Hypertension and Type 2 Diabetes—The Novel Treatment Possibilities
Abstract
:1. Introduction
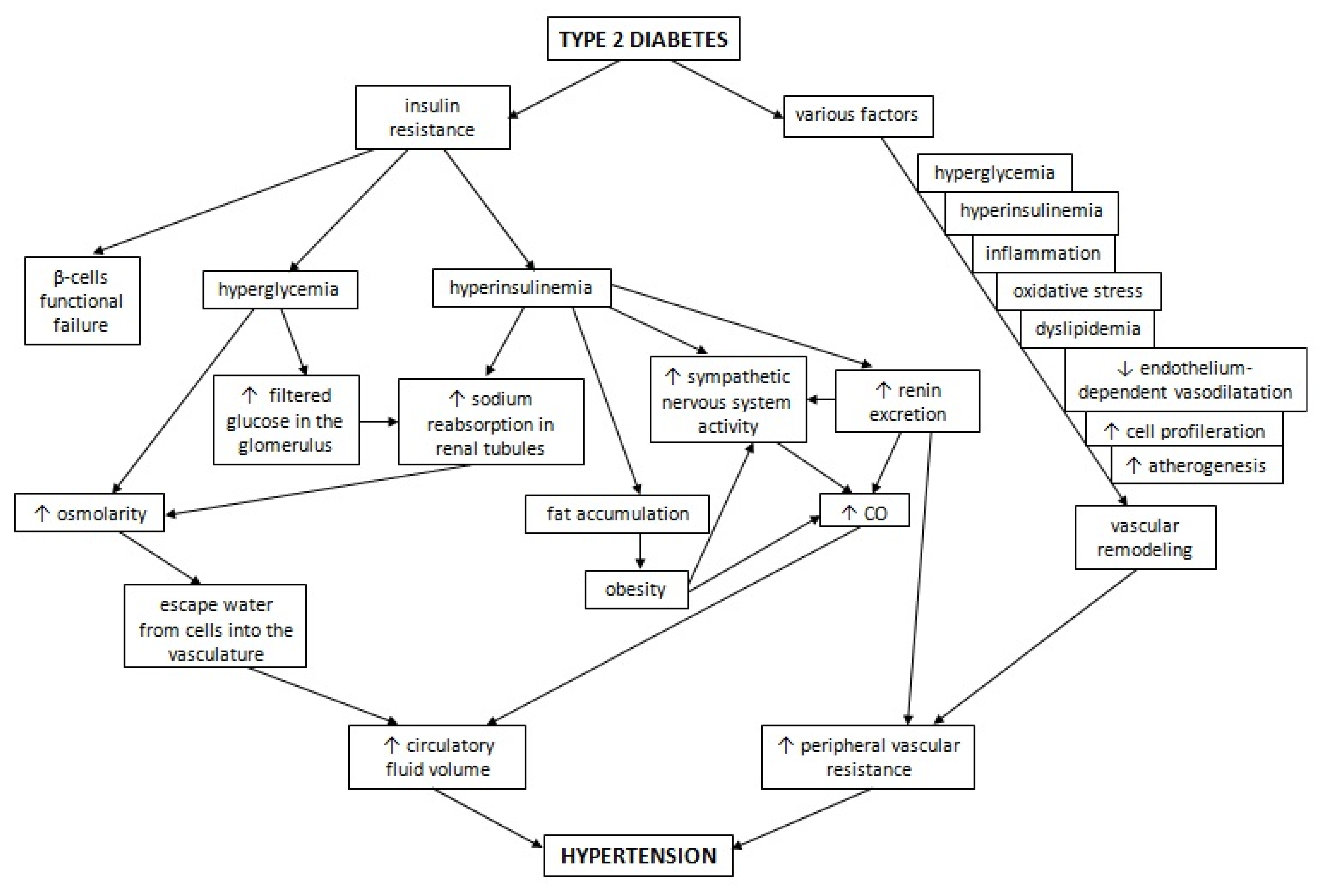
2. Pathophysiology of Hypertension in Diabetes
3. Attempts to Define Targets for Treatment of High Blood Pressure in Diabetic Patients
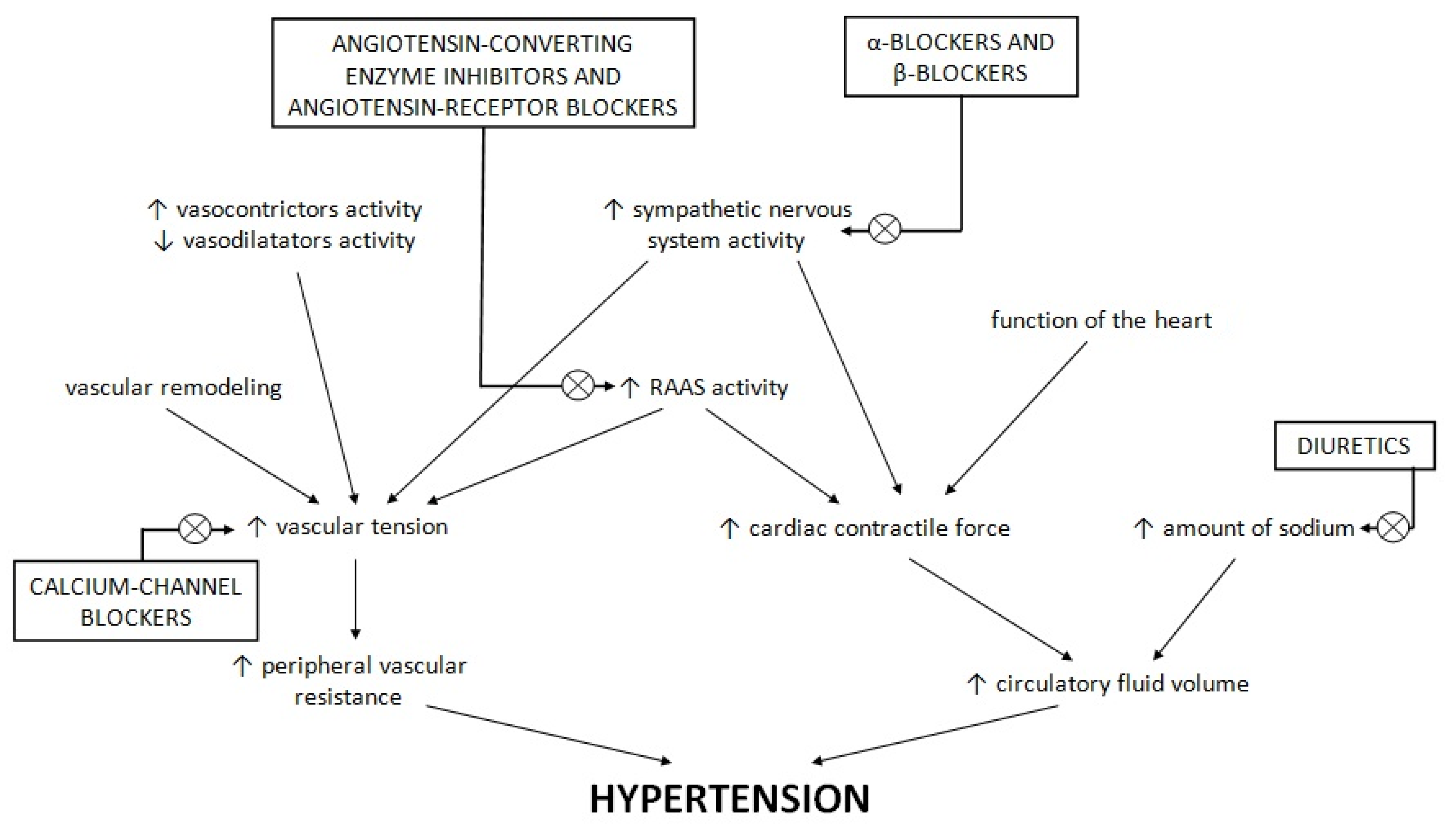
4. Standard Antihypertensive Drugs in the Therapy of Patients with Diabetes
5. Novel Antihypertensive Drugs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzo, R.; de Simone, G.; Chinali, M.; Iaccarino, G.; Trimarco, V.; Rozza, F.; Giudice, R.; Trimarco, B.; De Luca, N. Insufficient control of blood pressure and incident diabetes. Diabetes Care 2009, 32, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Medalie, J.H.; Papier, C.M.; Goldbourt, U.; Herman, J.B. Major factors in the development of diabetes mellitus in 10,000 men. Arch. Intern. Med. 1975, 135, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R. Diabetes mellitus and vascular disease. Hypertension 2013, 61, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.S.; Coady, S.A.; Goff, D.C., Jr.; Brancati, F.L.; Levy, D.; Selvin, E.; Vasan, R.S.; Fox, C.S. Blood pressure and the risk of developing diabetes in african americans and whites: ARIC, CARDIA, and the framingham heart study. Diabetes Care 2011, 34, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, T.; Kubo, M.; Doi, Y.; Yonemoto, K.; Tanizaki, Y.; Rahman, M.; Arima, H.; Tsuryuya, K.; Iida, M.; Kiyohara, Y. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: The Hisayama study. Stroke 2007, 38, 2063–2069. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; McAlister, F.A.; Walker, R.L.; Hemmelgarn, B.R.; Campbell, N.R. Cardiovascular outcomes in framingham participants with diabetes: The importance of blood pressure. Hypertension 2011, 57, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Solini, A.; Penno, G.; Bonora, E.; Fondelli, C.; Orsi, E.; Arosio, M.; Trevisan, R.; Vedovato, M.; Cignarelli, M.; Andreozzi, F.; et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: The renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care 2012, 35, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.B.; Nikoulina, S.E.; Ciaraldi, T.P.; Henry, R.R.; Kahn, B.B. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J. Clin. Investig. 1999, 104, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Folli, F.; Kahn, C.R.; Hansen, H.; Bouchie, J.L.; Feener, E.P. Angiotensin II inhibits insulin signalling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J. Clin. Investig. 1997, 100, 2158–2169. [Google Scholar] [CrossRef] [Green Version]
- Mulvany, M.J.; Aalkjaer, C. Structure and function of small arteries. Physiol. Rev. 1990, 70, 921–961. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Reactivity of small blood vessels in hypertension: Realtion with structural changes. State of the art lecture. Hypertension 1992, 19, Il1–Il9. [Google Scholar] [CrossRef] [Green Version]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfiels, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Virdis, A.; Taddei, S. Endothelial Dysfunction in Resistance Arteries of Hypertensive Humans: Old and New Conspirators. J. Cardiovasc. Phaarmacol. 2016, 67, 451–457. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Mattei, P.; Salvetti, A. Vasodilatation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 1993, 21, 929–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, B.; de Faria, A.P.; Ritter, A.M.V.; Yugar, L.B.T.; Ferreira-Melo, S.E.; Amorim, R.; Modolo, R.; Fattori, A.; Yugar-Toledo, J.C.; Coca, A.; et al. Glycated hemoglobin correlates with arterial stiffness and endothelial dysfunction in patients with resistant hypertension and uncontrolled diabetes mellitus. J. Clin. Hypertens. 2018, 20, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Duplain, H.; Burcelin, R.; Sartori, C.; Cook, S.; Egli, M.; Lepori, M.; Vollenweider, P.; Pedrazzini, T.; Nicod, P.; Thorens, B.; et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001, 104, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; O’donnell, C.J.; Tofler, G.H.; Benjamin, E.J.; Fox, C.S.; Lipinska, I.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B.; Wilson, P.W. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: The Framingham Offspring Study. Diabetes 2006, 55, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Fève, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Buzzigoli, G.; Bonadonna, R.; Giorico, M.A.; Oleggini, M.; Graziadei, L.; Pedrinelli, R.; Brandi, L.; Bevilacqua, S. Insulin resistance in essential hypertension. N. Engl. J. Med. 1987, 317, 350–357. [Google Scholar] [CrossRef]
- Shen, D.C.; Shieh, S.M.; Fuh, M.M.; Wu, D.A.; Chen, Y.D.; Reaven, G.M. Resistance to insulin-stimulated-glucose uptake in patients with hypertension. J. Clin. Endocrinol. Metab. 1988, 66, 580–583. [Google Scholar] [CrossRef]
- Lucas, C.P.; Estigarribia, J.A.; Darga, L.L.; Reaven, G.M. Insulin and blood pressure in obesity. Hypertension 1985, 7, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Manicardi, V.; Camellini, L.; Bellodi, G.; Coscelli, C.; Ferrannini, E. Evidence for an association of high blood pressyre and hyperinsulinemia in obese man. J. Clin. Endocrinol. Metab. 1986, 62, 1302–1304. [Google Scholar] [CrossRef] [PubMed]
- Kawasoe, S.; Maruguchi, Y.; Kajiya, S.; Uenomachi, H.; Miyata, M.; Kawasoe, M.; Kubozono, T.; Ohishi, M. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol. Toxicol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, M.; Sowers, J.R. Diabetes mellitus and hypertension. Hypertension 1992, 19, 403–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosadini, R.; Sambataro, M.; Thomaseth, K.; Pacini, G.; Cipollina, M.R.; Brocco, E.; Solini, A.; Carraro, A.; Velussi, M.; Frigato, F.; et al. Role of hyperglycemia and insulin resistance in determining sodium retention in non-insulin-dependent diabetes. Kidney Int. 1993, 44, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, F.J.; Sancho-Rof, J.M. Epidemiology of high blood pressure and obesity. Drugs 1993, 46 (Suppl. 2), 160–164. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Sympathetic Nervous System, Hypertension, Obesity and Metabolic Syndrome. High Blood Press Cardiovasc. Prev. 2016, 23, 175–179. [Google Scholar] [CrossRef]
- Aneja, A.; El-Atat, F.; McFarlane, S.I.; Sowers, J.R. Hypertension and obesity. Recent Prog. Horm. Res. 2004, 59, 169–205. [Google Scholar] [CrossRef] [Green Version]
- Kishida, K.; Funahashi, T.; Shimomura, I. Clinical importance of assessment of type 2 diabetes mellitus with visceral obesity. A Japanese perspective. Curr. Diabetes Rev. 2012, 8, 84–91. [Google Scholar] [CrossRef]
- Ferrannini, E.; Cushman, W.C. Diabetes and hypertension: The bad companions. Lancet 2012, 380, 601–610. [Google Scholar] [CrossRef]
- Emdin, M.; Gastaldelli, A.; Muscelli, E.; Macerata, A.; Natali, A.; Camastra, S.; Ferrannini, E. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: Effects of weight loss. Circulation 2001, 103, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Davy, K.P.; Hall, J.E. Obesity and hypertension: Two epidemics or one? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R803–R813. [Google Scholar] [CrossRef] [PubMed]
- Leighton, B.; Cooper, G.J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 1988, 335, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Dégano, P.; Silvestre, R.A.; Salas, M.; Peiró, E.; Marco, J. Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner. Study in the perfused rat pancreas. Regul. Pept. 1993, 43, 91–96. [Google Scholar] [CrossRef]
- Williams, B. Insulin resistance: The shape of things to come. Lancet 1994, 344, 521–524. [Google Scholar] [CrossRef]
- Young, A.A.; Rink, T.J.; Vine, W.; Gedulin, B. Amylin and Syndrome X. Drug Dev. Res. 1994, 32, 90–99. [Google Scholar] [CrossRef]
- Zweers, E.J.K.; Bravenboer, B.; van Hulst, K.L.; Lips, C.J.M.; Christiaens, G.C.M.L.; Hackend, W.H.L.; Erkelens, D.W. Glucose stimulated islet amyloid polypeptide and gestational diabetes mellitus. Diabetologia 1992, 35, A179. [Google Scholar]
- Young, A.A.; Vine, W.; Carlo, P.; Smith, P.; Rink, T.J.; Rumple, J.; Cooper, M.E. Amylin stimulation of renin activity in rats: A possible link between insulin resistance and hypertension. J. Hypertens. 1994, 12, S152. [Google Scholar]
- Cooper, M.E.; McNally, P.G.; Phillips, P.A.; Johnston, C.I. Amylin stimulates plasma renin concentration in humans. Hypertension 1995, 26, 460–464. [Google Scholar] [CrossRef]
- Fujita, T. Aldosterone in salt-sensitive hypertension and metabolic syndrome. J. Mol. Med. 2008, 86, 729–734. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Taddei, S.; Virdis, A.; Mattei, P.; Natali, A.; Ferrannini, E.; Salvetti, A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation 1995, 92, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Baron, A.D. Vascular function, insulin resistance and fatty acids. Diabetologia 2002, 45, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, A.M.; Heerkens, E.H.; Izzard, A.S. Small artery structure and function in hypertension. J. Cell Mol. Med. 2010, 14, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; Li, Q.; Freund, B.; Feather, D.; Abramov, R.; Wu, I.H.; Chen, K.; Yamamoto-Hiraoka, J.; Goldenbogen, J.; Sotiropoulos, K.B.; et al. Loss of insulin signalling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010, 11, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Rask-Madsen, C.; Buonomo, E.; Li, Q.; Park, K.; Clermont, A.C.; Yerokun, O.; Rekhter, M.; King, G.L. Hyperinsulinemia does not change atherosclerosis development in apolipoprotein E null mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998, 317, 703–713, Erratum in BMJ 1999, 318, 29. [Google Scholar]
- Buckley, L.F.; Dixon, D.L.; Wohlford, G.F., IV; Wijesinghe, D.S.; Baker, W.L.; Van Tassell, B.W. Effect of intensive blood pressure control in patients with type 2 diabetes mellitus over 9 years of follow-up: A subgroup analysis of high-risk ACCORDION trial participants. Diabetes Obes. Metab. 2018, 20, 1499–1502. [Google Scholar] [CrossRef]
- Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; SPRINT Research Group; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116, Erratum in N. Engl. J. Med. 2017, 377, 2506. [Google Scholar]
- Bress, A.P.; King, J.B.; Kreider, K.E.; Beddhu, S.; Simmons, D.L.; Cheung, A.K.; Zhang, Y.; Doumas, M.; Nord, J.; Sweeney, M.E.; et al. Effect of Intensive Versus Standard Blood Pressure Treatment According to Baseline Prediabetes Status: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2017, 40, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Atkins, E.; Lv, J.; Bennett, A.; Neal, B.; Ninomiya, T.; Woodward, M.; MacMahon, S.; Turnbull, F.; Hillis, G.S.; et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 2016, 387, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Scheen, A.J. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab. 2004, 30, 498–505. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S144–S174. [Google Scholar] [CrossRef]
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000, 355, 253–259, Erratum in Lancet 2000, 356, 860. [Google Scholar]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef] [Green Version]
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators; Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet 2008, 372, 1174–1183, Erratum in Lancet 2008, 372, 1384. [Google Scholar]
- Strawn, W.B.; Ferrario, C.M. Mechanisms linking angiotensin II and atherogenesis. Curr. Opin. Lipidol. 2002, 13, 505–512. [Google Scholar] [CrossRef]
- de Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstein, H.C.; Mann, J.F.; Pogue, J.; Dinneen, S.F.; Hallé, J.P.; Hoogwerf, B.; Joyce, C.; Rashkow, A.; Young, J.; Zinman, B.; et al. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care 2000, 23 (Suppl. 2), B35–B39. [Google Scholar] [PubMed]
- Patel, A.; ADVANCE Collaborative Group; MacMahon, S.; Chalmers, J.; Neal, B.; Woodward, M.; Billot, L.; Harrap, S.; Poulter, N.; Marre, M.; et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 2007, 370, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Jamerson, K.; Weber, M.A.; Bakris, G.L.; Dahlöf, B.; Pitt, B.; Shi, V.; Hester, A.; Gupte, J.; Gatlin, M.; Velazquez, E.J.; et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N. Engl. J. Med. 2008, 359, 2417–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.A.; Bakris, G.L.; Jamerson, K.; Weir, M.; Kjeldsen, S.E.; Devereux, R.B.; Velazquez, E.J.; Dahlöf, B.; Kelly, R.Y.; Hua, T.A.; et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J. Am. Coll. Cardiol. 2010, 56, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, M. Adding spironolactone to conventional antihypertensives reduces albuminuria in patients with diabetic nephropathy. Nat. Clin. Pract. Nephrol. 2006, 2, 310–311. [Google Scholar] [CrossRef]
- Mehdi, U.F.; Adams-Huet, B.; Raskin, P.; Vega, G.L.; Toto, R.D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Tuomilehto, J.; Rastenyte, D.; Birkenhäger, W.H.; Thijs, L.; Antikainen, R.; Bulpitt, C.J.; Fletcher, A.E.; Forette, F.; Goldhaber, A.; Palatini, P.; et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N. Engl. J. Med. 1999, 340, 677–684. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 2012, 61, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Effect of calcium channel blockers on incidence of diabetes: A meta-analysis. Diabetes Metab. Syndr. Obes. 2013, 6, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Q. Association of Thiazide-Type Diuretics With Glycemic Changes in Hypertensive Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J. Clin. Hypertens. 2016, 18, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Dahlof, B.; Dobson, J.; Sever, P.S.; Wedel, H.; Poulter, N.R.; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008, 31, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.J.; Chang, H.C.; Ku, C.T.; Chen, H.Y. Hydrochlorothiazide hypertension treatment induced metabolic effects in type 2 diabetes: A meta-analysis of parallel-design RCTs. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2926–2934. [Google Scholar]
- Dahlöf, B.; Sever, P.S.; Poulter, N.R.; Wedel, H.; Beevers, D.G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet 2005, 366, 895–906. [Google Scholar] [PubMed]
- Bangalore, S.; Parkar, S.; Grossman, E.; Messerli, F.H. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am. J. Cardiol. 2007, 100, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Grosse, J.; Simonsen, U.; Infanger, M.; Bauer, J.; Grimm, D. The effects of newer beta-adrenoceptor antagonists on vascular function in cardiovascular disease. Curr. Vasc. Pharmacol. 2012, 10, 378–390. [Google Scholar] [CrossRef]
- Cheng, J.W. Nebivolol: A third-generation beta-blocker for hypertension. Clin. Ther. 2009, 31, 447–462. [Google Scholar] [CrossRef]
- Marketou, M.; Gupta, Y.; Jain, S.; Vardas, P. Differential Metabolic Effects of Beta-Blockers: An Updated Systematic Review of Nebivolol. Curr. Hypertens. Rep. 2017, 19, 22. [Google Scholar] [CrossRef]
- Fongemie, J.; Felix-Getzik, E. A Review of Nebivolol Pharmacology and Clinical Evidence. Drugs 2015, 75, 1349–1371. [Google Scholar] [CrossRef] [Green Version]
- Kumar Saini, A.; Wali, P.; Verma, M.; Chandra, S.; Singh, A.; Yadav, S.; Bansode, H.; Nischaya, K.; Saraswat, N. Nebivolol: An Appealing, Awaited and Nitric Oxide Potentiator drug for the Treatment of Heart Failure. J. Young Pharm. 2018, 10, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Abelardo, N.; Buranakitjaroen, P.; Krittayaphong, R.; Lim, C.H.; Park, S.H.; Pham, N.V.; Rogelio, G.; Wong, B.; Low, L.P. Hypertension treatment in the Asia-Pacific: The role of and treatment strategies with nebivolol. Heart Asia 2016, 8, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisker, F.Y.; Grimm, D.; Wehland, M. Third-generation beta-adrenoceptor antagonists in the treatment of hypertension and heart failure. Basic Clin. Pharmacol. Toxicol. 2015, 117, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakris, G.L.; Fonseca, V.; Katholi, R.E.; McGill, J.B.; Messerli, F.H.; Phillips, R.A.; Raskin, P.; Wright, J.T., Jr.; Oakes, R.; Lukas, M.A.; et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: A randomized controlled trial. JAMA 2004, 292, 2227–2236. [Google Scholar] [CrossRef] [Green Version]
- Elliott, W.J.; Meyer, P.M. Incident diabetes in clinical trials of antihypertensive drugs: A network meta-analysis. Lancet 2007, 369, 201–207, Erratum in Lancet 2007, 369, 1518. [Google Scholar] [CrossRef]
- Rico-Mesa, J.S.; White, A.; Ahmadian-Tehrani, A.; Anderson, A.S. Mineralocorticoid Receptor Antagonists: A Comprehensive Review of Finerenone. Curr. Cardiol. Rep. 2020, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Schloemer, P.; Tornus, I.; Joseph, A.; et al. Finerenone and Cardiovascular Outcomes in Patients With Chronic Kidney Disease and Type 2 Diabetes. Circulation 2021, 143, 540–552. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef]
- Duggan, S. Esaxerenone: First Global Approval. Drugs. 2019, 79, 477–481. [Google Scholar] [CrossRef]
- Wan, N.; Rahman, A.; Nishiyama, A. Esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker (MRB) in hypertension and chronic kidney disease. J. Hum. Hypertens. 2021, 35, 148–156. [Google Scholar] [CrossRef]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Iijima, S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens. Res. 2021, 44, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Berra, C.; Manfrini, R.; Regazzoli, D.; Radaelli, M.G.; Disoteo, O.; Sommese, C.; Fiorina, P.; Ambrosio, G.; Folli, F. Blood pressure control in type 2 diabetes mellitus with arterial hypertension. The important ancillary role of SGLT2-inhibitors and GLP1-receptor agonists. Pharmacol. Res. 2020, 160, 105052. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamasi, A.A.; Elkaffash, R.; Mohamed, M.; Rayan, M.; Al-Khater, D.; Gadeau, A.P.; Ahmed, R.; Hasan, A.; Eldassouki, H.; Yalcin, H.C.; et al. Crosstalk between Sodium-Glucose Cotransporter Inhibitors and Sodium-Hydrogen Exchanger 1 and 3 in Cardiometabolic Diseases. Int. J. Mol. Sci. 2021, 22, 12677. [Google Scholar] [CrossRef]
- Pessoa, T.D.; Campos, L.C.; Carraro-Lacroix, L.; Girardi, A.C.; Malnic, G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J. Am. Soc. Nephrol. 2014, 25, 2028–2039. [Google Scholar] [CrossRef] [Green Version]
- Wichaiyo, S.; Saengklub, N. Alterations of sodium-hydrogen exchanger 1 function in response to SGLT2 inhibitors: What is the evidence? Heart Fail. Rev. 2022. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Filippatos, G.S.; Jamal, W.; Salsali, A.; Schnee, J.; Kimura, K.; Zeller, C.; George, J.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: Rationale for and design of the EMPEROR-Reduced trial. Eur. J. Heart Fail. 2019, 21, 1270–1278. [Google Scholar] [PubMed] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heerspink, H.J.L.; Stefansson, B.V.; Chertow, G.M.; Correa-Rotter, R.; Greene, T.; Hou, F.F.; Lindberg, M.; McMurray, J.; Rossing, P.; Toto, R.; et al. DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol. Dial. Transplant. 2020, 35, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.I.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J.; et al. VERTIS CV Investigators. Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Hüser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef]
- Madsbad, S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2016, 18, 317–332. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furtado, R.H.M.; Bonaca, M.P.; Raz, I.; Zelniker, T.A.; Mosenzon, O.; Cahn, A.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Previous Myocardial Infarction. Circulation 2019, 139, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.F.; Vaduganathan, M.; Solomon, S.D.; McMurray, J.J.V. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years after PARADIGM-HF. JACC Heart Fail. 2020, 8, 800–810, Erratum in JACC Heart Fail. 2020, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- De Vecchis, R.; Ariano, C.; Soreca, S. Antihypertensive effect of sacubitril/valsartan: A meta-analysis. Minerva. Cardioangiol. 2019, 67, 214–222. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan: The PARADIGM-HF Trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C.; et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, J.P.; Solomon, S.D.; Seely, E.W. Potential mechanisms of beneficial effect of sacubitril/valsartan on glycemic control. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820970444. [Google Scholar] [CrossRef]
- Frampton, J.E. Finerenone: First Approval. Drugs 2021, 81, 1787–1794. [Google Scholar] [CrossRef]
- Elkinson, S.; Scott, L.J. Canagliflozin: First global approval. Drugs 2013, 73, 979–988. [Google Scholar] [CrossRef]
- Dhillon, S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, J.E. Empagliflozin: A Review in Type 2 Diabetes. Drugs 2018, 78, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Salmanowicz, M.; Banach, B. Możliwości wykorzystania analogów glukagonopodobnego peptydu-1 w różnych jednostkach chorobowych. Farm. Pol. 2021, 77, 48–55. [Google Scholar] [CrossRef]
- Eadie, A.L.; Brunt, K.R.; Herder, M. Exploring the Food and Drug Administration’s review and approval of Entresto (sacubitril/valsartan). Pharmacol. Res. Perspect. 2021, 9, e00794. [Google Scholar] [CrossRef] [PubMed]
| Systolic Blood Pressure | Diastolic Blood Pressure | |
|---|---|---|
| Empagliflozin 25 mg/d | −4.78 mmHg | −1.90 mmHg |
| Canagliflozin | −3.93 mmHg | −1.39 mmHg |
| Dapagliflozin | −2.70 mmHg | −0.70 mmHg |
| Exenatide | −1.57 mmHg | +0.25 mmHg |
| Liraglutide | −1.20 mmHg | +0.60 mmHg |
| Dulaglutide | −1.70 mmHg | +0.12 mmHg |
| Semaglutide | −2.60 mmHg | +0.14 mmHg |
| Name of Drug | Mode of Action | Dosage | Method and Route of Administration | Indications | Contraindications | Side Effects |
|---|---|---|---|---|---|---|
| Finerenone | non-steroidal MRA | 10–20 mg | Oral use once daily | Diabetic kidney disease Heart failure | Hyperkalaemia Kidney failure Addison disease | Increased level of serum potassium |
| Esaxerenone | non-steroidal MRA | 1.25–5 mg | Oral use once daily | Hypertension Diabetic nephropathies | Hyperkalaemia | Increased level of serum potassium Hyperuricemia |
| Canagliflozin | SGLT-2i | 100–300 mg | Oral use once daily | Type 2 diabetes | Kidney failure Ketoacidosis Hospitalization Hypotension | Hypoglycaemia Candidiasis Genito-urinary tract infection |
| Dapagliflozin | SGLT-2i | 5–10 mg | Oral use once daily | Type 2 diabetes Chronic heart failure | Kidney failure Hypotension Liver failure Ketoacidosis | Hypoglycaemia Dizziness Dysuria Genito-urinary tract infection |
| Empagliflozin | SGLT-2i | 10–25 mg | Oral use once daily | Type 2 diabetes Chronic heart failure | Ketoacidosis Kidney failure Liver failure | Hypoglycaemia Dehydration Genito-urinary tract infection |
| Exenatide | Short-acting GLP-1 analogue | 5–10 µg | Subcutaneous injection twice daily | Type 2 diabetes | Type 1 diabetes and ketoacidosis Allergy and anaphylaxis Pregnancy and breast feeding Kidney failure Gastroparesis | Nausea Vomiting |
| Lixisenatide | Short-acting GLP-1 analogue | 10–20 µg | Subcutaneous injection once daily | Type 2 diabetes | Pancreatitis Kidney failure Dehydration | Hypoglycaemia Nausea Vomiting Diarrhoea Headache |
| Dulaglutide | Long-acting GLP-1 analogue | 0.75–1.5 mg | Subcutaneous injection once weekly | Type 2 diabetes | Type 1 diabetes and ketoacidosis End-stage renal disease Dehydration Gastroparesis Acute pancreatitis | Hypoglycaemia Nausea Vomiting Diarrhoea Stomach ache |
| Long-acting exenatide | Long-acting GLP-1 analogue | 2 mg | Subcutaneous injection once weekly | Type 2 diabetes | Type 1 diabetes and ketoacidosis Allergy and anaphylaxis Pregnancy and breast feeding Kidney failure Gastroparesis | Nausea Vomiting |
| Liraglutide | Long-acting GLP-1 analogue | 0.6–1.8 mg | Subcutaneous injection once daily | Type 2 diabetes Obesity and overweight with additional metabolic disease | Congestive heart failure Pancreatitis Dehydration Thyroid diseases Gastroparesis | Nausea Vomiting Diarrhoea |
| Semaglutide | Long-acting GLP-1 analogue | (0.25–1.0 mg)/(3–14 mg) | Subcutaneous injection once weekly/oral use once daily | Type 2 diabetes | Congestive heart failure State after bariatric operation Acute pancreatitis | Hypoglycaemia Nausea Diarrhoea |
| Sacubitril/valsartan | ARB and neprilysin inhibitor | (24 mg/26 mg)-(97 mg/103 mg) | Oral use twice daily | Chronic heart failure with reduced ejection fraction | Kidney failure Hyperkalaemia Liver failure Allergy and anaphylaxis Hypotension | Hyperkalaemia Hypotension Kidney function disorder |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przezak, A.; Bielka, W.; Pawlik, A. Hypertension and Type 2 Diabetes—The Novel Treatment Possibilities. Int. J. Mol. Sci. 2022, 23, 6500. https://doi.org/10.3390/ijms23126500
Przezak A, Bielka W, Pawlik A. Hypertension and Type 2 Diabetes—The Novel Treatment Possibilities. International Journal of Molecular Sciences. 2022; 23(12):6500. https://doi.org/10.3390/ijms23126500
Chicago/Turabian StylePrzezak, Agnieszka, Weronika Bielka, and Andrzej Pawlik. 2022. "Hypertension and Type 2 Diabetes—The Novel Treatment Possibilities" International Journal of Molecular Sciences 23, no. 12: 6500. https://doi.org/10.3390/ijms23126500






