1. Introduction
Most cell responses that have been biochemically characterized are specifically activated following ligand–receptor binding. However, mechanobiology studies have shown that cells can alternatively trigger specific biochemical pathways via a multitude of mechanical stimuli [
1]. Indeed, external biomechanical forces and matrix mechanics have been reported to be critical regulators of stem cell fate [
2,
3]. The application of loading forces (e.g., cyclic tensile forces) has been reported to promote anabolism by stimulating osteogenesis both in vitro and in vivo [
4,
5,
6]. Interestingly, mechanical factors are essential not only for the well-known preservation of bone quality and quantity but also because they contribute to the development of cancer [
7,
8,
9,
10]. Increasing evidence suggests that environmental mechanics has a significant impact on gene expression, cell fate, and function and ultimately gives rise to modifications of the malignancy of osteosarcoma [
7,
11,
12].
Osteosarcoma (OS) is the most common primary malignant bone tumor in children and adolescents, associated with locally aggressive growth and a high risk of metastases in early-stage cancer. During their metastatic journey, cancer cells are exposed to several biophysical challenges. In fact, they acquire genetic and epigenetic modifications that render them more competitive in the neoplastic microenvironment [
13,
14]. For instance, malignant cells show increased migration, reduced adhesion, and higher compliance [
3].
Most of the targeted therapies for osteosarcoma have focused on signaling pathways such as human epidermal growth factor receptor (HEGR2), insulin-like growth factor 1(IGF1), and mammalian target of rapamycin (mTOR), which appear overactive in osteosarcoma tumors [
12,
15]. Current data demonstrate that commonalities in cell responses to different types of mechanical stimuli are correlated with the differential activation of intracellular signaling [
16]. Regarding mechano-oncology, a few biophysical markers of malignancy have been discovered to be related to the mechanical forces they were subjected to [
17,
18]. Nonetheless, targeting molecules involved in mechano-transduction is believed to have promising innovative potential because the identification of mechano-sensor molecules, which regulate osteosarcoma cell pathology, could reveal new oncogenic targets that are not identified in conventional biochemical screening for chemotherapy [
11,
19,
20].
Following a number of studies regarding the effect of environmental mechanical cues on cell volume regulation, changes in cell volume are considered part of the mechanical signal mechano-transduction process for chondrocyte and mesenchymal cells [
20,
21]. Furthermore, dynamically applied mechanical forces have been reported to produce modifications in cellular shape [
22], and a stretch-induced elongated shape in mesenchymal cells can aid osteogenic differentiation [
4,
23]. Previous results concerning biomechanical changes have reported that low metastatic osteosarcoma cells display larger spreading sizes and generate higher forces than the highly malignant variants [
17]. Notably, a variation in cell volume can modify a cell’s ability to move and thus hinder cell migration during development and tumor progression [
24].
In this study, atomic force microscopy (AFM) and confocal microscopy, which are leading imaging techniques used in 3D cellular morphology, were used for an in vitro study of the mechanobiological responses of osteosarcoma SAOS-2 cells. Since during fast-walking activities bone cells are subjected to moderate elongation [
25], we hypothesized that cells might evolve a mechano-biological response to a physiological stretch. Thus, our culturing experimental model was stimulated with a medium-magnitude stretch (corresponding to 0.5% elongation). Ab initio, we screened for the frequency (Hz) that worked best in applying the 0.5% elongation cyclic in order to induce the widest biological response of SAOS-2 cells (for further information, see the
Supplementary Materials). Moreover, with cell shape being affected by the balance between external and internal forces, it was theorized that applying cyclic tensile forces to adherent cells would prompt a consequent change in cell morphology.
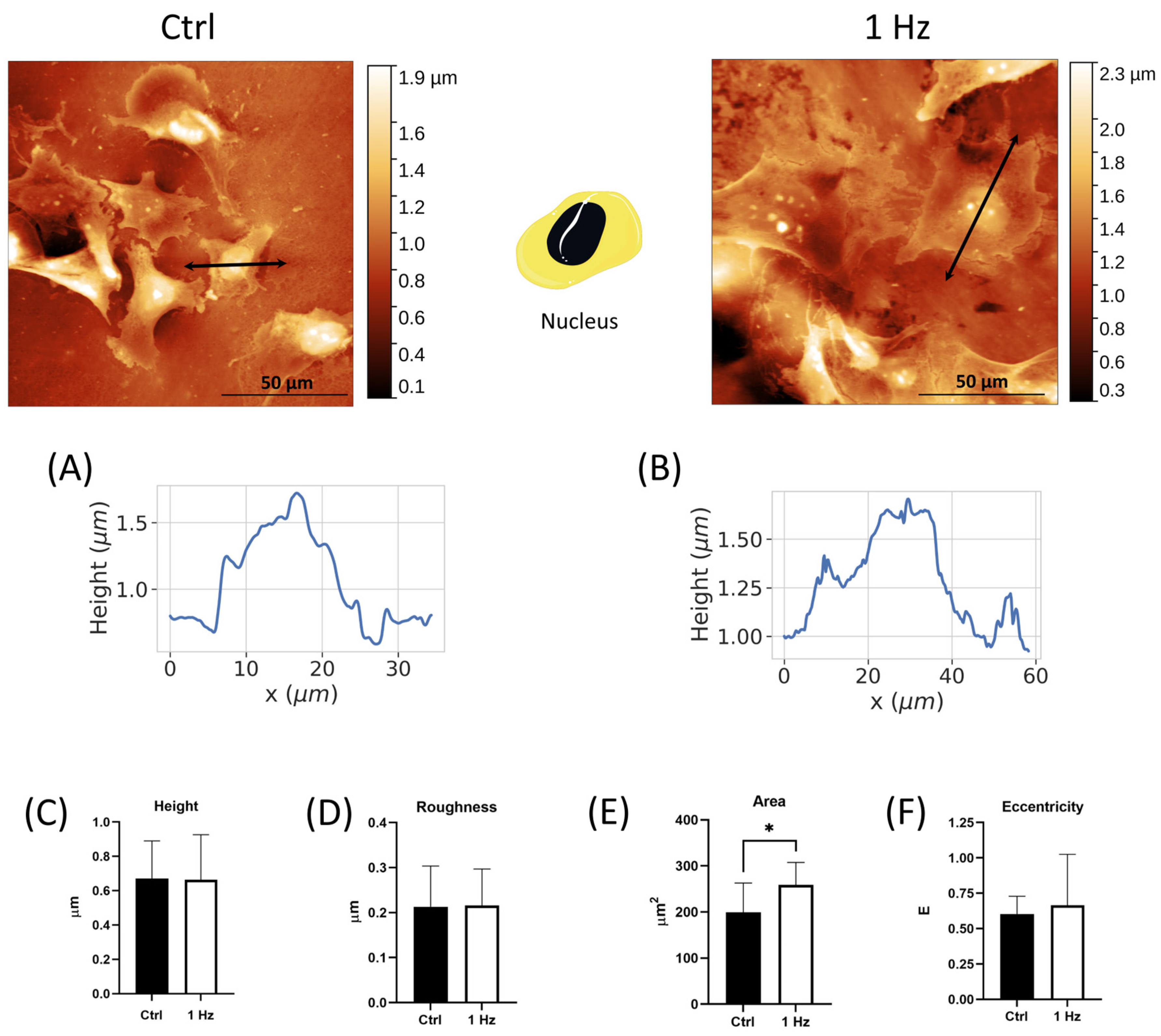
Since the enlargement of the nucleus (due to the increased amounts of chromatin present within malignant cells) and increases in the nuclear-to-cytoplasmic (N/C) ratio have been reported to be correlated with an increase in cell malignancy [
26,
27,
28], we looked for stretch-induced phenotypical changes by measuring the classical AFM morphological parameters (i.e., height, area, shape, and roughness) of the stretched and untreated SAOS-2 cells. Next, given that nuclear size changes may potentially impact gene expression through the modulation of intranuclear structures, we hypothesized that stretch-induced morphological changes could result in a variation of the osteoblastic differentiative grade. Therefore, the levels of the osteogenic gene markers (namely,
RUNX-
2,
COL1A1, and
ALPL) of 24 h 1 Hz-treated and untreated SAOS-2 cells were measured via quantitative real-time PCR (polymerase chain reaction).
Finally, we assessed the induced changes in cellular behavior by looking at the osteoblastic adhesiveness, the migration, and the transmigration (using a wound healing assay and a trans-well Boyden chamber) of pretreated or untreated SAOS-2 cells with the 24 h 1 Hz stimulation.
Since mechanical stresses from the microenvironment have been reported to regulate the PI3K/Akt signaling pathway (the most activated downstream effector in the oncogenic landscape) and the MMP-mediated degradation of the extracellular matrix [
29,
30,
31,
32], we decided to investigate whether these were sensible or not for our mechanical stimulus. Furthermore, cell volume regulation, which plays a role in cell migration, has been reported to be rapidly achieved through the participation of ion transportation across the cell membrane [
33,
34].
To select the class of molecules involved in the mechanism underlying the stretch-induced upregulation process, we tested whether or not soluble inhibitors could counteract the stretch-induced migration of SAOS-2 cells. Hence, considering that stretch-induced changes in cell motility could depend on force-sensitive ion-channels and/or MMP’s proteolytic activity, we screened MMP’s broad spectrum inhibitor and ion stretch-channel blocker.
3. Discussion
The mechanical properties of cells that depend on cytoskeleton architecture have been reported to play a critical role in the mechano-transduction process and have great potential in cancer diagnosis and therapy [
37]. Cell shape emerges from a balance between internal and external forces propagated through the cytoskeleton, the cell membrane, and cell–substrate adhesions. Even though many of these constituent elements have been studied in great detail at the molecular level, the mechanism by which global morphology is molded to address cell motility has yet to be fully understood.
This study analyzes stretch-induced changes in the morphological and motile properties of osteosarcoma cell biology. SAOS-2 cells were assessed using atomic force microscopy, confocal microscopy, and cell-based assays. As cyclic mechanical strain has been reported to affect osteosarcoma cell biology depending on the frequency by which the stretch is applied to cells [
38], we started the investigation by screening the best frequency capable of inducing the widest biological response. Our results demonstrated that a 1 Hz frequency of mild mechanical stretching (corresponding to 0.5% Ɛ elongation) applied for 3 days was able to increase the proliferation rate (
Supplementary Figure S1A). To characterize how the SAOS-2 cells respond to 1 Hz mechanical stimulation, the cell response was investigated in depth in a shortened process of one day. Our data demonstrated that changes in the proliferation rate following a 3-day stimulus were not significantly induced after a shortened treatment of 1 day (
Supplementary Figure S1B). However, the 24 h application of the 1 Hz cyclic tensile forces induced morphological changes in both the shape and dimension of the nucleus and cytoplasm (
Figure 1 and
Figure 2), which was accompanied by the elongation of the whole cell body (
Figure 2B,C), without inducing any preferential orientation in the cells (
Supplementary Figure S3). As most cell types orient themselves perpendicularly to (or along) the direction of uniaxial stretches when treated with high strain levels [
39,
40], we cannot exclude that SAOS-2 cells can orient themselves in response to prolonged mechanical stimulations and the elongation magnitude (Ɛ).
Metastatic cancer cells, in many cancers, have been reported to have altered cytoskeletal properties. In particular, the cells become more deformable and contractile. Consequently, the shape characteristics of more metastatic cancer cells would be expected to diverge from those of their parental cells [
41]. Interestingly, large and round tumor nuclei in osteosarcoma have been reported to be correlated with good clinical outcomes [
26]. In line with measurements reported in the literature for osteosarcoma cells [
11,
27], under our experimental conditions, the nuclear shape of SAOS-2 also displayed an elliptical geometry with an E-value of around 0.6. A comparison of cell morphology between cells that were or were not subjected to the 24 h 1 Hz treatment showed that the typical oval shape of osteosarcoma nuclei was retained, with the starting nuclear surface becoming even larger following mechanical stimulus (
Figure 1).
In addition, our quantitative morphological analysis demonstrated that the 24 h 1 Hz mechanical stimulation could decrease the nuclear-to-cytoplasmic (N/C) ratio of the cyclically stretched SAOS-2 cells by 22% when compared with unstretched cells (
Figure 2F). Interestingly, osteosarcoma cytoplasm changes in size have been reported to be correlated with increased metastasis, and the nuclear-to-cytoplasmic (N/C) ratio is believed to be essential for proper cell function [
26,
27]. Since the morphological abnormalities of the nucleus and N/C cell scaling are essential diagnostic features needed to distinguish benign and malignant cells in the daily practice of cytopathology [
28], we hypothesize that 24 h 1 Hz exogenous stimulation could be exploited to identify the cellular pathways underlying biological abnormalities in malignant cells. However, to gain insights into genetic and epigenetic abnormalities, further studies are needed regarding the 24 h 1 Hz induced changes in nuclear membrane irregularities, hyperchromasia, and abnormal chromatin distribution.
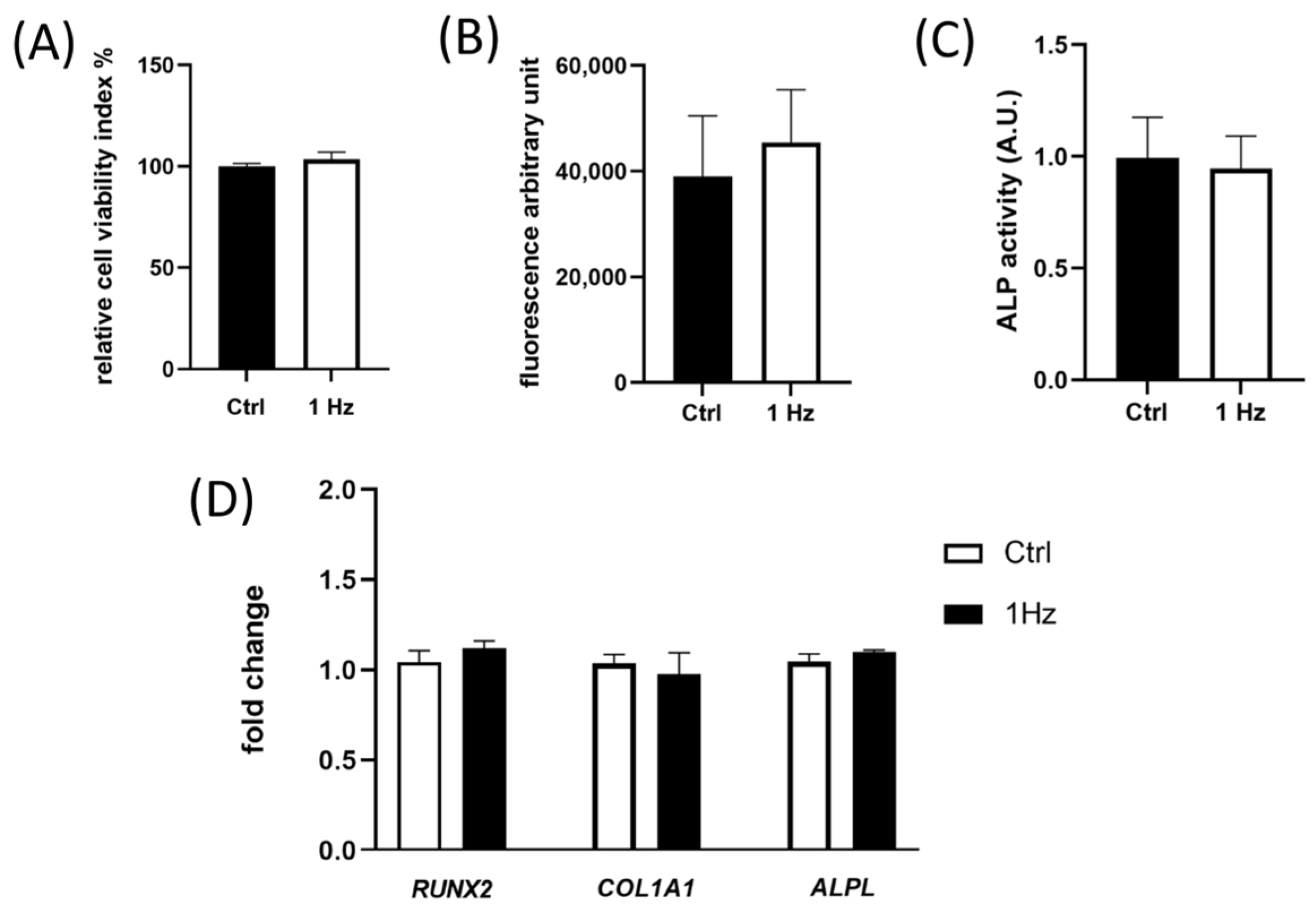
The expression of early osteogenic differentiation marker genes (i.e.,
ALPL,
RUNX-
2, and
COL1A1) and the ALP enzymatic activity, whose functionally mirrors the differentiation grades of osteoblastic cells, have been reported to be involved in metastasis in osteosarcoma patients [
36]. Our data demonstrated that both genes and the enzyme were not observed to be sensitive to the 24 h 1 Hz stretching stimulation (
Figure 4C,D), indicating that the mechanically induced morphology changes we observed do not seem to be linked to a modulation of the differentiative osteoblast phenotype. In any case, we cannot exclude that there could be an effect on the late osteogenic differentiation process after longer treatments.
Since cell volume regulation (a fundamental aspect of the homeostasis of the cell) and nuclear size dysregulation have been reported to affect cell migration by altering gene regulation, cell signaling, and function [
27,
42], we looked at the induced mechanical change in SAOS-2 cell motility capacity. Interestingly, we observed that the 24 h duration of the stimulus was long enough to profoundly change two relevant functions that influence the metastatic potential of osteosarcoma cells in vitro, namely, (i) cell motility and (ii) cell adhesion. On the one hand, the adhesion capability of mechanically pre-treated cells decreased by around 30% compared with untreated control cells (
Figure 5C,D). On the other hand, mechanically pre-treated cells could migrate and transmigrate faster than their control counterparts (
Figure 5A,B).
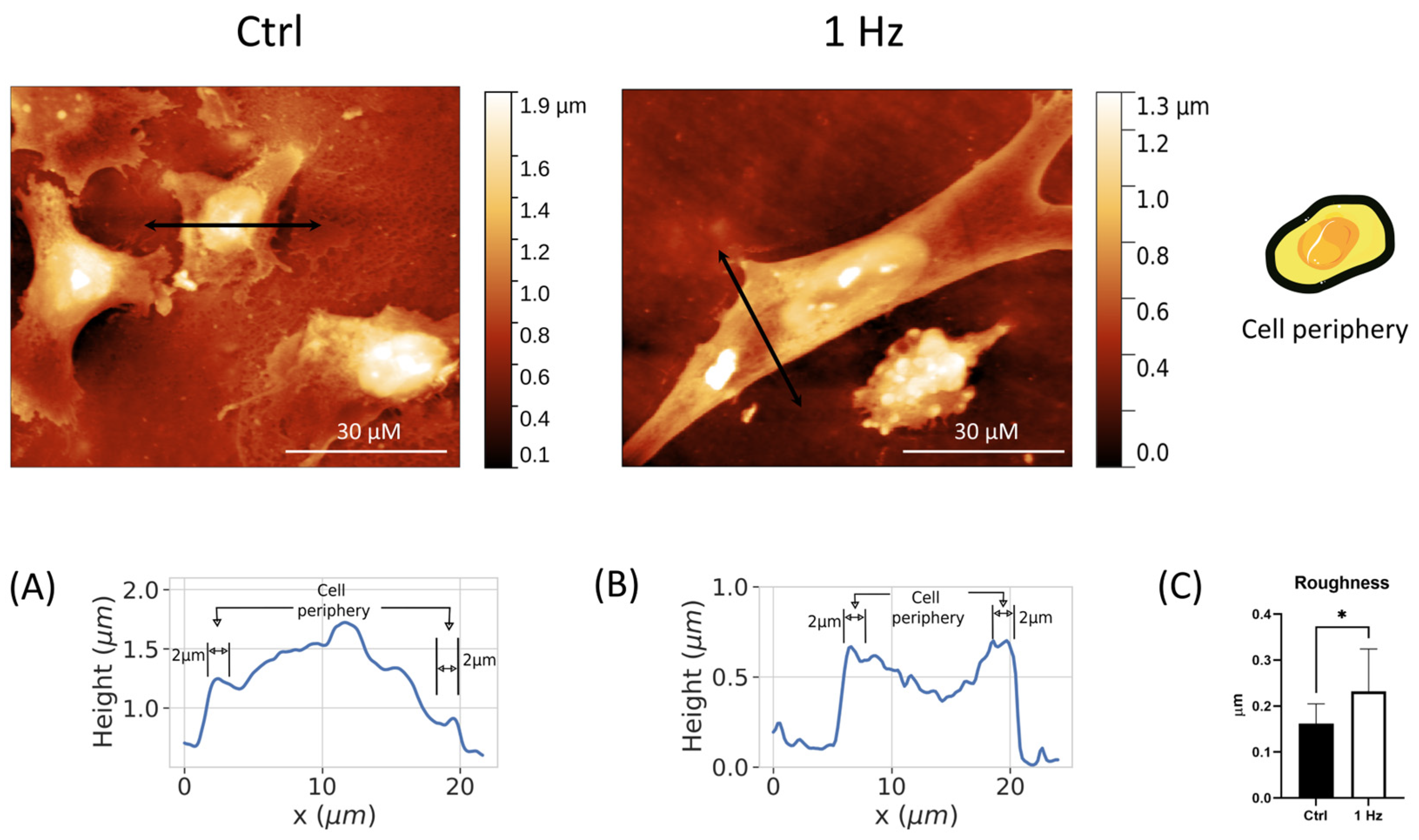
Despite the fact that the mechanisms and types of cell migration and invasion have been described and studied relatively well, there are currently no highly efficient and validated molecular markers for the identification of migrating/invading tumor cells in tumors and, therefore, for the assessment of their invasive potential [
29,
43]. In this regard, the differences in the complexity of boundary protrusions and cell peripheral roughness (
Figure S4 and
Figure 3) between 24 h 1 Hz treated versus untreated cells suggest it would be useful to focus future research on these regions in order to identify possible new markers for highly migratory cells.
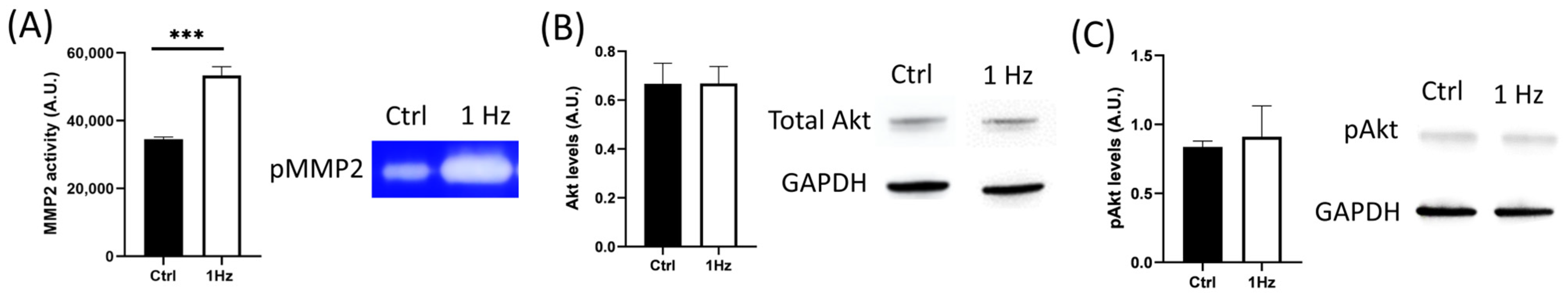
Besides the specific mechanical signals that have been reported to regulate cancer invasion and migration, the soluble extracellular context is also crucial for osteosarcoma cell biology in influencing metastatic cell journeys [
42]. Mechanical stresses from the microenvironment have been reported to regulate the PI3K/Akt signaling pathway (the most activated downstream effectors in the oncogenic landscape), promoting the expression of MMP-2 and MMP-9, thus degrading ECM and enabling osteosarcoma cell invasion and metastasis [
29,
30,
31]. Several MMPs have been associated with mature invadopodia [
38], including MMP-2, which was found to have an upregulated expression following mechanical stimulation [
44].
Although we did not observe any significant change in the total levels of Akt protein following mechanical stimulation, when the secretion of MMP-2 was examined using gelatin zymography of the conditioned medium, we found that 1 Hz mechanical stimulation was able to upregulate its enzymatic activity (
Figure 6). Despite the fact that mechanical stresses have been reported to affect MMP levels [
30] (
Figure 6), which, in turn, contribute to cell migration, invasion, and metastasis [
45], the 24 h 1 Hz induced upregulation of the motile capacity of the SAOS-2 cells was found to be independent of the activity of any type of MMP, given that both migration and transmigration assays were unaffected by the presence of ilomastat (a broad-spectrum inhibitor of MMPs) (
Figure 7). Itho et al. report that MMP-mediated cell migration could alternatively occur either through proteolytic or non-proteolytic mechanisms [
46]. However, our results indicate that the stretch-induced upregulation of migration does not happen through a proteolytic mechanism.
On the contrary, GsMTx4, a specific mechanosensitive stretch-activated ion-channel inhibitor, effectively counteracts the cell migrative upregulation induced by 24 h 1 Hz stimulation (
Figure 7E). Recently, the GsMTx4 peptide has also been reported to inhibit mechanically induced epithelial–mesenchymal transitions in keratinocytes [
47]. Indeed, the control gate function of ion stretch channels represents the best-known mechanosensory regulation that is induced by the tension forces of cell membranes [
40].
Considering that (i) the increase in membrane tension can activate specific mechanosensitive ion channels, including Piezo and transient receptor potential (TRP) channels [
48,
49,
50,
51]; (ii) mechanical compression has been reported to enhance the invasive phenotype of breast cancer cells [
52]; and (iii) TRP and/or Piezo channels have been shown to play an essential role in the regulation of cell migration [
24], it is reasonable to hypothesize that the 24 h 1 Hz stimulation stretches the cell membrane, possibly activating TRP and/or Piezo channels.
Understanding the underlying mechanisms of mechano-sensation and developing therapeutic applications for oncology treatments are current and future research goals. This study proves (though a single cell line) that some of the metastatic potential properties of osteosarcoma cells (such as the nuclear-to-cytoplasmic (N/C) ratio, cell shape, peripheral cell rugosity, cell adhesion, and cell migration) are mechanoresponsive.
From an oncological perspective, we believe that 24 h 1 Hz exogenous stimulation could be exploited to identify the cellular pathways underlying the biological abnormalities in malignant cells. These mechanobiology studies may represent an alternative approach to identifying cell signaling and new markers for the highly migratory cells that drive osteosarcoma progression and metastasis.
4. Materials and Methods
4.1. Cell Culture
Human SAOS-2 (HTB-85) cells were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (4.5 g/L glucose)/Ham F12 (1:1) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Euroclone s.p.a.,MI Milano, Italy), Penicillin–Streptomycin Solution 100X (Gibco, Life Technologies, Carlsbad, CA, USA), and Amphotericin B 100X (Biowest, Riverside, MO, USA) at 37 °C in an atmosphere of 5% CO2. The culture medium was changed twice a week, during which nonadherent cells were discarded.
4.2. Mechanical Stretch Application
A silicone well culturing plate (CellScale Biomaterials Testing, Waterloo, ON, Canada) is made of a deformable silicone rubber that displays a 10A Shore (on the Shore D hardness scale), which allows the culturing system to follow deformation applied (along the
x-axis) by the mechanical stretch MechanoCulture FX device (CellScale Biomaterials Testing, Waterloo, ON, Canada [
53]). The silicone wells were coated with rat type I collagen (Enzo Life Sciences, Farmingdale, NY, USA) at a 50 µg/mL concentration in PBS solution. SAOS-2 cells were trypsinized and seeded at a density of 2 × 10
5 cells per well (culture area: 16 wells, each well 8 mm × 8 mm). For each dataset, two silicon plates were seeded, and both were exposed to the same experimental conditions, but just one of the two was subjected to the mechanical stretch, while the spare one was used as a static unstimulated control. At 24 h after cell seeding, the mechanical stretch was applied to cells using the MechanoCulture FX mechanical stimulation system (CellScale Biomaterials Testing, Waterloo, ON, Canada) for 1 or 3 days. The MCFX software (
https://www.cellscale.com/products/mcfx/, accessed on 1 February 2023) was used to program the device with the phases, cycles, and sets of choices (
Supplementary Figure S8). The strain regimen was performed under the selected stretch, i.e., 0.5% elongation applied at 1 Hz cyclical frequency for 24 h, alternating 1 h of stretching and 3 h of rest (for further information, see
Supplementary Materials, Supplementary Figure S1). To measure the cell response of the adherent live cells immediately after the mechanical stretch, a cell-based experimental setup on a silicone deformable plate was developed to detect fluorescence and spectrometry on a multi-plate reader (see
Supplementary Materials, Supplementary Figures S6 and S7).
4.3. Cell Count and Protein Content
Immediately after the mechanical treatment, the stretched samples and control counterparts were processed as follows. The cell density was determined by counting the cells after trypsinization with an automated cell-counting chip
TM TECAN spark
® multimode reader (Tecan Group, Männedorf, Switzerland). A CellTiter 96
® AQ
ueousOne Solution Cell Proliferation Assay (MTS) (Promega, Milano, Italy), the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) colorimetric method (Merk Life Science s.r.l., Milano, Italia), and a CyQUANT™ NF Cell Proliferation Assay (Thermofisher Scientific Inc. Waltham, MA, USA) were used to count cells in a population and to measure proliferation in a microplate format (
Supplementary Figure S7). Protein content was measured in extracts of confluent cells grown on silicone well plates. The SAOS-2 cells were then detached from the plates and centrifuged. The supernatant was discarded, rinsed with PBS, and then lysed with RIPA buffer containing 150 nM NaCl, 50 mM Tris-HCl, 0.1% SDS, 1% Triton X-100, 1% Na cholate, 1% NaOV, 1 mM NaF, 1 mM EDTA, PMSF 1X, and Protease Inhibitor Cocktail (Cell Signalling Technology, Danvers, MA, USA). Protein quantifications were performed on the protein extracts using a Bradford assay (BioRad, Hercules, CA, USA). The protein content of cell lysates was determined with the Biorad protein assay and with bovine serum albumin (BSA) as a standard. Optical density was measured at 595 nm with a TECAN spark
R multimode reader (
Tecan Group
Ltd., Männedorf, Switzerland).
4.4. Cell Viability and Proliferation Assay
Rigid supports, which we customized on the flexible silicone well plate, were built to allow for fluorescence and spectrophotometric reading via the multi-plate TECAN spark
R reader (
Tecan Group
Ltd., Männedorf, Switzerland). The 3D model was designed with the e-Drawing program (version 30.1.0.0032; 1999–2022 Dassault Systèmes Corporation). As a 3D-printing filler, polylactic acid was used (see
Supplementary Materials, Figure S8).
4.4.1. MTS Assay on Flexible Silicone Plates
Cell viability was determined through a CellTiter 96® AQueousOne Solution Cell Proliferation Assay (MTS) (Promega, Milano, Italy) in the silicone well plate. Cells were seeded on a silicone plate at a density of 400 cells/mm2 and cultured in the growth medium as previously indicated. After mechanical treatment, 20 μL of MTS-PMS solution was added per well followed by 2 h of incubation at 37 °C with 5% CO2. Then, the absorbance of the formazan products of each well was recorded at 490 nm using an Infinite®200 PRO multi-well plate reader (Tecan Group Ltd., Männedorf, Switzerland).
4.4.2. Cell Viability Assay on 96-Well Plates
To examine cell proliferation ability, cells were seeded on silicone plates at a density of 110 cells/mm
2 and cultured in the growth medium as previously indicated. The cell number at each time-point was counted with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) colorimetric assay (Merk Life Science s.r.l., Milano, Italy). Briefly, 20 μL of MTT solution (5 mg/mL in PBS
w/
w Ca
2+ and Mg
2+) was added to each well followed by 2 h of incubation at 37 °C with 5% CO
2. Then, 100 μL of extraction buffer (5% SDS in N,N-Dimethylformamide) was added to each well followed by 2 h of incubation at 37 °C with 5% CO
2. In total, 150 μL per silicone well was transferred to a 96-well plate to measure the optical density of the formazan products. The absorbance of the formazan products was recorded at 570 nm using an Infinite
® 200 PRO multi-well plate reader (Tecan Group Ltd., Männedorf, Switzerland) (
Supplementary Figure S7).
4.5. Quantitative RT-PCR Analysis
The impact of the mechanical treatment on gene expression was evaluated as follows: following the 24-h mechanical stretch, treated SAOS-2 cells and the static control counterpart were trypsin-detached, pelleted, processed, and -analyzed for the following target genes: COL1A1, RUNX-2, BGLAP/OCC, and ALPL. RNA extraction from cellular pellets was prepared using the TRIZOL Reagent (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer’s protocol. RNA quality was examined by measuring the absorbance ratio at 260 nm and 280 nm through the NanoQuant Plate of an Infinite®200 PRO multi-well plate reader (Tecan Group Ltd., Männedorf, Switzerland). RNA was reverse-transcribed with SensiFAST™ cDNA Synthesis Kit (Bioline, Meridian Bioscience, London, UK) following the manufacturer’s specifications. Gene expression was measured using iTaq Universal SYBR Green Supermix (Biorad Laboratories, Hercules, CA, USA). qRT-PCR was performed using a LightCycler 96 Real-Time PCR System (Roche Diagnostics GmbH). For data analysis, the expressions of all genes were normalized using the ΔΔ cycle threshold method against human glyceraldehyde 3-phosphate dehydrogenase GAPDH gene expression. The sequences of primers used are as follows.
| Gene | Sequences (5′-3′) |
| h ALPL F | ACCTCGTTGACACCTGGAAG |
| h ALPL R | CCACCATCTCGGAGAGTGAC |
| h RUNX-2 F | GAGTGGACGAGGCAAGAGTT |
| h RUNX-2 R | AGCTTCTGTCTGTGCCTTCTG |
| h COL1A1 F | GTGCGATGACGTGATCTGTGA |
| h COL1A1 R | CGGTGGTTTCTTGGTCGGT |
| h BGLAP/OCC F | CACTCCTCGCCCTATTGGC |
| h BGLAP/OCC R | CCCTCCTGCTTGGACACAAAG |
| h GAPDH F | AGAAGGCTGGGGCTCATTT |
| h GAPDH R | AGGGGCCATCCACAGTCTT |
4.6. Alkaline Phosphatase Activity Assay on Silicone Plate
The ALP activity was performed within the silicone plate using an alkaline phosphatase blue microwell substrate containing Bromo-Chloro-Indolylphosphate (BCIP
®) (Sigma-Aldrich, Chemical Co., St. Louis, MO, USA) (
Supplementary Figure S7). Prior to any reaction with alkaline phosphatase, the BCIP
® reagent is a colorless to faint blue solution, and the Nitro Blue Tetrazolium (NBT/Thiazolyl Blue/Nitro BT) reagent is a yellow solution. The two components, when mixed, develop a bluish-purple product when reacting with alkaline phosphatase in microwell-type assays. The colorimetric reaction was assessed by using the TECAN spark
R multimode reader to measure the absorbance of the product at 520 nm within the first 10 min of incubation at 37 °C. The absorbances at t 10 min were normalized for the absorbances recorded at t 0.
4.7. Adhesion Assay
Right after the mechanical treatment, the stretched samples and control counterparts were trypsin-detached, pelleted, and processed (
Supplementary Figure S7). A cell adhesion assay after mechanical stimulus was performed on a 96-well plate that had been precoated with rat tail collagen I at a 50 µg/mL concentration and maintained at 37 °C for 2 h. SAOS-2 cells were seeded in DMEM high glucose at a density of 400 cells per well and maintained at 37 °C for 24 h under conventional tissue-growing conditions. The following day, the cells were washed and cell live confluence was recorded with the live imaging program of a TECAN spark
R multimode reader (
Tecan Group
Ltd., Männedorf, Switzerland). Cell monolayers were then crystal-violet-stained through 10 min of incubation in 0.5% crystal violet dissolved in 20%
v/
v methanol solution at 37 °C (Sigma Chemical Co., St. Louis, MO, USA). After several washes to remove the excess crystal violet, pictures were taken by an Olympus CKX53 inverted microscope equipped with an EP50 Microscope Digital Camera (Olympus Life Science, Waltham, MA, USA). Cell counts were based on counts of ten fields from digital images taken randomly at ×4 magnification by the ImageJ software ImageJ bundled with 64-bit Java 8 (ImageJ bundled with 64-bit Java 8,
http://rsb.info.nih.gov/ij/, accessed on 1 February 2023).
4.8. Morphological Microscopy
The measurement of the areas, perimeters, lengths, roughness, and heights was performed on subconfluent cells. Ab initio, to validate the SAOS-2 cell morphology, AFM and confocal microscopy were performed on cells seeded at a 110 cells/mm2 density on conventional glass coverslips precoated with collagen I cultured at 37 °C for 24 h and then morphologically inspected.
Furthermore, immediately after the mechanical treatment, the stretched cells and their control counterparts were trypsin-detached, pelleted, counted, and seeded at 110 cells/mm
2 density on conventional glass coverslips precoated with collagen I cultured at 37 °C for 24 h and then morphologically inspected (
Supplementary Figure S7).
Similarly, on the silicone plate, SAOS-2 cells were seeded at low density (110 cells/mm
2), cultured at 37 °C for 24 h, and treated or not with a mechanical uniaxial stretch. Adherent cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and then washed twice with bi-distilled water. The silicone wells were cut out, rinsed, and glued to a tailored glass coverslip for the AFM or confocal microscope inspections as described in the following paragraphs (
Supplementary Figure S7).
4.8.1. Nuclear Morphometric Analysis via Atomic Force Microscope (AFM)
AFM measurements were performed with the microscope working in the repulsive regime of contact mode in air at room temperature, as previously described [
54]. Bruker silicon nitride MSNL-10 cantilevers were employed. Constant force images with a force of 1 nN were acquired with a typical scan rate of 2–4 s/row (800–1600 points/row). Data were background-subtracted and then analyzed using the Gwyddion software. Nuclear shape was quantified by the eccentricity, E (according to Equation (1)). A high value for the E index indicates a round shape, while a low value refers to an elongated nuclear form. The nuclear surface was calculated from measurements of the major and minor axes according to Equation (2). Differentiation between areas corresponding to the nucleus and off-nucleus was based solely on topographical features. While it is assumed that areas marked as the “nucleus” indeed have a predominant height contribution from the nucleus of the cell, we cannot discard possible contributions to the measured height from other parts of the cell. However, for simplicity, we simply refer to these areas as the nucleus and off-nucleus. Cell height corresponds to the average value of the nucleus and off-nucleus areas of each measured cell. The roughness of SAOS-2 cells was calculated using AFM images, restricting the analysis to the surface areas near the cell edge (within 2 μm of the cell boundaries). The cell periphery was defined by those areas within approximately 2 μm of the cell perimeter, as shown in
Figure 4A). An average of the topographical roughness was then calculated for this area. Similar definitions of cell periphery have been used in past studies of cell morphology with AFM techniques [
55].
4.8.2. Confocal Microscopy
An Olympus LEXT OLS 4000 confocal microscope (Olympus Corporation, Tokyo, Japan) was used in confocal acquiring mode; ×20 or ×50 objective lenses were used (NA = 0.60, WD = 1 mm and NA = 0.95, WD = 0.35 mm, respectively) with a 405-nm laser. The images were acquired using the microscopy laser–confocal method without any DIC filter, with a size of 1024 × 1024 pixels. Using the ×20 lens, the xy area imaged was 648 × 648 μm, and using the ×50 lens, the xy area imaged was 258 × 258 μm, thus giving a lateral spatial resolution of 0.025 μm per pixel. The LEXT-specific files were exported in the jpg image format. The quantitative geometrical shape analysis for the identification of the stretch-induced morphological differences was performed as previously described [
22]. Areas, distances, and perimeters measured on cells and nucleus sizes were normalized with a reference scale using ImageJ software ImageJ bundled with 64-bit Java 8 (
http://rsb.info.nih.gov/ij/, accessed on 1February 2023). The number of pseudopodia per cell was counted, considering the cell processes with a measurement of more than 15 μm. The cell elongation was quantified not only by eccentricity, E, but also through roundness, R (Equation (1) and Equation (3), respectively). In both cases, a high value for these indexes indicates a round shape, while a low value reflects an elongated shape. Confocal microscopy pictures were inspected and analyzed to determine the N/C ratio: the whole cell surface and nuclear area were measured for each cell individually. The measurements of the cell area and the nuclear area were performed employing freehand and elliptical selections, respectively (ImageJ software). The circularity, C, shape descriptor was derived according to Equation (4). High values for C correlate with the absence of a star-like shape.
4.9. Western Blot
Immediately after the mechanical treatment, the stretched samples and control counterparts were trypsin-detached, pelleted, and processed as follows. To assess the protein expression level, SAOS-2 samples were lysed with RIPA buffer containing 150 nM NaCl, 50 mM Tris-HCl, 0.1% SDS, 1% Triton X-100, 1% Na cholate, 1% NaOV, 1 mM NaF, 1 mM EDTA, PMSF 1X, and a Protease Inhibitor Cocktail (Cell Signaling Technology, Danvers, MA, USA). From 30 to 60 µg of proteome was loaded per lane into precast gels (Mini-PROTEAN® TGX™ Precast Gels 4–20%, BioRad, Hercules, CA, USA) and then transferred to a PVDF membrane (Amersham, Buckinghamshire, UK). The membrane was blocked with 5% milk (Sigma Chemical Co., St. Louis, MO, USA) and probed with specific primary and secondary antibodies. The blots were developed by using Enhanced Chemiluminescence (ECL) detection systems (Amersham, UK). The following primary antibodies were used: GAPDH (dilution 1:10,000) (GeneText Irvine, Irvine, CA, USA, GTX100118) as a control, Phospo-Akt (Ser473) (D9E) (dilution 1:500) (Cell Signaling Technology, Danvers, MA, USA, #4060), and Akt (pan) (11E7) (dilution 1:1000) (Cell Signaling Technology, Danvers, MA, USA, #4685). The intensities of bands were measured through an image analysis program (ImageJ, public domain software; NIH, Bethesda, MD, USA) and quantified using a scale of arbitrary units (AU). The results obtained were analyzed using statistical software (Prism 9 ver. 9.0.0 (121), GraphPad Software).
4.10. Wound Healing Migration Assay
Following the 24 h mechanical treatment, treated SAOS-2 cells and the static control counterpart were seeded (at a density of 1 × 10
4 cells per well) on conventional culturing 96-well plates and incubated at 37 °C with 5% CO
2 for 24 h to allow a cell-confluent monolayer to firmly adhere to the rat type I collagen precoated bottom. Then, the 100% cell confluence monolayer was scratched to create a gap with a 10 µL pipette tip and rinsed with PBS to remove the suspended cells. Following the introduction of a scratch, cells were then incubated with serum-free DMEM supplemented or not with the indicated 1 μM GsMTx-4 (ab141871, Abcam, Cambridge, UK) or 1,6 μM ilomastat (CC10, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and were maintained at 37 °C with 5% CO
2. The wound closure was monitored for up to 48 h, and the gaps were photographed at 0 h and 20 h after scratching. The area of scratches was calculated using microscope measuring instruments (Olympus CKX53 equipped with an EP50 Microscope Digital Camera; Olympus Life Science, Waltham, MA, USA) and cell confluence (Tecan Group Ltd., Männedorf, Switzerland). The wound migration was expressed as wound closure (WC)% and as relative wound density (RWD) calculated according to Equations (5) and (6), respectively, as previously reported [
56]
where
A(t = 0) is the area of the wound measured immediately after scratching at t = 0.
And
A(
t∆
h) is the area of the wound measured h hours after the scratch is performed.
where
w(0) is the cell density of the wound area measured at time 0 immediately after the scratch is created.
w(t) is the cell density of the wound area measured at time t.
c(t) is the cell density of the cell area measured at time t.
Areas were calculated using the ImageJ public domain software (NIH, Bethesda, MD), whereas cell density was measured using the spark multi-mode plate reader (Tecan Group Ltd., Männedorf, Switzerland, Life Sciences).
4.11. Cell Transmigration Assay
The cell migration assay was assessed using a trans-well Boyden Chamber. After mechanical treatment, in serum-free DMEM, supplemented or not with the indicated compound, 1 μM GsMTx-4 (ab141871, Abcam, Cambridge, UK) or 1.6 μM ilomastat (CC10, Sigma-Aldrich Chemical Co., St. Louis, MO, USA), SAOS-2 cells were trypsin-detached and 4 × 104 cells in 100 μL of serum-free DMEM (supplemented or not with the compounds mentioned above) were seeded in the upper chamber of a trans-well 24-well plate (8.0 μm pore size, Corning-Costar Corporation, Cambridge, MA, USA). After 10 min of incubation at 37 °C, 600 μL of culture medium containing 10% FBS was added to the lower chamber as a chemoattractant. After 24 h at 37 °C with 5% CO2 for incubation, the trans-well chambers were removed. The cells that had migrated to the bottom of the wells were photographed with an optical microscope digital camera (Olympus CKX53 equipped with an EP50, Olympus Life Science, Waltham, MA, USA). Migrated cells were 0.1% crystal-violet-stained and subjected to a microscopic inspection. Cell counts were based on counts of ten fields from digital images taken randomly at ×4 magnification by the ImageJ software. The percentage of migrated cells was calculated using the average migrated cells in stretched group/average migrated cells in the control group, ×100%.
4.12. Zymography
Gelatin substrate zymography was used for the evaluation and characterization of the SAOS-2-cell-conditioned media, as previously described [
57]. Right after the mechanical treatment, the conditioned medium from the stretched samples and control counterparts were harvested and processed as follows: the confluent cell layers on the silicone plates were washed three times with a room-temperature PBS buffer. Media were replaced by serum-free DMEM, and cells were incubated at 37 °C overnight (mechanically treated or not). The cell-conditioned media were concentrated 10-fold via speed-vac centrivapor. Protein concentrations were determined using a Bradford assay (BioRad, Hercules, CA, USA) and confirmed by running SDS-PAGE gel electrophoresis in 10% polyacrylamide gels prior to zymography. Concentrated medium samples were diluted in an SDS-polyacrylamide gel electrophoresis sample buffer under nonreducing conditions without heating. Samples were separated by 10% SDS-polyacrylamide gels, which were co-polymerized with 1 mg/mL gelatin type B (Sigma Chemical Co., St. Louis, MO, USA). After electrophoresis runs, gels were washed twice for 30 min in 2.5% Triton X-100 and incubated overnight in an “activity buffer” (50 mmol/L Tris-HCl, pH 7.5, 10 mmol/L CaCl
2, 150 mmol/L NaCl) at 37 °C. As a control, an additional gel was incubated with an activity buffer that was supplemented with 1 mM of metalloproteinase inhibitor (GM6001), which completely abolishes the appearance of the clear transparent band. Gels were stained in Coomassie Blue R 250 (Bio-Rad, Milano, Italy) in a mixture of methanol: acetic acid: water (4:1:5) for 1 h and destained in the same solution without dye. Gelatinase activities were visualized as distinct bands, indicating the proteolysis of the substrate. The intensities of the gelatinolytic activity areas were measured through an image analysis program (ImageJ public domain software, NIH, Bethesda, MD, USA) and quantified using a scale of arbitrary units (AU). The results obtained were analyzed using statistical software (Prism 9 ver. 9.0.0 (121), GraphPad Software). Recombinant human MMP-2 (R&D Systems, Boston, MA, USA) was used as a reference standard for proMMP-2.
4.13. Data Analysis
The results are the means ± SEM, and differences between means were determined with the parametric t-test using the GraphPad Prism 9.01 software (San Diego, CA, USA).