Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides
Abstract
:1. Introduction
2. Plant Responses to Drought, from Morphological to Physiological Levels
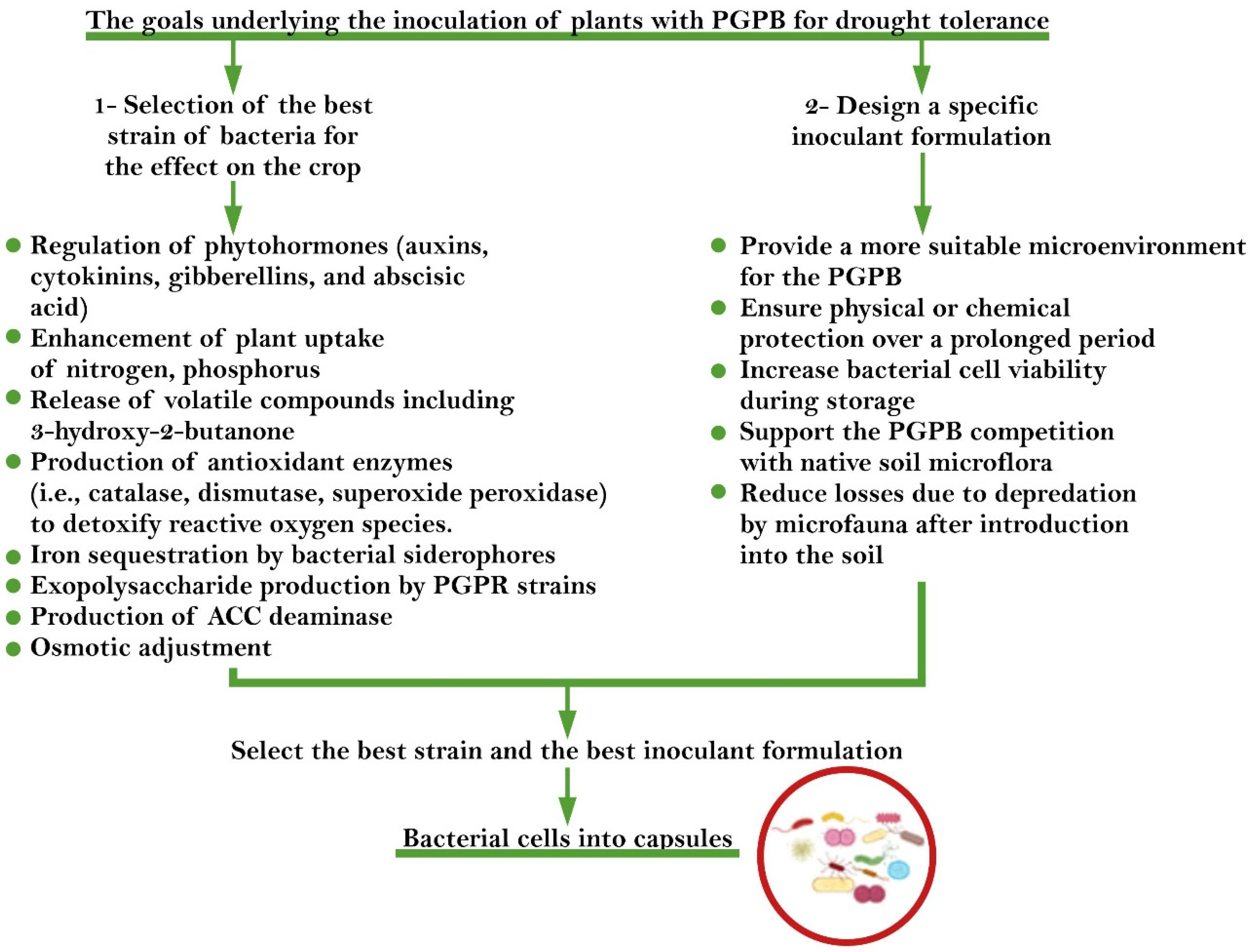
3. PGPB Mitigate the Adverse Effects of Drought on Plants
4. Encapsulation of PGPBs
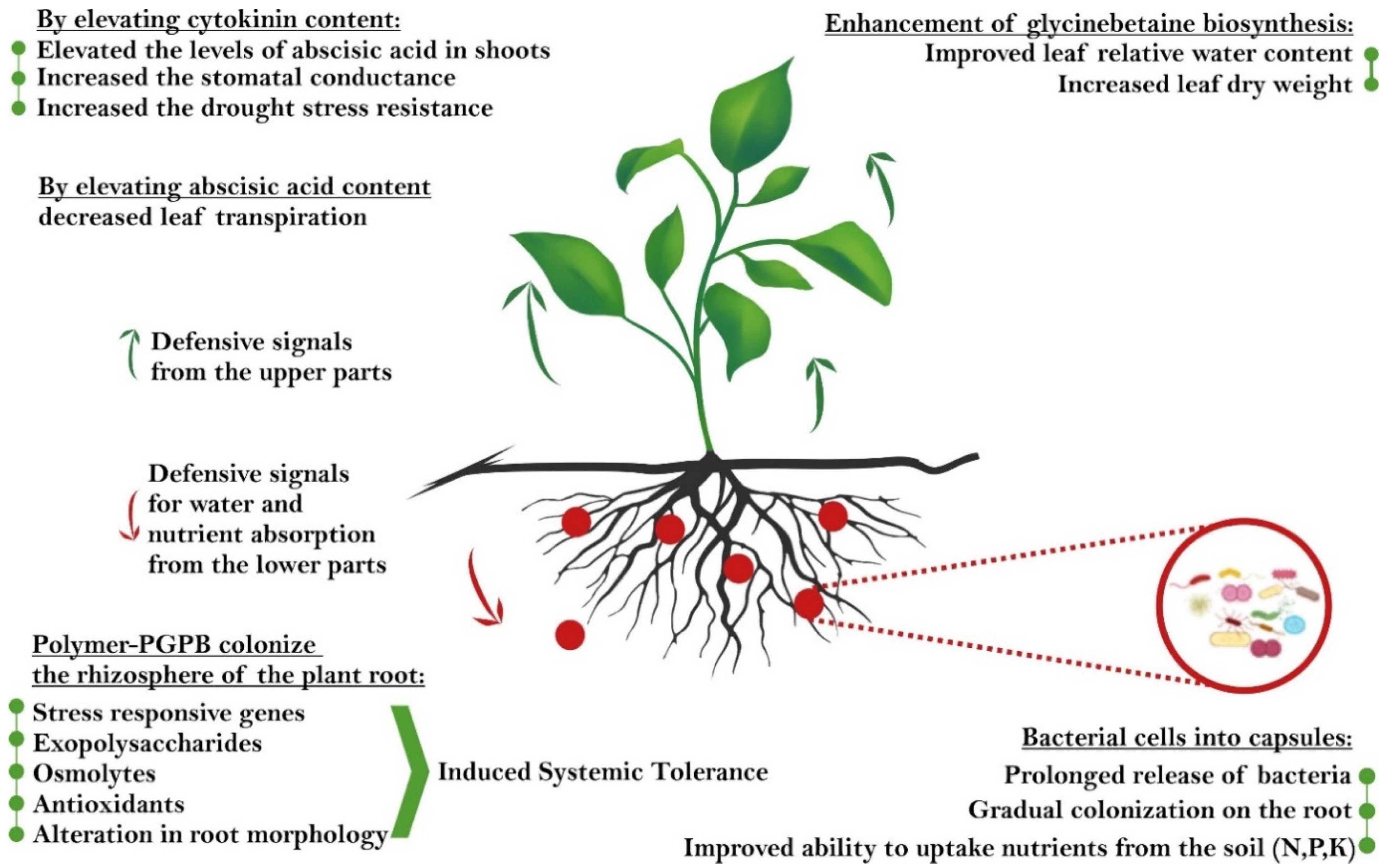
5. Enhancement of Drought Tolerance by Encapsulation of PGPBs
6. Polysaccharides for Encapsulation of PGPBs
6.1. Sodium Alginate
6.2. Chitosan
6.3. Other Polysaccharides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [Green Version]
- de Vries, F.T.; Shade, A. Controls on soil microbial community stability under climate change. Front. Microbiol. 2013, 4, 265. [Google Scholar] [CrossRef] [Green Version]
- Santos-Medellin, C.; Liechty, Z.; Edwards, J.; Nguyen, B.; Huang, B.; Weimer, B.C.; Sundaresan, V. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat. Plants 2021, 7, 1065–1077. [Google Scholar] [CrossRef]
- McHugh, T.A.; Compson, Z.; van Gestel, N.; Hayer, M.; Ballard, L.; Haverty, M.; Hines, J.; Irvine, N.; Krassner, D.; Lyons, T.; et al. Climate controls prokaryotic community composition in desert soils of the southwestern united states. FEMS Microbiol. Ecol. 2017, 93, 116. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K.; Berlemont, R.; Allison, S.D.; Martiny, A.C. Drought increases the frequencies of fungal functional genes related to carbon and nitrogen acquisition. PLoS ONE 2018, 13, e0206441. [Google Scholar] [CrossRef]
- Bouskill, N.J.; Wood, T.E.; Baran, R.; Hao, Z.; Ye, Z.; Bowen, B.P.; Lim, H.C.; Nico, P.S.; Holman, H.Y.; Gilbert, B.; et al. Belowground response to drought in a tropical forest soil. II. Change in microbial function impacts carbon composition. Front. Microbiol. 2016, 7, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Galván, A.E.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in guinea grass. Appl. Soil Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Jones, M.M.; Turner, N.C.; Osmond, C.B. Mechanisms of drought resistance. In Physiology and Biochemistry of Drought Resistance in Plants; Paleg, E.G., Aspinall, D., Eds.; Academic Press: Sydney, Australia, 1981; pp. 15–37. [Google Scholar]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, F1000 Faculty Rev-1554. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2017, 8, 2580. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.-P.; Leyva, L.A.; Bacilio, M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. N. Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. Aba transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [Green Version]
- Nonami, H. Plant water relations and control of cell elongation at low water potentials. J. Plant Res. 1998, 111, 373–382. [Google Scholar] [CrossRef]
- Meyer, R.F.; Boyer, J.S. Sensitivity of cell division and cell elongation to low water potentials in soybean hypocotyls. Planta 1972, 108, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Deak, K.I.; Malamy, J. Osmotic regulation of root system architecture. Plant J. 2005, 43, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The pin auxin efflux facilitator network controls growth and patterning in arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Sengupta, D.; Reddy, A.R. Water deficit as a regulatory switch for legume root responses. Plant Signal. Behav 2011, 6, 914–917. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Yamazaki, Y.; Kobayashi, A.; Higashitani, A.; Takahashi, H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of arabidopsis and radish. Plant Physiol. 2003, 132, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Plaut, Z. Plant exposure to water stress during specific growth stages. In Encyclopedia of Water Science; Stewart, B.A., Howell, T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 673–675. [Google Scholar]
- Singh, J.; Thakur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 27–46. [Google Scholar]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Zhou, Y.; Lam, H.M.; Zhang, J. Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J. Exp. Bot. 2007, 58, 1207–1217. [Google Scholar] [CrossRef]
- Garg, B. Nutrient uptake and management under drought: Nutrient-moisture interaction. Curr. Agric. 2003, 27, 1–8. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Samarah, N.; Mullen, R.; Cianzio, S. Size distribution and mineral nutrients of soybean seeds in response to drought stress. J. Plant Nutr. 2004, 27, 815–835. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Penuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Sili.icon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Shamim, F.; Johnson, G.N.; Saqlan, S.; Waheed, A. Higher antioxidant capacity protects photosynthetic activities as revealed by chl a fluorescence in drought tolerant tomato genotypes. Pak. J. Bot. 2013, 45, 1631–1642. [Google Scholar]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.; Alharby, H.; Bamagoos, A.; Kumar, N. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Jayant, K.S.; Sarangi, S.K. Effect of drought stress on proline accumulation in peanut genotypes. Int. J. Adv. Res. 2014, 2, 301–309. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Siddiqui, M.N.; Fujita, M.; Tran, L.P. Salicylic acid antagonizes selenium phytotoxicity in rice: Selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J. Hazard. Mater. 2020, 394, 122572. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, i: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia Ur Rehman, M.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Fahad, S.; Ullah, A.; Ali, U.; Ali, E.; Saud, S.; Hakeem, K.R.; Alharby, H.; Sabagh, A.E.; Barutcular, C.; Kamran, M. Drought tolerance in plantsrole of phytohormones and scavenging system of ros. In Plant Tolerance to Environmental Stress; Hasanuzzaman, M., Fujita, M., Oku, H., Tofazzal Islam, M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 103–114. [Google Scholar]
- Fang, L.; Su, L.; Sun, X.; Li, X.; Sun, M.; Karungo, S.K.; Fang, S.; Chu, J.; Li, S.; Xin, H. Expression of vitis amurensis nac26 in arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis. J. Exp. Bot. 2016, 67, 2829–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashan, Y.; de-Bashan, L.E. Chapter two—How the plant growth-promoting bacterium azospirillum promotes plant growth—a critical assessment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Sydney, Australia, 2010; Volume 108, pp. 77–136. [Google Scholar]
- de Brito, A.M.; Gagne, S.; Antoun, H. Effect of compost on rhizosphere microflora of the tomato and on the incidence of plant growth-promoting rhizobacteria. Appl. Env. Microbiol. 1995, 61, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Ghyselinck, J.; Velivelli, S.L.; Heylen, K.; O’Herlihy, E.; Franco, J.; Rojas, M.; De Vos, P.; Prestwich, B.D. Bioprospecting in potato fields in the central andean highlands: Screening of rhizobacteria for plant growth-promoting properties. Syst. Appl. Microbiol. 2013, 36, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Sen, S.; Mohapatra, S. Drought-mitigating pseudomonas putida gap-p45 modulates proline turnover and oxidative status in arabidopsis thaliana under water stress. Ann. Microbiol. 2018, 68, 579–594. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium azospirillum brasilense. N. Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Glick, B.R.; Bashan, Y.; Ryu, C.-M. Enhancement of plant drought tolerance by microbes. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 383–413. [Google Scholar]
- Mathur, P.; Roy, S. Insights into the plant responses to drought and decoding the potential of root associated microbiome for inducing drought tolerance. Physiol. Plant 2021, 172, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; Sk, Z.A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Gupta, A.; Mohapatra, S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in arabidopsis thaliana. World J. Microbiol. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Hassen, A.I.; Labuschagne, N. Rhizobacteria-induced systemic tolerance against drought stress in Sorghum bicolor (L.) moench. Microbiol. Res. 2020, 232, 126388. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; Enshasy, H.A.E.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M.; et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. During drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef]
- Prudent, M.; Salon, C.; Souleimanov, A.; Emery, R.J.N.; Smith, D.L. Soybean is less impacted by water stress using bradyrhizobium japonicum and thuricin-17 from bacillus thuringiensis. Agron. Sustain. Dev. 2015, 35, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Kohler, J.; Hernandez, J.A.; Caravaca, F.; Roldan, A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with burkholderia phytofirmans strain psjn. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Armada, E.; Leite, M.F.A.; Medina, A.; Azcon, R.; Kuramae, E.E. Native bacteria promote plant growth under drought stress condition without impacting the rhizomicrobiome. FEMS Microbiol. Ecol. 2018, 94, fiy092. [Google Scholar] [CrossRef] [Green Version]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by azospirillum sp. Under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin secreting rhizobacterium, pseudomonas putida h-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Ansary, M.H.; Rahmani, H.A.; Ardakani, M.R.; Paknejad, F.; Habibi, D.; Mafakheri, S. Effect of pseudomonas fluorescent on proline and phytohormonal status of maize (Zea mays L.) under water deficit stress. Ann. Biol. Res. 2012, 3, 1054–1062. [Google Scholar]
- Arshad, M.; Shaharoona, B.; Mahmood, T. Inoculation with pseudomonas spp. Containing acc-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The pgpr strain phyllobacterium brassicacearum stm196 induces a reproductive delay and physiological changes that result in improved drought tolerance in arabidopsis. N. Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Martinez, C.R.; Burity, H.A.; Chanway, C.P. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J. Microbiol. Biotechnol. 2008, 24, 1187–1193. [Google Scholar] [CrossRef]
- Sandhya, V.; Skz, A.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing pseudomonas putida strain gap-p45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Shintu, P.; Jayaram, K. Phosphate solubilising bacteria (Bacillus polymyxa)—An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill.). Trop. Plant Res. 2015, 2, 17–22. [Google Scholar]
- Schoebitz, M.; López Belchí, M.D. Encapsulation techniques for plant growth-promoting rhizobacteria. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 251–265. [Google Scholar]
- Kim, K.I.; Yoon, Y.H.; Baek, Y.J. Effects of rehydration media and immobilization in ca-alginate on the survival of lactobacillus casei and bifidobacterium bifidum. Korean J. Dairy Sci. 1996, 18, 193–198. [Google Scholar]
- Schoebitz, M.; Simonin, H.; Poncelet, D. Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J. Microencapsul. 2012, 29, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.; Schroth, M. Development of a powder formulation of rhizobacteria for inoculation of potato seed pieces. Phytopathology 1981, 71, 590–592. [Google Scholar] [CrossRef] [Green Version]
- Temprano, F.J.; Albareda, M.; Camacho, M.; Daza, A.; Santamaria, C.; Rodriguez-Navarro, D.N. Survival of several rhizobium/bradyrhizobium strains on different inoculant formulations and inoculated seeds. Int. Microbiol. 2002, 5, 81–86. [Google Scholar] [CrossRef]
- Dommergues, Y.R.; Diem, H.G.; Divies, C. Polyacrylamide-entrapped rhizobium as an inoculant for legumes. Appl. Environ. Microbiol. 1979, 37, 779–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. Biotechnol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Bashan, Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl. Environ. Microbiol. 1986, 51, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Young, C.C.; Rekha, P.D.; Lai, W.A.; Arun, A.B. Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnol. Bioeng. 2006, 95, 76–83. [Google Scholar] [CrossRef]
- Amiet-Charpentier, C.; Gadille, P.; Benoit, J.P. Rhizobacteria microencapsulation: Properties of microparticles obtained by spray-drying. J. Microencapsul. 1999, 16, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Denton, M.D.; Pearce, D.J.; Ballard, R.A.; Hannah, M.C.; Mutch, L.A.; Norng, S.; Slattery, J.F. A multi-site field evaluation of granular inoculants for legume nodulation. Soil Biol. Biochem. 2009, 41, 2508–2516. [Google Scholar] [CrossRef]
- Rose, M.T.; Deaker, R.; Potard, S.; Tran, C.K.T.; Vu, N.T.; Kennedy, I.R. The survival of plant growth promoting microorganisms in peat inoculant as measured by selective plate counting and enzyme-linked immunoassay. World J. Microbiol. Biotechnol. 2011, 27, 1649–1659. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Díaz-Zorita, M.; Fernández-Canigia, M.V. Field performance of a liquid formulation of azospirillum brasilense on dryland wheat productivity. Eur. J. Soil Biol. 2009, 45, 3–11. [Google Scholar] [CrossRef]
- Goss, G.R.; Baldwin, H.M.; Riepl, R.G. Clays As Biological Carriers; Downer, R.A., Mueninghoff, J.C., Volgas, G.C., Eds.; ASTM International: West Conshohocken, PA, USA, 2003; pp. 24–34. [Google Scholar]
- John, R.P.; Tyagi, R.; Brar, S.; Surampalli, R.; Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A. Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 C. J. Ind. Microbiol. Biotechnol. 2008, 35, 205–209. [Google Scholar] [CrossRef]
- Bashan, Y.; Gonzalez, L.E. Long-term survival of the plant-growth-promoting bacteria azospirillum brasilense and pseudomonas fluorescens in dry alginate inoculant. Appl. Microbiol. Biotechnol. 1999, 51, 262–266. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Heijnen, C.E.; Hok-A-Hin, C.H.; Van Veen, J.A. Improvements to the use of bentonite clay as a protective agent, increasing survival levels of bacteria introduced into soil. Soil Biol. Biochem. 1992, 24, 533–538. [Google Scholar] [CrossRef]
- Heijnen, C.E.; van Veen, J.A. A determination of protective microhabitats for bacteria introduced into soil. FEMS Microbiol. Lett. 1991, 85, 73–80. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R. Superior polymeric formulations and emerging innovative products of bacterial inoculants for sustainable agriculture and the environment. In Agriculturally Important Microorganisms: Commercialization and Regulatory Requirements in Asia; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; Springer: Singapore, 2016; pp. 15–46. [Google Scholar]
- Covarrubias, S.A.; de-Bashan, L.E.; Moreno, M.; Bashan, Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012, 93, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Souza-Alonso, P.; Rocha, M.; Rocha, I.; Ma, Y.; Freitas, H.; Oliveira, R.S. Encapsulation of pseudomonas libanensis in alginate beads to sustain bacterial viability and inoculation of vigna unguiculata under drought stress. 3 Biotech 2021, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, E.L.D.; Venkateswarlu, B.; Suseelendra, D.; Kumar, G.P.; Ahmed, S.K.M.H.; Meenakshi, T.; Sultana, U.; Pinisetty, S.; Narasu, L.M. Effect of polymeric additives, adjuvants, surfactants on survival, stability and plant growth promoting ability of liquid bioinoculants. J. Plant Physiol. Pathol. 2013, 1, 105. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Campaniello, D.; Bevilacqua, A.; Cataldi, M.P.; Sinigaglia, M.; Flagella, Z.; Corbo, M.R. Isolation, screening, and characterization of plant-growth-promoting bacteria from durum wheat rhizosphere to improve n and p nutrient use efficiency. Microorganisms 2019, 7, 541. [Google Scholar] [CrossRef] [Green Version]
- Diep, E.; Schiffman, J.D. Encapsulating bacteria in alginate-based electrospun nanofibers. Biomater. Sci. 2021, 9, 4364–4373. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.E.; Salama, D.M.; Morsi, S.M.M.; Youssef, A.M.; El-Sakhawy, M. Development of polymer composites and encapsulation technology for slow-release fertilizers. Rev. Chem. Eng. 2021, 2020, 44. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Labrador, J.; Romero-Perdomo, F.; Abril, J.; Hernández, J.-P.; Uribe-Vélez, D.; Buitrago, R.B. Bacillus strains immobilized in alginate macrobeads enhance drought stress adaptation of guinea grass. Rhizosphere 2021, 19, 100385. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia Del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusa, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of plant growth promoting rhizobacteria—prospects and potential in agricultural sector: A review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Rehim, H.A.; Hegazy, E.-S.A.; Ali, A.M.; Rabie, A.M. Synergistic effect of combining uv-sunlight-soil burial treatment on the biodegradation rate of ldpe/starch blends. J. Photochem. Photobiol. A Chem. 2004, 163, 547–556. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Wu, H.; Li, Y. Addition of modified bentonites in polymer gel formulation of 2,4-d for its controlled release in water and soil. J. Agric. Food Chem. 2009, 57, 2868–2874. [Google Scholar] [CrossRef]
- Woodhouse, J.; Johnson, M.S. Effect of superabsorbent polymers on survival and growth of crop seedlings. Agric. Water Manag. 1991, 20, 63–70. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug. Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Kumari, S.; Mahapatra, S.; Das, S. Ca-alginate as a support matrix for pb(ii) biosorption with immobilized biofilm associated extracellular polymeric substances of pseudomonas aeruginosa n6p6. Chem. Eng. J. 2017, 328, 556–566. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, L.A.; Federizzi, L.C.; Barbosa Neto, J.F. Correlation and path analysis of yield and its components and plant traits in wheat. Ciência Rural. 2004, 34, 1701–1708. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Gagne-Bourque, F.; Bertrand, A.; Claessens, A.; Aliferis, K.A.; Jabaji, S. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with bacillus subtilis b26. Front. Plant Sci. 2016, 7, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Castro, T.H.d.R.; Silva, E.F.d.; Rêgo, M.C.F.; Silva, G.B.d. Impacts of plant growth-promoting rhizobacteria on tropical forage grass in brazil. J. Agric. Stud. 2020, 8, 342–356. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: Effects on dry matter intake and milk yield of dairy cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Khan, N.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Induction of paenibacillus lentimorbus biofilm by sodium alginate and cacl2 alleviates drought stress in chickpea. Ann. Appl. Biol. 2011, 159, 372–386. [Google Scholar] [CrossRef]
- Fujishige, N.A.; Kapadia, N.N.; De Hoff, P.L.; Hirsch, A.M. Investigations of rhizobium biofilm formation. FEMS Microbiol. Ecol. 2006, 56, 195–206. [Google Scholar] [CrossRef] [Green Version]
- DasGupta, S.M.; Khan, N.; Nautiyal, C.S. Biologic control ability of plant growth-promoting paenibacillus lentimorbus nrrl b-30488 isolated from milk. Curr. Microbiol. 2006, 53, 502–505. [Google Scholar] [CrossRef]
- Kerchove, A.J.; Elimelech, M. Calcium and magnesium cations enhance the adhesion of motile and nonmotile pseudomonas aeruginosa on alginate films. Langmuir 2008, 24, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Andranovitš, S.; Skorik, Y.A. Chitosan and its derivatives: Vectors in gene therapy. Russ. Chem. Rev. 2017, 86, 231. [Google Scholar] [CrossRef]
- Ramírez, M.Á.; Rodríguez, A.T.; Alfonso, L.; Peniche, C. Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol. Apl. 2010, 27, 270–276. [Google Scholar]
- Chanratana, M.; Joe, M.M.; Roy Choudhury, A.; Anandham, R.; Krishnamoorthy, R.; Kim, K.; Jeon, S.; Choi, J.; Choi, J.; Sa, T. Physiological response of tomato plant to chitosan-immobilized aggregated methylobacterium oryzae cbmb20 inoculation under salinity stress. 3 Biotech 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-T.; Mau, J.-L. Selected physical properties of chitin prepared from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 558–563. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Corradini, E.; De Moura, M.; Mattoso, L. A preliminary study of the incorparation of npk fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Chanratana, M.; Han, G.H.; Melvin Joe, M.; Roy Choudhury, A.; Sundaram, S.; Halim, M.A.; Sa, T. Evaluation of chitosan and alginate immobilized methylobacterium oryzae cbmb20 on tomato plant growth. Arch. Agron. Soil Sci. 2018, 64, 1489–1502. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 2018, 3, 22–39. [Google Scholar]
- Priyaadharshini, M.; Sritharan, N.; Senthil, A.; Marimuthu, S. Physiological studies on effect of chitosan nanoemulsion in pearl millet under drought condition. J. Pharmacogn. Phytochem. 2019, 8, 3304–3307. [Google Scholar]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and nutrition of cowpea (Vigna unguiculata) under water deficit as influenced by microbial inoculation via seed coating. J. Agron. Crop. Sci. 2019, 205, 447–459. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Sci. Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Landis, T.D.; Haase, D.L. Applications of Hydrogels in the Nursery and during Outplanting. In National Proceedings: Forest and Conservation Nursery Associations; Haase, D.L., Pinto, J.R., Riley, L.E., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 53–58. [Google Scholar]
- Chain, J.M.; Tubert, E.; Graciano, C.; Castagno, L.N.; Recchi, M.; Pieckenstain, F.L.; Estrella, M.J.; Gudesblat, G.; Amodeo, G.; Baroli, I. Growth promotion and protection from drought in eucalyptus grandis seedlings inoculated with beneficial bacteria embedded in a superabsorbent polymer. Sci. Rep. 2020, 10, 18221. [Google Scholar] [CrossRef]

| PGPB | Host | Mechanism | Reference |
|---|---|---|---|
| Pseudomonas putida | Chickpea (Cicer arietinum) | osmolyte accumulation (proline, glycine betaine) and ROS scavenging | [68] |
| Bacillus thuringiensis | Soybean (Glycine max) | modification of root structures and increased root and nodule biomass, root length, and total nitrogen content | [69] |
| Pseudomonas mendocina | Lettuce (Lactuca sativa) | high antioxidant enzyme activity | [70] |
| Pseudomonas aeruginosa | Mung bean (Vigna radiata) | production of ROS; increased root length, shoot length, dry weight, relative water content; and upregulation of three drought stress-genes (dehydration-responsive element-binding protein, catalase, and dehydrin). | [71] |
| Burkholderia phytofirmans | Wheat (Triticum aestivum) | improved photosynthetic rate, water-use efficiency, chlorophyll content, nitrogen, phosphorus, potassium, and protein levels in the grains of wheat | [72] |
| Azospirillum lipoferum | Maize (Zea mays) | production of phytohormones, such as ABA and gibberellins | [73] |
| Bacillus thuringiensis | Autochthonous (species Thymus vulgaris, Santolina chamaecyparissus, and Lavandula dentata) | improved the ability to uptake nutrients, and increase the shoot length | [74] |
| Azospirillum sp. | Wheat (Triticum aestivum) | production of plant hormones IAA, increased root growth, and formation of lateral roots, and uptake of water and nutrients | [75] |
| Pseudomonas putida | Soybean (Glycine max) | increased plant growth and production gibberellins | [76] |
| Pseudomonas fluorescens | Maize (Zea mays) | increased leaf proline, ABA, auxin, gibberellin, and cytokinin. | [77] |
| Pseudomonas spp. | Pea (Pisum sativum) | better grain yield | [78] |
| Phyllobacterium brassicacearum | Arabidopsis thaliana | increased biomass, ABA content, higher water-use efficiency | [79] |
| Paenibacillus polymyxa and Rhizobium tropici | Bean (Phaseolus vulgaris) | increased plant growth, nitrogen content, and nodulation | [80] |
| Pseudomonas putida | Sunflower (Helianthus annuus) | increased plant biomass, adhesion of soil to roots, and formation of biofilm on the roots | [81] |
| Bacillus polymyxa | Tomato (Lycopersicon esculentum) | increased relative water content, chlorophyll, protein, proline accumulation, yield | [82] |
| Carriers | Advantages | Disadvantages | References |
|---|---|---|---|
| Peats | complex organic material with a high variability | decrease in cell concentration and adverse effects on the quality of the final product | [93,100] |
| Liquid inoculants | direct contact between seeds and microorganisms, increased survival of bacteria on roots | decrease in bacterial survival rates | [83,101] |
| Clays (as granules, suspensions, and powder) | storage for dried inoculants (large surface area, pore size distribution, and total porosity), increase the survival of rhizobia in the soil | inaccessible to predators | [83,102,103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Gholizadeh Vazvani, M.; Skorik, Y.A. Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. https://doi.org/10.3390/ijms222312979
Saberi Riseh R, Ebrahimi-Zarandi M, Gholizadeh Vazvani M, Skorik YA. Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. International Journal of Molecular Sciences. 2021; 22(23):12979. https://doi.org/10.3390/ijms222312979
Chicago/Turabian StyleSaberi Riseh, Roohallah, Marzieh Ebrahimi-Zarandi, Mozhgan Gholizadeh Vazvani, and Yury A. Skorik. 2021. "Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides" International Journal of Molecular Sciences 22, no. 23: 12979. https://doi.org/10.3390/ijms222312979
APA StyleSaberi Riseh, R., Ebrahimi-Zarandi, M., Gholizadeh Vazvani, M., & Skorik, Y. A. (2021). Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. International Journal of Molecular Sciences, 22(23), 12979. https://doi.org/10.3390/ijms222312979







