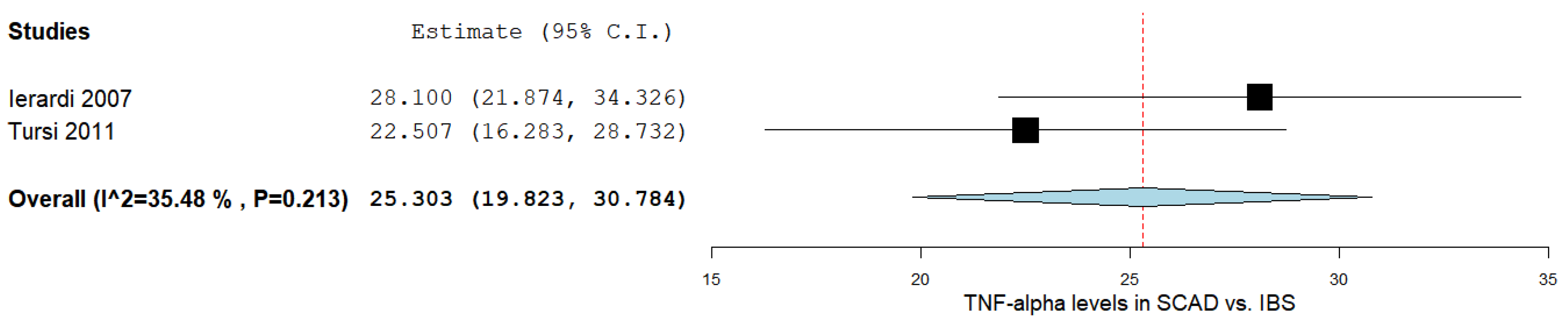Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Risk of Bias Assessment in Individual Studies
2.4. Summary Measure and Synthesis of Results
3. Results
3.1. General Results
3.2. Study Characteristics
3.3. Definition of Diverticulosis
3.4. TNF-α Levels in SUDD Patients vs. Healthy Controls
3.5. TNF-α Levels According to DD Status
3.6. TNF-α Levels in DD vs. IBS
3.7. TNF-α Levels between SCAD and IBS Patients
3.8. Qualitative Analysis
3.9. Bias Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Prim. 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Segmental colitis associated diverticulosis syndrome. World J. Gastroenterol. 2016, 22, 8067–8069. [Google Scholar] [CrossRef] [PubMed]
- Violi, A.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Capasso, M.; Leandro, G.; Meschi, T.; De’Angelis, G.L.; Di Mario, F. Epidemiology and risk factors for diverticular disease. Acta Bio-Med. Atenei Parm. 2018, 89, 107–112. [Google Scholar] [CrossRef]
- Imaeda, H.; Hibi, T. The Burden of Diverticular Disease and Its Complications: West versus East. Inflamm. Intest. Dis. 2018, 3, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 1282–1298.e1281. [Google Scholar] [CrossRef] [PubMed]
- Yeow, M.; Syn, N.; Chong, C.S. Elective surgical versus conservative management of complicated diverticulitis: A systematic review and meta-analysis. J. Dig. Dis. 2022, 23, 91–98. [Google Scholar] [CrossRef]
- Böhm, S.K. Risk Factors for Diverticulosis, Diverticulitis, Diverticular Perforation, and Bleeding: A Plea for More Subtle History Taking. Viszeralmedizin 2015, 31, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Matrana, M.R.; Margolin, D.A. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg 2009, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Fuschi, D.; Marasco, G. Pathophysiology of Diverticular Disease: From Diverticula Formation to Symptom Generation. Int. J. Mol. Sci. 2022, 23, 6698. [Google Scholar] [CrossRef] [PubMed]
- Munie, S.T.; Nalamati, S.P.M. Epidemiology and Pathophysiology of Diverticular Disease. Clin. Colon Rectal Surg. 2018, 31, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, M.; Lo Bianco, G.; Gianotti, L.; Nespoli, L. Inflammation management in acute diverticulitis: Current perspectives. J. Inflamm. Res. 2018, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Ali, S.; Stollman, N. Diverticular Disease: An Update on Pathogenesis and Management. Gut Liver 2018, 12, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Elisei, W. Role of Inflammation in the Pathogenesis of Diverticular Disease. Mediat. Inflamm. 2019, 2019, 8328490. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Ierardi, E. Tumour necrosis factor-alpha expression in segmental colitis associated with diverticulosis is related to the severity of the endoscopic damage. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2011, 43, 374–379. [Google Scholar] [CrossRef]
- Gareb, B.; Otten, A.T.; Frijlink, H.W. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E. Musosal tumour necrosis factor α in diverticular disease of the colon is overexpressed with disease severity. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2012, 14, e258–e263. [Google Scholar] [CrossRef]
- Ierardi, E.; Meucci, G.; Hassan, C.; Zullo, A.; Imperiali, G.; De Francesco, V.; Panella, C.; Morini, S.; Minoli, G. Tumour necrosis factor alpha in segmental colitis associated with diverticula. Dig. Dis. Sci. 2008, 53, 1865–1868. [Google Scholar] [CrossRef]
- Peery, A.F.; Keku, T.O.; Addamo, C.; McCoy, A.N.; Martin, C.F.; Galanko, J.A.; Sandler, R.S. Colonic Diverticula Are Not Associated With Mucosal Inflammation or Chronic Gastrointestinal Symptoms. Clin. Gastroenterol. Hepatol. 2018, 16, 884–891.e881. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 18 January 2023).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 15. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current (accessed on 17 January 2023).
- Hassan, C.; Zullo, A.; Ierardi, E.; Burattini, O.; De Francesco, V.; Morini, S. Tumour necrosis factor alpha downregulation and therapeutic response to infliximab in a case of segmental colitis associated with diverticula. Gut 2006, 55, 589–590. [Google Scholar] [CrossRef]
- Torii, Y.; Katano, Y.; Yoshino, J.; Inui, K.; Wakabayasi, T.; Kobayashi, T.; Kosaka, T. A case of diverticular colitis with lesions resembling ulcerative colitis and correlation of tumor necrosis factor-alpha staining with clinical manifestations. Clin. J. Gastroenterol. 2015, 8, 377–384. [Google Scholar] [CrossRef]
- Biancheri, P.; Di Sabatino, A.; Giuffrida, P.; Pender, S.L.; MacDonald, T.T.; Corazza, G.R. Immune and extracellular matrix remodelling alterations in peridiverticular inflamed mucosa of patients with diverticulitis. United Eur. Gastroenterol. J. 2013, 1, A246. [Google Scholar] [CrossRef]
- Biancheri, P.; Di Sabatino, A.; Kok, K.; Giuffrida, P.; Guerci, M.; Salvatore, C.; Massari, A.; Pasini, A.; Ubezio, C.; Macdonald, T.T.; et al. Marked activation of mucosal pro-inflammatory immune response in diverticulitis but not in diverticulosis. Dig. Liver Dis. 2013, 45, S180. [Google Scholar] [CrossRef]
- Ierardi, E.; Giorgio, F.; Piscitelli, D.; Fiore, M.G.; Rossi, R.; Cantatore, S.; Principi, M.; Di Leo, A.; Panella, C. The lack of tumour necrosis factor alpha downregulation on basic fibroblast growth factor/syndecan 1 link may be a relevant molecular pathway in crohn's disease fibrotic stenosis. Dig. Liver Dis. 2013, 45, S72. [Google Scholar] [CrossRef]
- Peery, A.; Keku, T.; Addamo, C.; McCoy, A.; Martin, C.; Galanko, J.; Sandler, R. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. United Eur. Gastroenterol. J. 2017, 5, A89. [Google Scholar] [CrossRef]
- Tursi, A.; Inchingolo, C.D.; Nenna, R.; Stoppino, G.; Zotti, M.; Panella, C.; Ierardi, E. Pattern of mucosal tumor necrosis factor-α expression in segmental colitis associated with diverticula suggests a truly autonomous clinical entity. Inflamm. Bowel Dis. 2008, 14, 1315–1317. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Ierardi, E.; Brandimarte, G. Chronic diverticulitis and Crohn’s disease share the same expression of basic fibroblastic growth factor, syndecan 1 and tumour necrosis factor-α. J. Clin. Pathol. 2014, 67, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chan, A.T. Does Subclinical Inflammation Play a Role in the Pathogenesis of Diverticulosis? Clin. Gastroenterol. Hepatol. 2018, 16, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Zullo, A.; De Francesco, V.; Campo, S.M.A.; Morini, S.; Panella, C.; Ierardi, E. Tumor necrosis factor alpha in ulcerative colitis and diverticular disease associated colitis. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 187–194. [Google Scholar] [CrossRef]
- Ghalyaie, N. Management of Diverticular Disease in the Setting of Other Colorectal Pathology: Data on Simultaneous Issues in Segmental Colitis, Inflammatory Bowel Disease, Cancer, and Complications. Clin. Colon Rectal Surg. 2018, 31, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Hassan, C.; Zullo, A.; De Francesco, V.; Valle, N.D.; Prencipe, S.; Rosania, R.; Morini, S.; Panella, C. Segmental colitis associated with diverticula: A rare clinical entity and a new challenge for the gastroenterologist. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2009, 41, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Roncoroni, L.; Bardella, M.T.; Terrani, C.; Bonura, A.; Ciulla, M.; Marconi, S.; Piodi, L. Absence of mucosal inflammation in uncomplicated diverticular disease. Dig. Dis. Sci. 2011, 56, 2098–2103. [Google Scholar] [CrossRef]
- Humes, D.J.; Simpson, J.; Smith, J.; Sutton, P.; Zaitoun, A.; Bush, D.; Bennett, A.; Scholefield, J.H.; Spiller, R.C. Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol. Motil. 2012, 24, 318-e163. [Google Scholar] [CrossRef]
- Potapova, V.B.; Levchenko, S.V.; Gudkova, R.B.; Rogozina, V.A.; Lazebnik, L.B. Regeneration of colorectal epithelium in diverticulosis. Bull. Exp. Biol. Med. 2012, 152, 760–763. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Ierardi, E. Tumour necrosis factor-alpha expression in segmental colitis associated with diverticulosis Down-Regulates after treatment. J. Gastrointest. Liver Dis. 2011, 20, 366–370. [Google Scholar]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E. Mucosal expression of basic fibroblastic growth factor, Syndecan 1 and tumor necrosis factor-alpha in diverticular disease of the colon: A case-control study. Neurogastroenterol. Motil. 2012, 24, 836-e396. [Google Scholar] [CrossRef]
- Tursi, A.; Elisei, W.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E.; Brandimarte, G. Expression of basic fibroblastic growth factor, syndecan 1 and tumour necrosis factor α in resected acute colonic diverticulitis. Color. Dis. 2014, 16, O98–O103. [Google Scholar] [CrossRef] [PubMed]
- Cossais, F.; Leuschner, S.; Barrenschee, M.; Lange, C.; Ebsen, M.; Vogel, I.; Böttner, M.; Wedel, T. Persistent Increased Enteric Glial Expression of S100β is Associated with Low-grade Inflammation in Patients with Diverticular Disease. J. Clin. Gastroenterol. 2019, 53, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Lahat, A.; Necula, D.; Yavzori, M.; Picard, O.; Halperin, S.; Eliakim, R.; Ben-Horin, S. Prolonged Recurrent Abdominal Pain is Associated With Ongoing Underlying Mucosal Inflammation in Patients who had an Episode of Acute Complicated Diverticulitis. J. Clin. Gastroenterol. 2019, 53, e178–e185. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, D.A.; Mack, T.M.; Beart, R.W., Jr.; Kaiser, A.M. Diverticulitis in the United States: 1998-2005: Changing patterns of disease and treatment. Ann. Surg. 2009, 249, 210–217. [Google Scholar] [CrossRef]
- Painter, N.S.; Burkitt, D.P. Diverticular disease of the colon: A deficiency disease of Western civilization. Br. Med. J. 1971, 2, 450–454. [Google Scholar] [CrossRef]
- Schwesinger, W.H.; Page, C.P.; Gaskill, H.V., 3rd; Steward, R.M.; Chopra, S.; Strodel, W.E.; Sirinek, K.R. Operative management of diverticular emergencies: Strategies and outcomes. Arch. Surg. 2000, 135, 558–562. [Google Scholar] [CrossRef]
- Oomen, J.L.; Engel, A.F.; Cuesta, M.A. Mortality after acute surgery for complications of diverticular disease of the sigmoid colon is almost exclusively due to patient related factors. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2006, 8, 112–119. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A.; Danese, S. Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 2015, 42, 664–684. [Google Scholar] [CrossRef]
- Elisei, W.; Tursi, A. The Pathophysiology of Colonic Diverticulosis: Inflammation versus Constipation? Inflamm. Intest. Dis. 2018, 3, 55–60. [Google Scholar] [CrossRef]
- Braegger, C.P.; Nicholls, S.; Murch, S.H.; Stephens, S.; MacDonald, T.T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1992, 339, 89–91. [Google Scholar] [CrossRef]
- Berner, B.; Akça, D.; Jung, T.; Muller, G.A.; Reuss-Borst, M.A. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J. Rheumatol. 2000, 27, 1128–1135. [Google Scholar] [PubMed]
- Tursi, A.; Brandimarte, G.; Elisei, W.; Giorgetti, G.M.; Inchingolo, C.D.; Danese, S.; Aiello, F. Assessment and grading of mucosal inflammation in colonic diverticular disease. J. Clin. Gastroenterol. 2008, 42, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Ierardi, E.; Burattini, O.; De Francesco, V.; Zullo, A.; Stoppino, G.; Panella, C.; Morini, S. Tumour necrosis factor alpha down-regulation parallels inflammatory regression in ulcerative colitis patients treated with infliximab. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2007, 39, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Macsharry, J.; O’Mahony, L.; Fanning, A.; Bairead, E.; Sherlock, G.; Tiesman, J.; Fulmer, A.; Kiely, B.; Dinan, T.G.; Shanahan, F.; et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand. J. Gastroenterol. 2008, 43, 1467–1476. [Google Scholar] [CrossRef] [PubMed]

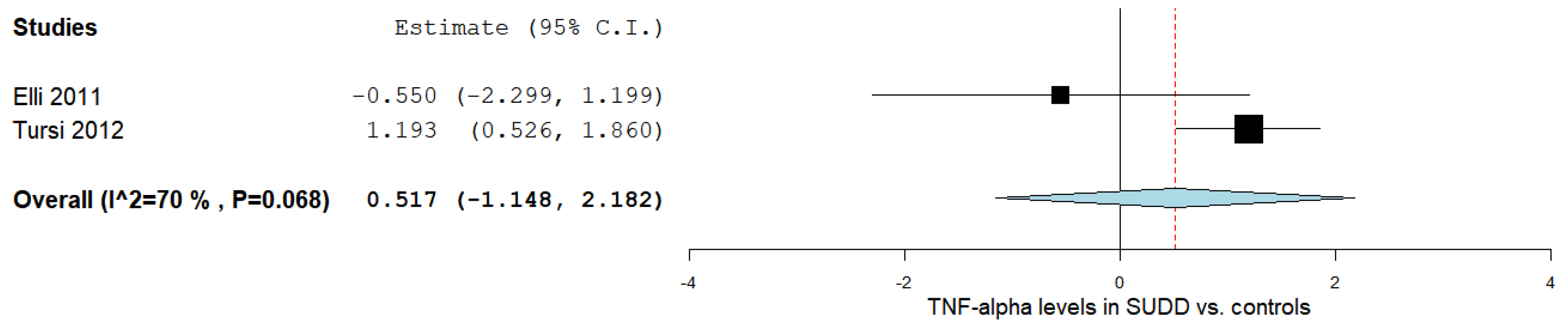

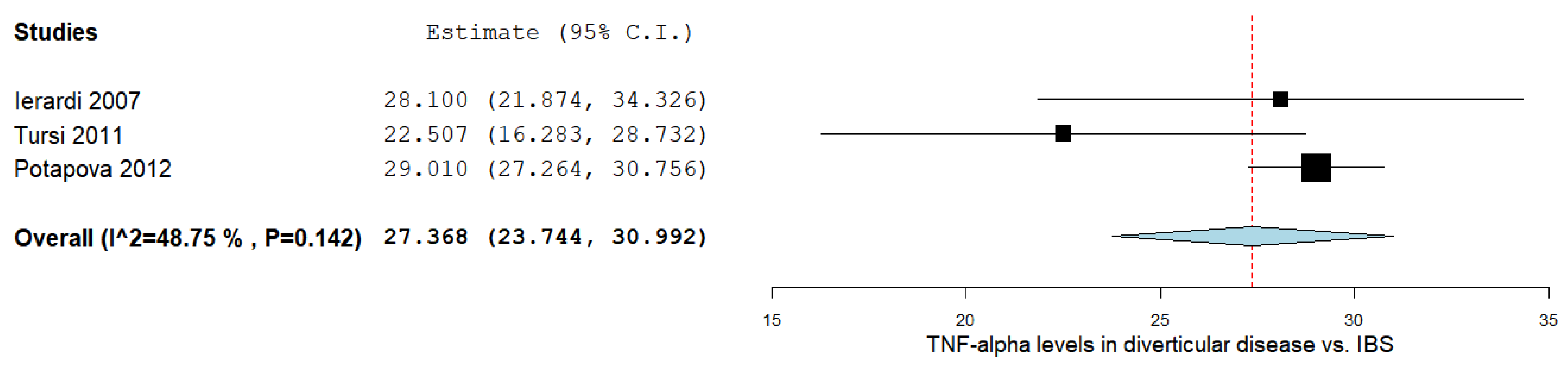
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabo, C.M.; Ismaiel, M.; Ismaiel, A.; Leucuta, D.-C.; Popa, S.-L.; Grad, S.; Dumitrascu, D.L. Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 9934. https://doi.org/10.3390/ijms24129934
Sabo CM, Ismaiel M, Ismaiel A, Leucuta D-C, Popa S-L, Grad S, Dumitrascu DL. Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(12):9934. https://doi.org/10.3390/ijms24129934
Chicago/Turabian StyleSabo, Cristina Maria, Mohamed Ismaiel, Abdulrahman Ismaiel, Daniel-Corneliu Leucuta, Stefan-Lucian Popa, Simona Grad, and Dan L. Dumitrascu. 2023. "Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 12: 9934. https://doi.org/10.3390/ijms24129934
APA StyleSabo, C. M., Ismaiel, M., Ismaiel, A., Leucuta, D.-C., Popa, S.-L., Grad, S., & Dumitrascu, D. L. (2023). Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(12), 9934. https://doi.org/10.3390/ijms24129934