Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma
Abstract
1. Introduction
2. Phytoestrogens: Chemical Classification and General Aspects
2.1. Flavonoids
2.2. Non-Flavonoids
2.3. Metabolism of Dietary Phytoestrogens
3. Phytoestrogen Mechanisms of Action—Anticancer Related Effects
4. Molecular Basis of Osteosarcoma Pathogeneses
5. Estrogen Receptors as a Potential Target for the Treatment of Osteosarcoma
6. Anti-Osteosarcoma Effects of Flavonoids
6.1. Genistein and Related Isoflavones
6.1.1. Daidzein
6.1.2. Biochanin A
6.1.3. Formononetin
6.2. Flavonols
6.2.1. Quercetin
6.2.2. Galangin
6.3. Apigenin
6.4. Naringenin
6.5. Catechins
7. Anti-Osteosarcoma Effects of Non-Flavonoids
7.1. Stilbenes
7.1.1. Resveratrol
| Phytoestrogen | Cell line/ In Vivo Model | Concentrations | Combined Treatment | Molecular Mechanism | Observed Effects | References |
|---|---|---|---|---|---|---|
| Resveratrol | MNNG/HOS, MG-63 tumor xenograft mouse | 10–40 μM 100 mg/kg/d | ↑ caspase-3, Bax, cleaved PARP, ↓ Bcl-2, Bcl-xL; ↓ cytokines ↓ JAK2/STAT3 pathway | ↑ apoptosis ↓ CSCs survival ↓ tumor growth | [284] | |
| U2OS | 6–24 μg/mL | ↓ Wnt/β-catenin pathway ↓ β-catenin, c-myc, cyclin D1, ↓ MMP-2/9 ↑ Cx43, E-cadherin | ↓ proliferation ↑ apoptosis ↓ migration, invasion | [290] | ||
| MG-63 | 10–40 µg/mL | ↓ β-catenin signaling | ↓ proliferation | [283] | ||
| HOS, MG-63, U2OS, SaOS-2, 143B HOS orthotopic graft model | 25–200 μM 40, 100 mg/kg/d | ↓ p38 MAPK/JNK pathways ↓ CREB, ↓ MMP-2, ↑ miR-328 | ↓ migration, invasion, adhesion ↓ tumor growth, metastasis | [296] | ||
| U2OS | 10–40 μM | ↓ VEGF | [299] | |||
| MG-63, SaOS-2, KHOS, U2OS | 50–100 µM | DOX 0.1–10 µM or CDDP 0.2–2 µg/mL for 24 h | ↓ pAKT, ↑ caspase-3 ↓ IL-6/8 ↑ Osx, OPN, ALP, Col I, OCN | ↓ proliferation ↑ apoptosis ↑ differentiation ↑ DOX/CDDP sensitivity | [286] | |
| Polydatin | 143B, MG-63 | 1–100 µM | ↓ β-catenin signaling ↑ Bax/Bcl-2, caspase-3 | ↓ proliferation ↑ apoptosis | [303] | |
| MG-63 | 10–160 µM | ↓ STAT3 signaling | ↑ apoptosis, ↑ autophagy | [304,305] | ||
| Polydatin | SaOS-2/DOX, MG-63/DOX MG-63/DOX xenograft model | 50–250 μM 150 mg/kg/d | ↓ TUG1/Akt signaling | ↓ proliferation ↑ apoptosis ↓ tumor growth | [306] | |
| U2OS, MG-63 | paclitaxel | ↓ proliferation ↓ migration ↑ cell cycle arrest | [304] | |||
| SaOS-2 | 1–150 μM | ionizing radiation | ↓ Wnt/β-catenin pathway ↑ lipid metabolite secretion | ↑ differentiation ↑ cell cycle arrest ↑ radiation sensitivity | [307] | |
| Enterodiol, Enterolactone | MG-63 | 0.1–10 mg/mL | ↑ ALP activity ↑ ON, Col I | ↓ proliferation ↑ differentiation | [308] |
7.1.2. Polydatin
7.2. Lignans
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Cancer Treatment and Research; Springer: Boston, MA, USA, 2009; Volume 152, pp. 3–13. [Google Scholar] [CrossRef]
- Denduluri, S.; Wang, Z.; Yan, Z.; Wang, J.; Wei, Q.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; He, T.C. Molecular pathogenesis and therapeutic strategies of human osteosarcoma. J. Biomed. Res. 2015, 30, 5–18. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, R.; Li, J.; Lu, X.; Zhang, Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: A network meta-analysis. J. Orthop. Surg. Res. 2020, 15, 51. [Google Scholar] [CrossRef]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Arima, Y.; Nobusue, H.; Saya, H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020, 111, 2689–2695. [Google Scholar] [CrossRef]
- Shukla, S.; Ohnuma, S.; Ambudkar, S.V. Improving Cancer Chemotherapy with Modulators of ABC Drug Transporters. Curr. Drug Targets 2011, 12, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Roca, P. Phytoestrogens for Cancer Prevention and Treatment. Biology 2020, 9, 427. [Google Scholar] [CrossRef]
- Hu, X.J.; Song, W.R.; Gao, L.Y.; Nie, S.P.; Eisenbrand, G.; Xie, M.Y. Assessment of dietary phytoestrogen intake via plant-derived foods in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Cipolletti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: The Modulation of Estrogen Receptors (ERs) Signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phytoestrogens: End of a tale? Ann. Med. 2005, 37, 423–438. [Google Scholar] [CrossRef]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Yang, M.-F.; Yu, W.; Tao, H.-M. Molecular mechanisms of estrogen receptor β-induced apoptosis and autophagy in tumors: Implication for treating osteosarcoma. J. Int. Med. Res. 2019, 47, 4644–4655. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Rajabi, A.; Raisi, A.; Mohajeri, M.; Yazdi, S.M.; Davoodvandi, A.; Aslanbeigi, F.; Vaziri, M.; Hamblin, M.R.; Mirzaei, H. Potential of natural products in osteosarcoma treatment: Focus on molecular mechanisms. Biomed. Pharmacother. 2021, 144, 112257. [Google Scholar] [CrossRef]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural Product Polyphenols of Relevance to Human Health. Pharm. Biol. 2004, 42, 46–63. [Google Scholar] [CrossRef]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.; Wennberg, T.; Vuorela, H. Natural Products in the Process of Finding New Drug Candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Miksicek, R.J. Estrogenic Flavonoids: Structural Requirements for Biological Activity. Exp. Biol. Med. 1995, 208, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, J.-C.; Champavier, Y.; Chulia, A.-J.; Habrioux, G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000, 66, 1281–1291. [Google Scholar] [CrossRef]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-J.; Wu, B.; Ding, T.; Chu, J.-H.; Li, C.-Y.; Zhang, J.; Wu, T.; Wu, J.; Liu, S.-J.; Liu, S.-L.; et al. Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode-array detection and quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2343–2358. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2015, 2016, 3128951. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Mapesa, J.O. Phenolics in Human Nutrition: Importance of the Intestinal Microbiome for Isoflavone and Lignan Bioavailability. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2433–2463. [Google Scholar] [CrossRef]
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients 2019, 11, 1709. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef] [PubMed]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Jordan, V.C. SERMs: Meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 2007, 99, 350–356. [Google Scholar] [CrossRef]
- Bedell, S.; Nachtigall, M.; Naftolin, F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2014, 139, 225–236. [Google Scholar] [CrossRef]
- van de Schans, M.G.; Vincken, J.-P.; de Waard, P.; Hamers, A.R.; Bovee, T.F.; Gruppen, H. Glyceollins and dehydroglyceollins isolated from soybean act as SERMs and ER subtype-selective phytoestrogens. J. Steroid Biochem. Mol. Biol. 2016, 156, 53–63. [Google Scholar] [CrossRef]
- Lee, G.-A.; Hwang, K.-A.; Choi, K.-C. Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis. Toxins 2016, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Isoflavones made simple-genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses 2006, 66, 1093–1114. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Jonsson, P.; Katchy, A.; Williams, C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr.-Relat. Cancer 2013, 21, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Warner, M.; Gustafsson, J.Å. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol. Cell. Endocrinol. 2015, 418, 240–244. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, H.; ter Veld, M.G.R.; Jacobs, N.; van der Saag, P.T.; Murk, A.J.; Rietjens, I.M.C.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tzagarakis-Foster, C.; Scharschmidt, T.C.; Lomri, N.; Leitman, D.C. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 2001, 276, 17808–17814. [Google Scholar] [CrossRef]
- McDonnell, D.P. The molecular determinants of estrogen receptor pharmacology. Maturitas 2004, 48 (Suppl. S1), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Stuart, J.A. Resveratrol interacts with estrogen receptor-β to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase. Free Radic. Biol. Med. 2011, 50, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell. Physiol. Biochem. 2017, 42, 1051–1062. [Google Scholar] [CrossRef]
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Microenvironmental regulation of estrogen signals in breast cancer. Breast Cancer 2007, 14, 175–181. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- Viñas, R.; Jeng, Y.-J.; Watson, C.S. Non-Genomic Effects of Xenoestrogen Mixtures. Int. J. Environ. Res. Public Health 2012, 9, 2694–2714. [Google Scholar] [CrossRef]
- Molina, L.; Bustamante, F.A.; Bhoola, K.D.; Figueroa, C.D.; Ehrenfeld, P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci. 2018, 132, 2583–2598. [Google Scholar] [CrossRef]
- Razandi, M.; Pedram, A.; Merchenthaler, I.; Greene, G.L.; Levin, E.R. Plasma membrane estrogen receptors exist and functions as dimers. Mol. Endocrinol. 2004, 18, 2854–2865. [Google Scholar] [CrossRef]
- Márquez, D.C.; Lee, J.; Lin, T.; Pietras, R.J. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine 2001, 16, 073–082. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Madak-Erdogan, Z.; Flaws, J.A.; Shapiro, D.J.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells. Mol. Cell. Endocrinol. 2016, 437, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 3203161. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Adhya, A.K.; Rath, A.K.; Reddy, P.B.; Mishra, S.K. Estrogen-related receptors alpha, beta and gamma expression and function is associated with transcriptional repressor EZH2 in breast carcinoma. BMC Cancer 2018, 18, 690. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Zheng, X.; Lee, S.-K.; Song, W.O.; Chun, O.K.; Hwang, I.; Shin, H.S.; Kim, B.-G.; Kim, K.S.; Lee, S.-Y.; et al. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Pandima Devi, K.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting MiRNAs by Polyphenols: Novel Therapeutic Strategy for Cancer. Semin. Cancer Biol. 2017, 46, 146–157. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Hsu, Y.-L.; Huang, Y.-F.; Tsai, E.-M. Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 323–332. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.-J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.-W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-C.; Peng, S.-F.; Lai, K.-C.; Liao, C.-L.; Huang, Y.-P.; Lin, C.-C.; Lin, M.-L.; Liu, K.-C.; Tsai, C.-C.; Ma, Y.-S.; et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The genetics of osteosarcoma. Sarcoma 2012, 2012, 627254. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; Fernandes, T.A.A.D.M.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Hameed, M.; Mandelker, D. Tumor Syndromes Predisposing to Osteosarcoma. Adv. Anat. Pathol. 2018, 25, 217–222. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef]
- Wang, L.L.; Gannavarapu, A.; Kozinetz, C.A.; Levy, M.L.; Lewis, R.A.; Chintagumpala, M.M.; Ruiz-Maldanado, R.; Contreras-Ruiz, J.; Cunniff, C.; Erickson, R.P.; et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J. Natl. Cancer Inst. 2003, 95, 669–674. [Google Scholar] [CrossRef]
- Kleinerman, R.A.; Schonfeld, S.J.; Tucker, M.A. Sarcomas in hereditary retinoblastoma. Clin. Sarcoma Res. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Rickel, K.; Fang, F.; Tao, J. Molecular genetics of osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Bartnik, E.; Sobczuk, P.; Teterycz, P.; Czarnecka, A.M. Molecular biology of sarcoma. Oncol. Clin. Pract. 2019, 14, 307–330. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Z.; Huang, Z.; Chen, D.-C.; Zhu, X.-X.; Wang, Y.-Z.; Yan, Y.-W.; Tang, S.; Madhavan, S.; Ni, W.; et al. Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma. Bone Res. 2018, 6, 11. [Google Scholar] [CrossRef]
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef]
- Hingorani, P.; Zhang, W.; Gorlick, R.; Kolb, E.A. Inhibition of Src Phosphorylation Alters Metastatic Potential of Osteosarcoma In Vitro but not In Vivo. Clin. Cancer Res. 2009, 15, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, M.L.; Clark, J.C.M.; Myers, D.E.; Dass, C.R.; Choong, P.F.M. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef] [PubMed]
- Gagiannis, S.; Müller, M.; Uhlemann, S.; Koch, A.; Melino, G.; Krammer, P.H.; Nawroth, P.P.; Brune, M.; Schilling, T. Parathyroid hormone-related protein confers chemoresistance by blocking apoptosis signaling via death receptors and mitochondria. Int. J. Cancer 2009, 125, 1551–1557. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- DeRoo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, B.; Hu, J.; Zhou, Y.; Jiang, M.; Wu, M.; Qin, L.; Yang, X. miR-421 is a diagnostic and prognostic marker in patients with osteosarcoma. Tumor Biol. 2016, 37, 9001–9007. [Google Scholar] [CrossRef]
- Ren, Z.; He, M.; Shen, T.; Wang, K.; Meng, Q.; Chen, X.; Zhou, L.; Han, Y.; Ji, C.; Liu, S.; et al. MiR-421 promotes the development of osteosarcoma by regulating MCPIP1 expression. Cancer Biol. Ther. 2020, 21, 231–240. [Google Scholar] [CrossRef]
- PosthumaDeBoer, J.; Witlox, M.A.; Kaspers, G.J.L.; van Royen, B.J. Molecular alterations as target for therapy in metastatic osteosarcoma: A review of literature. Clin. Exp. Metastasis 2011, 28, 493–503. [Google Scholar] [CrossRef]
- Felx, M.; Guyot, M.-C.; Isler, M.; Turcotte, R.E.; Doyon, J.; Khatib, A.-M.; Leclerc, S.; Moreau, A.; Moldovan, F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin. Sci. 2006, 110, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, M.; Tian, A.; Zhang, X.; Yao, Z.; Ma, X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015, 6, 17570–17583. [Google Scholar] [CrossRef]
- Fang, F.; VanCleave, A.; Helmuth, R.; Torres, H.; Rickel, K.; Wollenzien, H.; Sun, H.; Zeng, E.; Zhao, J.; Tao, J. Targeting the Wnt/β-catenin pathway in human osteosarcoma cells. Oncotarget 2018, 9, 36780–36792. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.L.; Lafleur, E.A.; Jia, S.-F.; Kleinerman, E.S. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol. Rep. 2002, 9, 823–827. [Google Scholar] [CrossRef]
- Lafleur, E.A.; Koshkina, N.V.; Stewart, J.; Jia, S.-F.; Worth, L.L.; Duan, X.; Kleinerman, E.S. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin. Cancer Res. 2004, 10, 8114–8119. [Google Scholar] [CrossRef]
- Alloisio, G.; Ciaccio, C.; Fasciglione, G.F.; Tarantino, U.; Marini, S.; Coletta, M.; Gioia, M. Effects of Extracellular Osteoanabolic Agents on the Endogenous Response of Osteoblastic Cells. Cells 2021, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Nørregaard, K.S.; Jürgensen, H.J.; Gårdsvoll, H.; Engelholm, L.H.; Behrendt, N.; Søe, K. Osteosarcoma and Metastasis Associated Bone Degradation—A Tale of Osteoclast and Malignant Cell Cooperativity. Int. J. Mol. Sci. 2021, 22, 6865. [Google Scholar] [CrossRef]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Osteoprotegerin (OPG) Ratio Is Increased in Severe Osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL blockade prevents and treats aggressive osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197. [Google Scholar] [CrossRef]
- Wagner, E.R.; Luther, G.; Zhu, G.; Luo, Q.; Shi, Q.; Kim, S.H.; Gao, J.-L.; Huang, E.; Gao, Y.; Yang, K.; et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma 2011, 2011, 325238. [Google Scholar] [CrossRef]
- Wagner, E.R.; He, B.-C.; Chen, L.; Zuo, G.-W.; Zhang, W.; Shi, Q.; Luo, Q.; Luo, X.; Liu, B.; Luo, J.; et al. Therapeutic Implications of PPARgamma in Human Osteosarcoma. PPAR Res. 2010, 2010, 956427. [Google Scholar] [CrossRef]
- Carpio, L.; Gladu, J.; Goltzman, D.; Rabbani, S.A.; Robichaux, W.G.; Cheng, X.; Agas, D.; Marchetti, L.; Capitani, M.; Sabbieti, M.G. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am. J. Physiol. Metab. 2001, 281, E489–E499. [Google Scholar] [CrossRef] [PubMed]
- Kallio, A.; Guo, T.; Lamminen, E.; Seppänen, J.; Kangas, L.; Väänänen, H.K.; Härkönen, P. Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Mol. Cell. Endocrinol. 2008, 289, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.J.; Wright, C.M.; Pearce, M.S.; Craft, A.W.; UKCCSG/MRC Bone Tumour Working Group. Stature of young people with malignant bone tumors. Pediatr. Blood Cancer 2004, 42, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Shih, L.-H.; Lee, J.-Y.; Shen, Y.-J.; Lee, H.-H. Estrogen enhances activity of Wnt signaling during osteogenesis by inducing Fhl1 expression. J. Cell. Biochem. 2015, 116, 1419–1430. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bodine, P.V.N.; Henderson, R.A.; Green, J.; Aronow, M.; Owen, T.; Stein, G.S.; Lian, J.B.; Komm, B.S. Estrogen receptor-alpha is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression. Endocrinology 1998, 139, 2048–2057. [Google Scholar] [CrossRef]
- Spelsberg, T.C.; Subramaniam, M.; Riggs, B.L.; Khosla, S. The Actions and Interactions of Sex Steroids and Growth Factors/Cytokines on the Skeleton. Mol. Endocrinol. 1999, 13, 819–828. [Google Scholar] [CrossRef]
- Monroe, D.G.; Getz, B.J.; Johnsen, S.A.; Riggs, B.L.; Khosla, S.; Spelsberg, T.C. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J. Cell. Biochem. 2003, 90, 315–326. [Google Scholar] [CrossRef]
- Tee, M.K.; Rogatsky, I.; Tzagarakis-Foster, C.; Cvoro, A.; An, J.; Christy, R.J.; Yamamoto, K.R.; Leitman, D.C. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol. Biol. Cell 2004, 15, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.D. Is Estrogen the Answer for Osteosarcoma? Cancer Res. 2019, 79, 1034–1035. [Google Scholar] [CrossRef]
- Lillo Osuna, M.A.; Garcia-Lopez, J.; El Ayachi, I.; Fatima, I.; Khalid, A.B.; Kumpati, J.; Slayden, A.V.; Seagroves, T.N.; Miranda-Carboni, G.A.; Krum, S.A. Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis. Cancer Res. 2019, 79, 1054–1068. [Google Scholar] [CrossRef]
- Stossi, F.; Barnett, D.H.; Frasor, J.; Komm, B.; Lyttle, C.R.; Katzenellenbogen, B.S. Transcriptional Profiling of Estrogen-Regulated Gene Expression via Estrogen Receptor (ER) α or ERβ in Human Osteosarcoma Cells: Distinct and Common Target Genes for These Receptors. Endocrinology 2004, 145, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Dohi, O.; Hatori, M.; Suzuki, T.; Ono, K.; Hosaka, M.; Akahira, J.-I.; Miki, Y.; Nagasaki, S.; Itoi, E.; Sasano, H. Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma. Cancer Sci. 2008, 99, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Chen, C.-M.; Chen, C.-F.; Wu, P.-K.; Chen, W.-M. Suppression of Estrogen Receptor Alpha Inhibits Cell Proliferation, Differentiation and Enhances the Chemosensitivity of P53-Positive U2OS Osteosarcoma Cell. Int. J. Mol. Sci. 2021, 22, 11238. [Google Scholar] [CrossRef]
- Lin, P.-I.; Tai, Y.-T.; Chan, W.P.; Lin, Y.-L.; Liao, M.-H.; Chen, R.-M. Estrogen/ERα signaling axis participates in osteoblast maturation via upregulating chromosomal and mitochondrial complex gene expressions. Oncotarget 2018, 9, 1169–1186. [Google Scholar] [CrossRef]
- Auld, K.L.; Berasi, S.P.; Liu, Y.; Cain, M.; Zhang, Y.; Huard, C.; Fukayama, S.; Zhang, J.; Choe, S.; Zhong, W.; et al. Estrogen-related receptor α regulates osteoblast differentiation via Wnt/β-catenin signaling. J. Mol. Endocrinol. 2012, 48, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.-X.; Li, X.-A. Inhibitory effects of tamoxifen and doxorubicin, alone and in combination, on the proliferation of the MG63 human osteosarcoma cell line. Oncol. Lett. 2013, 6, 970–976. [Google Scholar] [CrossRef]
- Quist, T.; Jin, H.; Zhu, J.-F.; Smith-Fry, K.; Capecchi, M.R.; Jones, K.B. The impact of osteoblastic differentiation on osteosarcomagenesis in the mouse. Oncogene 2015, 34, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef]
- Gorska, M.; Wyszkowska, R.M.; Kuban-Jankowska, A.; Wozniak, M. Impact of Apparent Antagonism of Estrogen Receptor β by Fulvestrant on Anticancer Activity of 2-Methoxyestradiol. Anticancer Res. 2016, 36, 2217–2226. [Google Scholar] [PubMed]
- Yang, M.; Liu, B.; Jin, L.; Tao, H.; Yang, Z. Estrogen receptor β exhibited anti-tumor effects on osteosarcoma cells by regulating integrin, IAP, NF-kB/BCL-2 and PI3K/Akt signal pathway. J. Bone Oncol. 2017, 9, 15–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Zhou, X.; Wu, Y.; Wang, L. Silencing of estrogen receptor β promotes the invasion and migration of osteosarcoma cells through activating Wnt signaling pathway. OncoTargets Ther. 2019, 12, 6779–6788. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, W.; Liu, B.; Yang, M.; Tao, H. Estrogen receptor β induces autophagy of osteosarcoma through the mTOR signaling pathway. J. Orthop. Surg. Res. 2020, 15, 50. [Google Scholar] [CrossRef]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Cassidy Aedin Bioavailability of Isoflavones in Humans. In Flavonoids and Related Compounds: Bioavail-Ability and Function; CRC Press: Boca Raton, FL, USA, 2012.
- Polkowski, K.; Popiołkiewicz, J.; Krzeczyński, P.; Ramza, J.; Pucko, W.; Zegrocka-Stendel, O.; Boryski, J.; Skierski, J.S.; Mazurek, A.P.; Grynkiewicz, G. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004, 203, 59–69. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kasparovska, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Hertrampf, T.; Gruca, M.; Seibel, J.; Laudenbach, U.; Fritzemeier, K.; Diel, P. The bone-protective effect of the phytoestrogen genistein is mediated via ER alpha-dependent mechanisms and strongly enhanced by physical activity. Bone 2007, 40, 1529–1535. [Google Scholar] [CrossRef]
- Kurzer, M.S. Hormonal effects of soy in premenopausal women and men. J. Nutr. 2002, 132, 570S–573S. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Met. Rev. 2002, 21, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fan, J.; Cheng, L.; Hu, P.; Liu, R. The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. OncoTargets Ther. 2018, 11, 8153–8163. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.; Monroe, D.; Ruesink, T.; Khosla, S.; Riggs, B.; Spelsberg, T. Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors alpha and beta. J. Cell. Biochem. 2003, 89, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Lambrinidis, G.; Halabalaki, M.; Katsanou, E.S.; Skaltsounis, A.-L.; Alexis, M.N.; Mikros, E. The estrogen receptor and polyphenols: Molecular simulation studies of their interactions, a review. Environ. Chem. Lett. 2006, 4, 159–174. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Koike, T.; Nozawa, Y. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol. Pharmacol. 1991, 39, 475–480. [Google Scholar] [PubMed]
- Degagné, E.; Pandurangan, A.; Bandhuvula, P.; Kumar, A.; Eltanawy, A.; Zhang, M.; Yoshinaga, Y.; Nefedov, M.; de Jong, P.J.; Fong, L.G.; et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Investig. 2014, 124, 5368–5384. [Google Scholar] [CrossRef]
- Renda, G.; Yalçın, F.N.; Nemutlu, E.; Akkol, E.K.; Süntar, I.; Keleş, H.; Ina, H.; Çalış, I.; Ersöz, T. Comparative assessment of dermal wound healing potentials of various Trifolium L. extracts and determination of their isoflavone contents as potential active ingredients. J. Ethnopharmacol. 2013, 148, 423–432. [Google Scholar] [CrossRef]
- Djiogue, S.; Njamen, D.; Halabalaki, M.; Kretzschmar, G.; Beyer, A.; Mbanya, J.-C.; Skaltsounis, A.-L.; Vollmer, G. Estrogenic properties of naturally occurring prenylated isoflavones in U2OS human osteosarcoma cells: Structure-activity relationships. J. Steroid Biochem. Mol. Biol. 2010, 120, 184–191. [Google Scholar] [CrossRef]
- Salvatori, L.; Caporuscio, F.; Coroniti, G.; Starace, G.; Frati, L.; Russo, M.A.; Petrangeli, E. Down-regulation of epidermal growth factor receptor induced by estrogens and phytoestrogens promotes the differentiation of U2OS human osteosarcoma cells. J. Cell. Physiol. 2009, 220, 35–44. [Google Scholar] [CrossRef]
- Chen, X.; Garner, S.; Anderson, J. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem. Biophys. Res. Commun. 2002, 295, 417–422. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, A.; Lieberherr, M.; Colin, C.; Pointillart, A. A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets. J. Cell. Physiol. 2004, 200, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.-L.; Wang, H.-Z.; Xie, L.-P.; Wang, X.-Y.; Zhang, R.-Q. Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production. Biochem. Pharmacol. 2003, 65, 709–715. [Google Scholar] [CrossRef]
- Morris, C.; Thorpe, J.; Ambrosio, L.; Santin, M. The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. J. Nutr. 2006, 136, 1166–1170. [Google Scholar] [CrossRef]
- Nakamura, A.; Aizawa, J.; Sakayama, K.; Kidani, T.; Takata, T.; Norimatsu, Y.; Miura, H.; Masuno, H. Genistein inhibits cell invasion and motility by inducing cell differentiation in murine osteosarcoma cell line LM8. BMC Cell Biol. 2012, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef]
- Birch, M.; Skerry, T. Differential regulation of syndecan expression by osteosarcoma cell lines in response to cytokines but not osteotropic hormones. Bone 1999, 24, 571–578. [Google Scholar] [CrossRef]
- Nikitovic, D.; Tsatsakis, A.M.; Karamanos, N.K.; Tzanakakis, G. The effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by two osteosarcoma cell lines depends on tyrosine kinase and the estrogen receptor density. Anticancer Res. 2003, 23, 459–464. [Google Scholar]
- Dang, Z.-C.; Audinot, V.; Papapoulos, S.E.; Boutin, J.A.; Löwik, C.W.G.M. Peroxisome Proliferator-activated Receptor γ (PPARγ) as a Molecular Target for the Soy Phytoestrogen Genistein. J. Biol. Chem. 2003, 278, 962–967. [Google Scholar] [CrossRef]
- Song, M.; Tian, X.; Lu, M.; Zhang, X.; Ma, K.; Lv, Z.; Wang, Z.; Hu, Y.; Xun, C.; Zhang, Z.; et al. Genistein exerts growth inhibition on human osteosarcoma MG-63 cells via PPARγ pathway. Int. J. Oncol. 2015, 46, 1131–1140. [Google Scholar] [CrossRef]
- Engel, N.; Adamus, A.; Schauer, N.; Kühn, J.; Nebe, B.; Seitz, G.; Kraft, K. Synergistic Action of Genistein and Calcitriol in Immature Osteosarcoma MG-63 Cells by SGPL1 Up-Regulation. PLoS ONE 2017, 12, e0169742. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Garland, C.F. Vitamin D has a greater impact on cancer mortality rates than on cancer incidence rates. BMJ 2014, 348, g2862. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Kunz, I.; Bendik, I.; Prudence, K.; Weber, P.; Recker, R.; Heaney, R.P. Effect of a combination of genistein, polyunsaturated fatty acids and vitamins D3 and K1 on bone mineral density in postmenopausal women: A randomized, placebo-controlled, double-blind pilot study. Eur. J. Nutr. 2013, 52, 203–215. [Google Scholar] [CrossRef]
- Ando, T.; Ichikawa, J.; Okamoto, A.; Tasaka, K.; Nakao, A.; Hamada, Y. Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. J. Orthop. Res. 2005, 23, 964–969. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Z.-L.; Liu, B.; Yan, X.-B.; Feng, J.; Tao, H.-M. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: The role of Akt and nuclear factor-kappaB. Anti-Cancer Drugs 2010, 21, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, C.; Toi, M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Shen, C.; Lai, J.; Shi, Z.; Liu, B.; Tao, H.-M. Genistein potentiates the anti-cancer effects of gemcitabine in human osteosarcoma via the downregulation of Akt and nuclear factor-κB pathway. Anti-Cancer Agents Med. Chem. 2012, 12, 554–563. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Xie, Y.; Yang, M.; Zhang, Y.; Deng, Z.; Cai, L. Investigation of inhibition effect of daidzein on osteosarcoma cells based on experimental validation and systematic pharmacology analysis. PeerJ 2021, 9, e12072. [Google Scholar] [CrossRef]
- Hsu, Y.-N.; Shyu, H.-W.; Hu, T.-W.; Yeh, J.-P.; Lin, Y.-W.; Lee, L.-Y.; Yeh, Y.-T.; Dai, H.-Y.; Perng, D.-S.; Su, S.-H.; et al. Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem. Toxicol. 2018, 112, 194–204. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Zhai, X.; Cui, T.; Wang, G.; Pang, Q. The effect of biochanin A on cell growth, apoptosis, and migration in osteosarcoma cells. Pharmazie 2018, 73, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xiao, Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell. Physiol. Biochem. 2015, 37, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Chen, X.; Li, J.; Shen, M.; Yu, W.; Yang, Y.; Xiao, Z. The Proapoptotic Effect of Formononetin in Human Osteosarcoma Cells: Involvement of Inactivation of ERK and Akt Pathways. Cell. Physiol. Biochem. 2014, 34, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shen, H.; Lu, M.; Chen, J.; Yin, Q.; Li, P. Formononetin inhibits osteosarcoma cell proliferation and promotes apoptosis by regulating the miR-214-3p/phosphatase and tensin homolog pathway. Transl. Cancer Res. 2020, 9, 4914–4921. [Google Scholar] [CrossRef]
- Hu, W.; Wu, X.; Tang, J.; Zhao, G.; Xiao, N.; Zhang, L.; Li, S. Anti-Cancer Targets of Formononetin and Molecular Mechanisms in Osteosarcoma: Findings of Bioinformatic and Experimental Assays. J. Cell Mol. Med. 2019, 23, 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, X.; Li, C.; Liao, L.; Gao, B.; Gan, H.; Yang, Z.; Liao, L.; Chen, X. Quercetin-Mediated Apoptosis via Activation of the Mitochondrial-Dependent Pathway in MG-63 Osteosarcoma Cells. Mol. Med. Rep. 2011, 4, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.K.; Lee, E.J.; Kim, H.C.; Kim, J.H. Induction of G(1)/S Phase Arrest and Apoptosis by Quercetin in Human Osteosarcoma Cells. Arch. Pharm. Res. 2010, 33, 781–785. [Google Scholar] [CrossRef]
- Xie, X.; Yin, J.; Jia, Q.; Wang, J.; Zou, C.; Brewer, K.J.; Colombo, C.; Wang, Y.; Huang, G.; Shen, J. Quercetin Induces Apoptosis in the Methotrexate-Resistant Osteosarcoma Cell Line U2-OS/MTX300 via Mitochondrial Dysfunction and Dephosphorylation of Akt. Oncol. Rep. 2011, 26, 687–693. [Google Scholar] [CrossRef]
- Berndt, K.; Campanile, C.; Muff, R.; Strehler, E.; Born, W.; Fuchs, B. Evaluation of quercetin as a potential drug in osteosarcoma treatment. Anticancer Res. 2013, 33, 1297–1306. [Google Scholar]
- Wu, B.; Zeng, W.; Ouyang, W.; Xu, Q.; Chen, J.; Wang, B.; Zhang, X. Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells. J. Clin. Biochem. Nutr. 2020, 67, 137–145. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef]
- Mohammadi, E.; Alemi, F.; Maleki, M.; Malakoti, F.; Farsad-Akhtar, N.; Yousefi, B. Quercetin and Methotrexate in Combination have Anticancer Activity in Osteosarcoma Cells and Repress Oncogenic MicroRNA-223. Drug Res. 2022, 72, 226–233. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Han, W.; Lu, X.; Jin, S.; Yang, W.; Li, J.; He, W.; Qian, Y. Galangin suppresses human osteosarcoma cells: An exploration of its underlying mechanism. Oncol. Rep. 2017, 37, 435–441. [Google Scholar] [CrossRef]
- Liu, C.; Ma, M.; Zhang, J.; Gui, S.; Zhang, X.; Xue, S. Galangin inhibits human osteosarcoma cells growth by inducing transforming growth factor-β1-dependent osteogenic differentiation. Biomed. Pharmacother. 2017, 89, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chuang, Y.-J.; Yu, C.-C.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Lin, J.-P.; Tang, N.-Y.; Huang, A.-C.; Chung, J.-G. Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo. J. Agric. Food Chem. 2012, 60, 11395–11402. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Lv, L.; Chen, D.; Shen, L.; Xie, Z. Apigenin inhibits the proliferation and invasion of osteosarcoma cells by suppressing the Wnt/β-catenin signaling pathway. Oncol. Rep. 2015, 34, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Huang, C.C.-Y.; Chi, M.-C.; Lee, K.-H.; Peng, K.-T.; Fang, M.-L.; Chiang, Y.-C.; Liu, J.-F. Naringenin Induces ROS-Mediated ER Stress, Autophagy, and Apoptosis in Human Osteosarcoma Cell Lines. Molecules 2022, 27, 373. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Shen, P.; Chen, Y.; Wang, J.; Wang, H. Epigallocatechin-3-gallate suppresses the growth of human osteosarcoma by inhibiting the Wnt/β-catenin signalling pathway. Bioengineered 2022, 13, 8490–8502. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumor Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Hönicke, A.-S.; Ender, S.A.; Radons, J. Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-induced tumorigenic factors in U-2 OS human osteosarcoma cells. Int. J. Oncol. 2012, 41, 753–758. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 37. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+)equol and S-(−)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Singh-Gupta, V.; Zhang, H.; Yunker, C.K.; Ahmad, Z.; Zwier, D.; Sarkar, F.H.; Hillman, G.G. Daidzein Effect on Hormone Refractory Prostate Cancer In Vitro and In Vivo Compared to Genistein and Soy Extract: Potentiation of Radiotherapy. Pharm. Res. 2010, 27, 1115–1127. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Qi, W.-T.; Guo, W.; Wang, C.-L.; Hu, Z.-B.; Li, A.-K. Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, P.; Lou, L.; Wang, Y. Perspectives Regarding the Role of Biochanin A in Humans. Front. Pharmacol. 2019, 10, 793. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Lai, W.-F. Chemical and Biological Properties of Biochanin A and Its Pharmaceutical Applications. Pharmaceutics 2023, 15, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Wu, Y.-Y.; Huang, H.; He, C.; Li, W.-Z.; Wang, H.-L.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015, 35, 391–398. [Google Scholar] [CrossRef]
- Jalaludeen, A.M.; Ha, W.T.; Lee, R.; Kim, J.H.; Do, J.T.; Park, C.; Heo, Y.T.; Lee, W.Y.; Song, H. Biochanin A Ameliorates Arsenic-Induced Hepato- and Hematotoxicity in Rats. Molecules 2016, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Caley, A.; Jones, R. The principles of cancer treatment by chemotherapy. Surgery 2012, 30, 186–190. [Google Scholar] [CrossRef]
- Lu, M.-H.; Fan, M.-F.; Yu, X.-D. NSD2 promotes osteosarcoma cell proliferation and metastasis by inhibiting E-cadherin expression. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 928–936. [Google Scholar]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Ong, S.K.L.; Shanmugam, M.K.; Fan, L.; Fraser, S.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Machado Dutra, J.; Espitia, P.J.P.; Andrade Batista, R. Formononetin: Biological Effects and Uses—A Review. Food Chem. 2021, 359, 129975. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Lin, F.; Wang, X.; Wang, Y.; Wang, J.; Wang, C. RNA Sequencing of Osteosarcoma Gene Expression Profile Revealed that miR-214-3p Facilitates Osteosarcoma Cell Proliferation via Targeting Ubiquinol-Cytochrome c Reductase Core Protein 1 (UQCRC1). Med. Sci. Monit. 2019, 25, 4982–4991. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Yoshitake, R.; Chan, Y.S.; Sadava, D.; Chen, S. Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts. Int. J. Mol. Sci. 2021, 22, 8798. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Virgili, F.; Acconcia, F.; Ambra, R.; Rinna, A.; Totta, P.; Marino, M. Nutritional flavonoids modulate estrogen receptor alpha signaling. IUBMB Life 2004, 56, 145–151. [Google Scholar] [CrossRef]
- Marino, M.; Pellegrini, M.; La Rosa, P.; Acconcia, F. Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes. Steroids 2012, 77, 910–917. [Google Scholar] [CrossRef]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol. Nutr. Food Res. 2009, 53, 699–708. [Google Scholar] [CrossRef]
- Bulzomi, P.; Marino, M. Environmental endocrine disruptors: Does a sex-related susceptibility exist? Front. Biosci. 2011, 16, 2478–2498. [Google Scholar] [CrossRef]
- Delepine, N.; Delepine, G.; Bacci, G.; Rosen, G.; Desbois, J.C. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. Analysis of the literature. Cancer 1996, 78, 2127–2135. [Google Scholar] [CrossRef]
- Yin, J.; Xie, X.; Jia, Q.; Wang, J.; Huang, G.; Zou, C.; Shen, J. Effect and mechanism of quercetin on proliferation and apoptosis of human osteosarcoma cell U-2OS/MTX300. Zhongguo Zhong Yao Za Zhi 2012, 37, 611–614. [Google Scholar] [PubMed]
- Yuan, J.; Ossendorf, C.; Szatkowski, J.P.; Bronk, J.T.; Maran, A.; Yaszemski, M.; Bolander, M.E.; Sarkar, G.; Fuchs, B. Osteoblastic and osteolytic human osteosarcomas can be studied with a new xenograft mouse model producing spontaneous metastases. Cancer Investig. 2009, 27, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Husmann, K.; Arlt, M.J.; Muff, R.; Langsam, B.; Bertz, J.; Born, W.; Fuchs, B. Matrix Metalloproteinase 1 promotes tumor formation and lung metastasis in an intratibial injection osteosarcoma mouse model. Biochim. Biophys. Acta 2013, 1832, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Bhat, P.; Kriel, J.; Priya, B.S.; Shivananju, N.S.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef]
- Martin, T.A.; Li, A.X.; Sanders, A.J.; Ye, L.; Frewer, K.; Hargest, R.; Jiang, W.G. NUPR1 and its potential role in cancer and pathological conditions (Review). Int. J. Oncol. 2021, 58, 21. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Li, H.Y.; Xie, T.; Sun, L.L.; Zhu, T.; Wang, S.D.; Ye, Z.M.; Zhang, Y.-H. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: A meta-analysis. OncoTargets Ther. 2016, 9, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Kavasi, R.-M.; Berdiaki, A.; Papachristou, D.J.; Tsiaoussis, J.; Spandidos, D.A.; Tsatsakis, A.M.; Tzanakakis, G.N. Parathyroid hormone/parathyroid hormone-related peptide regulate osteosarcoma cell functions: Focus on the extracellular matrix (Review). Oncol. Rep. 2016, 36, 1787–1792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oršolić, N.; Car, N. Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumor Biol. 2014, 35, 6445–6454. [Google Scholar] [CrossRef]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; GuiLian, Y.; Wei, G. Quercetin Enhances Apoptotic Effect of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) in Ovarian Cancer Cells through Reactive Oxygen Species (ROS) Mediated CCAAT Enhancer-binding Protein Homologous Protein (CHOP)-death Receptor 5 Pathway. Cancer Sci 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Benedetti Panici, P.; Bonanno, G.; De Vincenzo, R.; Piantelli, M.; Mancuso, S. Synergistic Antiproliferative Activity of Quercetin and Cisplatin on Ovarian Cancer Cell Growth. Anticancer Drugs 1990, 1, 45–48. [Google Scholar] [CrossRef]
- Guo, J.; Feng, Z.; Huang, Z.; Wang, H.; Lu, W. MicroRNA-217 functions as a tumour suppressor gene and correlates with cell resistance to cisplatin in lung cancer. Mol. Cells 2014, 37, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Kim, M.E.; Yoon, J.H.; Park, P.R.; Youn, H.-Y.; Lee, H.-W.; Lee, J.S. Anti-inflammatory effects of galangin on lipopolysaccharide-activated macrophages via ERK and NF-κB pathway regulation. Immunopharmacol. Immunotoxicol. 2014, 36, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.; Lamb, A. Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus. Phytomedicine 2006, 13, 187–191. [Google Scholar] [CrossRef]
- Meyer, J.; Afolayan, A.; Taylor, M.; Erasmus, D. Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 56, 165–169. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, Q.; Bi, J.; Ding, J.; Ge, S.; Chen, F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem. Biol. Interact. 2014, 224, 149–156. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Chen, F.; Fang, J.; Xu, A.; Xi, W.; Zhang, S.; Wu, G.; Wang, Z. Galangin inhibits cell invasion by suppressing the epithelial-mesenchymal transition and inducing apoptosis in renal cell carcinoma. Mol. Med. Rep. 2016, 13, 4238–4244. [Google Scholar] [CrossRef]
- Zou, W.-W.; Xu, S.-P. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed. Pharmacother. 2018, 97, 851–863. [Google Scholar] [CrossRef]
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-beta1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure−Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Way, T.-D.; Kao, M.-C.; Lin, J.-K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004, 279, 4479–4489. [Google Scholar] [CrossRef]
- Gonzalez-Mejia, M.E.; Voss, O.H.; Murnan, E.J.; Doseff, A.I. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein–27. Cell Death Dis. 2010, 1, e64. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, J.; Yang, D.; Tian, W.; Zhu, Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer 2014, 14, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, A.; Chen, J.; Yu, T.; Guo, F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac. J. Cancer Prev. 2011, 12, 239–245. [Google Scholar]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium Naringenin Prevents Osteosarcoma Progression and Recurrence in the Patients Who Underwent Osteosarcoma Surgery by Improving Antioxidant Capability. Oxidative Med. Cell. Longev. 2018, 2018, 8713263. [Google Scholar] [CrossRef]
- Kanno, S.-I.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef]
- Totta, P.; Acconcia, F.; Leone, S.; Cardillo, I.; Marino, M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: Involvement of estrogen receptor alpha and beta signalling. IUBMB Life 2004, 56, 491–499. [Google Scholar] [CrossRef]
- Xiang, L.-P.; Wang, A.; Ye, J.-H.; Zheng, X.-Q.; Polito, C.A.; Lu, J.-L.; Li, Q.-S.; Liang, Y.-R. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Wu, L.; Xie, J.; Chen, X.; Hu, B.; Wu, Z.; Yao, Q.; Li, Q. Effects of Tea-Polysaccharide Conjugates and Metal Ions on Precipitate Formation by Epigallocatechin Gallate and Caffeine, the Key Components of Green Tea Infusion. J. Agric. Food Chem. 2019, 67, 3744–3751. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Chen, C.-Y.; Chiou, Y.-H.; Shyu, H.-W.; Lin, K.-H.; Chou, M.-C.; Huang, M.-H.; Wang, Y.-F. Epigallocatechin-3-Gallate Suppresses Human Herpesvirus 8 Replication and Induces ROS Leading to Apoptosis and Autophagy in Primary Effusion Lymphoma Cells. Int. J. Mol. Sci. 2017, 19, 16. [Google Scholar] [CrossRef]
- Stadlbauer, S.; Steinborn, C.; Klemd, A.; Hattori, F.; Ohmori, K.; Suzuki, K.; Huber, R.; Wolf, P.; Gründemann, C. Impact of Green Tea Catechin ECG and Its Synthesized Fluorinated Analogue on Prostate Cancer Cells and Stimulated Immunocompetent Cells. Planta Medica 2018, 84, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S.; Netke, S.; Roomi, W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract: Quercetin/red onions increase bioavailability of egcg. Phytother. Res. 2009, 24, S48–S55. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Gabriel, L.P.; dos Santos, M.E.M.; Jardini, A.L.; Bastos, G.N.; Dias, C.G.; Webster, T.J.; Filho, R.M. Bio-based polyurethane for tissue engineering applications: How hydroxyapatite nanoparticles influence the structure, thermal and biological behavior of polyurethane composites. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, M.W.; Siddique, R.; Liu, Y.; Ullah, I.; Xue, M.; Yang, G.; Hou, H. Catechins-Modified Selenium-Doped Hydroxyapatite Nanomaterials for Improved Osteosarcoma Therapy Through Generation of Reactive Oxygen Species. Front. Oncol. 2019, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Wang, G.; Yang, B.; Ren, W.; Ma, L.; Yu, Q. Stereospecific determination ofcis- andtrans-resveratrol in rat plasma by HPLC: Application to pharmacokinetic studies. Biomed. Chromatogr. 2007, 21, 257–265. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From chemoprevention to chemotherapy: Common targets and common goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Sahebkar, A.; Zabetian-Targhi, F.; Maleki, V. The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review. Biofactors 2019, 45, 651–665. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Cal, C.; Garban, H.; Jazirehi, A.; Yeh, C.; Mizutani, Y.; Bonavida, B. Resveratrol and cancer: Chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr. Med. Chem.-Anti-Cancer Agents 2003, 3, 77–93. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Noh, K.T.; Chae, S.H.; Chun, S.H.; Jung, I.D.; Kang, H.K.; Park, Y.-M. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2013, 431, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.-H.; Meng, Y.-L.; Jiang, W.-J.; Li, Y.-P.; Ge, L.-P.; Zhang, C.-H.; Liu, L.-N.; Kang, Y.-F. Finding an efficient tetramethylated hydroxydiethylene of resveratrol analogue for potential anticancer agent. BMC Chem. 2020, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Horiguchi, H.; Oguma, E.; Kayama, F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J. Nutr. Biochem. 2010, 21, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Guisado, E.; Alvarez-Barrientos, A.; Mulero-Navarro, S.; Santiago-Josefat, B.; Fernandez-Salguero, P.M. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: Cell-specific alteration of the cell cycle. Biochem. Pharmacol. 2002, 64, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C. Resveratrol supplementation affects bone acquisition and osteoporosis: Pre-clinical evidence toward translational diet therapy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1186–1194. [Google Scholar] [CrossRef]
- Bellavia, D.; Caradonna, F.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Giavaresi, G. Non-flavonoid polyphenols in osteoporosis: Preclinical evidence. Trends Endocrinol. Metab. 2021, 32, 515–529. [Google Scholar] [CrossRef]
- Li, Y.; Bäckesjö, C.-M.; Haldosén, L.-A.; Lindgren, U. Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur. J. Pharmacol. 2009, 609, 13–18. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wu, Y.; Lv, C.; Li, X.; Cao, X.; Yang, M.; Feng, D.; Luo, Z. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology 2013, 304, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, J.; Jiang, D. Resveratrol inhibits canonical Wnt signaling in human MG-63 osteosarcoma cells. Mol. Med. Rep. 2015, 12, 7221–7226. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE 2018, 13, e0205918. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Fu, S.; Jiang, B. Resveratrol is cytotoxic and acts synergistically with NF-κB inhibition in osteosarcoma MG-63 cells. Arch. Med. Sci. 2021, 17, 166–176. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals 2022, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Mengie Ayele, T.; Tilahun Muche, Z.; Behaile Teklemariam, A.; Bogale Kassie, A.; Chekol Abebe, E. Role of JAK2/STAT3 Signaling Pathway in the Tumorigenesis, Chemotherapy Resistance, and Treatment of Solid Tumors: A Systemic Review. J. Inflamm. Res. 2022, 15, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-F.; Chang, Y.-W.; Kuo, K.-T.; Shen, Y.-S.; Liu, C.-Y.; Yu, Y.-H.; Cheng, C.-C.; Lee, K.-Y.; Chen, F.-C.; Hsu, M.-K.; et al. NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl. Acad. Sci. USA 2016, 113, E2526–E2535. [Google Scholar] [CrossRef]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef]
- Xie, D.; Zheng, G.-Z.; Xie, P.; Zhang, Q.-H.; Lin, F.-X.; Chang, B.; Hu, Q.-X.; Du, S.-X.; Li, X.-D. Antitumor activity of resveratrol against human osteosarcoma cells: A key role of Cx43 and Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 111419–111432. [Google Scholar] [CrossRef]
- Shen, Y.; Khusial, P.R.; Li, X.; Ichikawa, H.; Moreno, A.P.; Goldberg, G.S. Src Utilizes Cas to Block Gap Junctional Communication Mediated by Connexin43. J. Biol. Chem. 2007, 282, 18914–18921. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef]
- Yoon, S.-O.; Park, S.-J.; Yun, C.-H.; Chung, A.-S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. BMB Rep. 2003, 36, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Lee, W.-J.; Tan, P.; Tang, C.-H.; Hsiao, M.; Hsieh, F.-K.; Chien, M.-H. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget 2015, 6, 2736–2753. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Patruno, R.; Ruggieri, E.; Montemurro, S.; Valerio, P.; Ribatti, D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: From the biology to the clinic. Curr. Med. Chem. 2006, 13, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.C.J.; Boult, J.K.R.; Walker-Samuel, S.; Chung, Y.-L.; Jamin, Y.; Ashcroft, M.; Robinson, S.P. The HIF-pathway inhibitor NSC-134754 induces metabolic changes and anti-tumour activity while maintaining vascular function. Br. J. Cancer 2012, 106, 1638–1647. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Yang, R. Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation. Oncol. Lett. 2012, 4, 837–839. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Baron, V.T.; Pio, R.; Jia, Z.; Mercola, D. Early Growth Response 3 regulates genes of inflammation and directly activates IL6 and IL8 expression in prostate cancer. Br. J. Cancer 2015, 112, 755–764. [Google Scholar] [CrossRef]
- Jayatilaka, H.; Tyle, P.; Chen, J.J.; Kwak, M.; Ju, J.; Kim, H.J.; Lee, J.S.H.; Wu, P.-H.; Gilkes, D.M.; Fan, R.; et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat. Commun. 2017, 8, 15584. [Google Scholar] [CrossRef]
- Xu, G.; Kuang, G.; Jiang, W.; Jiang, R.; Jiang, D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. Am. J. Transl. Res. 2016, 8, 922–931. [Google Scholar]
- Zhao, W.; Chen, Z.; Guan, M. Polydatin enhances the chemosensitivity of osteosarcoma cells to paclitaxel. J. Cell. Biochem. 2019, 120, 17481–17490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Q.; Ma, L.-L.; Lv, Z.-D.; Feng, F.; Chen, Z.; Liu, Z.-D. Polydatin induces apoptosis and autophagy via STAT3 signaling in human osteosarcoma MG-63 cells. J. Nat. Med. 2020, 74, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Fei, Z.; Su, H.; Xie, R.; Chen, L. Polydatin inhibits proliferation and promotes apoptosis of doxorubicin-resistant osteosarcoma through LncRNA TUG1 mediated suppression of Akt signaling. Toxicol. Appl. Pharmacol. 2019, 371, 55–62. [Google Scholar] [CrossRef]
- Luce, A.; Lama, S.; Millan, P.C.; Itro, A.; Sangiovanni, A.; Caputo, C.; Ferranti, P.; Cappabianca, S.; Caraglia, M.; Stiuso, P. Polydatin Induces Differentiation and Radiation Sensitivity in Human Osteosarcoma Cells and Parallel Secretion through Lipid Metabolite Secretion. Oxidative Med. Cell. Longev. 2021, 2021, 3337013. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Z.; Ye, Z. Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biol. Pharm. Bull. 2008, 31, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 615–625. [Google Scholar] [CrossRef]
- Barros, A.S.; Costa, E.C.; Nunes, A.S.; De Melo-Diogo, D.; Correia, I.J. Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models. Int. J. Pharm. 2018, 551, 76–83. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sánchez, E.; Uddin, S.; Bru, R.; Martínez-Márquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Rasekhian, M.; et al. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021, 38, 1282–1329. [Google Scholar] [CrossRef]
- Chen, S.; Tao, J.; Zhong, F.; Jiao, Y.; Xu, J.; Shen, Q.; Wang, H.; Fan, S.; Zhang, Y. Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell. PLoS ONE 2017, 12, e0176501. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L.; et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017, 7, 11895. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, D.-Q.; Liao, Z.; Wang, B.; Gong, S.; Wang, C.; Zhang, M.-Z.; Wang, G.-H.; Cai, H.; Liao, F.-F.; et al. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol. Neurodegener. 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, D.; Gao, Z.; Zhang, C. Enzymatic transformation of polydatin to resveratrol by piceid-β-d-glucosidase from Aspergillus oryzae. Bioprocess Biosyst. Eng. 2013, 37, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-J.; Wu, K.; Wang, C.; Wan, D.-M. Polydatin-induced cell apoptosis and cell cycle arrest are potentiated by Janus kinase 2 inhibition in leukemia cells. Mol. Med. Rep. 2016, 13, 3297–3302. [Google Scholar] [CrossRef]
- Wang, H.-L.; Gao, J.-P.; Han, Y.-L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.-H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Zhang, H.; Park, S.; Huang, H.; Kim, E.; Yi, J.; Choi, S.-K.; Ryoo, Z.; Kim, M. Anticancer effects and potential mechanisms of ginsenoside Rh2 in various cancer types (Review). Oncol. Rep. 2021, 45, 33. [Google Scholar] [CrossRef]
- Shah, M.A.; Hamid, A.; Faheem, H.I.; Rasul, A.; Baokbah, T.A.S.; Haris, M.; Yousaf, R.; Saleem, U.; Iqbal, S.; Alves, M.S.; et al. Uncovering the Anticancer Potential of Polydatin: A Mechanistic Insight. Molecules 2022, 27, 7175. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Mao, Z.; Yu, D.; Gao, C. Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracellular release of zinc ions. J. Nanosci. Nanotechnol. 2014, 14, 5688–5696. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A.; et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef]
- Kota, V.; Hama, H. 2′-Hydroxy ceramide in membrane homeostasis and cell signaling. Adv. Biol. Regul. 2014, 54, 223–230. [Google Scholar] [CrossRef]
- Matsuzaki, E.; Hiratsuka, S.; Hamachi, T.; Takahashi-Yanaga, F.; Hashimoto, Y.; Higashi, K.; Kobayashi, M.; Hirofuji, T.; Hirata, M.; Maeda, K. Sphingosine-1-phosphate promotes the nuclear translocation of β-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone 2013, 55, 315–324. [Google Scholar] [CrossRef]
- Mostafa, F.; Abdel-Moneim, A.; Abdul-Hamid, M.; Galaly, S.R.; Mohamed, H.M. Polydatin and polydatin-loaded chitosan nanoparticles attenuate diabetic cardiomyopathy in rats. J. Mol. Histol. 2021, 52, 135–152. [Google Scholar] [CrossRef]
- Guan, Q.; Chen, W.; Hu, X.; Wang, X.; Li, L. Novel nanoliposomal delivery system for polydatin: Preparation, characterization, and in vivo evaluation. Drug Des. Dev. Ther. 2015, 9, 1805–1813. [Google Scholar] [CrossRef]
- Lin, L.; Gong, H.; Li, R.; Huang, J.; Cai, M.; Lan, T.; Huang, W.; Guo, Y.; Zhou, Z.; An, Y.; et al. Nanodrug with ROS and pH Dual-Sensitivity Ameliorates Liver Fibrosis via Multicellular Regulation. Adv. Sci. 2020, 7, 1903138. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Liu, J.; Wang, L.; Kai, G.; Yu, X. Effect of ZnO#ZnS QDs heterojunctures on the stilbenes–plasma proteins interactions. Mol. Biosyst. 2011, 7, 2452–2458. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Ślusarczyk, J.; Szczepanowicz, K.; Warszyński, P.; Leśkiewicz, M.; Regulska, M.; Trojan, E.; Lasoń, W. Protective effects of polydatin in free and nanocapsulated form on changes caused by lipopolysaccharide in hippocampal organotypic cultures. Pharmacol. Rep. 2019, 71, 603–613. [Google Scholar] [CrossRef]
- El-Hameed, A.M.A.; Yousef, A.I.; El-Twab, S.M.A.; El-Shahawy, A.A.G.; Abdel-Moneim, A. Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers. Biochemistry 2021, 86, 179–189. [Google Scholar] [CrossRef]
- Moyers-Montoya, E.D.; Escobedo-González, R.G.; Vargas-Requena, C.L.; Garcia-Casillas, P.E.; Martínez-Pérez, C.A. Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation. Polymers 2021, 13, 1303. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.; Luce, A.; Bitti, G.; Chacon-Millan, P.; Itro, A.; Ferranti, P.; D’auria, G.; Cammarota, M.; Nicoletti, G.F.; Ferraro, G.A.; et al. Polydatin Incorporated in Polycaprolactone Nanofibers Improves Osteogenic Differentiation. Pharmaceuticals 2022, 15, 727. [Google Scholar] [CrossRef]
- Peirotén, Á.; Bravo, D.; Landete, J.M. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit. Rev. Food Sci. Nutr. 2020, 60, 1922–1937. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.H. Frontiers in Polyphenols and Cancer Prevention3. J. Nutr. 2007, 137, 267S–269S. [Google Scholar] [CrossRef] [PubMed]
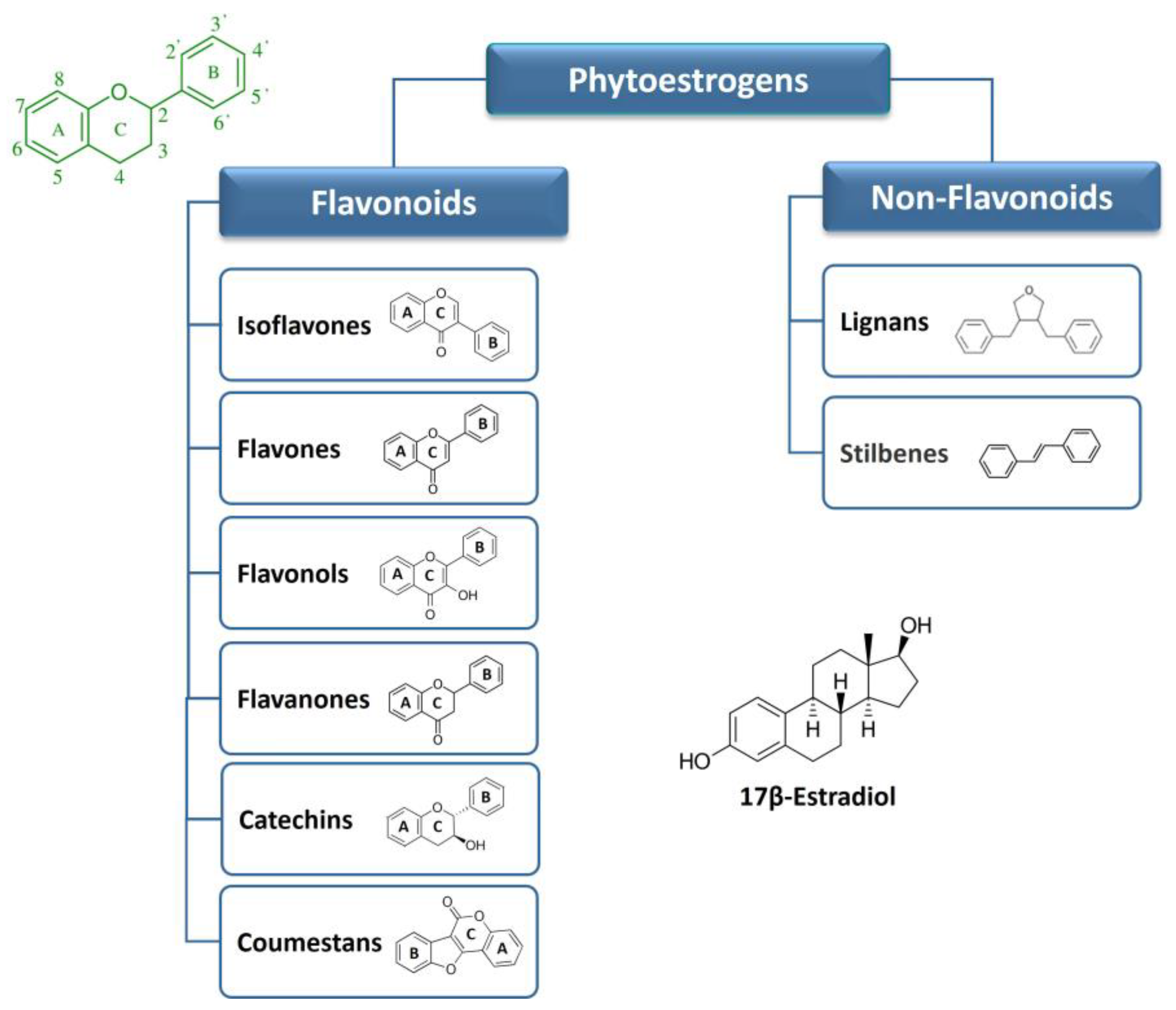

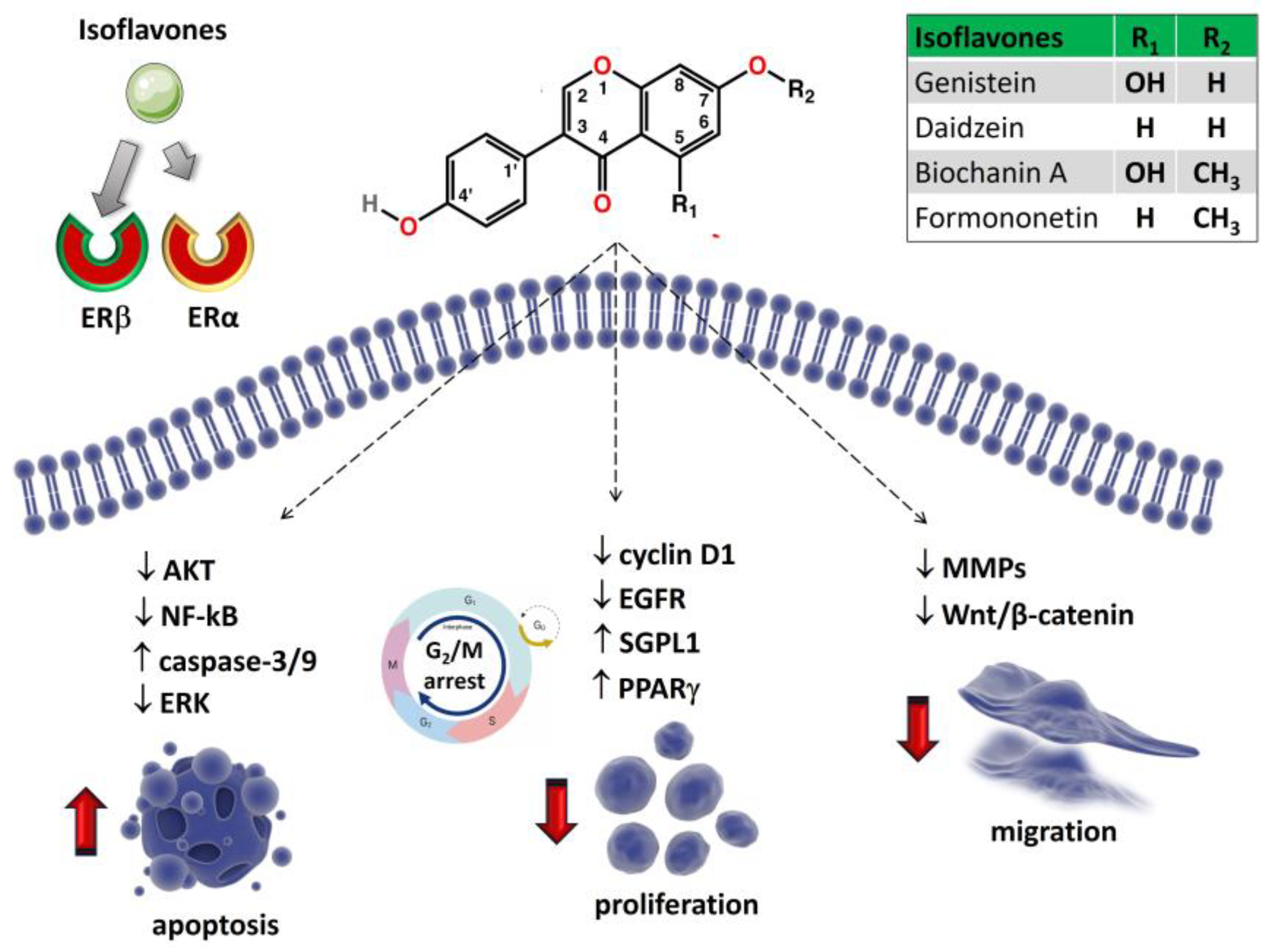
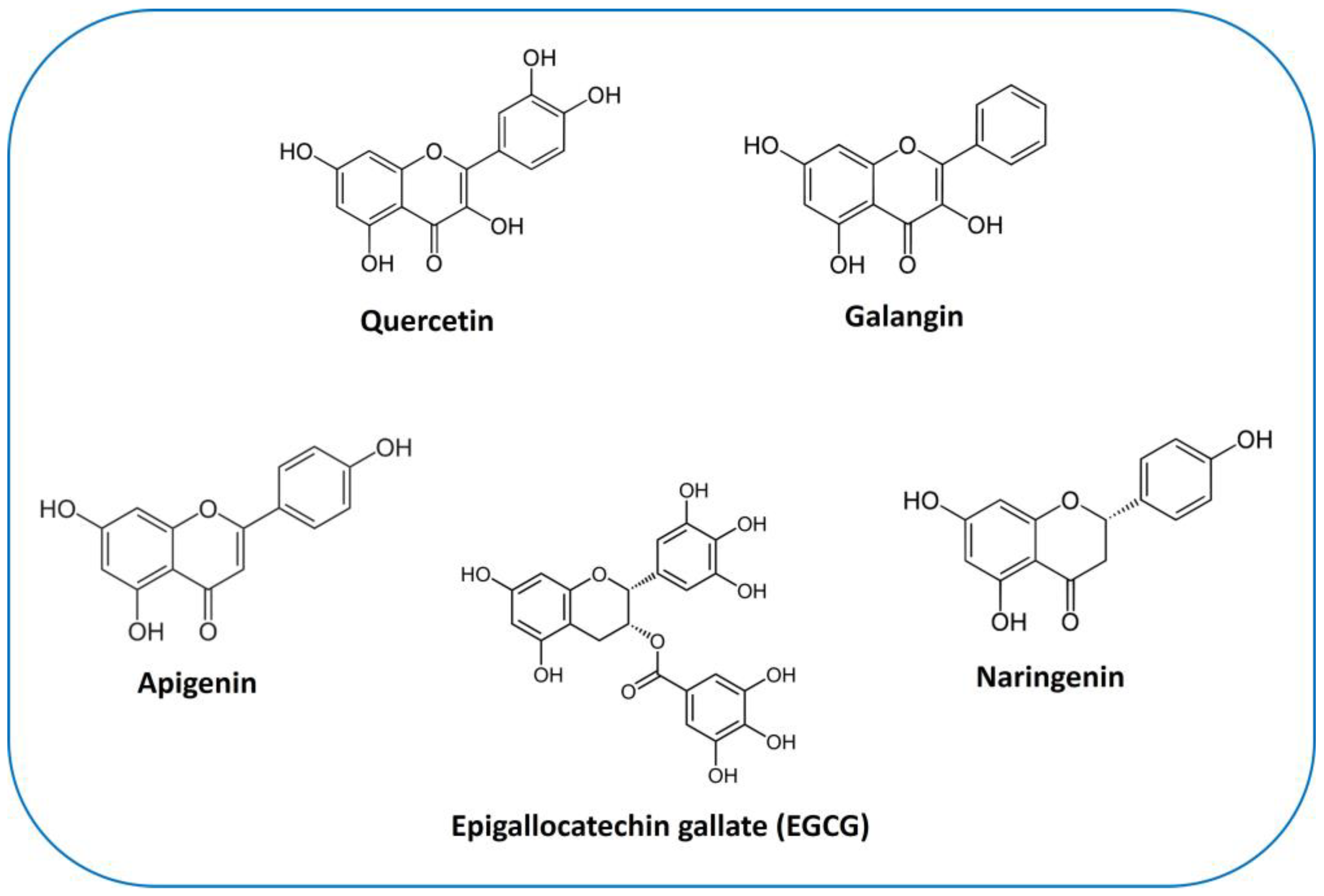

| Phytoestrogen | Relative Binding Affinity | References | |
|---|---|---|---|
| ERα | ERβ | ||
| Isoflavones | |||
| Genistein | 4 | 87 | |
| Daidzein | 0.1 | 0.5 | [19] |
| Biochanin A | <0.01 | <0.01 | |
| Formononetin | <0.01 | <0.01 | |
| Flavonols | |||
| Quercetin | 0.01 | 0.04 | [19] |
| Galangin | ND | ND | |
| Flavones | |||
| Apigenin | 0.3 | 0.6 | [19] |
| Flavanones | |||
| Naringenin | 0.01 | 0.11 | [19] |
| Stilbenes | |||
| Resveratrol | 6.11–11.2 a | 4.7–15.66 a | [52] |
| Polydatin | ND | ND | |
| Phytoestrogen | Cell Line/ In Vivo Model | Concentrations | Combined Treatment | Molecular Mechanism | Observed Effects | References |
|---|---|---|---|---|---|---|
| Genistein | U2OS | 1 µM | ER-mediated downregulation of EGFR | ↑ differentiation ↑ apoptosis ↑ cell cycle arrest | [154] | |
| MG-63 | 2.5–30 μmol/L | ↑ matrix vesicles, ↑ ALP activity | ↑ differentiation ↑ mineralized bone noduli ↓ proliferation | [158] | ||
| MG-63, SaOS-2 | 10–30 µM | ↓ PTK, ↑ ER | ↓ synthesis and secretion of GAGs/PGs | [162] | ||
| MG-63 | 40–80 µM | ↑ PPARγ pathway | ↑ cell cycle arrest ↓ proliferation | [164] | ||
| SaOS-2, MG-63 | 1–100 µM | calcitriol 10 nM for 48 h | ↑ SGPL1, VDR, ERβ | ↑ calcitriol sensitivity ↓ proliferation ↓ extracellular acidification ↓ mitochondrial respiration | [165] | |
| MG-63, U2OS | 10–100 μmol/L | gemcitabine 0.5 µmol/L for 72 h | ↓ Akt/NF-κB pathway | ↑ gemcitabine sensitivity ↑ apoptosis | [169] | |
| Daidzein | U2OS | 1 µM | ER-mediated downregulation of EGFR | ↑ differentiation ↑ apoptosis ↑cell cycle arrest | [154] | |
| 143B, U2OS xenograft mouse model | 10–500 μmol/L 20 mg/kg every 2 days | ↓ Src-ERK pathway | ↑ apoptosis ↑ cell cycle arrest ↓ migration ↓ tumor weights | [172] | ||
| Biochanin A | MG-63, U2OS | 5–30 μg/mL | DOX 1 μg/mL for 24 h | ↑ caspase-3/9, ↑ Bax: Bcl-2/Bcl-XL ratio | ↑ DOX sensitivity ↓ proliferation ↑ apoptosis | [173] |
| MG-63, U2OS | 5–80 μM | ↓ PCNA, cyclin D1, Bcl-2, ↑ Bax, caspase-3; ↓ MMP-9, N-cadherin, ↑ E-cadherin | ↑ cell cycle arrest ↑ apoptosis ↓ migration, invasion | [174] | ||
| Formononetin | U2OS, tumor-bearing nude mice | 20–80 μM, 80 mg/kg/d | ↓ Bcl-2, miR-375, ↑ Bax | ↑ apoptosis ↓ tumor weights | [175] | |
| U2OS | 5–100 μM | ↑ caspase-3 and Bax, ↓ Bcl-2 ↓ ERK and PI3K/AKT pathway | ↑ apoptosis | [176] | ||
| MG-63 | 15–45 μM | ↑ miR-214-3p/PTEN pathway | ↓ proliferation ↑ apoptosis | [177] | ||
| tumor-bearing nude mice | 25–100 mg/kg/d | ↓ ESR1, p53, ERBB2 | ↓ tumor weights | [178] | ||
| Quercetin | MG-63 | 20–320 μM | ↑ cytochrome C, caspase-3/9, Bax, ↓ Bcl-2 | ↑ apoptosis | [179] | |
| HOS, ATCC 1543 | 10–1000 µM | ↓ cyclin D1, ↑ caspase-3, cleaved PARP | ↑ cell cycle arrest ↓ proliferation ↑ apoptosis | [180] | ||
| U2OS/MTX300 | 10–50 µM | ↑ cytochrome C, caspase-3, Bax, cleaved PARP; ↓ Bcl-2, Akt | ↑ apoptosis ↓ proliferation | [181] | ||
| 143B | 10–100 µM | ↑ caspase-3, cleaved PARP | ↑ cell cycle arrest ↑ apoptosis ↓ adhesion ↓ migration | [182] | ||
| MG-63 | 50–200 µM | ↑ LC3B-II/LC3B-I ratio, ↓ ROS-NUPR1 pathway | ↑ autophagy | [183] | ||
| HOS, MG-63, tumor-bearing nude mice | 20–100 µM 25–100 mg/kg/d | ↓ HIF-1α, VEGF, MMP-2/9 | ↓ migration, invasion ↓ tumor growth | [184] | ||
| U2OS, SaOS-2 | ↓ PTHR1 | ↓ proliferation ↓ adhesion ↓ migration, invasion | [185] | |||
| 143B | 5 μM | CDDP 5 μM for 24 h | ↑ miR-217- KRAS axis | ↑ CDDP sensitivity ↓ proliferation ↓ migration, invasion | [186] | |
| SaOS-2 | 10–200 μM | MTX 10–200 μMfor 48 h | ↑ p53, CBX7, CYLD, ↓ Bcl-2, miR-223 | ↑ MTX sensitivity ↓ proliferation ↑ apoptosis | [187] | |
| Galangin | MG-63, U2OS | 5–300 µM | ↓ PI3K/Akt pathway ↓ cyclin D1, MMP-2/9, ↑ p27Kip1, caspase-3/8 | ↓ proliferation ↑ apoptosis ↓ invasion | [188] | |
| MG-63, U2OS tumor xenograft mouse | 25–100 μM 50, 100 mg/kg/d | ↑ TGF-β1/Smad2/3 pathway ↑ Col I, ALP, OPN, OCN | ↑ differentiation ↓ tumor growth | [189] | ||
| Apigenin | U2OS tumor xenograft mouse | 50–200 μM 2 mg/kg every 3 days | ↑ caspase-3/8/9, BAX, AIF | ↑ apoptosis ↓ tumor growth | [190] | |
| U2OS, MG-63 | 20–100 μg/mL | ↓ Wnt/β-catenin pathway | ↓ proliferation ↓ invasion | [191] | ||
| Naringenin | HOS, U2OS | 100–500 μM | ↑ ROS-Mediated ER Stress | ↑ autophagy ↑ apoptosis | [192] | |
| EGCG | MG-63, 143B, SaOS-2 tumor-bearing nude mice | 10–50 μM 10–40 mg/kg every 2 days | ↓ Wnt/β-catenin pathway | ↓ proliferation ↓ migration, invasion ↓ tumor growth | [193] | |
| MG-63, U2OS tumor-bearing nude mice | 0.0125–0.1 g/L 30 mg/kg/d | ↑ miR-1 | ↓ proliferation ↓ tumor growth | [194] | ||
| U2OS | 5–50 μM | IL-1Ra1 ng/mL | ↓ MMP-2, VEGF ↓ IL-6/8 | ↓ proliferation ↓ invasion | [195] | |
| U2OS, SaOS-2 | 20 μg/mL | DOX 1–2.5 μM | ↓ SOX2OT | ↑ autophagy | [196] (p. 7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, A.; Fasciglione, G.F.; Gioia, M.; Marini, S.; Ciaccio, C. Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma. Int. J. Mol. Sci. 2023, 24, 13344. https://doi.org/10.3390/ijms241713344
Cimmino A, Fasciglione GF, Gioia M, Marini S, Ciaccio C. Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma. International Journal of Molecular Sciences. 2023; 24(17):13344. https://doi.org/10.3390/ijms241713344
Chicago/Turabian StyleCimmino, Alessio, Giovanni Francesco Fasciglione, Magda Gioia, Stefano Marini, and Chiara Ciaccio. 2023. "Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma" International Journal of Molecular Sciences 24, no. 17: 13344. https://doi.org/10.3390/ijms241713344
APA StyleCimmino, A., Fasciglione, G. F., Gioia, M., Marini, S., & Ciaccio, C. (2023). Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma. International Journal of Molecular Sciences, 24(17), 13344. https://doi.org/10.3390/ijms241713344







