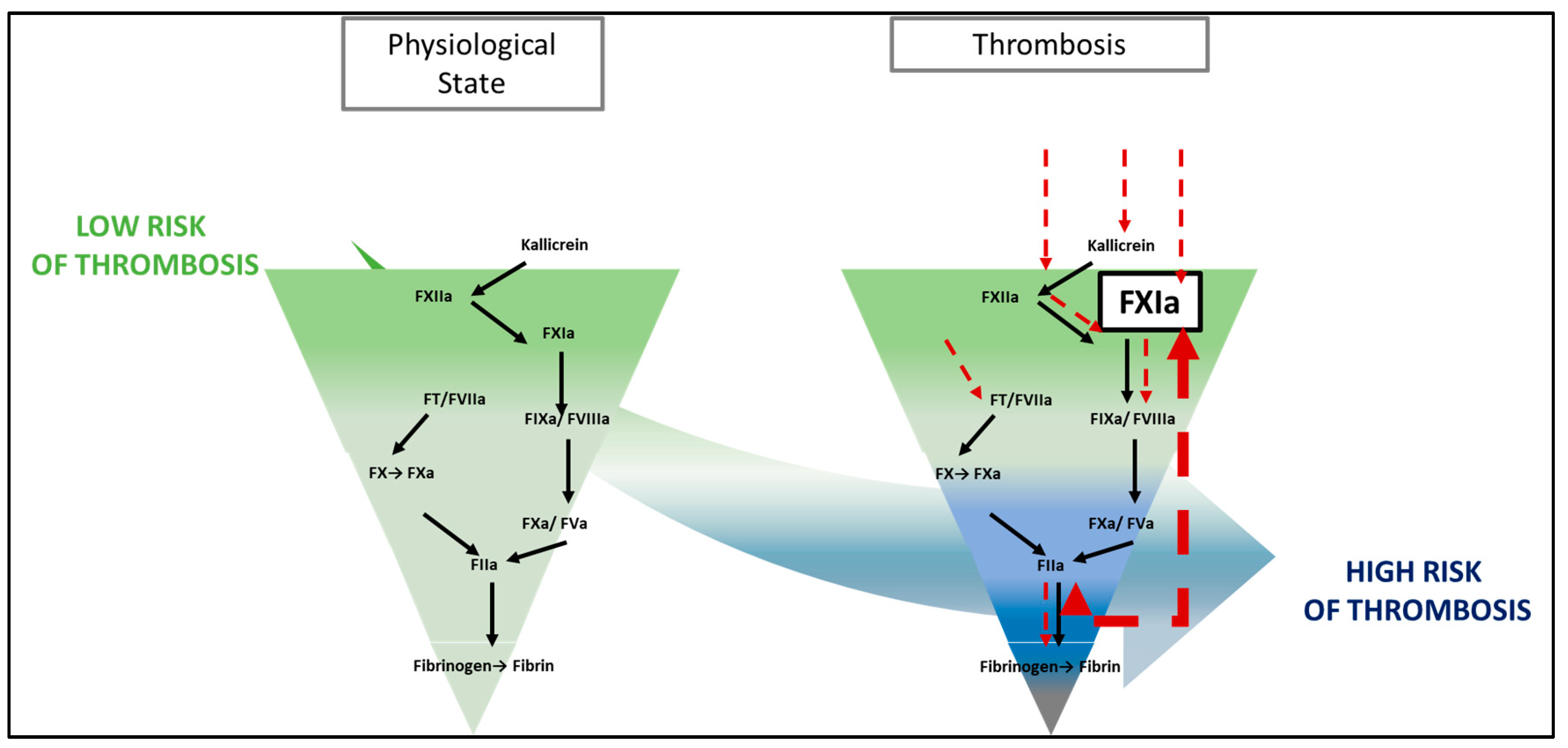
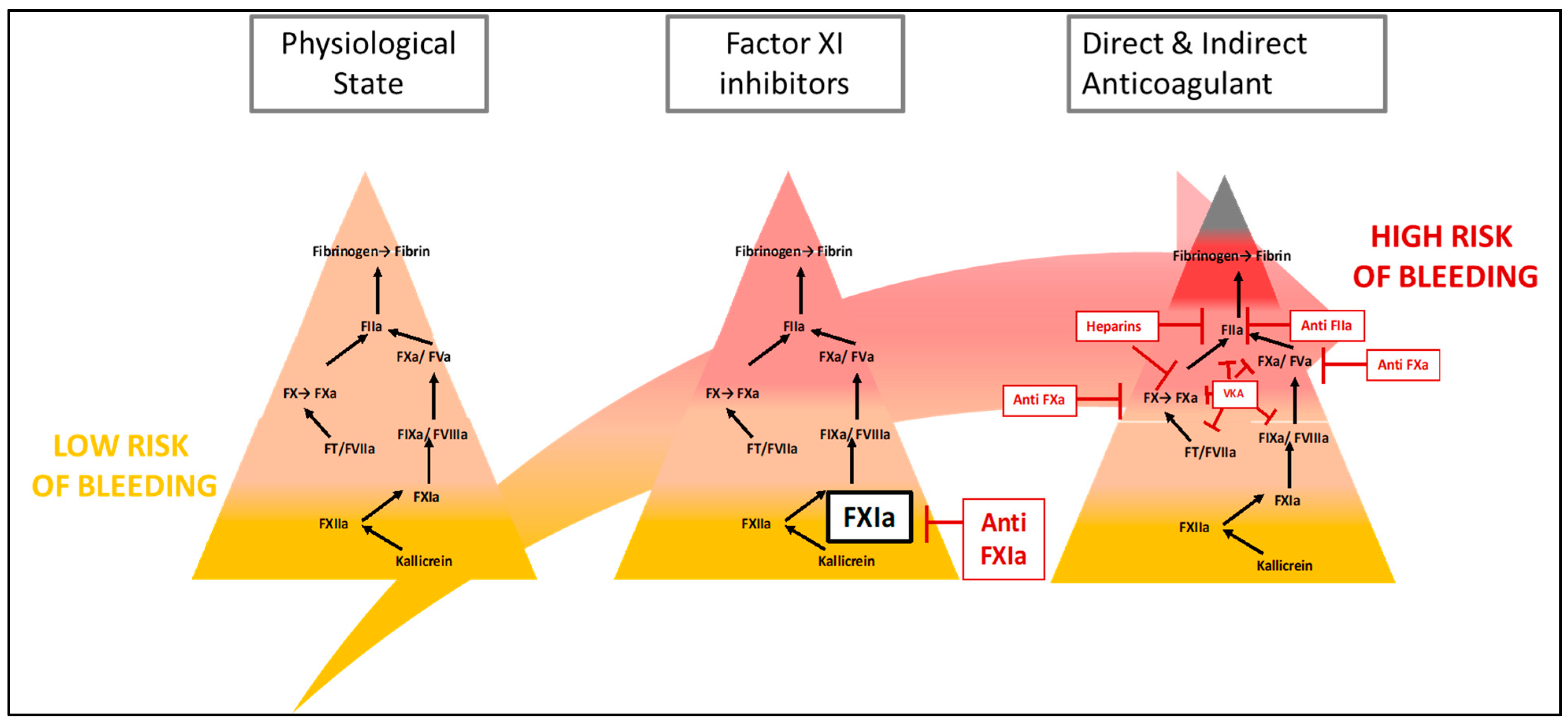
2. General Information about FXI Inhibitors
2.1. The Inhibition of Contact Pathway as a New Therapeutic Target
2.2. FXI Inhibitor Monitoring
2.3. Antidote
3. Potential of FXI Inhibitors in Venous Thromboembolic Events
3.1. Physiopathology of Venous Thrombosis
3.2. Management of Cancer-Associated Thrombosis
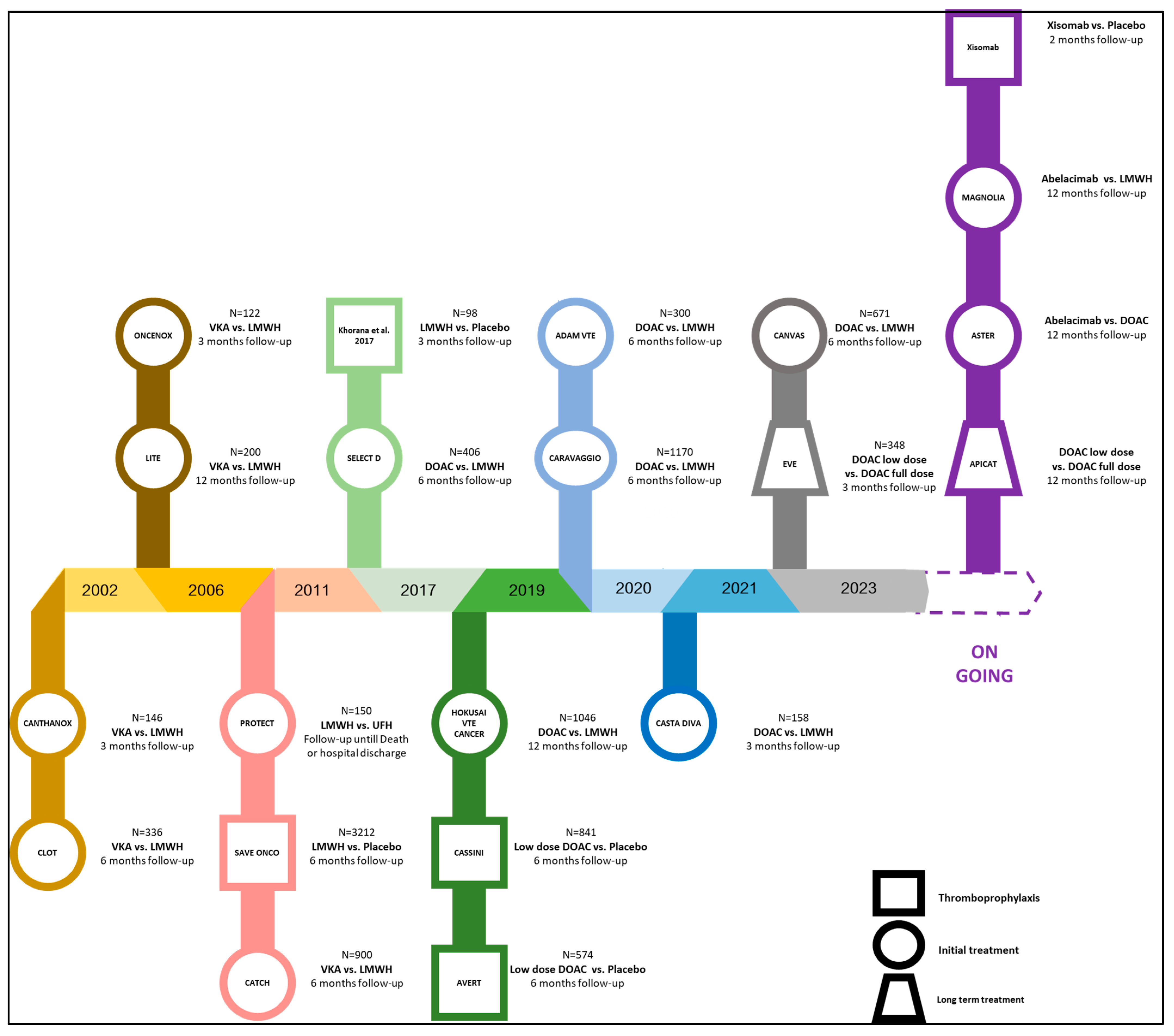
3.3. FXI Inhibitors to Prevent Venous Thromboembolic Events
3.4. FXI Inhibitors to Treat Venous Thromboembolic Events
3.5. FXI Inhibitors to Prevent Catheter-Related Thrombosis
4. Other Clinical Needs of Cancer Patients
4.1. FXI Inhibitors to Prevent Arterial Thromboembolic Events
4.2. Chronic Complications of Cancer-Associated Thrombosis (Post-Thrombotic Syndrome, Chronic Thromboembolic Pulmonary Hypertension, and Recurrence)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heestermans, M.; Poenou, G.; Hamzeh-Cognasse, H.; Cognasse, F.; Bertoletti, L. Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells 2022, 11, 3214. [Google Scholar] [CrossRef]
- Link, K.P. The discovery of dicumarol and its sequels. Circulation 1959, 19, 97–107. [Google Scholar] [CrossRef]
- Mclean, J. The Discovery of Heparin. Circulation 1959, 19, 75–78. [Google Scholar] [CrossRef]
- Svendsen, E.; Karwinski, B. Prevalence of pulmonary embolism at necropsy in patients with cancer. J. Clin. Pathol. 1989, 42, 805–809. [Google Scholar] [CrossRef]
- Perzborn, E.; Roehrig, S.; Straub, A.; Kubitza, D.; Misselwitz, F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat. Rev. Drug Discov. 2011, 10, 61–75. [Google Scholar] [CrossRef]
- Sanchez, O.; Benhamou, Y.; Bertoletti, L.; Constans, J.; Couturaud, F.; Delluc, A.; Elias, A.; Fischer, A.-M.; Frappé, P.; Gendron, N.; et al. Recommandations de bonne pratique pour la prise en charge de la maladie veineuse thromboembolique chez l’adulte—Version longue. Rev. Mal. Respir. 2021, 38, e1–e6. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 Guidelines for Management of Venous Thromboembolism: Treatment of Deep Vein Thrombosis and Pulmonary Embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Pengo, V.; Denas, G.; Zoppellaro, G.; Jose, S.P.; Hoxha, A.; Ruffatti, A.; Andreoli, L.; Tincani, A.; Cenci, C.; Prisco, D.; et al. Rivaroxaban vs Warfarin in High-Risk Patients with Antiphospholipid Syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Weitz, J.I. Warfarin faring better: Vitamin K antagonists beat rivaroxaban and apixaban in the INVICTUS and PROACT Xa trials. J. Thromb. Haemost. 2023, S1538783623005238. [Google Scholar] [CrossRef]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020, 19, 333–352. [Google Scholar] [CrossRef]
- Hsu, C.; Hutt, E.; Bloomfield, D.M.; Gailani, D.; Weitz, J.I. Factor XI Inhibition to Uncouple Thrombosis From Hemostasis. J. Am. Coll. Cardiol. 2021, 78, 625–631. [Google Scholar] [CrossRef]
- Sharman Moser, S.; Chodick, G.; Ni, Y.G.; Chalothorn, D.; Wang, M.-D.; Shuldiner, A.R.; Morton, L.; Salomon, O.; Jalbert, J.J. The Association between Factor XI Deficiency and the Risk of Bleeding, Cardiovascular, and Venous Thromboembolic Events. Thromb. Haemost. 2022, 122, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Poenou, G.; Dimitru Dimitru, T.; Lafaie, L.; Mismetti, V.; Heestermans, M.; Bertoletti, L. Factor XI Inhibition for the Prevention of Venous Thromboembolism: An Update on Current Evidence and Future Perspectives. Vasc. Health Risk Manag. 2022, 18, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Ratnoff, O.D. Waterfall Sequence for Intrinsic Blood Clotting. Science 1964, 145, 1310–1312. [Google Scholar] [CrossRef]
- Ignjatovic, V. Activated partial thromboplastin time. Methods Mol. Biol. Clifton NJ 2013, 992, 111–120. [Google Scholar]
- Renné, T.; Pozgajová, M.; Grüner, S.; Schuh, K.; Pauer, H.-U.; Burfeind, P.; Gailani, D.; Nieswandt, B. Defective Thrombus Formation in Mice Lacking Coagulation Factor XII. J. Exp. Med. 2005, 202, 271–281. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, J.; Zhai, Z.; Yang, Y.; Wan, J.; Xie, W.; Wang, C. Comparison of Prediction Value of Four Bleeding Risk Scores for Pulmonary Embolism with Anticoagulation: A Real-World Study in Chinese Patients. Clin. Respir. J. 2019, 13, 139–147. [Google Scholar] [CrossRef]
- Cheng, Q.; Tucker, E.I.; Pine, M.S.; Sisler, I.; Matafonov, A.; Sun, M.; White-Adams, T.C.; Smith, S.A.; Hanson, S.R.; McCarty, O.J.T.; et al. A Role for Factor XIIa–Mediated Factor XI Activation in Thrombus Formation in Vivo. Blood 2010, 116, 3981–3989. [Google Scholar] [CrossRef]
- Maas, C.; Oschatz, C.; Renné, T. The Plasma Contact System 2.0. Semin. Thromb. Hemost. 2011, 37, 375–381. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet Polyphosphates Are Proinflammatory and Procoagulant Mediators In Vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Mailer, R.K.; Rangaswamy, C.; Konrath, S.; Emsley, J.; Renné, T. An Update on Factor XII-Driven Vascular Inflammation. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119166. [Google Scholar] [CrossRef]
- Gailani, D.; Broze, G.J. Factor XI Activation in a Revised Model of Blood Coagulation. Science 1991, 253, 909–912. [Google Scholar] [CrossRef]
- Yi, B.A.; Freedholm, D.; Widener, N.; Wang, X.; Simard, E.; Cullen, C.; Al-Saady, N.M.; Lepor, N.E.; Coulter, S.; Lovern, M.; et al. Pharmacokinetics and Pharmacodynamics of Abelacimab (MAA868), a Novel Dual Inhibitor of Factor XI and Factor XIa. J. Thromb. Haemost. 2022, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Bethune, C.; Smyth, A.; Tyrwhitt, J.; Jung, S.W.; Yu, R.Z.; Wang, Y.; Geary, R.S.; Weitz, J.; Bhanot, S.; et al. Phase 2 Study of the Factor XI Antisense Inhibitor IONIS-FXIRx in Patients With ESRD. Kidney Int. Rep. 2022, 7, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Perera, V.; Wang, Z.; Luettgen, J.; Li, D.; DeSouza, M.; Cerra, M.; Seiffert, D. First-in-Human Study of Milvexian, an Oral, Direct, Small Molecule Factor XIa Inhibitor. Clin. Transl. Sci. 2021, 15, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Heckmann, M.; Distler, J.; Koechel, A.; Schwers, S.; Kanefendt, F. Pharmacokinetics, Pharmacodynamics and Safety of BAY 2433334, a Novel Activated Factor XI Inhibitor, in Healthy Volunteers: A Randomized Phase 1 Multiple-dose Study. Br. J. Clin. Pharmacol. 2022, bcp.15230. [Google Scholar] [CrossRef]
- Zaslavsky, A.; Adams, M.; Coa, X.; Yamaguchi, A.; Henderson, J.; Busch-Østergren, P.; Udager, A.; Sethuramasundaram Pitchiaya, S.; Tourdot, B.; Kasputis, T.; et al. Antisense Oligonucleotides and Nucleic Acids Generate Hypersensitive Platelets. Thromb. Res. 2021, 200, 64–71. [Google Scholar] [CrossRef]
- Ragni, M.V.; Sinha, D.; Seaman, F.; Lewis, J.H.; Spero, J.A.; Walsh, P.N. Comparison of Bleeding Tendency, Factor XI Coagulant Activity, and Factor XI Antigen in 25 Factor XI-Deficient Kindreds. Blood 1985, 65, 719–724. [Google Scholar] [CrossRef]
- Bertaggia Calderara, D.; Zermatten, M.G.; Aliotta, A.; Alberio, L. How to Capture the Bleeding Phenotype in FXI-Deficient Patients. Hamostaseologie 2020, 40, 491–499. [Google Scholar] [CrossRef]
- Depasse, F.; Binder, N.B.; Mueller, J.; Wissel, T.; Schwers, S.; Germer, M.; Hermes, B.; Turecek, P.L. Thrombin Generation Assays Are Versatile Tools in Blood Coagulation Analysis: A Review of Technical Features, and Applications from Research to Laboratory Routine. J. Thromb. Haemost. 2021, 19, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Livnat, T.; Tamarin, I.; Mor, Y.; Winckler, H.; Horowitz, Z.; Korianski, Y.; Bar-Zakay, B.; Seligsohn, U.; Salomon, O. Recombinant Activated Factor VII and Tranexamic Acid Are Haemostatically Effective during Major Surgery in Factor XI-Deficient Patients with Inhibitor Antibodies. Thromb. Haemost. 2009, 102, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Bauduer, F.; de Raucourt, E.; Boyer-Neumann, C.; Trossaert, M.; Beurrier, P.; Faradji, A.; Peynet, J.; Borg, J.-Y.; Chamouni, P.; Chatelanaz, C.; et al. Factor XI Replacement for Inherited Factor XI Deficiency in Routine Clinical Practice: Results of the HEMOLEVEN Prospective 3-Year Postmarketing Study. Haemoph. Off. J. World Fed. Hemoph. 2015, 21, 481–489. [Google Scholar] [CrossRef]
- Salomon, O.; Gailani, D. A Proposal for Managing Bleeding in Patients on Therapeutic Factor XI(a) Inhibitors. J. Thromb. Haemost. 2022, 20, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; DeGuzman, F.R.; Hollenbach, S.J.; Karbarz, M.J.; Abe, K.; Lee, G.; Luan, P.; Hutchaleelaha, A.; Inagaki, M.; Conley, P.B.; et al. A Specific Antidote for Reversal of Anticoagulation by Direct and Indirect Inhibitors of Coagulation Factor Xa. Nat. Med. 2013, 19, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V.; Reilly, P.A.; Eikelboom, J.; Glund, S.; Verhamme, P.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kamphuisen, P.W.; et al. Idarucizumab for Dabigatran Reversal. N. Engl. J. Med. 2015, 373, 511–520. [Google Scholar] [CrossRef]
- Ansell, J.E.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Grosso, M.; Brown, K.; Dishy, V.; Noveck, R.J.; Costin, J.C. Use of PER977 to Reverse the Anticoagulant Effect of Edoxaban. N. Engl. J. Med. 2014, 371, 2141–2142. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Rosendaal, F.R.; Weitz, J.I.; Jaffer, I.H.; Agnelli, G.; Baglin, T.; Mackman, N. Venous thrombosis. Nat. Rev. Dis. Prim. 2015, 1, 15006. [Google Scholar]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Levin, J.; Conley, C.L. Thrombocytosis associated with malignant disease. Arch. Intern. Med. 1964, 114, 497–500. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gurewich, Y.; Gallant, L.A.; Pinkhas, J. Leukocytosis in non hematological malignancies—A possible tumor-associated marker. J. Cancer Res. Clin. Oncol. 1986, 111, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Rickles, F.R.; Moritz, T.E.; Henderson, W.G.; Zacharski, L.R.; Forman, W.B.; Cornell, C.J.; Forcier, R.J.; O’Donnel, J.F.; Headley, E. Abnormalities of blood coagulation in patients with cancer. Mononuclear cell tissue factor generation. J. Lab. Clin. Med. 1987, 98, 917–928. [Google Scholar]
- Thalin, C.; Hisada, Y.; Lundstrom, S.; Mackmen, N.; Wallen, H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.H.; Rondon, A.M.R.; Gomes, T.; Monteiro, R. Q Novel Aspects of Extracellular Vesicles as Mediators of Cancer-Associated Thrombosis. Cells 2019, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Kubala, M.H.; DeClerck, Y.A. The plasminogen activator inhibitor-1 paradox in cancer: A mechanistic understanding. Cancer Metastasis Rev. 2019, 38, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Yang, E.H.; Iliescu, C.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. 2020, 17, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W.H.; Pineo, G.F.; Heit, J.A.; Bergqvist, D.; Lassen, M.R.; Colwell, C.W.; Ray, J.G. Prevention of Venous Thromboembolism. Chest 2004, 126, 338S–400S. [Google Scholar] [CrossRef]
- Weitz, J.I.; Bauersachs, R.; Becker, B.; Berkowitz, S.D.; Freitas, M.C.S.; Lassen, M.R.; Metzig, C.; Raskob, G.E. Effect of Osocimab in Preventing Venous Thromboembolism Among Patients Undergoing Knee Arthroplasty: The FOXTROT Randomized Clinical Trial. JAMA 2020, 323, 130. [Google Scholar] [CrossRef]
- Weitz, J.I.; Strony, J.; Ageno, W.; Gailani, D.; Hylek, E.M.; Lassen, M.R.; Mahaffey, K.W.; Notani, R.S.; Roberts, R.; Segers, A.; et al. Milvexian for the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 2161–2172. [Google Scholar] [CrossRef]
- Verhamme, P.; Yi, B.A.; Segers, A.; Salter, J.; Bloomfield, D.; Büller, H.R.; Raskob, G.E.; Weitz, J.I. Abelacimab for Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 609–617. [Google Scholar] [CrossRef]
- Büller, H.R.; Bethune, C.; Bhanot, S.; Gailani, D.; Monia, B.P.; Raskob, G.E.; Segers, A.; Verhamme, P.; Weitz, J.I. Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis. N. Engl. J. Med. 2015, 372, 232–240. [Google Scholar] [CrossRef]
- Nopp, S.; Kraemmer, D.; Ay, C. Factor XI Inhibitors for Prevention and Treatment of Venous Thromboembolism: A Review on the Rationale and Update on Current Evidence. Front. Cardiovasc. Med. 2022, 9, 903029. [Google Scholar] [CrossRef]
- Presume, J.; Ferreira, J.; Ribeiras, R.; Mendes, M. Achieving Higher Efficacy without Compromising Safety with Factor XI Inhibitors versus Low Molecular Weight Heparin for the Prevention of Venous Thromboembolism in Major Orthopedic Surgery—Systematic Review and Meta-analysis. J. Thromb. Haemost. 2022, 20, 2930–2938. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Kuderer, N.M.; Carrier, M.; Ortel, T.L.; Wun, T.; Rubens, D.; Hobbs, S.; Iyer, R.; Peterson, D.; et al. Dalteparin Thromboprophylaxis in Cancer Patients at High Risk for Venous Thromboembolism: A Randomized Trial. Thromb. Res. 2017, 151, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Key, N.S. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Lee, A.Y.Y. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Ageno, W.; Vedovati, M.C.; Cohen, A.; Huisman, M.; Bauersachs, R.; Gussoni, G.; Becattini, C.; Agnelli, G. Bleeding with Apixaban and Dalteparin in Patients with Cancer-Associated Venous Thromboembolism: Results from the Caravaggio Study. Thromb. Haemost. 2021, 121, 616–624. [Google Scholar] [CrossRef]
- McBane, R.D.; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and Dalteparin in Active Malignancy-associated Venous Thromboembolism: The ADAM VTE Trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs Dalteparin in Cancer-Associated Thromboembolism. Chest 2021, S0012369221040794. [Google Scholar] [CrossRef] [PubMed]
- Poénou, G.; Tolédano, E.; Helfer, H.; Plaisance, L.; Happe, F.; Versini, E.; Diab, N.; Djennaoui, S.; Mahé, I. In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients. Cancers 2022, 14, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 Guidelines for Management of Venous Thromboembolism: Prevention and Treatment in Patients with Cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.; Floege, J.; Thadhani, R.; Weitz, J.I.; Winkelmayer, W.C. Anticoagulation in Patients with Kidney Failure on Dialysis: Factor XI as a Therapeutic Target. Kidney Int. 2021, 100, 1199–1207. [Google Scholar] [CrossRef]
- Pollack, C.V.; Kurz, M.A.; Hayward, N.J. EP-7041, a Factor XIa Inhibitor as a Potential Antithrombotic Strategy in Extracorporeal Membrane Oxygenation: A Brief Report. Crit. Care Explor. 2020, 2, e0196. [Google Scholar] [CrossRef]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.A.; Prins, M.H.; Girolami, A. An Association between Atherosclerosis and Venous Thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef]
- Naschitz, J.E. Cancer-Associated Atherothrombosis: The Challenge. Int. J. Angiol. 2021, 30, 249–256. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Zilberman-Rudenko, J.; Itakura, A.; Wiesenekker, C.P.; Vetter, R.; Maas, C.; Gailani, D.; Tucker, E.I.; Gruber, A.; Gerdes, C.; McCarty, O.J.T. Coagulation Factor XI Promotes Distal Platelet Activation and Single Platelet Consumption in the Bloodstream Under Shear Flow. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 510–517. [Google Scholar] [CrossRef]
- Arockiam, S.; Staniforth, B.; Kepreotis, S.; Maznyczka, A.; Bulluck, H. A Contemporary Review of Antiplatelet Therapies in Current Clinical Practice. Int. J. Mol. Sci. 2023, 24, 11132. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.F.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.-S.; Hirsch, A.T.; Mas, J.-L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients with Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Shestakovska, O.; Aboyans, V.; Alings, M.; Anand, S.S.; Avezum, A.; Berkowitz, S.D.; Bhatt, D.L.; et al. Bleeding and New Cancer Diagnosis in Patients With Atherosclerosis. Circulation 2019, 140, 1451–1459. [Google Scholar] [CrossRef]
- Klok, F.A.; Ageno, W.; Ay, C.; Bäck, M.; Barco, S.; Bertoletti, L.; Becattini, C.; Carlsen, J.; Delcroix, M.; van Es, N.; et al. Optimal Follow-up after Acute Pulmonary Embolism: A Position Paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in Collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, Endorsed by the European Respiratory Society. Eur. Heart J. 2022, 43, 183–189. [Google Scholar] [CrossRef]
- Jordan, K.R.; Wyatt, C.R.; Fallon, M.E.; Woltjer, R.; Neuwelt, E.A.; Cheng, Q.; Gailani, D.; Lorentz, C.; Tucker, E.I.; McCarty, O.J.T.; et al. Pharmacological Reduction of Coagulation Factor XI Reduces Macrophage Accumulation and Accelerates Deep Vein Thrombosis Resolution in a Mouse Model of Venous Thrombosis. J. Thromb. Haemost. 2022, 20, 2035–2045. [Google Scholar] [CrossRef]
- Catella-Chatron, J.; Merah, A.; De Magalhaes, E.; Moulin, N.; Accassat, S.; Duvillard, C.; Mismetti, P.; Bertoletti, L. Chronic Thromboembolic Pulmonary Hypertension Suspicion after Pulmonary Embolism in Cancer Patients. Respir. Med. Res. 2019, 76, 34–37. [Google Scholar] [CrossRef]
| Drug Type | Molecule | Target | Mechanism | Oral Route Available | Half Life | Action | Renal Excretion | Cytochrome p450 Metabolism | Potential for Drug-Drug Interaction | Need for Reversion Strategy | Is It a Suitable Option for Cancer Associated Thrombosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antisense oligonucleotides or ASO | Fesomersen IONIS FXI-LRx (ISIS 416858) FXI-ASO (ISIS416858) | FXI mRNA | Specific protein synthesis blocking | No | Long: weekly administration | Slow and long acting | No | No | No | Yes: FXI replacement | The slow setting makes it less likely; however, it can be interesting for the prevention of CAT |
| Aptamers | / | FXIa | Specific protein binding | No | Short: daily administration | Fast and short acting | No | No | No | No | There is not enough evidence to make an opinion |
| Monoclonal antibodies | Abelacimab (MAA868), Osocimab (BAY1213790), FXI-175, FXI-203 (preclinical), 14E11 (preclinical), Xisomab 3G3 (AB023), MK 2060 | FXI or FXI synthesis | Specific protein binding and decreases its concentration | No | Long: monthly administration | Fast and long acting | No | No | No | Yes: no existing failure of FXI replacement | Yes |
| Natural inhibitors | IrCPI | FXIa or FXIa + FXIIa | Specific protein binding | No | Short: daily administration | Fast and short acting | Unknown | No | Unknown | No | There is not enough evidence to make an opinion |
| Small peptidomimetic molecules | Asundexian (oral). Milvexian (oral)/Frunexian EP-7041a, BMS-962212, ONO-7684 (oral), BMS-654457 (preclinical), ONO-5450598 (preclinical),BMS-262084 (preclinical), SHR2285 (oral) | FXIa or FXI + plasma kallicrein | Specific protein binding | Yes | Short: daily administration | Fast and short acting | Biliary and 15% renal excretion | CYP3A4 | Midazolam Rifampicin Verapamil, Ketoconazole… | No | The risk of interaction makes it less likely |
| Recommendation | Thromboprophylaxis | Intital Treatment | Long-Term Treatement |
|---|---|---|---|
| ACCP 2016 | / | LMWH | LMWH or DOAC or VKA |
| ASCO 2019 | LMWH or DOAC | LMWH, Fondaparinux or DOAC | LMWH or DOAC |
| ISTH 2019 | Khorana ≥ 2 LMWH or DOAC Khorana ≥ 2 LMWH | ||
| ITAC 2019 | LMWH or Fondaparinux | UFH, LMWH, DOAC or VKA | |
| ASH 2021 | LMWH | LMWH or DOAC | |
| French recommendations 2021 | No thromboprophylaxis | LMWH | LMWH or DOAC or VKA |
| ACCP = American College of Chest Physicians ASCO = American Society of Clinical Oncology | ASH = American Society of Hematology ISTH = International Society on Thrombosis and Haemostasis | ITAC = International Initiative on Thrombosis and Cancer | |
| Venous Thromboembolism | Study Name | Registration Number | Drug Name | Comparator | Status |
|---|---|---|---|---|---|
| Orthopedic-surgery-related thrombosis prevention | FXI-ASO TKA trial | NCT01713361 | IONIS FXI-LRx (ISIS 416858) 200 mg or 300 mg SC | Enoxaparin 40 mg | COMPLETED |
| AXIOMATIC-TKR trial | NCT03891524 | Milvexian (BMS-986177) (JNJ70033093) oral 5 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily | Enoxaparin 40 mg | COMPLETED | |
| FOXTROT trial | NCT03276143 | Osocimab (BAY1213790) Single IV postoperative doses of 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, or 1.8 mg/kg Single IV preoperative doses of 0.3 mg/kg or 1.8 mg/kg | Enoxaparin 40 mg | COMPLETED | |
| ANT-005 TKA trial | EudraCT number, 2019-003756-37 | Abelacimab (MAA868) 30 mg, 75 mg, or 150 mg | Enoxaparin 40 mg | COMPLETED | |
| COVID-19-related thrombosis prevention | COVID-19 ThromboprophylaXIs | NCT05040776 | Frunexian EP-7041a | Clinician choice of thromboprophylaxis | ONGOING |
| Cancer-associated thrombosis treatment | MAGNOLIA trial | NCT05171075 | Abelacimab (MAA868) 150 mg | Dalteparin 20 mg | ONGOING |
| ASTER trial | NCT05171049 | Abelacimab (MAA868) 150 mg | Apixaban 5 mg bid | ONGOING |
| Catheter-Related Thrombosis | Name of the Study | Registration Number | Drug Name | Comparator | Status |
|---|---|---|---|---|---|
| End-stage renal disease | / | NCT04534114 | Factor XI LICA (BAY 2976217) | Placebo | UPCOMING |
| ESMERALD trial | NCT03358030 | IONIS FXI-LRx (ISIS 416858) 200 mg, 250 mg or 300 mg | Placebo | ONGOING | |
| / | NCT02553889 | IONIS FXI-LRx (ISIS 416858) | Placebo | COMPLETED | |
| / | NCT03196206 | Milvexian (BMS-986177) | Enoxaparin | COMPLETED | |
| NCT02902679 | Milvexian (BMS-986177) | None | COMPLETED | ||
| MK-2060-004 | NCT03873038 | MK-2060 | Placebo | COMPLETED | |
| MK-2060-007 | NCT05027074 | MK-2060 | Placebo | ON GOING | |
| / | NCT03787368 | Osocimab (BAY1213790) | Placebo | COMPLETED | |
| / | NCT04523220 | Osocimab (BAY1213790) | Placebo | UPCOMING | |
| / | NCT04510987 | BAY2433334 (Asundexian) | None | COMPLETED | |
| Catheter-related thrombosis prevention | NCT04465760 | Xisomab 3G3 (AB023) | Placebo | ONGOING |
| Arterial | Registration Number | Study | Drug Name | Comparator | Status |
|---|---|---|---|---|---|
| AOMI | AXIOMATIC-SSPtrial | NCT03766581 | Oral Milvexian (BMS-986177) (JNJ70033093) in association with aspirin and clopidogrel | Placebo | COMPLETED |
| FA | PACIFIC-AF | NCT04218266 | Asundexian 20 or 50 mg | Apixaban 5 or 2.5 mg | COMPLETED |
| Stroke | OCEANIC-STROKE | NCT04304508 | Asundexian 10, 20 or 50 mg | Placebo | COMPLETED |
| IDM | PACIFIC-AMI | NCT04304534 | Asundexian in association with aspirineand clopidogrel | Placebo | COMPLETED |
| OCEANIC-AF | NCT05643573 | Asundexian | Apixaban 5 or 2.5 mg | ONGOING |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poenou, G.; Heestermans, M.; Lafaie, L.; Accassat, S.; Moulin, N.; Rodière, A.; Petit, B.; Duvillard, C.; Mismetti, P.; Bertoletti, L. Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients? Int. J. Mol. Sci. 2023, 24, 14433. https://doi.org/10.3390/ijms241914433
Poenou G, Heestermans M, Lafaie L, Accassat S, Moulin N, Rodière A, Petit B, Duvillard C, Mismetti P, Bertoletti L. Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients? International Journal of Molecular Sciences. 2023; 24(19):14433. https://doi.org/10.3390/ijms241914433
Chicago/Turabian StylePoenou, Géraldine, Marco Heestermans, Ludovic Lafaie, Sandrine Accassat, Nathalie Moulin, Alexandre Rodière, Bastien Petit, Cécile Duvillard, Patrick Mismetti, and Laurent Bertoletti. 2023. "Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients?" International Journal of Molecular Sciences 24, no. 19: 14433. https://doi.org/10.3390/ijms241914433
APA StylePoenou, G., Heestermans, M., Lafaie, L., Accassat, S., Moulin, N., Rodière, A., Petit, B., Duvillard, C., Mismetti, P., & Bertoletti, L. (2023). Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients? International Journal of Molecular Sciences, 24(19), 14433. https://doi.org/10.3390/ijms241914433





