Comparison of Body Composition, Muscle Strength and Cardiometabolic Profile in Children with Prader-Willi Syndrome and Non-Alcoholic Fatty Liver Disease: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Demography, Anthropometry, and Body Composition
2.2. Markers of Cardiometabolic Dysregulation and Liver Dysfunction
2.3. Measures of Handgrip Strength, Six Minute-Walk-Test, Blood Pressure, Muscle Quality and Habitual Physical Activity
2.4. Associations between Anthropometric, Body Composition, and CardioMetabolic Markers
2.5. Associations between Genotype and Outcome Measures
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Anthropometric Measurements
4.3. Body Composition
4.3.1. Dual-Energy X-ray Absorptiometry (DXA)
4.3.2. Skinfold and Bone Breadth Tests
4.3.3. Somatotyping
4.4. Surrogate Markers of Liver and Metabolic Disease
4.5. Muscle Strength, Six Minute-Walk-Test, Blood Pressure, Muscle Quality and Habitual Physical Activity
4.6. PWS Genotyping
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | Six minute-walk-test |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| BP | Blood pressure |
| CRP | C-reactive protein |
| DXA | Dual-energy x-ray absorptiometry |
| FISH | Fluorescence in situ hybridization |
| FMI | Fat mass index |
| ISAK | International Society for the Advancement of Kinanthropometry |
| ϒGT | Gamma-glutamyl transferase |
| HDL-C | High density lipoprotein cholesterol |
| HOMA-IR | Homeostasis model assessment for insulin resistance |
| LDL-C | Low density lipoprotein cholesterol |
| miRNA | microRNA |
| NAFLD | Non-alcoholic fatty liver disease |
| NS | Not significant |
| PCR | Polymerase chain reaction |
| PNPLA3 | Patatin-like Phospholipase Domain-containing 3 |
| PWS | Prader-Willi syndrome |
| SSM | Skeletal muscle mass |
| SD | Standard deviation |
| TC | Total cholesterol |
| TER | Trunk-to-extremity ratio |
| TG | Fasting triglycerides |
| UPD | Uniparental disomy |
| WC | Waist circumference |
| WHtR | Waist to height ratio |
References
- World Health Organization. Overweight and Obesity. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 August 2022).
- Freemark, M.S. Pediatric Obesity: Etiology, Pathogenesis and Treatment; Humana Press: Totowa, NJ, USA, 2018. [Google Scholar]
- Murphy, S. Understanding childhood and adolescent obesity. Clin. Integr. Care 2022, 13, 100114. [Google Scholar] [CrossRef]
- Dhaliwal, K.K.; Avedzi, H.M.; Richard, C.; Zwaigenbaum, L.; Haqq, A.M. Brief Report: Plasma Leptin and Mealtime Feeding Behaviors Among Children with Autism Spectrum Disorder: A Pilot Study. J. Autism Dev. Disord. 2022, 1–8. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Beales, P.L. Genetic Obesity Syndromes. Front. Horm. Res. 2008, 36, 37–60. [Google Scholar]
- Huvenne, H.; Dubern, B.; Clément, K.; Poitou, C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes. Facts 2016, 9, 158–173. [Google Scholar] [CrossRef]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Charakida, M.; E Deanfield, J. BMI trajectories from childhood: The slippery slope to adult obesity and cardiovascular disease. Eur. Heart J. 2018, 39, 2271–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Falconer, C.; Viner, R.M.; Kinra, S. The impact of childhood obesity on morbidity and mortality in adulthood: A systematic review. Obes. Rev. 2012, 13, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Turer, C.B.; Brady, T.M.; de Ferranti, S.D. Obesity, Hypertension, and Dyslipidemia in Childhood Are Key Modifiable Antecedents of Adult Cardiovascular Disease: A Call to Action. Circulation 2018, 137, 1256–1259. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Wiegand, S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018, 7, 254–259. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, R.; Cecchi, N.; Carbone, M.G.; Dinardo, M.; Gaudino, G.; Del Giudice, E.M.; Umano, G.R. Pediatric obesity: Prevention is better than care. Ital. J. Pediatr. 2020, 46, 103. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Mulder, C.; Twisk, J.W.R.; Van Mechelen, W.; Chinapaw, M.J.M. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Au, N. The Health Care Cost Implications of Overweight and Obesity during Childhood. Health Serv. Res. 2012, 47, 655–676. [Google Scholar] [CrossRef] [Green Version]
- Trasande, L.; Chatterjee, S. The Impact of Obesity on Health Service Utilization and Costs in Childhood. Obes. (Silver Spring) 2009, 17, 1749–1754. [Google Scholar] [CrossRef]
- World Health Organization. National Institute for Health and Clinical Excellence: Guidance London. 2006. Available online: https://www.ncbi.nlm.nih.gov/pubmed/22497033 (accessed on 25 August 2022).
- Flynn, M.A.T.; McNeil, D.A.; Maloff, B.; Mutasingwa, D.; Wu, M.; Ford, C.; Tough, S.C. Reducing obesity and related chronic disease risk in children and youth: A synthesis of evidence with ‘best practice’ recommendations. Obes. Rev. 2006, 7 (Suppl. 1), 7–66. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S. Obesity in midlife: Lifestyle and dietary strategies. Climacteric 2020, 23, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Mackenzie, M.; Alberga, A.S.; Sharma, A.M.; Richer, L.; Rubin, D.A.; Prado, C.M.; Haqq, A.M. The use of magnetic resonance imaging to characterize abnormal body composition phenotypes in youth with Prader–Willi syndrome. Metabolism 2017, 69, 67–75. [Google Scholar] [CrossRef]
- Tovo, C.; A Fernandes, S.; Buss, C.; A De Mattos, A. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? A systematic review. World J. Hepatol. 2017, 9, 326–332. [Google Scholar] [CrossRef]
- Cassidy, S.B.; Forsythe, M.; Heeger, S.; Nicholls, R.D.; Schork, N.; Benn, P.; Schwartz, S. Comparison of phenotype between patients with Prader-Willi syndrome due to deletion 15q and uniparental disomy 15. Am. J. Med. Genet. 1997, 68, 433–440. [Google Scholar] [CrossRef]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar]
- Costa, R.A.; Ferreira, I.R.; Cintra, H.A.; Gomes, L.H.F.; Guida, L.D.C. Genotype-Phenotype Relationships and Endocrine Findings in Prader-Willi Syndrome. Front. Endocrinol. 2019, 10, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlSaif, M.; A Elliot, S.; Mackenzie, M.; Prado, C.M.; Field, C.J.; Haqq, A.M. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Adv. Nutr. Int. Rev. J. 2017, 8, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Crinò, A.; Bedogni, G.; Bosio, L.; Cappa, M.; Corrias, A.; Delvecchio, M.; Di Candia, S.; Gargantini, L.; Grechi, E. Metabolic syndrome in children with Prader–Willi syndrome: The effect of obesity. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Inzaghi, E.; Colajacomo, M.; Bocchini, S.; Grugni, G.; Brufani, C.; Cappa, M.; Nobili, V.; Cianfarani, S.; Crinò, A. Non-Alcoholic Fatty Liver Disease (NAFLD) in children and adolescents with Prader-Willi Syndrome (PWS). Pediatr. Obes. 2016, 11, 235–238. [Google Scholar] [CrossRef]
- Gross, I.; Hirsch, H.J.; Constantini, N.; Nice, S.; Pollak, Y.; Genstil, L.; Eldar-Geva, T.; Tsur, V.G. Physical activity and maximal oxygen uptake in adults with Prader–Willi syndrome. Eat. Weight Disord. 2018, 23, 615–620. [Google Scholar] [CrossRef]
- Lam, M.Y.; Rubin, D.A.; Duran, A.T.; Chavoya, F.A.; White, E.; Rose, D.J. A Characterization of Movement Skills in Obese Children With and Without Prader-Willi Syndrome. Res. Q. Exerc. Sport 2016, 87, 245–253. [Google Scholar] [CrossRef]
- Nicholls, R.D. Genomic imprinting and uniparental disomy in Angelman and Prader-Willi syndromes: A review. Am. J. Med. Genet. 1993, 46, 16–25. [Google Scholar] [CrossRef]
- Duker, A.L.; Ballif, B.C.; Bawle, E.V.; Person, R.E.; Mahadevan, S.; Alliman, S.; Thompson, R.; Traylor, R.; Bejjani, B.A.; Shaffer, L.G.; et al. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur. J. Hum. Genet. 2010, 18, 1196–1201. [Google Scholar] [CrossRef]
- Jauregi, J.; Arias, C.; Vegas, O.; Alén, F.; Martinez, S.; Copet, P.; Thuilleaux, D. A neuropsychological assessment of frontal cognitive functions in Prader? Willi syndrome. J. Intellect. Disabil. Res. 2007, 51, 350–365. [Google Scholar] [CrossRef]
- Sahoo, T.; Del Gaudio, D.; German, J.R.; Shinawi, M.; Peters, S.U.; Person, R.E.; Garnica, A.; Cheung, S.W.; Beaudet, A.L. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat. Genet. 2008, 40, 719–721. [Google Scholar] [CrossRef] [Green Version]
- Gariani, K.; Philippe, J.; Jornayvaz, F. Non-alcoholic fatty liver disease and insulin resistance: From bench to bedside. Diabetes Metab. 2013, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Patterson, C.; So, S.; Rogenstein, C.D.; Wykes, L.J.; A Roberts, E. Dietary and physical activity patterns in children with fatty liver. Eur. J. Clin. Nutr. 2010, 64, 628–635. [Google Scholar] [CrossRef]
- Mager, D.R.; Yap, J.; Rodriguez-Dimitrescu, C.; Mazurak, V.; Ball, G.; Gilmour, S. Anthropometric Measures of Visceral and Subcutaneous Fat Are Important in the Determination of Metabolic Dysregulation in Boys and Girls at Risk for Nonalcoholic Fatty Liver Disease. Nutr. Clin. Pract. 2013, 28, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [PubMed] [Green Version]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Duran, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trépo, E.; Valenti, L. Update on NAFLD genetics: From new variants to the clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fargion, S.; Fracanzani, A.L. miRNA Signature in NAFLD: A Turning Point for a Non-Invasive Diagnosis. Int. J. Mol. Sci. 2018, 19, 3966. [Google Scholar] [CrossRef] [Green Version]
- Umano, G.R.; Martino, M.; Santoro, N. The Association between Pediatric NAFLD and Common Genetic Variants. Children 2017, 4, 49. [Google Scholar] [CrossRef]
- Sahota, A.K.; Shapiro, W.L.; Newton, K.P.; Kim, S.T.; Chung, J.; Schwimmer, J.B. Incidence of Nonalcoholic Fatty Liver Disease in Children: 2009–2018. Pediatrics 2020, 146, e20200771. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rivera, C.; Hadjiyannakis, S.; Davila, J.; Hurteau, J.; Aglipay, M.; Barrowman, N.; Adamo, K.B. Prevalence and risk factors for non-alcoholic fatty liver in children and youth with obesity. BMC Pediatr. 2017, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Del Col, A.; Agosti, F.; Mazzilli, G.; Bellentani, S.; Tiribelli, C.; Bedogni, G. Predictors of non-alcoholic fatty liver disease in obese children. Eur. J. Clin. Nutr. 2007, 61, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Castner, D.M.; Rubin, D.A.; Judelson, D.A.; Haqq, A.M. Effects of Adiposity and Prader-Willi Syndrome on Postexercise Heart Rate Recovery. J. Obes. 2013, 2013, 384167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castner, D.M.; Tucker, J.M.; Wilson, K.S.; Rubin, D.A. Patterns of habitual physical activity in youth with and without Prader-Willi Syndrome. Res. Dev. Disabil. 2014, 35, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.G.; Knehans, A.; Arnold, S.; Dionne, C.; Hoffman, L.; Turner, P.; Baldwin, J. The associations between diet and physical activity with body composition and walking a timed distance in adults with Prader–Willi syndrome. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.S.; Carson, V.; Chaput, J.-P.; Gorber, S.C.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41 (Suppl. 3), S311–S327. [Google Scholar] [CrossRef]
- Theodoro, M.F.; Talebizadeh, Z.; Butler, M.G. Body Composition and Fatness Patterns in Prader-Willi Syndrome: Comparison with Simple Obesity. Obesity 2006, 14, 1685–1690. [Google Scholar] [CrossRef]
- Lafortuna, C.; Minocci, A.; Capodaglio, P.; Gondoni, L.; Sartorio, A.; Vismara, L.; Rizzo, G.; Grugni, G. Skeletal Muscle Characteristics and Motor Performance After 2-Year Growth Hormone Treatment in Adults With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Edge, R.; la Fleur, P.; Adcock, L. Human Growth Hormone Treatment for Children with Prader-Willi Syndrome: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. In CADTH Rapid Response Reports; NCBI Bookshelf: Ottawa, ON, Canada, 2018. [Google Scholar]
- Rubin, D.A.; Castner, D.; Pham, H.; Ng, J.; Adams, E.; Judelson, D.A. Hormonal and Metabolic Responses to a Resistance Exercise Protocol in Lean Children, Obese Children, and Lean Adults. Pediatr. Exerc. Sci. 2014, 26, 444–454. [Google Scholar] [CrossRef]
- Forsberg, A.M.; Nilsson, E.; Werneman, J.; Bergström, J.; Hultman, E. Muscle composition in relation to age and sex. Clin. Sci. 1991, 81, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragala, M.S.; Kenny, A.M.; Kuchel, G.A. Muscle Quality in Aging: A Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports Med. 2015, 45, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. (1985) 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Samsell, L.; Regier, M.; Walton, C.; Cottrell, L. Importance of Android/Gynoid Fat Ratio in Predicting Metabolic and Cardiovascular Disease Risk in Normal Weight as well as Overweight and Obese Children. J. Obes. 2014, 2014, 846578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aucouturier, J.; Meyer, M.; Thivel, D.; Taillardat, M.; Duché, P. Effect of Android to Gynoid Fat Ratio on Insulin Resistance in Obese Youth. Arch. Pediatr. Adolesc. Med. 2009, 163, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Sweeny, K.F.; Lee, C.K. Nonalcoholic Fatty Liver Disease in Children. Gastroenterol. Hepatol. (N. Y.) 2021, 17, 579–587. [Google Scholar] [PubMed]
- Adab, P.; Pallan, M.; Whincup, P.H. Is BMI the best measure of obesity? BMJ 2018, 360, k1274. [Google Scholar] [CrossRef] [Green Version]
- Kipping, R.R.; Jago, R.; Lawlor, D.A. Obesity in children. Part 1: Epidemiology, measurement, risk factors, and screening. BMJ 2008, 337, a1824. [Google Scholar] [CrossRef]
- Buśko, K.; Lewandowska, J.; Lipińska, M.; Michalski, R.; Pastuszak, A. Somatotype-variables related to muscle torque and power output in female volleyball players. Acta Bioeng. Biomech. 2013, 15, 119–126. [Google Scholar]
- Carter, J.E. The somatotypes of athletes—A review. Hum. Biol. 1970, 42, 535–569. [Google Scholar]
- Stepnicka, J. Somatotype in Relation to Physical Performance, Sports and Body Posture; E and FN Spon: London, UK, 1986. [Google Scholar]
- Lewandowska, J.; Buśko, K.; Pastuszak, A.; Boguszewska, K. Somatotype Variables Related to Muscle Torque and Power in Judoists. J. Hum. Kinet. 2011, 30, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Stewart, H.; Faulkner, J.; Jobson, S. The influence of somatotype on anaerobic performance. PLoS ONE 2018, 13, e0197761. [Google Scholar] [CrossRef] [PubMed]
- Silva CAD, d.S.M.D.; Oliveira, E.; Almeida, H.A.; Ascenso, R.M.T. BodyShifter–Software to Determine and Optimize an Individual’s Somatotype. Procedia Technol. 2014, 16, 1456–1461. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.E.; Phillips, W.H. Structural changes in exercising middle-aged males during a 2-year period. J. Appl. Physiol. 1969, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.O.T.; Olive, S.; Craig, N. Anthropometry and Sports Performance; CBS Publishers & Distributors: Delhi, India, 1996. [Google Scholar]
- Heath, B.H.; Carter, J.E.L. A modified somatotype method. Am. J. Phys. Anthropol. 1967, 27, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tan, Q.; Afhami, S.; Deehan, E.; Liang, S.; Gantz, M.; Triador, L.; Madsen, K.; Walter, J.; Tun, H.; et al. The Gut Microbiota Profile in Children with Prader–Willi Syndrome. Genes 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Johnson, J.S.; Angeles, J.E.; Behling, C.; Belt, P.H.; Borecki, I.; Bross, C.; Durelle, J.; Goyal, N.P.; Hamilton, G.; et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157, 1109–1122. [Google Scholar] [CrossRef] [Green Version]
- Bochukova, E.G.; Lawler, K.; Croizier, S.; Keogh, J.M.; Patel, N.; Strohbehn, G.; Lo, K.K.; Humphrey, J.; Hokken-Koelega, A.; Damen, L.; et al. A Transcriptomic Signature of the Hypothalamic Response to Fasting and BDNF Deficiency in Prader-Willi Syndrome. Cell Rep. 2018, 22, 3401–3408. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef]
- Pascut, D.; Tamini, S.; Bresolin, S.; Giraudi, P.; Basso, G.; Minocci, A.; Tiribelli, C.; Grugni, G.; Sartorio, A. Differences in circulating microRNA signature in Prader–Willi syndrome and non-syndromic obesity. Endocr. Connect. 2018, 7, 1262–1274. [Google Scholar] [CrossRef] [Green Version]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, R.; Wu, H.; Xiao, H.; Chen, X.; Willenbring, H.; Steer, C.J.; Song, G. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology 2014, 60, 554–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patry-Parisien, J.; Shields, M.; Bryan, S. Comparison of waist circumference using the World Health Organization and National Institutes of Health protocols. Health Rep. 2012, 23, 53–60. [Google Scholar] [PubMed]
- Webber, C.E.; Barr, R.D. Age- and gender-dependent values of skeletal muscle mass in healthy children and adolescents. J. Cachex. Sarcopenia Muscle 2011, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Bittel, D.C.; Kibiryeva, N.; Talebizadeh, Z.; Thompson, T. Behavioral Differences Among Subjects With Prader-Willi Syndrome and Type I or Type II Deletion and Maternal Disomy. Pediatrics 2004, 113, 565–573. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Dunn, W.; Norman, G.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology 2010, 138, 1357–1364.e2. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Geiger, R.; Strasak, A.; Treml, B.; Gasser, K.; Kleinsasser, A.; Fischer, V.; Geiger, H.; Loeckinger, A.; Stein, J.I. Six-Minute Walk Test in Children and Adolescents. J. Pediatr. 2007, 150, 395–399.e2. [Google Scholar] [CrossRef]
- McQuiddy, V.A.; Scheerer, C.R.; Lavalley, R.; McGrath, T.; Lin, L. Normative Values for Grip and Pinch Strength for 6- to 19-Year-Olds. Arch. Phys. Med. Rehabil. 2015, 96, 1627–1633. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. 2), 555–576. [CrossRef]
- Chiles Shaffer, N.; Fabbri, E.; Ferrucci, L.; Shardell, M.; Simonsick, E.M.; Studenski, S. Muscle Quality, Strength, and Lower Extremity Physical Performance in the Baltimore Longitudinal Study of Aging. J. Frailty Aging 2017, 6, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.A.; University, B.; Cairney, J. Development of the Habitual Activity Estimation Scale for Clinical Research: A Systematic Approach. Pediatr. Exerc. Sci. 2006, 18, 193–202. [Google Scholar] [CrossRef]
- Smith, A.; Hung, D. The dilemma of diagnostic testing for Prader-Willi syndrome. Transl. Pediatr. 2017, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
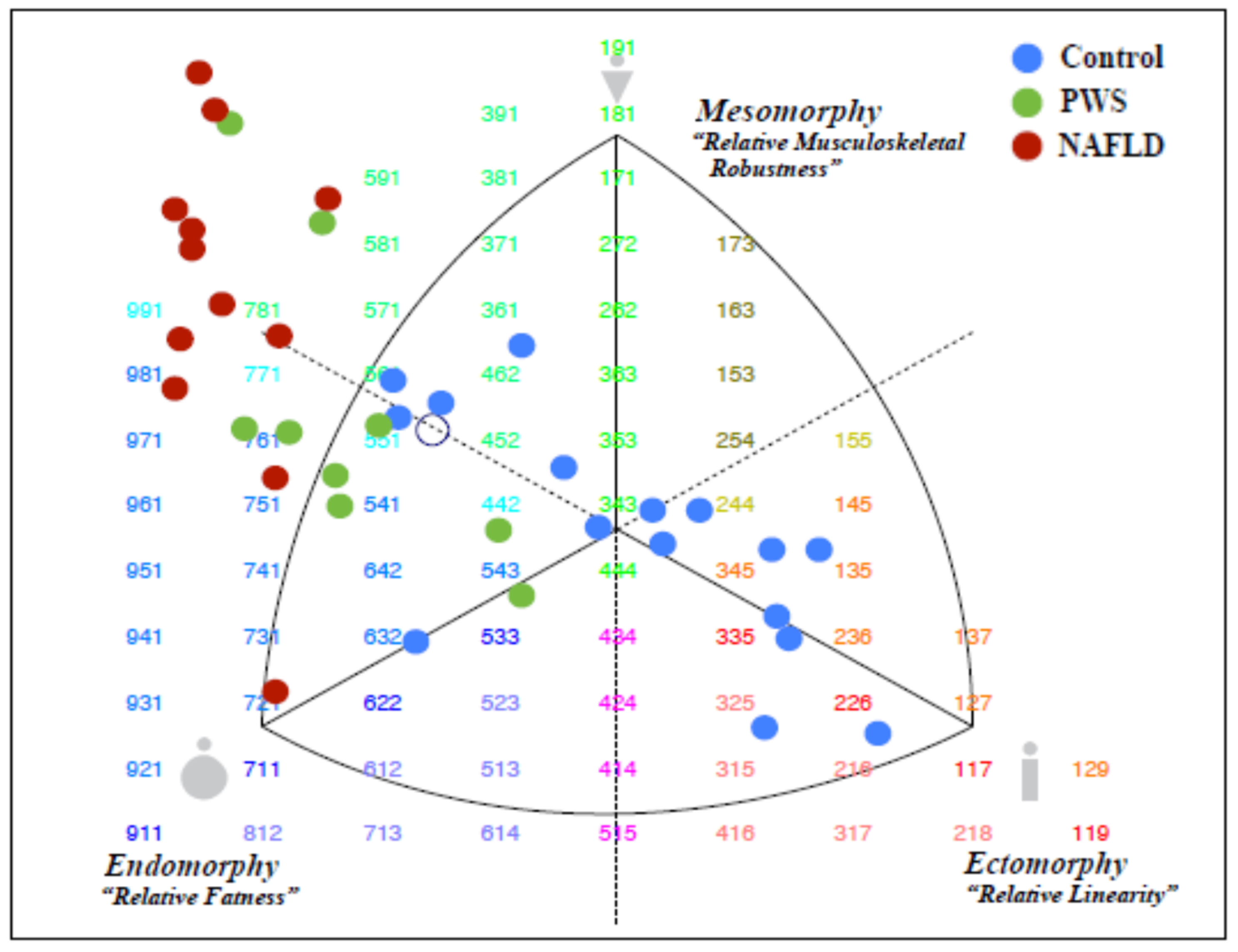

| HC (n = 16) 1 | PWS (n = 9) 1 | NAFLD (n = 14) 1 | HC vs. PWS p Value 2 | HC vs. NAFLD p Value 2 | PWS vs. NAFLD p Value 2 | |
|---|---|---|---|---|---|---|
| Gender (M:F) | 9:7 | 2:7 | 8:6 | NS | NS | NS |
| Age (years) | 12.7 | 13.0 | 13.6 | NS | NS | NS |
| (10.8, 14.5) | (9.8, 15.1) | (11.6, 15.4) | ||||
| Weight (kg) | 41.7 | 42.5 | 88.4 | NS | <0.0001 | 0.002 |
| (37.3, 55.7) | (33.2, 63.2) | (67.8, 101.7) | ||||
| Height (cm) | 154 | 143 | 162 | NS | NS | 0.007 |
| (144, 167) | (129, 154) | (151, 168) | ||||
| BMI (kg/m2) | 17.9 | 21.3 | 32.8 | 0.02 | <0.0001 | 0.007 |
| (16.8, 20.1) | (18.5, 28.1) | (27.8, 37.3) | ||||
| Weight | 0.51 | 0.6 | 3 | <0.0001 | <0.0001 | NS |
| Z-score 3 | (−0.19, 0.87) | (−0.22, 1.5) | (2.2, 2.9) | |||
| Height | 0.48 | −1.3 | 0.05 | 0.0007 | NS | 0.002 |
| Z-score 3 | (−0.11, 1.43) | (−1.9, −0.37) | (−0.13, 1.5) | |||
| BMI | −0.1 | 1.2 | 2.9 | 0.004 | <0.0001 | 0.0003 |
| Z-score 3 | (−0.79, 0.56) | (−0.67, 2.22) | (2.5, 2.9) | |||
| Waist (cm) | 65.7 | 75.5 | 95.7 | 0.04 | <0.0001 | 0.003 |
| (62, 71.4) | (66.4, 87.6) | (88.9, 114.7) | ||||
| Waist | −0.3 | 0.8 | 1.8 | 0.005 | <0.0001 | 0.0002 |
| Z-score 3 | (−0.52, 0.27) | (0.31, 1.3) | (1.5, 2.1) | |||
| WHtR 4 | 0.42 | 0.5 | 0.6 | <0.0001 | <0.0001 | 0.04 |
| (0.41, 0.45) | (0.5, 0.6) | (0.5, 07) | ||||
| WHtR 4 | −0.67 | 1.0 | 1.8 | <0.0001 | <0.0001 | 0.01 |
| Z-score 3 | (−1.0, −0.09) | (0.5, 1.6) | (1.4, 2.0) | |||
| Systolic BP Z-score | 0.78 | 1.2 | 1.6 | NS | NS | 0.006 |
| (0.019, 1.0) | (0.4, 2.5) | (1.2, 1.9) | ||||
| Diastolic BP Z-score | 0.15 | 0.9 | 1.0 | NS | 0.01 | NS |
| (−0.2, 0.57) | (0.6, 1.6) | (0.7, 1.3) | ||||
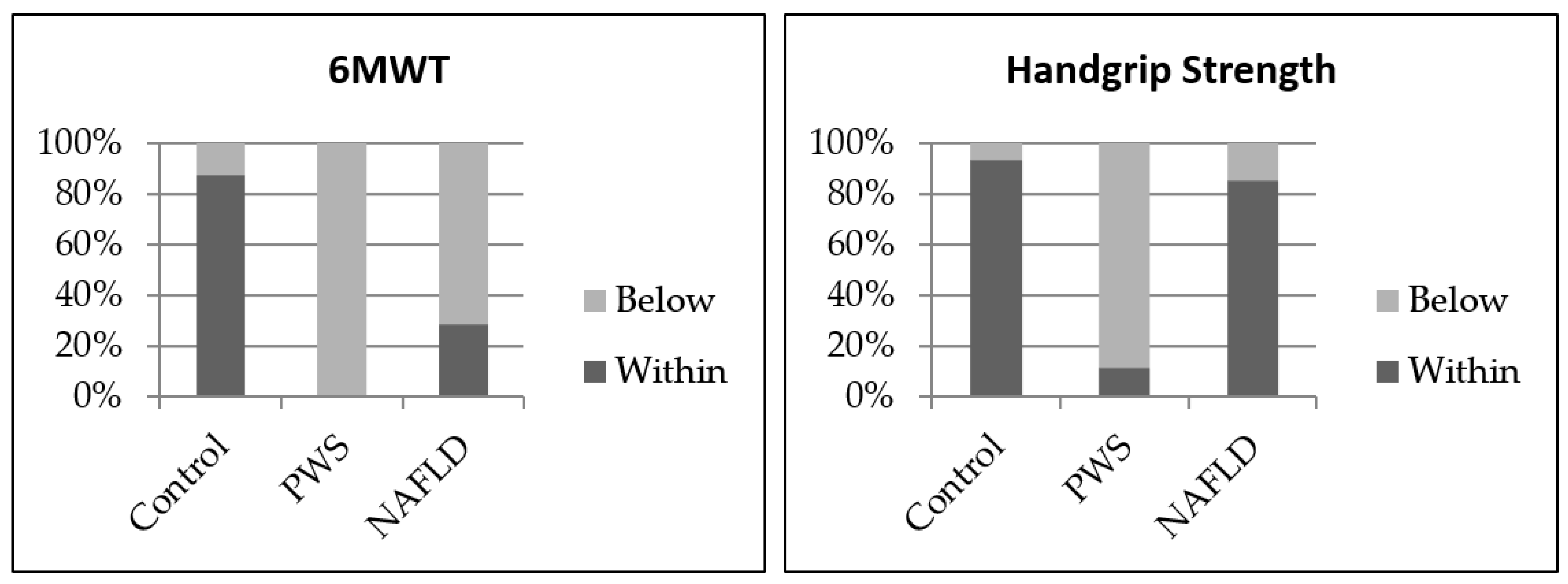
| Handgrip Strength (kg) | 18.3 | 9 | 23.7 | 0.002 | 0.001 | NS |
| (16.7, 29.2) | (3.9, 12.8) | (17.7, 32.2) | ||||
| 6MWT (m) | 603 | 436 | 489 | NS | NS | NS |
| (569, 634) | (379, 459) | (460, 522) | ||||
| Muscle Quality 5 | n/a | 3.4 | 4.4 | n/a | n/a | 0.05 |
| (3.1, 3.6) | (3.0, 5.8) |
| HC (n = 16) 1 | PWS (n = 9) 1 | NAFLD (n = 12) 1 | HC vs. PWS p Value 2 | HC vs. NAFLD p Value 2 | PWS vs. NAFLD p Value 2 | |
|---|---|---|---|---|---|---|
| Endomorph | 3.2 | 5.7 | 7.1 | 0.0085 | <0.001 | <0.001 |
| (2.8–4.1) | (5.0–6.2) | (6.4–7.4) | ||||
| Ectomorph | 3.9 | 0.9 | 0.1 | <0.001 | <0.001 | 0.21 |
| (2.8–4.7) | (0.3–1.9) | (0.04–0.6) | ||||
| Mesomorph | 3.5 | 4.7 | 7.6 | 0.002 | <0.001 | 0.06 |
| (2.9–4.2) | (3.5–6.7) | (5.4–8.8) |
| Variable | PWS (n = 8) 1 | NAFLD (n = 7) 1 | p Value |
|---|---|---|---|
| Adipose Indices | |||
| Fat mass total (kg) | 22.9 ± 11.0 | 35.4 ± 14.4 | NS |
| Fat mass/Height2 (kg/m2) | 11.1 ± 4.2 | 13.7 ± 3.6 | NS |
| Fat mass/Height2 Z-score | 1.2 ± 0.6 | 1.7 ± 0.3 | NS |
| Android/Gynoid ratio | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.006 |
| Trunk/Limb fat mass ratio | 0.8 ± 0.2 | 0.9 ± 0.2 | NS |
| Trunk/Limb fat mass ratio z-score | 0.5 ± 1.1 | 1.5 ± 0.6 | NS |
| Fat Mass Index | 10.8 ± 4.1 | 13.5 ± 3.4 | NS |
| Lean Indices | |||
| Lean mass total (kg) | 27.3 ± 8.8 | 43.6 ± 13.0 | 0.01 |
| Lean Mass/Height2 (kg/m2) | 13.2 ± 2.4 | 17.2 ± 2.6 | 0.009 |
| Lean Mass/Height z-score | −0.2 ± 0.9 | 1.2 ± 1.1 | 0.03 |
| Lean Body Mass Index | 12.9 ± 2.4 | 16.9 ± 2.5 | 0.008 |
| Skeletal Muscle Mass (kg) | 11.5 ± 4.7 | 20.9 ± 7.2 | 0.009 |
| Skeletal Muscle Mass Z-score | −1.7 ± 0.9 | 0.9 ± 0.9 | 0.0001 |
| Appendicular Lean/Height2 (kg/m2) | 5.3 ± 1.2 | 7.6 ± 1.2 | 0.003 |
| Appendicular Lean/Height2 Z-score | −0.7 ± 0.9 | 1.3 ± 0.5 | 0.0003 |
| Lean Mass to Fat Mass Ratio | |||
| Left Arm | 1.0 ± 0.2 | 1.1 ± 0.2 | NS |
| Right Arm | 0.9 ± 0.2 | 1.0 ± 0.2 | NS |
| Trunk | 1.5 ± 0.5 | 1.4 ± 0.3 | NS |
| Left Leg | 1.0 ± 0.1 | 1.2 ± 0.1 | 0.01 |
| Right Leg | 0.9 ± 0.1 | 1.2 ± 0.1 | 0.001 |
| Total | 1.3 ± 0.2 | 1.3 ± 0.2 | NS |
| HC (n = 16) 1 | PWS (n = 9) 1 | NAFLD (n = 14) 1 | HC vs. PWS p Value 2 | HC vs. NAFLD p Value 2 | PWS vs. NAFLD p Value 2 | Reference Values 3 | |
|---|---|---|---|---|---|---|---|
| ALT (U/L) | 15 | 20 | 45 | NS | <0.0001 | 0.0003 | <20 |
| (14, 16.5) | (13, 28) | (37, 84) | |||||
| AST (U/L) | 23 | 26 | 32 | NS | 0.001 | NS | 2–9 yrs: <50 |
| (21, 26) | (21, 33) | (27, 51) | ≥10 yrs: <40 | ||||
| GGT (U/L) | 5 | 5 | 7 | NS | 0.005 | NS | Male: <70 |
| (5, 5) | (4, 9) | (4.9, 28) | Female: <55 | ||||
| ALP (U/L) | 230 | 169 | 152 | NS | NS | NS | 5–17 yrs |
| (181, 274) | (123, 223) | (117, 227) | 100–500 | ||||
| Glucose (mmol/L) | 5.1 | 4.9 | 4.9 | NS | NS | NS | 3.3–6.0 |
| (4.7, 5.2) | (4.7, 5.1) | (4.6, 5.4) | |||||
| Insulin (mU/L) | 5.9 | 13.5 | 29 | NS | <0.0001 | 0.009 | 5.0–20.0 |
| (4. 2, 9.4) | (9.9, 21.4) | (21, 50) | |||||
| HOMA-IR | 1.2 | 3.0 | 5.9 | NS | <0.0001 | 0.01 | 3.16 |
| (0.9, 2.1) | (2.1, 4.8) | (3.9, 12.8) | |||||
| TG (mmol/L) | 0.7 | 1.1 | 1.4 | NS | 0.02 | NS | <1.5 |
| (0.6, 1.0) | (0.6, 1.5) | (1.0, 2.3) | |||||
| TC (mmol/L) | 3.9 | 4.2 | 4.4 | NS | NS | NS | <4.4 |
| (3.5, 4.2) | (3.7, 5.3) | (3.7, 4.7) | |||||
| HDL-C (mmol/L) | 1.4 | 1.2 | 1.1 | NS | 0.001 | 0.02 | >1.0 |
| (1.3, 1.6) | (1.1, 1.7) | (0.9, 2.3) | |||||
| LDL-C (mmol/L) | 2.0 | 2.4 | 2.5 | NS | NS | NS | <2.8 |
| (1.8, 2.4) | (1.8, 3.4) | (2.1, 2.7) | |||||
| Albumin (g/L) | 47 | 46 | 43 | NS | 0.02 | NS | 35–50 |
| (45, 48) | (43, 47) | (42, 46) | |||||
| Urate (umol/L) | 244 | 310 | 346 | NS | 0.0003 | NS | ≤9 yrs: 100–300 |
| 10–17 yrs: Male: 135–510 Female: 180–450 | |||||||
| ≥18 yrs: Male: 180–500 Female: 150–400 | |||||||
| (211, 294) | (243, 365) | (321, 411) | |||||
| CRP (mg/L) | 0.4 | 2.0 | 2.2 | NS | <0.0001 | NS | ≤10 |
| (0.1, 0.7) | (0.6, 6.9) | (1.6, 3.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mager, D.R.; MacDonald, K.; Duke, R.L.; Avedzi, H.M.; Deehan, E.C.; Yap, J.; Siminoski, K.; Haqq, A.M. Comparison of Body Composition, Muscle Strength and Cardiometabolic Profile in Children with Prader-Willi Syndrome and Non-Alcoholic Fatty Liver Disease: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 15115. https://doi.org/10.3390/ijms232315115
Mager DR, MacDonald K, Duke RL, Avedzi HM, Deehan EC, Yap J, Siminoski K, Haqq AM. Comparison of Body Composition, Muscle Strength and Cardiometabolic Profile in Children with Prader-Willi Syndrome and Non-Alcoholic Fatty Liver Disease: A Pilot Study. International Journal of Molecular Sciences. 2022; 23(23):15115. https://doi.org/10.3390/ijms232315115
Chicago/Turabian StyleMager, Diana R., Krista MacDonald, Reena L. Duke, Hayford M. Avedzi, Edward C. Deehan, Jason Yap, Kerry Siminoski, and Andrea M. Haqq. 2022. "Comparison of Body Composition, Muscle Strength and Cardiometabolic Profile in Children with Prader-Willi Syndrome and Non-Alcoholic Fatty Liver Disease: A Pilot Study" International Journal of Molecular Sciences 23, no. 23: 15115. https://doi.org/10.3390/ijms232315115






