Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle
Abstract
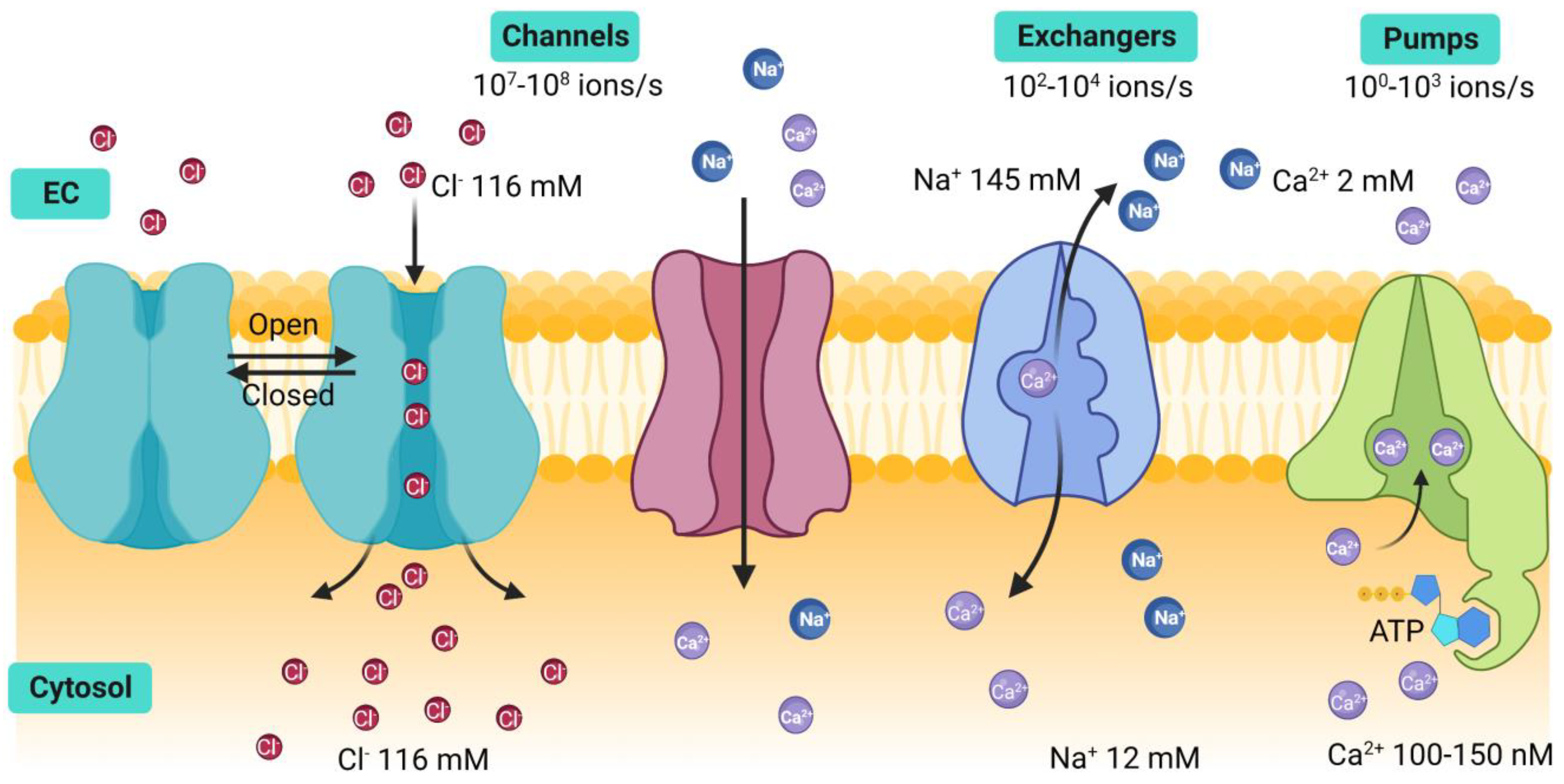
1. Introduction
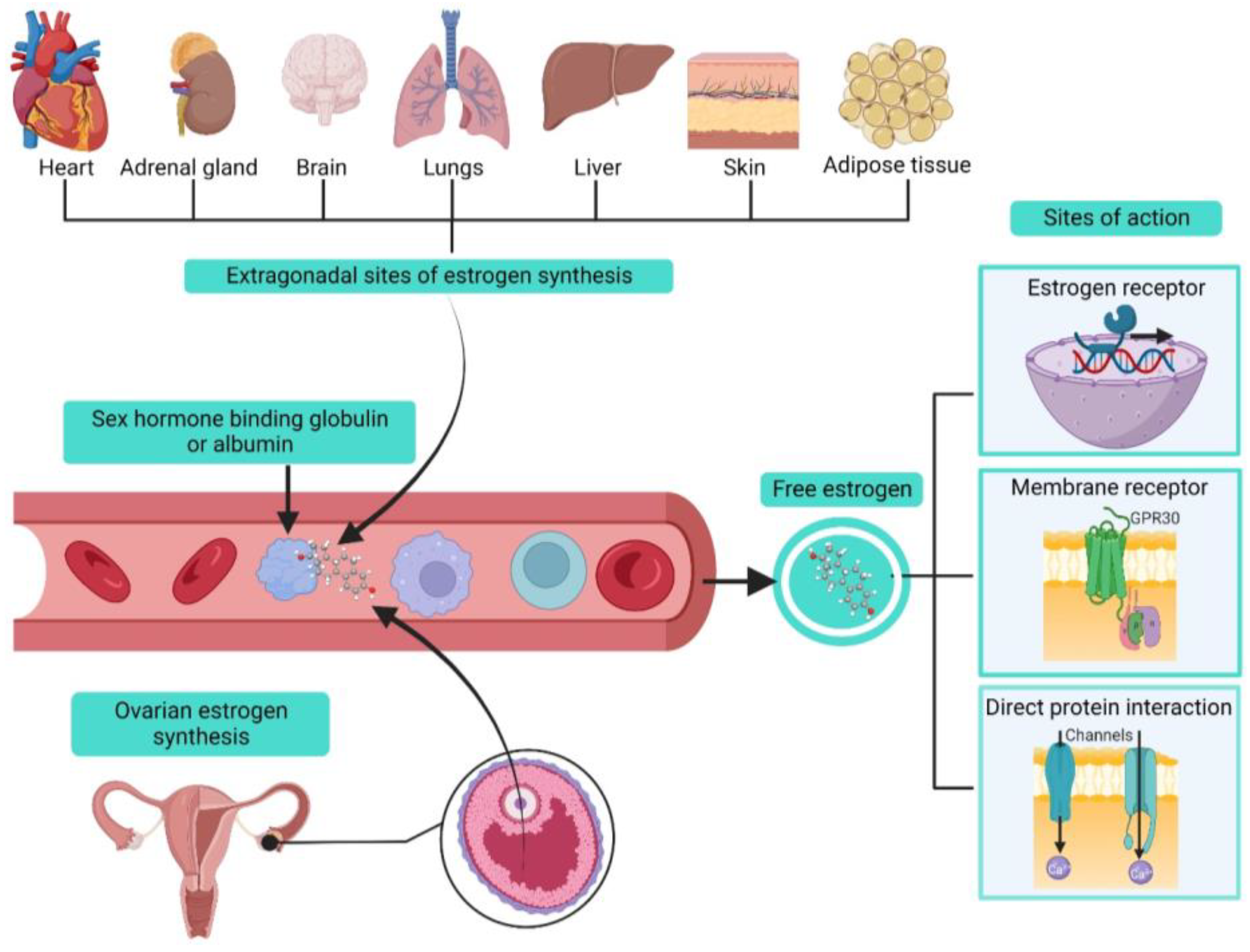
2. Estrogens Biosynthesis and Modes of Action
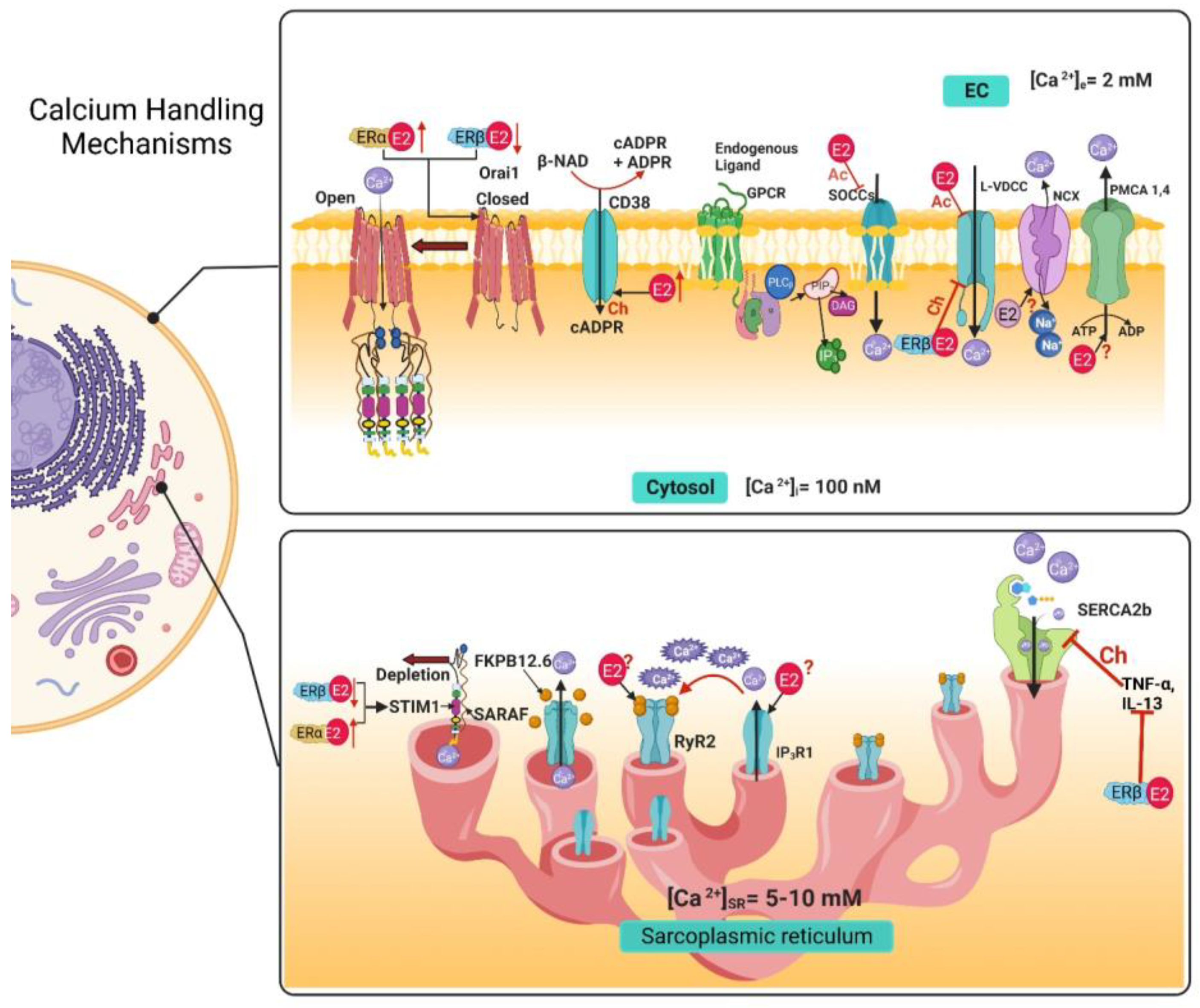
3. Airway Smooth Muscle Calcium Handling Mechanisms and Estrogens
3.1. Voltage-Dependent Ca2+ Channels
3.2. Store-Operated Calcium Channels
3.3. Ryanodine Receptor
3.4. IP3 Receptor
3.5. Na+/Ca2+ Exchanger
3.6. Plasma Membrane Ca2+ ATPase
3.7. Sarcoplasmic Reticulum Ca2+ ATPase
| Acute | Chronic | |||
|---|---|---|---|---|
| Calcium Handling Mechanisms | Pathway | Effect | Pathway | Effect |
| Voltage-dependent Ca2+ channels (VDCCs) | ERα | Inhibition [20,34] | ERβ | Inhibition [35] |
| Store-Operated Calcium Channels (SOCCs) | ERα | Inhibition via STIM1 phosphorylation [20,50] | ERβ | Downregulated STIM1 and Orai1 expression [49] |
| ERα | Upregulated STIM1 and Orai1 expression [49] | |||
| Ryanodine Receptor (RyR) | Unknown | Unknown | ERs | Upregulates CD38 expression [64] |
| IP3 Receptor (IP3R) | Unknown | Unknown | Unknown | Unknown |
| Na+/Ca2+ Exchanger (NCX) | No effect | No effect [20] | Unknown | Unknown |
| Plasma Membrane Ca2+ ATPase (PMCA) | Unknown | Unknown | Unknown | Unknown |
| Sarcoplasmic Reticulum Ca2+ ATPase (SERCA) | No effect | No effect | ERβ | Upregulates SERCA2 expression [35] |
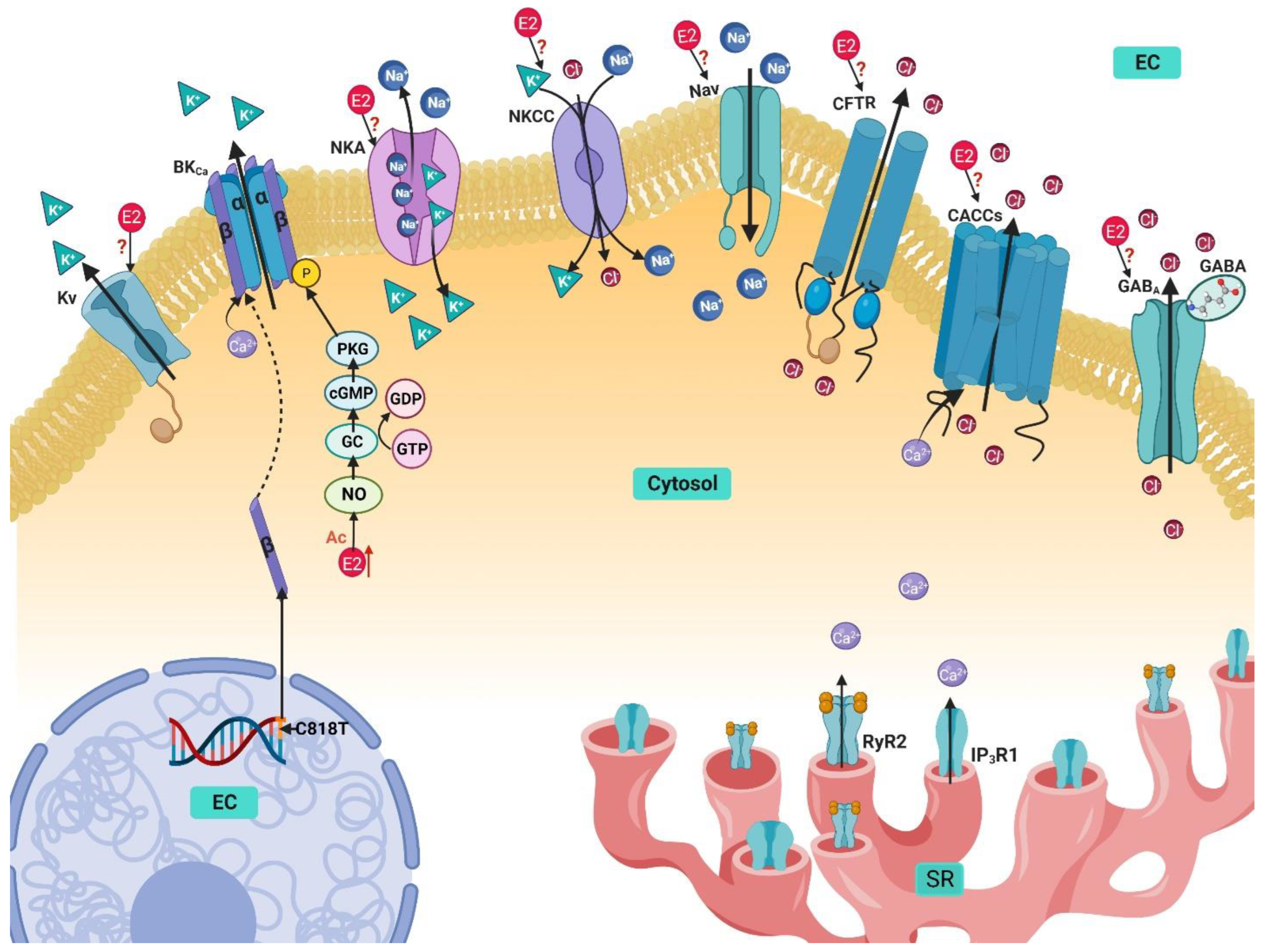
4. Potassium Handling Mechanisms in Airway Smooth Muscle and Their Modulation by Estrogens
4.1. Ca2+-Activated K+ Channels
4.2. Voltage-Activated K+ Channels
4.3. Na+/K+ ATPase
4.4. Na+/K+/Cl− Cotransporter
5. Sodium Handling in Airway Smooth Muscle and Its Modulation by Estrogen
Voltage-Gated Na+ Channels
6. Chlorine Handling Mechanisms in Airway Smooth Muscle and Their Modulation by Estrogen
6.1. Ca2+ activated Cl− Channels
6.2. Cystic Fibrosis Transmembrane Conductance Regulator
6.3. GABA-Activated Cl− Channels
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nehrke, K. Membrane ion transport in non-excitable tissues. WormBook 2014, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.J. Calcium Handling in Airway Smooth Muscle: Mechanisms and Therapeutic Implications. Can. Respir. J. 1998, 5, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C. Ion channels versus ion pumps: The principal difference, in principle. Nat. Rev. Mol. Cell Biol. 2009, 10, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Dascal, N. Ion-channel regulation by G proteins. Trends Endocrinol. Metab. 2001, 12, 391–398. [Google Scholar] [CrossRef]
- Song, S.; Luo, L.; Sun, B.; Sun, D. Roles of glial ion transporters in brain diseases. Glia 2020, 68, 472–494. [Google Scholar] [CrossRef]
- Rosati, B.; Mckinnon, D. Regulation of Ion Channel Expression. Circ. Res. 2004, 94, 874–883. [Google Scholar] [CrossRef]
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Martin, Y.N.; Pabelick, C.M. Sex differences in the pulmonary circulation: Implications for pulmonary hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H1253–H1264. [Google Scholar] [CrossRef]
- Sathish, V.; Martin, Y.N.; Prakash, Y.S. Sex steroid signaling: Implications for lung diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, J.; Kodama, M.; Clancy, C.E.; Furukawa, T. Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol. Ther. 2016, 168, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, H.; Barman, S.A.; Liu, A.T.; Sellers, M.; Stallone, J.N.; Prossnitz, E.R.; White, R.E.; Han, G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E882–E888. [Google Scholar] [CrossRef]
- Tran, Q.K. Reciprocality Between Estrogen Biology and Calcium Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 11, 568203. [Google Scholar] [CrossRef]
- Mah, V.; Seligson, D.B.; Li, A.; Márquez, D.C.; Wistuba, I.I.; Elshimali, Y.; Fishbein, M.C.; Chia, D.; Pietras, R.J.; Goodglick, L. Aromatase Expression Predicts Survival in Women with Early-Stage Non–Small Cell Lung Cancer. Cancer Res. 2007, 67, 10484–10490. [Google Scholar] [CrossRef]
- Mair, K.M.; Wright, A.F.; Duggan, N.; Rowlands, D.J.; Hussey, M.J.; Roberts, S.; Fullerton, J.; Nilsen, M.; Loughlin, L.; Thomas, M.; et al. Sex-Dependent Influence of Endogenous Estrogen in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2014, 190, 456–467. [Google Scholar] [CrossRef]
- Martin, Y.N.; Manlove, L.; Dong, J.; Carey, W.A.; Thompson, M.A.; Pabelick, C.M.; Pandya, H.C.; Martin, R.J.; Wigle, D.A.; Prakash, Y.S. Hyperoxia-induced changes in estradiol metabolism in postnatal airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L141–L146. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Townsend, E.A.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L521–L530. [Google Scholar] [CrossRef]
- Aravamudan, B.; Goorhouse, K.J.; Unnikrishnan, G.; Thompson, M.A.; Pabelick, C.M.; Hawse, J.R.; Prakash, Y.S.; Sathish, V. Differential Expression of Estrogen Receptor Variants in Response to Inflammation Signals in Human Airway Smooth Muscle. J. Cell. Physiol. 2017, 232, 1754–1760. [Google Scholar] [CrossRef]
- Kow, L.-M.; Pfaff, D.W. Rapid estrogen actions on ion channels: A survey in search for mechanisms. Steroids 2016, 111, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Flores-Soto, E.; Carbajal-García, A.; Sommer, B.; Montaño, L. Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review). Int. J. Mol. Med. 2018, 42, 2998–3008. [Google Scholar] [CrossRef]
- Janssen, L.J. T-type and L-type Ca2+ currents in canine bronchial smooth muscle: Characterization and physiological roles. Am. J. Physiol. 1997, 272, C1757–C1765. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Barajas-Lopez, C.; Daniel, E.E. Canine bronchial sustained contraction in Ca2+-free medium: Role of intracellular Ca2+. Can. J. Physiol. Pharmacol. 1996, 74, 1236–1248. [Google Scholar] [CrossRef]
- Sommer, B.; Flores-Soto, E.; Reyes-García, J.; Díaz-Hernández, V.; Carbajal, V.; Montaño, L.M. Na+ permeates through L-type Ca2+ channel in bovine airway smooth muscle. Eur. J. Pharmacol. 2016, 782, 77–88. [Google Scholar] [CrossRef]
- Bean, B.P. Classes of calcium channels in vertebrate cells. Annu. Rev. Physiol. 1989, 51, 367–384. [Google Scholar] [CrossRef]
- Yu, J.; Bose, R. Calcium channels in smooth muscle. Gastroenterology 1991, 100, 1448–1460. [Google Scholar] [CrossRef]
- Green, K.A.; Small, R.C.; Foster, R.W. The properties of voltage-operated Ca2+-channels in bovine isolated trachealis cells. Pulm. Pharmacol. 1993, 6, 49–62. [Google Scholar] [CrossRef]
- Hisada, T.; Kurachi, Y.; Sugimoto, T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflug. Arch. Eur. J. Physiol. 1990, 416, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kotlikoff, M.I. Calcium currents in isolated canine airway smooth muscle cells. Am. J. Physiol. 1988, 254, C793–C801. [Google Scholar] [CrossRef] [PubMed]
- Marthan, R.; Martin, C.; Amédée, T.; Mironneau, J. Calcium channel currents in isolated smooth muscle cells from human bronchus. J. Appl. Physiol. 1989, 66, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, E.; Reyes-García, J.; Carbajal-García, A.; Campuzano-González, E.; Perusquía, M.; Sommer, B.; Montaño, L.M. Sex steroids effects on guinea pig airway smooth muscle tone and intracellular Ca2+ basal levels. Mol. Cell. Endocrinol. 2017, 439, 444–456. [Google Scholar] [CrossRef]
- Bhallamudi, S.; Connell, J.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Estrogen receptors differentially regulate intracellular calcium handling in human nonasthmatic and asthmatic airway smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L112–L124. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.Y.; Yao, X.; Wong, C.M.; Chan, F.L.; Chen, Z.Y.; Huang, Y. Differential regulation of K+ and Ca2+ channel gene expression by chronic treatment with estrogen and tamoxifen in rat aorta. Eur. J. Pharmacol. 2004, 483, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Peel, S.E.; Liu, B.; Hall, I.P. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir. Res. 2006, 7, 119. [Google Scholar] [CrossRef]
- Peel, S.E.; Liu, B.; Hall, I.P. ORAI and Store-Operated Calcium Influx in Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 744–749. [Google Scholar] [CrossRef]
- Palty, R.; Raveh, A.; Kaminsky, I.; Meller, R.; Reuveny, E. SARAF Inactivates the Store Operated Calcium Entry Machinery to Prevent Excess Calcium Refilling. Cell 2012, 149, 425–438. [Google Scholar] [CrossRef]
- Albarran, L.; Regodón, S.; Salido, G.M.; Lopez, J.J.; Rosado, J.A. Role of STIM1 in the surface expression of SARAF. Channels 2017, 11, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Erxleben, C.; Yildirim, E.; Abramowitz, J.; Armstrong, D.L.; Birnbaumer, L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 4682–4687. [Google Scholar] [CrossRef]
- Xiao, J.-H.; Zheng, Y.-M.; Liao, B.; Wang, Y.-X. Functional Role of Canonical Transient Receptor Potential 1 and Canonical Transient Receptor Potential 3 in Normal and Asthmatic Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2010, 43, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yocum, G.T.; Chen, J.; Choi, C.H.; Townsend, E.A.; Zhang, Y.; Xu, D.; Fu, X.W.; Sanderson, M.J.; Emala, C.W. Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L812–L821. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Kuang, H.; Wu, J.; Guo, Y.; Ma, L. Effect of TRPV1 channel on the proliferation and apoptosis in asthmatic rat airway smooth muscle cells. Exp. Lung Res. 2013, 39, 283–294. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Varty, L.; Rizzo, C.; Yang, R.; Correll, C.; Phelps, P.; Egan, R.; Hey, J. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L272–L278. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Izumizaki, M. Effect of menstrual cycle and female hormones on TRP and TREK channels in modifying thermosensitivity and physiological functions in women. J. Therm. Biol. 2021, 100, 103029. [Google Scholar] [CrossRef]
- Flores-Soto, E.; Reyes-García, J.; Sommer, B.; Montaño, L.M. Sarcoplasmic reticulum Ca2+ refilling is determined by L-type Ca2+ and store operated Ca2+ channels in guinea pig airway smooth muscle. Eur. J. Pharmacol. 2013, 721, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.; Ambhore, N.; Thompson, M.; Pabelick, C.; Prakash, Y.; Venkatachalem, S. Differential estrogen receptor signaling regulates store operated calcium entry in human airway smooth muscle. Am. J. Respir. Crit. Care Med. 2018, 197, A7259. [Google Scholar]
- Sathish, V.; Freeman, M.R.; Long, E.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Cigarette Smoke and Estrogen Signaling in Human Airway Smooth Muscle. Cell. Physiol. Biochem. 2015, 36, 1101–1115. [Google Scholar] [CrossRef]
- Sheridan, J.T.; Gilmore, R.C.; Watson, M.J.; Archer, C.B.; Tarran, R. 17β-Estradiol Inhibits Phosphorylation of Stromal Interaction Molecule 1 (STIM1) Protein. J. Biol. Chem. 2013, 288, 33509–33518. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; So, W.Y.; Law, S.K.; Leung, F.P.; Yau, K.L.; Yao, X.; Huang, Y.; Li, X.; Tsang, S.Y. Estrogen controls embryonic stem cell proliferation via store-operated calcium entry and the nuclear factor of activated T-cells (NFAT). J. Cell. Physiol. 2012, 227, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Miao, C.; Liu, M.; Wang, X.; Wang, L.; Wang, D.J. 17β-Estradiol via Orai1 activates calcium mobilization to induce cell proliferation in epithelial ovarian cancer. J. Biochem. Mol. Toxicol. 2020, 34, e22603. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, K.; Li, J.; Hao, X.; Chu, W.; Luo, C.; Zhu, Y.; Xie, R.; Chen, B. Estrogen enhances the proliferation and migration of ovarian cancer cells by activating transient receptor potential channel C3. J. Ovarian Res. 2020, 13, 20. [Google Scholar] [CrossRef]
- Méndez-Reséndiz, K.A.; Enciso-Pablo, Ó.; González-Ramírez, R.; Juárez-Contreras, R.; Rosenbaum, T.; Morales-Lázaro, S.L. Steroids and TRP Channels: A Close Relationship. Int. J. Mol. Sci. 2020, 21, 3819. [Google Scholar] [CrossRef]
- Yang, H.; Choi, K.C.; Hyun, S.H.; Jeung, E.B. Coexpression and estrogen-mediated regulation of TRPV6 and PMCA1 in the human endometrium during the menstrual cycle. Mol. Reprod. Dev. 2011, 78, 274–282. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.-M.; Ahn, C.; Lee, J.-H.; Yoo, Y.-M.; Jeung, E.-B. Effects of Bisphenol A and 4-tert-Octylphenol on Embryo Implantation Failure in Mouse. Int. J. Environ. Res. Public Health 2018, 15, 1614. [Google Scholar] [CrossRef]
- Lifshitz, L.M.; Carmichael, J.D.; Lai, F.A.; Sorrentino, V.; Bellvé, K.; Fogarty, K.E.; Zhuge, R. Spatial organization of RYRs and BK channels underlying the activation of STOCs by Ca2+ sparks in airway myocytes. J. Gen. Physiol. 2011, 138, 195–209. [Google Scholar] [CrossRef]
- Jude, J.A.; Dileepan, M.; Panettieri, R.A.; Walseth, T.F.; Kannan, M.S. Altered CD38/Cyclic ADP-Ribose Signaling Contributes to the Asthmatic Phenotype. J. Allergy 2012, 2012, 289468. [Google Scholar] [CrossRef]
- Fritz, N.; Macrez, N.; Mironneau, J.; Jeyakumar, L.H.; Fleischer, S.; Morel, J.-L. Ryanodine receptor subtype 2 encodes Ca2+ oscillations activated by acetylcholine via the M2 muscarinic receptor/cADP-ribose signalling pathway in duodenum myocytes. J. Cell Sci. 2005, 118, 2261–2270. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Walseth, T.F.; Panettieri, R.A.; Kannan, M.S. CD38-cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyperresponsiveness. FASEB J. 2003, 17, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liang, Q.; Chen, Y.; Wang, H.-S. Molecular Mechanisms Underlying the Rapid Arrhythmogenic Action of Bisphenol A in Female Rat Hearts. Endocrinology 2013, 154, 4607–4617. [Google Scholar] [CrossRef]
- Gao, X.; Ma, J.; Chen, Y.; Wang, H. Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: Evidence of female-specific proarrhythmic effects. Environ. Health Perspect. 2015, 123, 571–578. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Huang, W.; Deng, K.Y.; Qian, Y.; Xin, H.B. 17β-Estradiol Promotes Apoptosis in Airway Smooth Muscle Cells Through CD38/SIRT1/p53 Pathway. Front. Endocrinol. 2018, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Song, R.; Romero, M.; Dasgupta, C.; Huang, X.; Holguin, M.A.; Williams, V.; Xiao, D.; Wilson, S.M.; Zhang, L. Pregnancy Increases Ca2+ Sparks/Spontaneous Transient Outward Currents and Reduces Uterine Arterial Myogenic Tone. Hypertension 2019, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Machuki, J.; Wu, Q.; Shi, M.; Fu, L.; Adekunle, A.; Tao, X.; Xu, C.; Hu, X.; Yin, Z.; et al. Estrogen and calcium handling proteins: New discoveries and mechanisms in cardiovascular diseases. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H820–H829. [Google Scholar] [CrossRef]
- Tappia, P.S.; Dent, M.R.; Aroutiounova, N.; Babick, A.P.; Weiler, H. Gender differences in the modulation of cardiac gene expression by dietary conjugated linoleic acid isomers. Can. J. Physiol. Pharmacol. 2007, 3–4, 465–475. [Google Scholar] [CrossRef]
- Yaras, N.; Tuncay, E.; Purali, N.; Sahinoglu, B.; Vassort, G.; Turan, B. Sex-related effects on diabetes-induced alterations in calcium release in the rat heart. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H3584–H3592. [Google Scholar] [CrossRef]
- Bell, J.R.; Raaijmakers, A.J.; Curl, C.L.; Reichelt, M.E.; Harding, T.W.; Bei, A.; Ng, D.C.; Erickson, J.R.; Vila Petroff, M.; Harrap, S.B.; et al. Cardiac CaMKIIδ splice variants exhibit target signaling specificity and confer sex-selective arrhythmogenic actions in the ischemic-reperfused heart. Int. J. Cardiol. 2015, 181, 288–296. [Google Scholar] [CrossRef]
- Farrell, S.R.; Ross, J.L.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H36–H45. [Google Scholar] [CrossRef]
- Rybalchenko, V.; Grillo, M.A.; Gastinger, M.J.; Rybalchenko, N.; Payne, A.J.; Koulen, P. The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+. J. Recept. Signal Transduct. Res. 2009, 29, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H2190–H2210. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hao, Q.; Zheng, Y.M.; Liu, Q.H.; Wang, Y.X. Inositol 1,4,5-trisphosphate activates TRPC3 channels to cause extracellular Ca2+ influx in airway smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L1455–L1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zheng, Y.M.; Mei, Q.B.; Wang, Q.S.; Collier, M.L.; Fleischer, S.; Xin, H.B.; Kotlikoff, M.I. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004, 286, C538–C546. [Google Scholar] [CrossRef]
- Montaño, L.M.; Flores-Soto, E.; Reyes-García, J.; Díaz-Hernández, V.; Carbajal-García, A.; Campuzano-González, E.; Ramírez-Salinas, G.L.; Velasco-Velázquez, M.A.; Sommer, B. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol. Cell. Endocrinol. 2018, 473, 17–30. [Google Scholar] [CrossRef]
- Romero-Martínez, B.S.; Montaño, L.M.; Solís-Chagoyán, H.; Sommer, B.; Ramírez-Salinas, G.L.; Pérez-Figueroa, G.E.; Flores-Soto, E. Possible Beneficial Actions of Caffeine in SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 5460. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Wang, W.C.H.; Mcilmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Reuquén, P.; Oróstica, M.L.; Rojas, I.; Díaz, P.; Parada-Bustamante, A.; Orihuela, P.A. Estradiol increases IP3 by a nongenomic mechanism in the smooth muscle cells from the rat oviduct. Reproduction 2015, 150, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Pallottini, V.; Trentalance, A. Estrogens cause rapid activation of IP3-PKC-α signal transduction pathway in HEPG2 cells. Biochem. Biophys. Res. Commun. 1998, 245, 254–258. [Google Scholar] [CrossRef]
- Ekstein, J.; Nasatzky, E.; Boyan, B.D.; Ornoy, A.; Schwartz, Z. Growth-plate chondrocytes respond to 17β-estradiol with sex-specific increases in IP3 and intracellular calcium ion signalling via a capacitative entry mechanism. Steroids 2005, 70, 775–786. [Google Scholar] [CrossRef]
- Le Mellay, V.; Grosse, B.; Lieberherr, M. Phospholipase C β and membrane action of calcitriol and estradiol. J. Biol. Chem. 1997, 272, 11902–11907. [Google Scholar] [CrossRef]
- Micevych, P.; Soma, K.K.; Sinchak, K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res. Rev. 2008, 57, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.L.; Homick, K.; Dragon, M.B.; Bradford, P.G. Cloning and characterization of the type I inositol 1,4,5-trisphosphate receptor gene promoter. Regulation by 17β-estradiol in osteoblasts. J. Biol. Chem. 1997, 272, 22425–22431. [Google Scholar] [CrossRef] [PubMed]
- Tomás, J.; Santos, C.R.A.; Duarte, A.C.; Maltez, M.; Quintela, T.; Lemos, M.C.; Gonçalves, I. Bitter taste signaling mediated by Tas2r144 is down-regulated by 17β-estradiol and progesterone in the rat choroid plexus. Mol. Cell. Endocrinol. 2019, 495, 110521. [Google Scholar] [CrossRef]
- Sommer, B.; Flores-Soto, E.; Gonzalez-Avila, G. Cellular Na+ handling mechanisms involved in airway smooth muscle contraction (Review). Int. J. Mol. Med. 2017, 40, 3–9. [Google Scholar] [CrossRef] [PubMed]
- DiPolo, R.; Beaugé, L. Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol. Rev. 2006, 86, 155–203. [Google Scholar] [CrossRef]
- Philipson, K.D.; Nicoll, D.A. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 2000, 62, 111–133. [Google Scholar] [CrossRef]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Algara-Suárez, P.; Mejía-Elizondo, R.; Sims, S.; Saavedra-Alanis, V.; Espinosa-Tanguma, R. The 1.3 isoform of Na+-Ca2+ exchanger expressed in guinea pig tracheal smooth muscle is less sensitive to KB-R7943. J. Physiol. Biochem. 2010, 66, 117–125. [Google Scholar] [CrossRef]
- Janssen, L.J.; Walters, D.K.; Wattie, J. Regulation of [Ca2+]i in canine airway smooth muscle by Ca2+-ATPase and Na+/Ca2+ exchange mechanisms. Am. J. Physiol. 1997, 273, L322–L330. [Google Scholar] [CrossRef]
- Wen, J.; Meng, X.; Xuan, B.; Zhou, T.; Gao, H.; Dong, H.; Wang, Y. Na+/Ca2+ Exchanger 1 in Airway Smooth Muscle of Allergic Inflammation Mouse Model. Front. Pharmacol. 2018, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, P.; Zhang, W.-J.; Qiu, J.-Y.; Tan, L.; Liu, X.-C.; Wang, Q.; Luo, X.; She, Y.-S.; Zang, D.-A.; et al. Generation and Role of Oscillatory Contractions in Mouse Airway Smooth Muscle. Cell. Physiol. Biochem. 2018, 47, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Delmotte, P.F.; Thompson, M.A.; Pabelick, C.M.; Sieck, G.C.; Prakash, Y.S. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS ONE 2011, 6, e23662. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Inman, M.; Kiss, L.; Janssen, L.J. Reverse-mode NCX current in mouse airway smooth muscle: Na+ and voltage dependence, contributions to Ca2+ influx and contraction, and altered expression in a model of allergen-induced hyperresponsiveness. Acta Physiol. 2012, 205, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, D.; Ahn, C.; Kang, H.Y.; An, B.S.; Seong, Y.H.; Jeung, E.B. Effects of estrogen on esophageal function through regulation of Ca2+-related proteins. J. Gastroenterol. 2017, 52, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.H.; Goldspink, P.; Kowalski, J.; Beck, J.; Schwertz, D.W. Effect of estrogen on calcium-handling proteins, β-adrenergic receptors, and function in rat heart. Life Sci. 2006, 79, 1257–1267. [Google Scholar] [CrossRef]
- Sims, C.; Reisenweber, S.; Viswanathan, P.C.; Choi, B.R.; Walker, W.H.; Salama, G. Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ. Res. 2008, 102, e86–e100. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Alber, S.; Shusterman, V.; Salama, G. Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen. J. Physiol. 2011, 589, 1061–1080. [Google Scholar] [CrossRef]
- Kravtsov, G.M.; Kam, K.W.; Liu, J.; Wu, S.; Wong, T.M. Altered Ca2+ handling by ryanodine receptor and Na+-Ca2+ exchange in the heart from ovariectomized rats: Role of protein kinase A. Am. J. Physiol. Cell Physiol. 2007, 292, C1625–C1635. [Google Scholar] [CrossRef]
- Yang, H.Y.; Firth, J.M.; Francis, A.J.; Alvarez-Laviada, A.; MacLeod, K.T. Effect of ovariectomy on intracellular Ca2+ regulation in guinea pig cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H1031–H1043. [Google Scholar] [CrossRef]
- Sugishita, K.; Su, Z.; Li, F.; Philipson, K.D.; Barry, W.H. Gender influences [Ca2+]i during metabolic inhibition in myocytes overexpressing the Na+-Ca2+ exchanger. Circulation 2001, 104, 2101–2106. [Google Scholar] [CrossRef]
- Cross, H.R.; Lu, L.; Steenbergen, C.; Philipson, K.D.; Murphy, E. Overexpression of the Cardiac Na+/Ca2+ Exchanger Increases Susceptibility to Ischemia/Reperfusion Injury in Male, but Not Female, Transgenic Mice. Circ. Res. 1998, 83, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.C.; López-Zapata, D.F.; Francis, L.; De Los Reyes, L. Effects of estradiol and IGF-1 on the sodium calcium exchanger in rat cultured cortical neurons. Cell. Mol. Neurobiol. 2011, 31, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, G.J.; Meloni, B.P.; Bakker, A.J.; Knuckey, N.W. The role of the Na+/Ca2+ exchanger (NCX) in neurons following ischaemia. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2007, 14, 507–514. [Google Scholar] [CrossRef]
- Chen, Y.F.; Cao, J.; Zhong, J.N.; Chen, X.; Cheng, M.; Yang, J.; Gao, Y.D. Plasma membrane Ca2+-ATPase regulates Ca2+ signaling and the proliferation of airway smooth muscle cells. Eur. J. Pharmacol. 2014, 740, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Varga, K.; Hollósi, A.; Pászty, K.; Hegedűs, L.; Szakács, G.; Tímár, J.; Papp, B.; Enyedi, Á.; Padányi, R. Expression of calcium pumps is differentially regulated by histone deacetylase inhibitors and estrogen receptor alpha in breast cancer cells. BMC Cancer 2018, 18, 1029. [Google Scholar] [CrossRef]
- El-Beialy, W.; Galal, N.; Deyama, Y.; Yoshimura, Y.; Suzuki, K.; Tei, K.; Totsuka, Y. Effects of Estrogen on PMCA 2 and 4 in Human Fibroblast-like Synovial Cells and Mouse Macrophage-like Cells. Endocr. J. 2010, 57, 93–97. [Google Scholar] [CrossRef]
- Dick, I.M.; Liu, J.; Glendenning, P.; Prince, R.L. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol. Cell. Endocrinol. 2003, 212, 11–18. [Google Scholar] [CrossRef]
- Khariv, V.; Acioglu, C.; Ni, L.; Ratnayake, A.; Li, L.; Tao, Y.-X.; Heary, R.F.; Elkabes, S. A link between plasma membrane calcium ATPase 2 (PMCA2), estrogen and estrogen receptor α signaling in mechanical pain. Sci. Rep. 2018, 8, 17260. [Google Scholar] [CrossRef]
- Bobe, R.; Bredoux, R.; Corvazier, E.; Andersen, J.P.; Clausen, J.D.; Dode, L.; Kovács, T.; Enouf, J. Identification, Expression, Function, and Localization of a Novel (Sixth) Isoform of the Human Sarco/Endoplasmic Reticulum Ca2+ ATPase 3 Gene. J. Biol. Chem. 2004, 279, 24297–24306. [Google Scholar] [CrossRef]
- Mahn, K.; Hirst, S.J.; Ying, S.; Holt, M.R.; Lavender, P.; Ojo, O.O.; Siew, L.; Simcock, D.E.; Mcvicker, C.G.; Kanabar, V.; et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc. Natl. Acad. Sci. USA 2009, 106, 10775–10780. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Sathish, V.; Thompson, M.A.; Pabelick, C.M.; Sieck, G.C. Asthma and sarcoplasmic reticulum Ca2+ reuptake in airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 297, L794. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, V.; Vargas, M.H.; lores-Soto, E.F.; Martínez-Cordero, E.; Bazán-Perkins, B.; Montaño, L.M. LTD4 induces hyperresponsiveness to histamine in bovine airway smooth muscle: Role of SR-ATPase Ca2+ pump and tyrosine kinase. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 288, L84–L92. [Google Scholar] [CrossRef] [PubMed]
- Kotlikoff, M.I. Potassium channels in airway smooth muscle: A tale of two channels. Pharmacol. Ther. 1993, 58, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adda, S.; Fleischmann, B.K.; Freedman, B.D.; Yu, M.; Hay, D.W.; Kotlikoff, M.I. Expression and function of voltage-dependent potassium channel genes in human airway smooth muscle. J. Biol. Chem. 1996, 271, 13239–13243. [Google Scholar] [CrossRef]
- Knox, A.J.; Tattersfield, A.E. Airway smooth muscle relaxation. Thorax 1995, 50, 894–901. [Google Scholar] [CrossRef]
- Brueggemann, L.I.; Kakad, P.P.; Love, R.B.; Solway, J.; Dowell, M.L.; Cribbs, L.L.; Byron, K.L. Kv7 potassium channels in airway smooth muscle cells: Signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L120–L132. [Google Scholar] [CrossRef]
- Isaac, L.; Mcardle, S.; Miller, N.M.; Foster, R.W.; Small, R.C. Effects of some K+-channel inhibitors on the electrical behaviour of guinea-pig isolated trachealis and on its responses to spasmogenic drugs. Br. J. Pharmacol. 1996, 117, 1653–1662. [Google Scholar] [CrossRef]
- Janssen, L.J.; Nana, R. Na+/K+ ATPase mediates rhythmic spontaneous relaxations in canine airway smooth muscle. Respir. Physiol. 1997, 108, 187–194. [Google Scholar] [CrossRef]
- Dodson, A.M.; Rhoden, K.J. Bradykinin increases Na+-K+pump activity in cultured guinea-pig tracheal smooth muscle cells. Br. J. Pharmacol. 2001, 133, 1339–1345. [Google Scholar] [CrossRef]
- Rhoden, K.J.; Douglas, J.S. Evidence of Na-K-Cl cotransport in airway smooth muscle. Am. J. Physiol. 1995, 268, L551–L557. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Hall, I.P.; Washabau, R.J.; Takagi, K.; Kotlikoff, M.I. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Investig. 1994, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; O’Connell, R.J.; Pietrzykowski, A.Z.; Treistman, S.N.; Ethier, M.F.; Madison, J.M. Interleukin-4 activates large-conductance, calcium-activated potassium (BKCa) channels in human airway smooth muscle cells. Exp. Physiol. 2008, 93, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.; Yanase, N.; Smulian, G.; Gildea, L.; Orekov, T.; Potter, C.; Brombacher, F.; Aronow, B.; Wills-Karp, M.; Finkelman, F.D. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J. Exp. Med. 2011, 208, 853–867. [Google Scholar] [CrossRef]
- Shepherd, M.C.; Duffy, S.M.; Harris, T.; Cruse, G.; Schuliga, M.; Brightling, C.E.; Neylon, C.B.; Bradding, P.; Stewart, A.G. KCa3.1 Ca2+-Activated K+ Channels Regulate Human Airway Smooth Muscle Proliferation. Am. J. Respir. Cell Mol. Biol. 2007, 37, 525–531. [Google Scholar] [CrossRef]
- Yu, Z.H.; Wang, Y.X.; Song, Y.; Lu, H.Z.; Hou, L.N.; Cui, Y.Y.; Chen, H.Z. Up-regulation of KCa3.1 promotes human airway smooth muscle cell phenotypic modulation. Pharmacol. Res. 2013, 77, 30–38. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Xu, J.-R.; Wang, Y.-X.; Xu, G.-N.; Xu, Z.-P.; Yang, K.; Wu, D.-Z.; Cui, Y.-Y.; Chen, H.-Z. Targeted Inhibition of KCa3.1 Channel Attenuates Airway Inflammation and Remodeling in Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 685–693. [Google Scholar] [CrossRef]
- Dimitropoulou, C.; White, R.E.; Ownby, D.R.; Catravas, J.D. Estrogen Reduces Carbachol-Induced Constriction of Asthmatic Airways by Stimulating Large-Conductance Voltage and Calcium-Dependent Potassium Channels. Am. J. Respir. Cell Mol. Biol. 2005, 32, 239–247. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wang, B.; Eng, C.; Kumar, G.; Beckman, K.B.; Sen, S.; Choudhry, S.; Meade, K.; Lenoir, M.; Watson, H.G.; et al. An african-specific functional polymorphism in KCNMB1 shows sex-specific association with asthma severity. Hum. Mol. Genet. 2008, 17, 2681–2690. [Google Scholar] [CrossRef]
- Tsang, S.Y.; Yao, X.; Chan, H.Y.; Wong, C.M.; Chen, Z.Y.; Au, C.L.; Huang, Y. Contribution of K+ channels to relaxation induced by 17β-estradiol but not by progesterone in isolated rat mesenteric artery rings. J. Cardiovasc. Pharmacol. 2003, 41, 4–13. [Google Scholar] [CrossRef]
- Wong, C.M.; Tsang, S.Y.; Yao, X.; Chan, F.L.; Huang, Y. Differential effects of estrogen and progesterone on potassium channels expressed in Xenopus oocytes. Steroids 2008, 73, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Pregnancy Upregulates Large-Conductance Ca2+-Activated K+-Channel Activity and Attenuates Myogenic Tone in Uterine Arteries. Hypertension 2011, 58, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Qiu, X.Y. 17β-Estradiol Upregulated Expression of α and β Subunits of Larger-Conductance Calcium-Activated K+ Channels (BK) via Estrogen Receptor β. J. Mol. Neurosci. 2015, 56, 799–807. [Google Scholar] [CrossRef]
- Nishimura, I.; Ui-Tei, K.; Saigo, K.; Ishii, H.; Sakuma, Y.; Kato, M. 17β-Estradiol at Physiological Concentrations Augments Ca2+-Activated K+ Currents via Estrogen Receptor β in the Gonadotropin-Releasing Hormone Neuronal Cell Line GT1-7. Endocrinology 2008, 149, 774–782. [Google Scholar] [CrossRef]
- Shi, J.; Jin, L.; Leng, J.; Lang, J. Response of potassium channels to estrogen and progesterone in the uterine smooth muscle cells of adenomyosis in vitro. Zhonghua Fu Chan Ke Za Zhi 2015, 50, 843–847. [Google Scholar]
- Restrepo-Angulo, I.; Bañuelos, C.; Camacho, J. Ion Channel Regulation by Sex Steroid Hormones and Vitamin D in Cancer: A Potential Opportunity for Cancer Diagnosis and Therapy. Front. Pharmacol. 2020, 11, 152. [Google Scholar] [CrossRef]
- Coiret, G.; Matifat, F.; Hague, F.; Ouadid-Ahidouch, H. 17-β-Estradiol activates maxi-K channels through a non-genomic pathway in human breast cancer cells. FEBS Lett. 2005, 579, 2995–3000. [Google Scholar] [CrossRef]
- Ramírez, A.; Vera, E.; Gamboa-Domínguez, A.; Lambert, P.; Gariglio, P.; Camacho, J. Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol. Lett. 2018, 15, 7249–7254. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pinna, J.; Marroqui, L.; Hmadcha, A.; Lopez-Beas, J.; Soriano, S.; Villar-Pazos, S.; Alonso-Magdalena, P.; Dos Santos, R.S.; Quesada, I.; Martin, F.; et al. Oestrogen receptor β mediates the actions of bisphenol-A on ion channel expression in mouse pancreatic beta cells. Diabetologia 2019, 62, 1667–1680. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.-A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef]
- Mohr, C.J.; Steudel, F.A.; Gross, D.; Ruth, P.; Lo, W.-Y.; Hoppe, R.; Schroth, W.; Brauch, H.; Huber, S.M.; Lukowski, R. Cancer-Associated Intermediate Conductance Ca2+-Activated K+ Channel KCa3.1. Cancers 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Dhakal, I.B.; Beggs, M.; Kadlubar, S.; Luo, D. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer 2012, 12, 29. [Google Scholar] [CrossRef]
- Davis, J.S.; Sun, M.; Kho, A.T.; Moore, K.G.; Sylvia, J.M.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Circulating microRNAs and association with methacholine PC20 in the Childhood Asthma Management Program (CAMP) cohort. PLoS ONE 2017, 12, e0180329. [Google Scholar] [CrossRef]
- Yu, B.; Yao, L.; Liu, C.; Tang, L.; Xing, T. Upregulation of microRNA-16 alters the response to inhaled β-agonists in patients with asthma though modulating expression of ADRB2. Mol. Med. Rep. 2019, 19, 4027–4034. [Google Scholar] [CrossRef]
- Taura, J.; Kircher, D.M.; Gameiro-Ros, I.; Slesinger, P.A. Comparison of K+ Channel Families. In Pharmacology of Potassium Channels; Gamper, N., Wang, K., Eds.; Springer: Cham, Switzerland, 2021; Volume 267. [Google Scholar]
- Evseev, A.I.; Semenov, I.; Archer, C.R.; Medina, J.L.; Dube, P.H.; Shapiro, M.S.; Brenner, R. Functional effects of KCNQ K+ channels in airway smooth muscle. Front. Physiol. 2013, 4, 277. [Google Scholar] [CrossRef] [PubMed]
- Drici, M.D.; Burklow, T.R.; Haridasse, V.; Glazer, R.I.; Woosley, R.L. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 1996, 94, 1471–1474. [Google Scholar] [CrossRef]
- Roepke, T.A.; Malyala, A.; Bosch, M.A.; Kelly, M.J.; Rønnekleiv, O.K. Estrogen Regulation of Genes Important for K+ Channel Signaling in the Arcuate Nucleus. Endocrinology 2007, 148, 4937–4951. [Google Scholar] [CrossRef]
- Roepke, T.A.; Qiu, J.; Smith, A.W.; Ronnekleiv, O.K.; Kelly, M.J. Fasting and 17β-Estradiol Differentially Modulate the M-Current in Neuropeptide Y Neurons. J. Neurosci. 2011, 31, 11825–11835. [Google Scholar] [CrossRef]
- Anneken, L.; Baumann, S.; Vigneault, P.; Biliczki, P.; Friedrich, C.; Xiao, L.; Girmatsion, Z.; Takac, I.; Brandes, R.P.; Kissler, S.; et al. Estradiol regulates human QT-interval: Acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking. Eur. Heart J. 2016, 37, 640–650. [Google Scholar] [CrossRef]
- Kim, J.G.; Leem, Y.-E.; Kwon, I.; Kang, J.-S.; Bae, Y.M.; Cho, H. Estrogen modulates serotonin effects on vasoconstriction through Src inhibition. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Díaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chávez, E.; García-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and Human Papilloma Virus Oncogenes Regulate Human Ether-à-go-go-1 Potassium Channel Expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Brelidze, T.I.; Zagotta, W.N. Flavonoid regulation of EAG1 channels. J. Gen. Physiol. 2013, 141, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Souhrada, M.; Souhrada, J.F.; Cherniack, R.M. Evidence for a sodium electrogenic pump in airway smooth muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Chideckel, E.W.; Frost, J.L.; Mike, P.; Fedan, J.S. The effect of ouabain on tension in isolated respiratory tract smooth muscle of humans and other species. Br. J. Pharmacol. 1987, 92, 609–614. [Google Scholar] [CrossRef]
- Gunst, S.J.; Stropp, J.Q. Effect of Na-K adenosinetriphosphatase activity on relaxation of canine tracheal smooth muscle. J. Appl. Physiol. 1988, 64, 635–641. [Google Scholar] [CrossRef]
- Obradovic, M.; Zafirovic, S.; Jovanovic, A.; Milovanovic, E.S.; Mousa, S.A.; Labudovic-Borovic, M.; Isenovic, E.R. Effects of 17β-estradiol on cardiac Na+/K+-ATPase in high fat diet fed rats. Mol. Cell. Endocrinol. 2015, 416, 46–56. [Google Scholar] [CrossRef]
- Liu, C.G.; Xu, K.Q.; Xu, X.; Huang, J.J.; Xiao, J.C.; Zhang, J.P.; Song, H.P. 17β-oestradiol regulates the expression of Na+/K+-ATPase β1-subunit, sarcoplasmic reticulum Ca2+-ATPase and carbonic anhydrase iv in H9C2 cells. Clin. Exp. Pharmacol. Physiol. 2007, 34, 998–1004. [Google Scholar] [CrossRef]
- Obradovic, M.; Stewart, A.J.; Pitt, S.J.; Labudovic-Borovic, M.; Sudar, E.; Petrovic, V.; Zafirovic, S.; Maravic-Stojkovic, V.; Vasic, V.; Isenovic, E.R. In vivo effects of 17β-estradiol on cardiac Na+/K+-ATPase expression and activity in rat heart. Mol. Cell. Endocrinol. 2014, 388, 58–68. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Li, S.; Zhang, J.; Zheng, J.; Hou, W.; Zhao, H.; Guo, Y.; Liu, X.; Dou, K.; et al. N-myc Downstream-regulated Gene 2, a Novel Estrogen-targeted Gene, Is Involved in the Regulation of Na+/K+-ATPase. J. Biol. Chem. 2011, 286, 32289–32299. [Google Scholar] [CrossRef]
- Melis, M.G.; Troffa, C.; Manunta, P.; Pinna Parpaglia, P.; Soro, A.; Pala, F.; Madeddu, P.; Pazzola, A.; Tonolo, G.; Patteri, G. Influenze degli ormoni del ciclo mestruale sui trasporti cationici di membrana dei globuli rossi [Effect of menstrual cycle hormones on cation transport in the red-cell membrane]. Boll. Della Soc. Ital. Di Biol. Sper. 1990, 66, 679–684. [Google Scholar]
- Palacios, J.; Marusic, E.T.; Lopez, N.C.; Gonzalez, M.; Michea, L. Estradiol-induced expression of N+-K+-ATPase catalytic isoforms in rat arteries: Gender differences in activity mediated by nitric oxide donors. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1793–H1800. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Junior, R.F.; Fiorim, J.; Marques, V.B.; de Sousa Ronconi, K.; Botelho, T.; Grando, M.D.; Bendhack, L.M.; Vassallo, D.V.; Stefanon, I. Vascular activation of K+ channels and Na+-K+ ATPase activity of estrogen-deficient female rats. Vasc. Pharmacol. 2017, 99, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, L.M.; Fujiwara, N.; Nakamura, K.T.; Wada, R.K. Na-K-2Cl cotransporter inhibition impairs human lung cellular proliferation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L510–L514. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, Y.Y.; Ellis, R.; Wattie, J.; Feng, M.; Inman, M.D.; Lu, W.Y. Effects of furosemide on allergic asthmatic responses in mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011, 41, 1456–1467. [Google Scholar] [CrossRef]
- O’Donnell, M.E.; Lam, T.I.; Tran, L.Q.; Foroutan, S.; Anderson, S.E. Estradiol Reduces Activity of the Blood–Brain Barrier Na–K–Cl Cotransporter and Decreases Edema Formation in Permanent Middle Cerebral Artery Occlusion. J. Cereb. Blood Flow Metab. 2006, 26, 1234–1249. [Google Scholar] [CrossRef]
- Chang, E.; O’Donnell, M.E.; Barakat, A.I. Shear stress and 17β-estradiol modulate cerebral microvascular endothelial Na-K-Cl cotransporter and Na/H exchanger protein levels. Am. J. Physiol. Cell Physiol. 2008, 294, C363–C371. [Google Scholar] [CrossRef]
- Nakamura, N.H.; Rosell, D.R.; Akama, K.T.; McEwen, B.S. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology 2004, 80, 308–323. [Google Scholar] [CrossRef]
- Nugent, B.M.; Valenzuela, C.V.; Simons, T.J.; Mccarthy, M.M. Kinases SPAK and OSR1 Are Upregulated by Estradiol and Activate NKCC1 in the Developing Hypothalamus. J. Neurosci. 2012, 32, 593–598. [Google Scholar] [CrossRef]
- Palacios, J.; Espinoza, F.; Munita, C.; Cifuentes, F.; Michea, L. Na+-K+-2Cl− cotransporter is implicated in gender differences in the response of the rat aorta to phenylephrine. Br. J. Pharmacol. 2006, 148, 964–972. [Google Scholar] [CrossRef]
- Bers, D. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc. Res. 2003, 57, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Webb, T.I.; Hollywood, M.A.; Sergeant, G.P.; McHale, N.G.; Thornbury, K.D. The cardiac sodium current Na(v)1.5 is functionally expressed in rabbit bronchial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2013, 305, C427–C435. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, V.; Hirst, S.; Ward, J. Ion channels in freshly isolated and cultured human bronchial smooth muscle cells. Exp. Physiol. 1996, 81, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Nagata, T.; Iida, H.; Imuta, H.; Iwasawa, K.; Ma, J.; Hara, K.; Omata, M.; Nagai, R.; Takizawa, H.; et al. Voltage-gated sodium channel expressed in cultured human smooth muscle cells: Involvement of SCN9A. FEBS Lett. 2004, 567, 339–343. [Google Scholar] [CrossRef]
- Nakajima, T.; Jo, T.; Meguro, K.; Oonuma, H.; Ma, J.; Kubota, N.; Imuta, H.; Takano, H.; Iida, H.; Nagase, T.; et al. Effect of dexamethasone on voltage-gated Na+ channel in cultured human bronchial smooth muscle cells. Life Sci. 2008, 82, 1210–1215. [Google Scholar] [CrossRef]
- Fraser, S.P.; Pardo, L.A. Ion channels: Functional expression and therapeutic potential in cancer. Colloquium on Ion Channels and Cancer. EMBO Rep. 2008, 9, 512–515. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Onkal, R.; Diss, J.K.; Latchman, D.S.; Djamgoz, M.B. Estrogen and non-genomic upregulation of voltage-gated Na+ channel activity in MDA-MB-231 human breast cancer cells: Role in adhesion. J. Cell. Physiol. 2010, 224, 527–539. [Google Scholar] [CrossRef]
- Fouda, M.A.; Ruben, P.C. Protein Kinases Mediate Anti-Inflammatory Effects of Cannabidiol and Estradiol Against High Glucose in Cardiac Sodium Channels. Front. Pharmacol. 2021, 12, 668657. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Q.; Wang, P.; Wang, W.; Qian, W.; Xiao, H.; Wang, L. 17β-Estradiol regulates the gene expression of voltage-gated sodium channels: Role of estrogen receptor α and estrogen receptor β. Endocrine 2012, 41, 274–280. [Google Scholar] [CrossRef]
- Bi, R.-Y.; Meng, Z.; Zhang, P.; Wang, X.-D.; Ding, Y.; Gan, Y.-H. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ. PLoS ONE 2017, 12, e0178589. [Google Scholar] [CrossRef]
- Kow, L.M.; Devidze, N.; Pataky, S.; Shibuya, I.; Pfaff, D.W. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006, 1116, 1–11. [Google Scholar] [CrossRef]
- Gallos, G.; Yim, P.; Emala, C.W. Chloride in airway smooth muscle: The ignored anion no longer? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L733–L735. [Google Scholar] [CrossRef] [PubMed]
- Bulley, S.; Jaggar, J.H. Cl− channels in smooth muscle cells. Pflügers Arch.-Eur. J. Physiol. 2014, 466, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.J.; Sims, S.M. Ca2+-dependent Cl− current in canine tracheal smooth muscle cells. Am. J. Physiol. 1995, 269, C163–C169. [Google Scholar] [CrossRef]
- Kotlikoff, M.I.; Wang, Y.-X. Calcium Release and Calcium-Activated Chloride Channels in Airway Smooth Muscle Cells. Am. J. Respir. Crit. Care Med. 1998, 158, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, J.; Yim, P.; Rinderspacher, A.; Fu, X.W.; Zhang, Y.; Landry, D.W.; Emala, C.W. Chloride channel blockade relaxes airway smooth muscle and potentiates relaxation by β-agonists. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 307, L273–L282. [Google Scholar] [CrossRef]
- Hirota, S.; Trimble, N.; Pertens, E.; Janssen, L.J. Intracellular Cl− fluxes play a novel role in Ca2+ handling in airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L1146–L1153. [Google Scholar] [CrossRef] [PubMed]
- Gallos, G.; Remy, K.E.; Danielsson, J.; Funayama, H.; Fu, X.W.; Chang, H.Y.; Yim, P.; Xu, D.; Emala, C.W., Sr. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 305, L625–L634. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef]
- Danielsson, J.; Kuforiji, A.S.; Yocum, G.T.; Zhang, Y.; Xu, D.; Gallos, G.; Emala, C.W. Agonism of the TMEM16A calcium-activated chloride channel modulates airway smooth muscle tone. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L287–L295. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Takayama, Y.; Sunagawa, M. The Calcium-Activated Chloride Channel TMEM16A is Inhibitied by Liquiritigenin. Front. Pharmacol. 2021, 12, 628968. [Google Scholar] [CrossRef] [PubMed]
- Coakley, R.D.; Sun, H.; Clunes, L.A.; Rasmussen, J.E.; Stackhouse, J.R.; Okada, S.F.; Fricks, I.; Young, S.L.; Tarran, R. 17β-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J. Clin. Investig. 2008, 118, 4025–4035. [Google Scholar] [CrossRef]
- Imberti, R.; Garavaglia, M.L.; Verduci, I.; Cannavale, G.; Balduzzi, G.; Papetti, S.; Mazzanti, M. Antiestrogen- and tamoxifen-induced effects on calcium-activated chloride currents in epithelial cells carrying the ∆F508-CFTR point mutation. Respir. Res. 2018, 19, 198. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Melin, P.; Norez, C.; Robert, R.; Guibert, C.; Mettey, Y.; Becq, F. Evidence that CFTR is expressed in rat tracheal smooth muscle cells and contributes to bronchodilation. Respir. Res. 2006, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Michoud, M.-C.; Robert, R.; Hassan, M.; Moynihan, B.; Haston, C.; Govindaraju, V.; Ferraro, P.; Hanrahan, J.W.; Martin, J.G. Role of the Cystic Fibrosis Transmembrane Conductance Channel in Human Airway Smooth Muscle. Am. J. Respir. Cell Mol. Biol. 2009, 40, 217–222. [Google Scholar] [CrossRef]
- Cook, D.P.; Rector, M.V.; Bouzek, D.C.; Michalski, A.S.; Gansemer, N.D.; Reznikov, L.R.; Li, X.; Stroik, M.R.; Ostedgaard, L.S.; Abou Alaiwa, M.H.; et al. Cystic Fibrosis Transmembrane Conductance Regulator in Sarcoplasmic Reticulum of Airway Smooth Muscle. Implications for Airway Contractility. Am. J. Respir. Crit. Care Med. 2016, 193, 417–426. [Google Scholar] [CrossRef]
- Norez, C.; Jayle, C.; Becq, F.; Vandebrouck, C. Bronchorelaxation of the human bronchi by CFTR activators. Pulm. Pharmacol. Ther. 2014, 27, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bazett, M.; Haston, C.K. Airway hyperresponsiveness in FVB/N delta F508 cystic fibrosis transmembrane conductance regulator mice. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014, 13, 378–383. [Google Scholar] [CrossRef]
- Huang, J.; Lam, H.; Koziol-White, C.; Limjunyawong, N.; Kim, D.; Kim, N.; Karmacharya, N.; Rajkumar, P.; Firer, D.; Dalesio, N.M.; et al. The odorant receptor OR2W3 on airway smooth muscle evokes bronchodilation via a cooperative chemosensory tradeoff between TMEM16A and CFTR. Proc. Natl. Acad. Sci. USA 2020, 117, 28485–28495. [Google Scholar] [CrossRef]
- Johannesson, M.; Lúdvíksdóttir, D.; Janson, C. Lung function changes in relation to menstrual cycle in females with cystic fibrosis. Respir. Med. 2000, 94, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Schultz, B.D.; Katzenellenbogen, J.A.; Price, E.M.; Bridges, R.J.; Bradbury, N.A. Estrogen inhibition of cystic fibrosis transmembrane conductance regulator-mediated chloride secretion. J. Pharmacol. Exp. Ther. 2000, 295, 195–204. [Google Scholar] [PubMed]
- Ajonuma, L.C.; Tsang, L.L.; Zhang, G.H.; Wong, C.H.Y.; Lau, M.C.; Ho, L.S.; Rowlands, D.K.; Zhou, C.X.; Ng, C.P.; Chen, J.; et al. Estrogen-Induced Abnormally High Cystic Fibrosis Transmembrane Conductance Regulator Expression Results in Ovarian Hyperstimulation Syndrome. Mol. Endocrinol. 2005, 19, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wen, G.; Deng, S.; Wan, S.; Xu, J.; Liu, X.; Xie, R.; Dong, H.; Tuo, B. Oestrogen upregulates the expression levels and functional activities of duodenal mucosal CFTR and SLC26A6. Exp. Physiol. 2016, 101, 1371–1382. [Google Scholar] [CrossRef]
- Jin, P.-Y.; Lu, Y.-C.; Li, L.; Han, Q.-F. Co action of CFTR and AQP1 increases permeability of peritoneal epithelial cells on estrogen-induced ovarian hyper stimulation syndrome. BMC Cell Biol. 2012, 13, 23. [Google Scholar] [CrossRef]
- Sweezey, N.B.; Gauthier, C.; Gagnon, S.; Ferretti, E.; Kopelman, H. Progesterone and estradiol inhibit CFTR-mediated ion transport by pancreatic epithelial cells. Am. J. Physiol. 1996, 271, G747–G754. [Google Scholar] [CrossRef]
- Goodstadt, L.; Powell, T.; Figtree, G.A. 17β-estradiol potentiates the cardiac cystic fibrosis transmembrane conductance regulator chloride current in guinea-pig ventricular myocytes. J. Physiol. Sci. 2006, 56, 29–37. [Google Scholar] [CrossRef]
- Mizuta, K.; Xu, D.; Pan, Y.; Comas, G.; Sonett, J.R.; Zhang, Y.; Panettieri, R.A.; Yang, J.; Emala, C.W. GABA<sub>A</sub>receptors are expressed and facilitate relaxation in airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L1206–L1216. [Google Scholar] [CrossRef]
- Gallos, G.; Yim, P.; Chang, S.; Zhang, Y.; Xu, D.; Cook, J.M.; Gerthoffer, W.T.; Emala, C.W., Sr. Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L248–L256. [Google Scholar] [CrossRef]
- Gallos, G.; Yocum, G.T.; Siviski, M.E.; Yim, P.D.; Fu, X.W.; Poe, M.M.; Cook, J.M.; Harrison, N.; Perez-Zoghbi, J.; Emala, C.W., Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L931–L942. [Google Scholar] [CrossRef]
- Yocum, G.T.; Turner, D.L.; Danielsson, J.; Barajas, M.B.; Zhang, Y.; Xu, D.; Harrison, N.L.; Homanics, G.E.; Farber, D.L.; Emala, C.W. GABAA receptor α4 -subunit knockout enhances lung inflammation and airway reactivity in a murine asthma model. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L406–L415. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.; Fenelon, V. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J. Neurosci. 1995, 15, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Maggi, A.; Perez, J. Estrogen-induced up-regulation of gamma-aminobutyric acid receptors in the CNS of rodents. J. Neurochem. 1986, 47, 1793–1797. [Google Scholar] [CrossRef]
- François-Bellan, A.M.; Segu, L.; Héry, M. Regulation by estradiol of GABAA and GABAB binding sites in the diencephalon of the rat: An autoradiographic study. Brain Res. 1989, 503, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Locci, A.; Porcu, P.; Talani, G.; Santoru, F.; Berretti, R.; Giunti, E.; Licheri, V.; Sanna, E.; Concas, A. Neonatal estradiol exposure to female rats changes GABAA receptor expression and function, and spatial learning during adulthood. Horm. Behav. 2017, 87, 35–46. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Martínez, B.S.; Sommer, B.; Solís-Chagoyán, H.; Calixto, E.; Aquino-Gálvez, A.; Jaimez, R.; Gomez-Verjan, J.C.; González-Avila, G.; Flores-Soto, E.; Montaño, L.M. Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 7879. https://doi.org/10.3390/ijms24097879
Romero-Martínez BS, Sommer B, Solís-Chagoyán H, Calixto E, Aquino-Gálvez A, Jaimez R, Gomez-Verjan JC, González-Avila G, Flores-Soto E, Montaño LM. Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle. International Journal of Molecular Sciences. 2023; 24(9):7879. https://doi.org/10.3390/ijms24097879
Chicago/Turabian StyleRomero-Martínez, Bianca S., Bettina Sommer, Héctor Solís-Chagoyán, Eduardo Calixto, Arnoldo Aquino-Gálvez, Ruth Jaimez, Juan C. Gomez-Verjan, Georgina González-Avila, Edgar Flores-Soto, and Luis M. Montaño. 2023. "Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle" International Journal of Molecular Sciences 24, no. 9: 7879. https://doi.org/10.3390/ijms24097879
APA StyleRomero-Martínez, B. S., Sommer, B., Solís-Chagoyán, H., Calixto, E., Aquino-Gálvez, A., Jaimez, R., Gomez-Verjan, J. C., González-Avila, G., Flores-Soto, E., & Montaño, L. M. (2023). Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle. International Journal of Molecular Sciences, 24(9), 7879. https://doi.org/10.3390/ijms24097879





