Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update
Abstract
:1. Introduction
1.1. Vitamin D Metabolism
1.2. Breast Cancer Disease and VD
2. Calcitriol in Combination with Chemo/Radiotherapy in BC
2.1. Enhancement of BC Responsiveness to Chemotherapeutic Agents and Radiation by Calcitriol
2.2. Mechanism of Action of Calcitriol and Its Analogs to Potentiate the Response to Ionizing Radiation in BC Cells
3. Combined Antitumoral Effect of Calcitriol with Natural Compounds in BC
3.1. Increased Antitumoral Effect of Calcitriol Combined with Resveratrol in BC
3.2. Combined Antitumoral Effect of Calcitriol and Curcumin in BC
3.3. Combination of Melatonin and Calcitriol in BC
3.4. Relationship between Genistein and VD Derivatives in BC
3.5. Synergistic Antitumoral Effects of Retinoids and Calcitriol in BC
3.6. Vitamin K3 (Menadione) Sensitizes BC Cells to the Growth Inhibitory Effects of Calcitriol
3.7. Combined Use of Calcitriol and Fatty Acid in BC
4. Calcitriol in Combination with Endocrine Therapy
4.1. Calcitriol or Its Analogs in Combination with AIs
4.2. Calcitriol and Resistance to Endocrine Therapy
5. Calcitriol in Combination with Histone Modifiers
6. Calcitriol in Combination with Kinase Inhibitors in BC
7. Calcitriol in Combination with Non-Steroidal Analgesic Drugs in BC
8. Calcitriol in Combination with Immunomodulatory Agents in BC
9. Calcitriol in Combination with Histamine Inhibitors in BC
10. In Vivo Preclinical and Clinical Studies with Calcitriol-Based Regimens in BC
| Preclinical In Vivo Models | |||||
|---|---|---|---|---|---|
| Drug | Model | Doses | Aim | Results | Ref. |
| Calcitriol/calcipotriol/alfacalcidol | Rat mammary cancer model induced by N-Methyl-nitrosourea | Intraperitoneal administration of 0.25 μg/kg and 1.25 μg/kg thrice weekly for 28 days of calcitriol, and administration of calcipotriol (50 μg/kg) in the same time | To evaluate the effects on calcium metabolism and mammary tumor growth in adult female rats, and compare the antitumoral effects of calcitriol and its analogs calcipotriol and alfacalcidol. | All VD3 metabolites inhibited tumor growth of mammary carcinoma. However, calcitriol and alfacalcidol at the doses tested provoked hypercalcemia | [214] |
| Calcitriol, anastrozole, and letrozole | Murine model (control and ovariectomized mice) | Anastrozole was administered at 5 µg and letrozole at 2.5 µg six days a week. Calcitriol was administered at 0.025, 0.05, and 0.1 µg doses three times a week. All substances were given intraperitoneally for four weeks. | To investigate whether calcitriol would enhance AIs activity in vivo to inhibit the growth of MCF-7 tumor xenografts. | All three concentrations of calcitriol tested exerted significant tumor inhibitory effects, and maximal inhibition was seen with the highest dose used (0.1 µg/mouse). Of note, the combined treatments caused higher inhibition of estrogen synthesis in the tumor microenvironment as reflected by estrogen levels measured in the tumors and surrounding mammary fat. Calcitriol decreased aromatase expression in various tissues. | [154] |
| VD3 and calcitriol | Murine model (control and ovariectomized mice) | Oral VD3 supplemented diet (5000 IU/kg) and injections of calcitriol 0.025, 0.05, or 0.1 μg/mouse, three times a week). | To investigate the beneficial effects dietary VD3 in comparison with injections of calcitriol using xenograft models of ER-positive BC. | Both treatments displayed similar effects in the inhibition of tumor growth in mice. Both calcitriol and dietary VD3 were equipotent in suppressing estrogen synthesis and signaling, and reduction of proinflammatory factors and growth signaling pathways. | [37] |
| EB1089 | Six-week-old ovariectomized female NCr-nu mice | EB1089 was administered in a daily subcutaneous injection (45 pmol EB1089 in propylene glycol/PBS, 4:1) or via implanted continuous release pellets delivering either 60 or 120 pmol of EB1089 per day. The total treatment lasted 4–5 weeks. | To determine the effects of calcitriol and EB1089 on the ER-negative, cell line SUM-159PT, in vitro. To determine whether EB1089 could modulate growth and/or apoptosis of ER-xenografts. | In mice implanted with EB1089 pellets, average tumor volume decreased gradually over the four weeks of treatment. The treatment with EB1089 decreased PCNA protein expression. Both forms of administration of EB1089 showed to reduce tumor growth; however, the data suggested that pellet delivery may minimize the calcemic side effects. | [216] |
| Calcitriol, PRI-2191, or PRI-2205 | Immune-competent BALB/c female mice | The analogs of calcitriol were administered subcutaneously thrice a week starting from day 7 after tumor cell inoculation. The single dose of compounds was as follows: calcitriol, 0.5 µg/kg; PRI-2191, 1.0 µg/kg; and PRI-2205, 10.0 µg/kg. | To investigate the effect of calcitriol and its analogs on the growth and metastasis of murine mammary cancer at various progression stages (days 14, 21, 28, and 33) | Treatment with calcitriol at initial stages showed moderate lung metastasis as compared with its analogs. Nevertheless, the treatment with calcitriol or both analogs resulted in the stimulation of lung metastases. The treatments did not alter antiangiogenic and angiogenic factors thrombospondin 1 (TSP-1) and VEGF, respectively. However, they positively affected the protein expression of OPN, TGF-β, serum levels of E2 and diminished the expression of VDR. Calcitriol or its analogs downregulated the expression of some genes encoding for growth factors. | [217] |
| Calcitriol + curcumin Calcitriol + resveratrol | TNBC xenografts performed in nude female mice | Calcitriol was intraperitoneally administrated 0.25 µg in 100 µL once a week. Curcumin was administered daily in the drinking water 40 mg/kg throughout the experiment. Resveratrol was given orally (1.2 g/kg) three times a week. All treatments alone or in combination were given for three weeks. | To determine the antiproliferative and antitumoral effect of the combination of calcitriol with two phytochemicals, curcumin or resveratrol. | In vitro: The combined treatment presented better antiproliferative properties than treatments alone In vivo: tumor onset, volume and micro-vessel density were significantly reduced in mice co-administered with calcitriol and curcumin Vessel count was also reduced in mice simultaneously treated with calcitriol and resveratrol The concomitant administration of calcitriol with curcumin or resveratrol synergistically promoted anticancer effects in vitro and in vivo in the human mammary tumor cell model. | [74] |
| Calcitriol alone or with dovitinib | Six-week-old female athymic female nude mice | Calcitriol was intraperitoneally administered 0.25 μg/100 μL each week. Dovitinib was intraperitoneally administered 20 mg/kg twice a week. | To evaluate whether an improved antineoplastic effect could be achieved in vitro and in vivo in TNBC by combining dovitinib, a multi-kinase inhibitor, with calcitriol. | In vitro and in vivo, the drug combination elicited a synergistically improved antiproliferative effect in TNBC-derived cells, which allowed a 7-fold dovitinib dose-reduction. | [22] |
| Clinical Trials | |||||
| Drugs | Clinical Trial | Doses | Aim | Results | Ref. |
| Calcitriol | Phase I (Patients with advanced malignancy) | 2 to 10 μg of calcitriol subcutaneously for 4 months. | To determine if a subcutaneous administration of calcitriol can achieve tolerable toxicity in order to ameliorate the hypercalcemia as a major side effect. | The subcutaneous administration led to three pharmacokinetic phases: the initial rapid absorption (Cpmax at two h) of calcitriol from s.c. tissues, a second phase in which plasma calcitriol remained constant for ~6 h, and a third phase starting 8 h after administration in which calcitriol plasma levels declined. The half-life of s.c. calcitriol administration was significantly longer than that reported after oral administration. This study demonstrated that s.c. calcitriol can be administered safely at doses up to 4–5-fold higher than the usual oral dose of 1.5–2.0 μg per day. The MTD for this trial was >5 times the 1.5 μg oral daily dose. No significant antitumor responses were demonstrated in this trial. | [218] |
| Calcitriol | Phase I trial patients with refractory malignancies | Four weeks of oral, weekly treatment of calcitriol from 0.06–2.8 μg/kg. | To determine the range of escalation doses of calcitriol administrated orally and to establish an ideal dose of it for future evaluations. | The dose of 0.5 microg/kg was selected for future evaluation in Phase II studies. | [219] |
| Calcitriol/Paclitaxel | Phase I | Calcitriol was given orally for three consecutive days each week at escalating doses, and paclitaxel (80 mg/m2) was given intravenously weekly. The starting dose of calcitriol was 4 μg for three consecutive days each week, and the maximum dose administered was 38 μg for three consecutive days each week. | To determine the MTDand pharmacokinetics of calcitriol when administered with paclitaxel in patients with advanced cancer. To evaluate the relationship between calcitriol dose and hypercalcemia. | Calcitriol plasma concentrations of 600 to 1440 pg/mL were achieved. No dose-limiting toxicity occurred in this trial. Despite variability in absorption, very high doses of calcitriol can be safely administered with paclitaxel. No dose-limiting hypercalcemia or other toxicity was observed in patients with cancer who received high doses of calcitriol plus paclitaxel | [220] |
| Calcitriol | Phase I: Patients were divided into two cohorts: (A) calcitriol + paclitaxel in patients with advanced solid tumors; (B) calcitriol ± dexamethasone in patients with androgen-independent prostate cancer | Oral administration of 12 μg to 21 μg/capsule of calcitriol were tested in 12 patients with advanced solid tumor, while doses from 13 μg to 36 μg of the liquid formulation of calcitriol were tested in 16 patients advanced solid tumor. Cohort A received calcitriol QDx3 (day l–3) + paclitaxel 80 mg/m2 on day 3; cohort B received calcitriol alone QDx3 on week 1, and in subsequent weeks, calcitriol QDx3 (days 1–3) and dexamethasone QDx4 (days 0–3). Treatment was continued until disease progression or occurrence of dose-limiting toxicity. Serum calcium, phosphorus, creatinine, BUN, albumin, and glucose were determined weekly. | To determine whether a liquid calcitriol formulation had a more favorable pharmacokinetic profile than a caplet formulation. | There were no differences in Cmax and AUC0–24h between the two formulations. The result of the use of calcitriol in capsule or liquid form was indistinct; however, at some point, the liquid formulation had the disadvantage of causing transient episodes of diarrhea. The use of dexamethasone is based on previous articles where it is shown that this agent decreases 1,25-D3-induced hypercalcemia and enhances 1,25-D3 antitumor activity. The combination with paclitaxel is based on the fact that no dose-limiting hypercalcemia or other toxicity was observed in patients with cancer who received calcitriol plus paclitaxel in a previous study. | [220,221] |
| High dose formulation of calcitriol (DN-101) | Patients with different adenocarcinomas including prostate, colon, rectum, gastric, squamous cell carcinoma) | Different oral, weekly doses of a high dose of a commercial presentation of calcitriol (DN-101) were given to patients with cancer (15, 30, 45, 60, 75, 90, 105, 135, 165, 210, 270, and 345 μg) | To establish a safe dose for weekly repeat dosing of DN-101. To compare the pharmacokinetic profile of DN-101 and rocaltrol. | Calcium and serum chemistry were monitored every two weeks. In general, DN-101 was very well tolerated on a weekly schedule. However, hypercalcemia was found at 60 μg. Thus, 45 μg is recommended as a safe dose for phase II studies in patients with different adenocarcionamas. Of note, this study did not include patients with BC. | [222] |
| Calcitriol/Gefitinib | Phase I | Calcitriol was given i.v. over 1 h on weeks 1, 3, and weekly after that. Gefitinib was given at a fixed oral daily dose of 250 mg starting at week 2 (day 8) | To evaluate MTD of this combination. | High doses of weekly i.v. calcitriol can be administered safely in combination with gefitinib. The MTD for calcitriol was 74 μg. The study design did not permit the evaluation of the effects of calcitriol on gefitinib. | [178] |
| Calcitriol/Gefitinib/Dexamethazone | Phase I | A fixed oral dose of dexamethasone of 4 mg/day was given. Calcitriol was administered i.v. over 1 h on weeks 1, 3, and weekly after that. The starting calcitriol dose level was 57 μg, and escalation occurred in cohorts of three patients until the MTD was defined. Gefitinib was given at a fixed oral daily dose of 250 mg starting at week 2 (day 8). | To determine the MTD of i.v. calcitriol administered in combination with a fixed oral dose of dexamethasone and gefitinib in patients with refractory solid tumors including, colorectal, head and neck, prostate, sarcoma, breast, stomach, non-small cell lung cancer, gastrointestinal stromal tumor and urachal. | The addition of a low dose of dexamethasone allowed the safe escalation of calcitriol to the MTD of 125 μg/week. However, no antitumor activity was observed in patients with different solid tumors. Of note, the study included only one patient with BC. | [179] |
| Alendronate and calcitriol | Double-blind, prospective, placebo-controlled 24-week trial with a daily combination of alendronate and calcitriol in Hormone- positive patients with early BC. | Daily, oral administration of Maxmarvil®® (5 mg of alendronate and 0.5 μg of calcitriol) for 24 weeks. | To determine whether a lower dosage of alendronate in oral form combined with calcitriol can effectively manage AI-induced bone loss. | The study demonstrated that a combination of 5 mg alendronate and 0.5 μg calcitriol is effective to prevent bone loss due to aromatase inhibitor regimen in post-menopausal women with early BC. | [230] |
| Calcitriol | Post-menopausal patients (33) with operable BC, without distant metastasis. | Oral administration of 0.50 μg/day (Rocaltrol). | To evaluate the antitumor effects of a short period of VD3 supplementation. | The blood analysis demonstrated that 87.5% of patients had a deficiency of calcitriol, as determined by calcidiol serum levels. Interestingly in paired samples collected before and after calcitriol supplementation, no differences were detected in calcidiol serum concentration. Data from pre- and post- calcitriol supplementation showed a modest reduction, around 35%, of Ki67 expression. Enriched molecular probes demonstrated that target genes of calcitriol were not modulated after the calcitriol supplementation. | [227] |
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 15-PGDH | 15-hydroxyprostaglandin dehydrogenase |
| AIs | Aromatase inhibitors |
| AKT | Protein kinase B |
| ALDH1 ATRA | Aldehyde dehydrogenase 1 All-trans-retinoic acid |
| BC | Breast cancer |
| Bcl-2 | B cell CLL/lymphoma-2 |
| BCSC | Breast cancer stem cells |
| CCND1 | Cyclin D1 |
| CD | Cluster of differentiation |
| CDK | Cyclin-dependent kinases |
| COX | Cyclooxygenase |
| CSCs DBP | Cancer stem cells Vitamin D binding protein |
| DNMT | DNA methyltransferase |
| EAG1 | Ether-a-go-go-1 potassium channel |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| EP | Prostaglandin E receptor |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinases |
| FP | Prostaglandin F receptor |
| HDAC | Histone deacetylases |
| HDACI | Histone deacetylase inhibitors |
| HER2 | Epidermal growth factor receptor type 2 |
| HERG | Human EAG related genes |
| i.v. | Intravenous |
| IGF-1 | Insulin-like growth factor 1 |
| IGFBP-3 | Insulin-like growth factor binding protein 3 |
| JAK | Janus kinase |
| MAPK | Mitogen-activated protein kinase |
| MDM2 | Murine double minute 2 |
| MMP | Metalloproteinase |
| MTD | Maximum tolerated dose |
| NFκβ | Nuclear factor-kappa beta |
| NMU | N-methylnitrosourea |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OVX | Ovariectomized |
| PARP | Poly (ADP-ribose) polymerase |
| PI3K | Phosphatidylinositol 3-kinase |
| PLA | Phospholipase A2 |
| PR | Progesterone receptor |
| QDR | Days a week |
| RARs | Retinoid acid receptors |
| RAREs | Retinoic-Acid-Response-Elements |
| ROS | Reactive oxygen species |
| RXR | Retinoid-X receptor |
| SERD | Selective estrogen receptor down- |
| SERM | Selective estrogen receptor modulator |
| STAT | Activator of transcription |
| TGF-β1 | Transforming growth factor-beta 1 |
| TIL | Tumor-infiltrating lymphocytes |
| TKI | Tyrosine kinase inhibitors |
| TNBC | Triple-negative breast cancer |
| TNFα | Tumor necrosis factor alpha |
| TSA | Trichostatin A |
| TSP-1 | Thrombospondin 1 |
| VD | Vitamin D |
| VD3 | Vitamin D3 |
| VDR | Vitamin D receptor |
| VDREs | Vitamin D response elements |
| VEGF | Vascular endothelial growth factor |
References
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.; Bishop, K.A. Regulation of target gene expression by the vitamin D receptor—An update on mechanisms. Rev. Endocr. Metab. Disord. 2012, 13, 45–55. [Google Scholar] [CrossRef]
- Larriba, M.J.; Valle, N.; Álvarez, S.; Muñoz, A. Vitamin D3 and Colorectal Cancer. In Hormonal Carcinogenesis V; Advances in Experimental Medicine and Biology Series; Springer: Berlin/Heidelberg, Germany, 2008; Volume 617, pp. 271–280. [Google Scholar] [CrossRef]
- Liu, M.; Lee, M.-H.; Cohen, M.; Bommakanti, M.; Freedman, L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996, 10, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.M.; Jones, J.B.; Studzinski, G.P. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996, 56, 264–267. [Google Scholar] [PubMed]
- McGuire, T.F.; Trump, D.L.; Johnson, C.S. Vitamin D3-induced Apoptosis of Murine Squamous Cell Carcinoma Cells: Selective induction of caspase-dependent mek cleavage and up-regulation of MEKK-1. J. Biol. Chem. 2001, 276, 26365–26373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.Y.; Mackay, A.G.; Colston, K.W. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J. Steroid Biochem. Mol. Biol. 1996, 58, 395–401. [Google Scholar] [CrossRef]
- Slater, S.J.; Kelly, M.B.; Taddeo, F.J.; Larkin, J.D.; Yeager, M.D.; McLane, J.A.; Ho, C.; Stubbs, C.D. Direct Activation of Protein Kinase C by 1α,25-Dihydroxyvitamin D3. J. Biol. Chem. 1995, 270, 6639–6643. [Google Scholar] [CrossRef] [Green Version]
- De Boland, A.R.; Morelli, S.; Boland, R. 1,25(OH)2-vitamin D3 signal transduction in chick myoblasts involves phosphatidylcholine hydrolysis. J. Biol. Chem. 1994, 269, 8675–8679. [Google Scholar] [CrossRef]
- De Boland, A.R.; Norman, A. Evidence for involvement of protein kinase C and cyclic adenosine 3′,5′ monophosphate-dependent protein kinase in the 1,25-dihydroxy-vitamin D3-mediated rapid stimulation of intestinal calcium transport, (transcaltachia). Endocrinology 1990, 127, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; De Pietro, S.; Andreini, B.; Bianchi, A.M.; Migliori, M.; Taccola, D.; Giovannini, L.; Tetta, C.; Palla, R. Calcitriol modulates in vivo and in vitro cytokine production: A role for intracellular calcium. Kidney Int. 1998, 54, 1463–1469. [Google Scholar] [CrossRef]
- Vuolo, L.; Faggiano, A.; Colao, A.A. Vitamin D and Cancer. Front. Endocrinol. 2012, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, J.A.; Akman, S.A.; Melin, S.A.; Case, L.D.; Schwartz, G.G. Oral paricalcitol (19-nor-1,25-dihydroxyvitamin D2) in women receiving chemotherapy for metastatic breast cancer: A feasibility trial. Cancer Biol. Ther. 2013, 14, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Gerstmeier, J.; Possmayer, A.L.; Bozkurt, S.; Hoffmann, M.E.; Dikic, I.; Herold-Mende, C.; Burger, M.C.; Münch, C.; Kögel, D.; Linder, B. Calcitriol Promotes Differentiation of Glioma Stem-Like Cells and Increases Their Susceptibility to Temozolomide. Cancers 2021, 13, 3577. [Google Scholar] [CrossRef]
- Audo, I.; Darjatmoko, S.R.; Schlamp, C.L.; Lokken, J.M.; Lindstrom, M.J.; Albert, D.M.; Nickells, R.W. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Investig. Opthalmology Vis. Sci. 2003, 44, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.V.; Feldman, D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: Implications for prostate cancer chemoprevention and treatment. Endocrine-Related Cancer 2010, 17, R19–R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, Y.M.; Hammam, O.A.; Ammar, R.A.; Mansour, M.T.; Elmazar, M.M. Crosstalk between aldehyde dehydrogenase-1 and chemoresistance in breast cancer: Insights into the role of vitamin D3. Life Sci. 2020, 253, 117733. [Google Scholar] [CrossRef]
- Yu, W.D.; Ma, Y.; Flynn, G.; Muindi, J.R.; Kong, R.X.; Trump, D.L.; Johnson, C.S. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 2010, 9, 3022–3029. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Mendoza, M.; Díaz, L.; González-González, M.E.; Martínez-Reza, I.; García-Quiroz, J.; Prado-Garcia, H.; Ibarra-Sánchez, M.J.; Esparza-López, J.; Larrea, F.; García-Becerra, R. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 148, 122–131. [Google Scholar] [CrossRef]
- García-Becerra, R.; Díaz, L.; Camacho, J.; Barrera, D.; Ordaz-Rosado, D.; Morales, A.; Ortiz, C.S.; Avila, E.; Bargallo, E.; Arrecillas, M.; et al. Calcitriol inhibits Ether-à go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp. Cell Res. 2010, 316, 433–442. [Google Scholar] [CrossRef]
- García-Quiroz, J.; Cárdenas-Ochoa, N.; García-Becerra, R.; Morales-Guadarrama, G.; Méndez-Pérez, E.A.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Segovia-Mendoza, M.; Prado-García, H.; Avila, E.; et al. Antitumoral effects of dovitinib in triple-negative breast cancer are synergized by calcitriol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2021, 214, 105979. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L. Calcitriol and cancer therapy: A missed opportunity. Bone Rep. 2018, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Wang, J.; Cui, Y.; Kato, F.; Shirato, H.; Ikeda, D.; Li, R. Identifying relations between imaging phenotypes and molecular subtypes of breast cancer: Model discovery and external validation. J. Magn. Reson. Imaging 2017, 46, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 2016, 35, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.; LaPorta, E.; Zinser, G.M.; Narvaez, C.J.; Welsh, J. Genomic vitamin D signaling in breast cancer: Insights from animal models and human cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J. Vitamin D and breast cancer: Insights from animal models. Am. J. Clin. Nutr. 2004, 80, 1721S–1724S. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Albertson, D.G.; Ylstra, B.; Segraves, R.; Collins, C.; Dairkee, S.; Kowbel, D.; Kuo, W.-L.; Gray, J.W.; Pinkel, D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat. Genet. 2000, 25, 144–146. [Google Scholar] [CrossRef]
- Zhalehjoo, N.; Shakiba, Y.; Panjehpour, M. Gene expression profiles of CYP24A1 and CYP27B1 in malignant and normal breast tissues. Mol. Med. Rep. 2017, 15, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Segersten, U.; Holm, P.K.; Björklund, P.; Hessman, O.; Nordgren, H.; Binderup, L.; Åkerström, G.; Hellman, P.; Westin, G. 25-Hydroxyvitamin D31α-hydroxylase expression in breast cancer and use of non-1α-hydroxylated vitamin D analogue. Breast Cancer Res. 2005, 7, R980–R986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisman, J.A.; Macintyre, I.; Martin, T.J.; Frampton, R.J.; King, R. Normal and malignant breast tissue is a target organ for 1, 25-(oh)2vitamin D3. Clin. Endocrinol. 1980, 13, 267–272. [Google Scholar] [CrossRef]
- Colston, K.; Berger, U.; Coombes, R. Possible role for vitamin D in controlling breast cancer cell proliferation. Lancet 1989, 333, 188–191. [Google Scholar] [CrossRef]
- Bortman, P.; Folgueira, M.; Katayama, M.; Snitcovsky, I.; Brentani, M. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on breast cells: A mini review. Braz. J. Med Biol. Res. 2002, 35, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.; Feldman, D. Dietary Vitamin D3 and 1,25-Dihydroxyvitamin D3 (Calcitriol) Exhibit Equivalent Anticancer Activity in Mouse Xenograft Models of Breast and Prostate Cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Paul, S.; Atalla, N.; Thomas, P.E.; Lin, X.; Yang, I.; Buckley, B.; Lu, G.; Zheng, X.; Lou, Y.-R.; et al. Gemini Vitamin D Analogues Inhibit Estrogen Receptor–Positive and Estrogen Receptor–Negative Mammary Tumorigenesis without Hypercalcemic Toxicity. Cancer Prev. Res. 2008, 1, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Axt-Fliedner, R.; Villena-Heinsen, C.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of Vitamin D-receptor (VDR) and Retinoid X-receptor α in Breast Cancer. J. Mol. Histol. 2002, 34, 35–40. [Google Scholar] [CrossRef]
- Bilani, N.; Elson, L.; Szuchan, C.; Elimimian, E.; Saleh, M.; Nahleh, Z. Newly-identified Pathways Relating Vitamin D to Carcinogenesis: A Review. In Vivo 2021, 35, 1345–1354. [Google Scholar] [CrossRef]
- Díaz, L.; Díaz-Muñoz, M.; García-Gaytán, A.C.; Méndez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Żmijewski, M.A.; Liu, Y.; Chen, J. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2020, 207, 112738. [Google Scholar] [CrossRef]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, J.; Loibl, S. Optimal Systemic Treatment for Early Triple-Negative Breast Cancer. Breast Care 2020, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Soljic, M.; Mrklic, I.; Tomic, S.; Omrcen, T.; Sutalo, N.; Bevanda, M.; Vrdoljak, E. Prognostic value of vitamin D receptor and insulin-like growth factor receptor 1 expression in triple-negative breast cancer. J. Clin. Pathol. 2018, 71, 34–39. [Google Scholar] [CrossRef]
- Turner, T.; Alzubi, M.A.; Harrell, J.C. Identification of synergistic drug combinations using breast cancer patient-derived xenografts. Sci. Rep. 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Albogami, S.M.; Asiri, Y.; Asiri, A.; Alnefaie, A.A.; Alnefaie, S. Effects of neoadjuvant therapies on genetic regulation of targeted pathways in ER+ primary ductal breast carcinoma: A meta-analysis of microarray datasets. Saudi Pharm. J. 2021, 29, 656–669. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dai, X.; Ji, J.; Liu, H.; Shi, G.; Yeung, S.-C.J. Nine-Year Median Follow-up of Cardiotoxicity and Efficacy of Trastuzumab Concurrently With Anthracycline-Based and Anthracycline-Free Neoadjuvant Chemotherapy in HER2-Positive Breast Cancer Patients. Clin. Breast Cancer 2021, in press. [Google Scholar] [CrossRef]
- Koshizuka, K.; Koike, M.; Asou, H.; Cho, S.K.; Stephen, T.; Rude, R.K.; Binderup, L.; Uskokovic, M.; Koeffler, H.P. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res. Treat. 1999, 53, 113–120. [Google Scholar] [CrossRef]
- Koshizuka, K.; Koike, M.; Kubota, T.; Said, J.; Binderup, L.; Koeffler, H.P. Novel vitamin D3 analog (CB1093) when combined with paclitaxel and cisplatin inhibit growth of MCF-7 human breast cancer cells in vivo. Int. J. Oncol. 1998, 13, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, W.; Uytingco, M.S.; Christakos, S.; Wieder, R. 1,25-Dihydroxyvitamin D3 and all-trans-retinoic acid sensitize breast cancer cells to chemotherapy-induced cell death. Cancer Res. 2000, 60, 2040–2048. [Google Scholar]
- Wilhelm, C.A.; Clor, Z.J.; Kelts, J.L. Effect of Vitamin D on Paclitaxel Efficacy in Triple-negative Breast Cancer Cell Lines. Anticancer. Res. 2018, 38, 5043–5048. [Google Scholar] [CrossRef] [PubMed]
- Klopotowska, D.; Matuszyk, J. VDR Agonists Increase Sensitivity of MCF-7 and BT-474 Breast Cancer Cells to 5 FU. Anticancer. Res. 2020, 40, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Dabiri, Y.; Almouhanna, F.; Blagojevic, B.; Theobald, J.; Büttner, M.; Wölfl, S. Activation of pro-survival metabolic networks by 1,25(OH)2D3 does not hamper the sensitivity of breast cancer cells to chemotherapeutics. Cancer Metab. 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Lang, T.; Huang, X.; Wang, G.; Lee, R.J.; Teng, L.; Yin, Q.; Lin, Y. Calcitriol-Loaded Dual-pH-Sensitive Micelle Counteracts Pro-Metastasis Effect of Paclitaxel in Triple-Negative Breast Cancer Therapy. Adv. Healthc. Mater. 2020, 9, e2000392. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, J.S.; Miele, L. Breast Cancer Stem Cells. Biomedicines 2018, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009, 13, 2236–2252. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Wahler, J.; So, J.Y.; Cheng, L.; Maehr, H.; Uskokovic, M.; Suh, N. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 148, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.L.; Wahler, J.; Lee, H.J.; Bak, M.J.; Das Gupta, S.; Maehr, H.; Suh, N. Vitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2017, 173, 122–129. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chem. Biol. Interactions 2020, 315, 108865. [Google Scholar] [CrossRef]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015, 14, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Khramtsov, A.I.; Khramtsova, G.F.; Tretiakova, M.; Huo, D.; Olopade, O.I.; Goss, K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010, 176, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Bristol, M.L.; Di, X.; Beckman, M.J.; Wilson, E.N.; Henderson, S.C.; Maiti, A.; Fan, Z.; Gewirtz, D.A. Dual functions of autophagy in the response of breast tumor cells to radiation: Cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D3. Autophagy 2012, 8, 739–753. [Google Scholar] [CrossRef] [Green Version]
- DeMasters, G.; Di, X.; Newsham, I.; Shiu, R.; Gewirtz, D.A. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol. Cancer Ther. 2006, 5, 2786–2797. [Google Scholar] [CrossRef] [Green Version]
- Wilson, E.N.; Bristol, M.L.; Di, X.; Maltese, W.A.; Koterba, K.; Beckman, M.J.; Gewirtz, D.A. A Switch Between Cytoprotective and Cytotoxic Autophagy in the Radiosensitization of Breast Tumor Cells by Chloroquine and Vitamin D. Horm. Cancer 2011, 2, 272–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gewirtz, D.A.; Hilliker, M.L.; Wilson, E.N. Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother. Oncol. 2009, 92, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Xu, Z.; Qian, P.; Sun, W.; Wang, X.; Jian, Z.; Xia, T.; Xu, Y.; Tang, J. RelB sustains endocrine resistant malignancy: An insight of noncanonical NF-kappaB pathway into breast Cancer progression. Cell Commun. Signal. 2020, 18, 128. [Google Scholar] [CrossRef]
- Mineva, N.D.; Wang, X.; Yang, S.; Ying, H.; Xiao, Z.-X.J.; Holick, M.F.; Sonenshein, G.E. Inhibition of RelB by 1,25-dihydroxyvitamin D3promotes sensitivity of breast cancer cells to radiation. J. Cell. Physiol. 2009, 220, 593–599. [Google Scholar] [CrossRef] [Green Version]
- DeMasters, G.A.; Gupta, M.S.; Jones, K.R.; Cabot, M.; Wang, H.; Gennings, C.; Park, M.; Bratland, Å.; Ree, A.H.; Gewirtz, D.A. Potentiation of cell killing by fractionated radiation and suppression of proliferative recovery in MCF-7 breast tumor cells by the Vitamin D3 analog EB 1089. J. Steroid Biochem. Mol. Biol. 2004, 92, 365–374. [Google Scholar] [CrossRef]
- Radityamurti, F.; Herdian, F.; Permata, T.B.M.; Handoko, H.; Kodrat, H.; Nuryadi, E.; Wibowo, H.; Gondhowiardjo, S.A. Vitamin D as Radiosensitizer: A Review in Cell Line. J. Pharm. Nutr. Sci. 2020, 10, 315–324. [Google Scholar] [CrossRef]
- García-Quiroz, J.; García-Becerra, R.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Morales-Guadarrama, G.; Cárdenas-Ochoa, N.; Segovia-Mendoza, M.; Prado-Garcia, H.; Ordaz-Rosado, D.; Avila, E.; et al. Synergistic Antitumorigenic Activity of Calcitriol with Curcumin or Resveratrol is Mediated by Angiogenesis Inhibition in Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, S.; Cucina, A.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Lisi, E.; Bizzarri, M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFbeta-1-dependent growth inhibition of breast cancer cells. J. Pineal Res. 2011, 50, 150–158. [Google Scholar] [PubMed]
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; DiNicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J. Pineal Res. 2014, 57, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Marchionatti, A.M.; Picotto, G.; Narvaez, C.J.; Welsh, J.; de Talamoni, N.G.T. Antiproliferative action of menadione and 1,25(OH)2D3 on breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009, 113, 227–232. [Google Scholar] [CrossRef]
- Guizzardi, S.; Picotto, G.; Rodriguez, V.; Welsh, J.; Narvaez, C.; Bohl, L.; De Talamoni, N.T. Combined treatment of menadione and calcitriol increases the antiproliferative effect by promoting oxidative/nitrosative stress, mitochondrial dysfunction, and autophagy in breast cancer MCF-7 cells. Can. J. Physiol. Pharmacol. 2020, 98, 548–556. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, S.; Lin, G.; Song, C.; He, Z. Vitamin D enhances omega-3 polyunsaturated fatty acids-induced apoptosis in breast cancer cells. Cell Biol. Int. 2017, 41, 890–897. [Google Scholar] [CrossRef]
- Thill, M.; Cordes, T.; Hoellen, F.; Becker, S.; Dittmer, C.; Kümmel, S.; Salehin, D.; Friedrich, M.; Diedrich, K.; Köster, F. Influence of calcitriol on prostaglandin- and vitamin D-metabolising enzymes in benign and malignant breast cell lines. Anticancer. Res. 2012, 32, 359–365. [Google Scholar]
- Thill, M.; Reichert, K.; Woeste, A.; Polack, S.; Fischer, D.; Hoellen, F.; Rody, A.; Friedrich, M.; Köster, F. Combined treatment of breast cancer cell lines with vitamin D and COX-2 inhibitors. Anticancer. Res. 2015, 35, 1189–1195. [Google Scholar]
- Saunders, D.E.; Christensen, C.; Williams, J.R.; Wappler, N.L.; Lawrence, W.D.; Malone, J.M.; Malviya, V.K.; Deppe, G. Inhibition of breast and ovarian carcinoma cell growth by 1,25-dihydroxyvitamin D3 combined with retinoic acid or dexamethasone. Anti-Cancer Drugs 1995, 6, 562–569. [Google Scholar] [CrossRef]
- Garcia-Quiroz, J.; García-Becerra, R.; Barrera, D.; Santos, N.; Avila, E.; Ordaz-Rosado, D.; Rivas-Suárez, M.; Halhali, A.; Rodríguez, P.; Gamboa-Domínguez, A.; et al. Astemizole Synergizes Calcitriol Antiproliferative Activity by Inhibiting CYP24A1 and Upregulating VDR: A Novel Approach for Breast Cancer Therapy. PLoS ONE 2012, 7, e45063. [Google Scholar] [CrossRef] [PubMed]
- Sharabani, H.; Izumchenko, E.; Wang, Q.; Kreinin, R.; Steiner, M.; Barvish, Z.; Kafka, M.; Sharoni, Y.; Levy, J.; Uskokovic, M.; et al. Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int. J. Cancer 2006, 118, 3012–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.-H.; Chen, J.-J.; An, H.-P. Enhancement of differentiation induction of HL-60 cells by 1,25-dihydroxyvitamin D3 in combination with carnosic acid. Zhongguo Dang Dai Er Ke Za Zhi 2008, 10, 55–59. [Google Scholar]
- Steiner, M.; Priel, I.; Giat, J.; Levy, J.; Sharoni, Y.; Danilenko, M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr. Cancer 2001, 41, 135–144. [Google Scholar] [CrossRef]
- Nachliely, M.; Sharony, E.; Bolla, N.R.; Kutner, A.; Danilenko, M. Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2016, 17, 1068. [Google Scholar] [CrossRef] [Green Version]
- Clément, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Cancer Chemopreventive Activity of Resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Choi, B.T.; Lee, Y.T.; Park, D.I.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol Rep. 2004, 11, 441–446. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Serrero, G. and R. Lu, Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid. Redox Signal 2001, 3, 969–979. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H.; Way, T.D.; Chen, W.J. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar] [CrossRef]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Shen, M. Resveratrol and Its Analogs: Potent Agents to Reverse Epithelial-to-Mesenchymal Transition in Tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef]
- Shi, X.-P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.-F.; Ma, H.-Z.; Xin, H.-L.; Feng, J.; Wen, A.-D.; Li, Y. Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features. Int. J. Mol. Sci. 2013, 14, 15655–15668. [Google Scholar] [CrossRef]
- Wietzke, J.A.; Welsh, J. Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1,25-dihydroxyvitamin D3 actions in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2003, 84, 149–157. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Cui, R.; Lin, J.; Ding, X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016, 21, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear Factor-κB–Regulated Gene Products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [Green Version]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, A.P.N.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin Synergizes with Resveratrol to Inhibit Colon Cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; De Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Poeggeler, B.; Reiter, R.J.; Tan, D.-X.; Chen, L.-D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, M.D.; Sanchez-Barcelo, E.J.; Tan, D.X.; Manchester, L.; Reiter, R.J. Basic Mechanisms Involved in the Anti-Cancer Effects of Melatonin. Curr. Med. Chem. 2010, 17, 4462–4481. [Google Scholar] [CrossRef] [PubMed]
- Cucina, A.; Proietti, S.; D’Anselmi, F.; Coluccia, P.; Dinicola, S.; Frati, L.; Bizzarri, M. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J. Pineal Res. 2009, 46, 172–180. [Google Scholar] [CrossRef]
- Veiga, E.C.D.A.; Simões, R.; Valenti, V.E.; Cipolla-Neto, J.; Abreu, L.; Barros, E.P.M.; Sorpreso, I.C.E.; Baracat, M.C.P.; Baracat, E.C.; Junior, J.M.S. Repercussions of melatonin on the risk of breast cancer: A systematic review and meta-analysis. Rev. Assoc. Méd. Bras. (1992) 2019, 65, 699–705. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, G.; Barnes, S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 1993, 22, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, F.; Darbon, J.-M. p21CIP1 Is Dispensable for the G2 Arrest Caused by Genistein in Human Melanoma Cells. Exp. Cell Res. 2000, 258, 101–108. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Marui, N.; Sakai, T.; Satomi, Y.; Yoshida, M.; Matsumoto, K.; Nishino, H.; Aoike, A. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993, 53, 1328–1331. [Google Scholar]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, P.; Fan, S.; Wang, H.; Mao, J.; Shi, Y.; Ibrahim, M.M.; Ma, W.; Yu, X.; Hou, Z.; Wang, B.; et al. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res. Ther. 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakla, M.S.; Shenouda, N.S.; Ansell, P.J.; MacDonald, R.S.; Lubahn, D.B. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine 2007, 32, 69–78. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-alpha (ERalpha) by genistein enhances hormonal therapy sensitivity in ERalpha-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Lechner, D.; Bajna, E.; Adlercreutz, H.; Cross, H.S. Genistein and 17beta-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer. Res. 2006, 26, 2597–2603. [Google Scholar]
- Wang, Y.R.; Wigington, D.P.; Strugnell, S.A.; Knutson, J.C. Growth inhibition of cancer cells by an active metabolite of a novel vitamin D prodrug. Anticancer. Res. 2005, 25, 4333–4339. [Google Scholar]
- Siddikuzzaman; Guruvayoorappan, C.; Grace, V.B. All Trans Retinoic Acid and Cancer. Immunopharmacol. Immunotoxicol. 2011, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Garattini, E.; Bolis, M.; Garattini, S.K.; Fratelli, M.; Centritto, F.; Paroni, G.; Gianni’, M.; Zanetti, A.; Pagani, A.; Fisher, J.N.; et al. Retinoids and breast cancer: From basic studies to the clinic and back again. Cancer Treat. Rev. 2014, 40, 739–749. [Google Scholar] [CrossRef]
- Di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Aspects Med. 2015, 41, 1–115. [Google Scholar]
- Okuno, M.; Kojima, S.; Matsushima-Nishiwaki, R.; Tsurumi, H.; Muto, Y.; Friedman, S.; Moriwaki, H. Retinoids in Cancer Chemoprevention. Curr. Cancer Drug Targets 2004, 4, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.L. Retinoids—“Differentiation agents” for cancer treatment and prevention. Am. J. Med. Sci. 1992, 304, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ye, Y.; Chen, S.; Chai, J.; Lu, J.; Zhoa, L.; Gu, L.; Wang, Z. Use of All-Trans Retinoic Acid in the Treatment of Acute Promyelocytic Leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Koga, M.; Sutherland, R.L. Retinoic acid acts synergistically with 1,25-dihydroxyvitamin D3 or antioestrogen to inhibit T-47D human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 1991, 39, 455–460. [Google Scholar] [CrossRef]
- Wang, Q.; Lee, D.; Sysounthone, V.; Chandraratna, R.A.; Christakos, S.; Korah, R.; Wieder, R. 1,25-dihydroxyvitamin D3 and retonic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Res. Treat. 2001, 67, 157–168. [Google Scholar] [CrossRef]
- Lamson, D.W.; Plaza, S.M. The anticancer effects of vitamin K. Altern. Med. Rev 2003, 8, 303–318. [Google Scholar]
- Yamada, A.; Osada, S.; Tanahashi, T.; Matsui, S.; Sasaki, Y.; Tanaka, Y.; Okumura, N.; Matsuhashi, N.; Takahashi, T.; Yamaguchi, K.; et al. Novel therapy for locally advanced triple-negative breast cancer. Int. J. Oncol. 2015, 47, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Ali, S.M.; Zheng, G.; Chen, A.; Dontaraju, V.S.; Bosland, M.C.; Kajdacsy-Balla, A.; Munirathinam, G. Vitamin K and its analogs: Potential avenues for prostate cancer management. Oncotarget 2017, 8, 57782–57799. [Google Scholar] [CrossRef] [Green Version]
- Bohl, L.; Guizzardi, S.; Rodríguez, V.; Hinrichsen, L.; Rozados, V.; Cremonezzi, D.; de Talamoni, N.T.; Picotto, G. Combined calcitriol and menadione reduces experimental murine triple negative breast tumor. Biomed. Pharmacother. 2017, 94, 21–26. [Google Scholar] [CrossRef]
- Borrie, A.; Kim, R.B. Molecular basis of aromatase inhibitor associated arthralgia: Known and potential candidate genes and associated biomarkers. Expert Opin. Drug Metab. Toxicol. 2016, 13, 149–156. [Google Scholar] [CrossRef]
- Reinbolt, R.E.; Mangini, N.; Hill, J.L.; Levine, L.B.; Dempsey, J.L.; Singaravelu, J.; Koehler, K.A.; Talley, A.; Lustberg, M.B. Endocrine Therapy in Breast Cancer: The Neoadjuvant, Adjuvant, and Metastatic Approach. Semin. Oncol. Nurs. 2015, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K. Tamoxifen in the Treatment of Breast Cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef]
- Mirkin, S.; Pickar, J.H. Selective estrogen receptor modulators (SERMs): A review of clinical data. Maturitas 2015, 80, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; O’Malley, B.W. Coregulator Function: A Key to Understanding Tissue Specificity of Selective Receptor Modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: An oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer 2004, 90 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Jones, S.E. Fulvestrant: An estrogen receptor antagonist that downregulates the estrogen receptor. Semin. Oncol. 2003, 30 (Suppl. S16), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, T.V.-V.; Pols, H.A.; Buurman, C.J.; Birkenhäger, J.C.; Van Leeuwen, J.P. Combined effects of 1,25-dihydroxyvitamin D3 and tamoxifen on the growth of MCF-7 and ZR-75-1 human breast cancer cells. Breast Cancer Res. Treat. 1994, 29, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem. Cell Biol. 1994, 72, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, T.V.-V.; Pols, H.A.; Buurman, C.J.; Bemd, G.J.V.D.; Dorssers, L.C.; Birkenhäger, J.C.; Van Leeuwen, J.P. Inhibition of breast cancer cell growth by combined treatment with vitamin D3 analogues and tamoxifen. Cancer Res. 1994, 54, 5711–5717. [Google Scholar]
- Escaleira, M.T.F.; Sonohara, S.; Brentani, M.M. Sex steroids induced up-regulation of 1,25-(OH)2 vitamin D3 receptors in T 47D breast cancer cells. J. Steroid Biochem. Mol. Biol. 1993, 45, 257–263. [Google Scholar] [CrossRef]
- James, S.Y.; Mackay, A.G.; Binderup, L.; Colston, K.W. Effects of a new synthetic vitamin D analogue, EB1089, on the oestrogen-responsive growth of human breast cancer cells. J. Endocrinol. 1994, 141, 555–563. [Google Scholar] [CrossRef]
- Abe, J.; Nakano, T.; Nishii, Y.; Matsumoto, T.; Ogata, E.; Ikeda, K. A Novel Vitamin D 3 Analog, 22-Oxa-1, 25- Dihydroxyvitamin D3, Inhibits the Growth of Human Breast Cancer in Vitro and in Vivo without Causing Hypercalcemia. Endocrinology 1991, 129, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Abe-Hashimoto, J.; Kikuchi, T.; Matsumoto, T.; Nishii, Y.; Ogata, E.; Ikeda, K. Antitumor effect of 22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic mice implanted with human breast carcinoma and its synergism with tamoxifen. Cancer Res. 1993, 53, 2534–2537. [Google Scholar] [PubMed]
- Anzano, M.A.; Smith, J.M.; Uskoković, M.R.; Peer, C.W.; Mullen, L.T.; Letterio, J.J.; Welsh, M.C.; Shrader, M.W.; Logsdon, D.L.; Driver, C.L. 1 alpha,25-Dihydroxy-16-ene-23-yne-26,27-hexafluorocholecalciferol (Ro24-5531), a new deltanoid (vitamin D analogue) for prevention of breast cancer in the rat. Cancer Res. 1994, 54, 1653–1656. [Google Scholar]
- Mathis, K.M.; Sturgeon, K.M.; Winkels, R.M.; Wiskemann, J.; De Souza, M.J.; Schmitz, K.H. Bone resorption and bone metastasis risk. Med. Hypotheses 2018, 118, 36–41. [Google Scholar] [CrossRef]
- Wijngaarden, T.V.-V.; Birkenhäger, J.C.; Kleinekoort, W.M.; Bemd, G.J.V.D.; Pols, H.A.; Van Leeuwen, J.P. Antiestrogens inhibit in vitro bone resorption stimulated by 1,25-dihydroxyvitamin D3 and the vitamin D3 analogs EB1089 and KH1060. Endocrinology 1995, 136, 812–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.; Miller, W.R. Role of aromatase inhibitors in breast cancer. Br. J. Cancer 2005, 93 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Harbeck, N.; Jeschke, U.; Doisneau-Sixou, S. Influence of vitamin D signaling on hormone receptor status and HER2 expression in breast cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1107–1122. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J. Steroid Biochem. Mol. Biol. 2010, 121, 343–348. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-Selective Regulation of Aromatase Expression by Calcitriol: Implications for Breast Cancer Therapy. Endocrinology 2010, 151, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, J.; Hansen, S.K.; Lykkesfeldt, A.E. Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter—A new regulatory pathway for aromatase. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1833, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Peng, L.; Albertelli, M.; Feldman, D. Inhibitory Effects of Calcitriol on the Growth of MCF-7 Breast Cancer Xenografts in Nude Mice: Selective Modulation of Aromatase Expression in vivo. Horm. Cancer 2011, 2, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Filip-Psurska, B.; Psurski, M.; Anisiewicz, A.; Libako, P.; Zbrojewicz, E.; Maciejewska, M.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Vitamin D Compounds PRI-2191 and PRI-2205 Enhance Anastrozole Activity in Human Breast Cancer Models. Int. J. Mol. Sci. 2021, 22, 2781. [Google Scholar] [CrossRef]
- García-Becerra, R.; Santos, N.; Díaz, L.; Camacho, J. Mechanisms of Resistance to Endocrine Therapy in Breast Cancer: Focus on Signaling Pathways, miRNAs and Genetically Based Resistance. Int. J. Mol. Sci. 2012, 14, 108–145. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.S.; Heiberg, I.; Lykkesfeldt, A.E. Anti-oestrogen resistant human breast cancer cell lines are more sensitive towards treatment with the vitamin D analogue EB1089 than parent MCF-7 cells. Br. J. Cancer 2001, 84, 686–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, E.; Donepudi, M.; VanWeelden, K.; Flanagan, L.; Welsh, J. Dissociation of vitamin D3 and anti-estrogen mediated growth regulation in MCF-7 breast cancer cells. Mol. Cell Biochem. 1998, 188, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.L.; Jepsen, J.; Fog, C.; Christensen, I.; Lykkesfeldt, A. Sequential Versus Combined Treatment of Human Breast Cancer Cells with Antiestrogens and the Vitamin D Analogue EB1089 and Evaluation of Predictive Markers for Vitamin D Treatment. Breast Cancer Res. Treat. 2004, 85, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Yde, C.W.; Lykkesfeldt, A.E. 1α,25-dihydroxyvitamin D3 inhibits cell growth and NFκB signaling in tamoxifen-resistant breast cancer cells. Steroids 2014, 85, 30–35. [Google Scholar] [CrossRef]
- Simboli-Campbell, M.; Narvaez, C.J.; VanWeelden, K.; Tenniswood, M.; Welsh, J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res. Treat. 1997, 42, 31–41. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Feldman, D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin. Cancer Res. 2000, 6, 3371–3379. [Google Scholar]
- Swami, S.; Krishnan, A.V.; Peng, L.; Lundqvist, J.; Feldman, D. Transrepression of the estrogen receptor promoter by calcitriol in human breast cancer cells via two negative vitamin D response elements. Endocr. Relat. Cancer 2013, 20, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Stoica, A.; Saceda, M.; Fakhro, A.; Solomon, H.B.; Fenster, B.D.; Martin, M.B. Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J. Cell. Biochem. 1999, 75, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Santos-Martínez, N.; Díaz, L.; Ordaz-Rosado, D.; García-Quiroz, J.; Barrera, D.; Avila, E.; Halhali, A.; Medina-Franco, H.; Ibarra-Sánchez, M.J.; Esparza-López, J.; et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: A potential new therapeutic approach. BMC Cancer 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Martínez, N.; Díaz, L.; Ortiz-Ortega, V.M.; Ordaz-Rosado, D.; Prado-Garcia, H.; Avila, E.; Larrea, F.; García-Becerra, R. Calcitriol induces estrogen receptor α expression through direct transcriptional regulation and epigenetic modifications in estrogen receptor-negative breast cancer cells. Am. J. Cancer Res. 2021, 11, 1–14. [Google Scholar]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, M. DNA methylation in cancer: Too much, but also too little. Oncogene 2002, 21, 5400–5413. [Google Scholar] [CrossRef] [Green Version]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar]
- Brooke, S. Effects of Histone Deacetylase Inhibitors on Vitamin D Activity in Human Breast Cancer Cells. Master’s Thesis, Universtity of Massachusetts, Amherst, MA, USA, 2013; pp. 1–66. [Google Scholar]
- Santen, R.J.; Song, R.X.; McPherson, R.; Kumar, R.; Adam, L.; Jeng, M.-H.; Yue, W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 239–256. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Mendoza, M.; Díaz, L.; Prado-Garcia, H.; Reginato, M.J.; Larrea, F.; García-Becerra, R. The addition of calcitriol or its synthetic analog EB1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am. J. Cancer Res. 2017, 7, 1486–1500. [Google Scholar]
- Koga, M.; Eisman, J.A.; Sutherland, R.L. Regulation of epidermal growth factor receptor levels by 1,25-dihydroxyvitamin D3 in human breast cancer cells. Cancer Res. 1988, 48, 2734–2739. [Google Scholar]
- McGaffin, K.R.; Chrysogelos, S.A. Identification and characterization of a response element in the EGFR promoter that mediates transcriptional repression by 1,25-dihydroxyvitamin D3 in breast cancer cells. J. Mol. Endocrinol. 2005, 35, 117–133. [Google Scholar] [CrossRef]
- Cordes, T.; Diesing, D.; Becker, S.; Diedrich, K.; Reichrath, J.; Friedrich, M. Modulation of MAPK ERK1 and ERK2 in VDR-positive and -negative breast cancer cell lines. Anticancer. Res. 2006, 26, 2749–2753. [Google Scholar]
- Fakih, M.G.; Trump, D.L.; Muindi, J.R.; Black, J.D.; Bernardi, R.J.; Creaven, P.J.; Schwartz, J.; Brattain, M.G.; Hutson, A.; French, R.; et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of Intravenous Calcitriol in Combination with Oral Gefitinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Muindi, J.R.; Johnson, C.S.; Trump, D.L.; Christy, R.; Engler, K.L.; Fakih, M.G. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009, 65, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.T.; Jeon, Y.W.; Gwak, H.; Kim, S.Y.; Suh, Y.J. Synergistic anticancer effects of ruxolitinib and calcitriol in estrogen receptor positive, human epidermal growth factor receptor 2 positive breast cancer cells. Mol. Med. Rep. 2018, 17, 5581–5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maj, E.; Filip-Psurska, B.; Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D derivatives potentiate the anticancer and anti-angiogenic activity of tyrosine kinase inhibitors in combination with cytostatic drugs in an A549 non-small cell lung cancer model. Int. J. Oncol. 2018, 52, 337–366. [Google Scholar] [PubMed]
- García Rodríguez, L.A.; Huerta-Alvarez, C. Reduced Incidence of Colorectal Adenoma among Long-Term Users of Nonsteroidal Antiinflammatory Drugs: A Pooled Analysis of Published Studies and a New Population-Based Study. Epidemiology 2000, 11, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Harris, R.E. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): Results of a case-control study. Oncol. Rep. 2000, 7, 169–170. [Google Scholar] [CrossRef]
- Zha, S.; Yegnasubramanian, V.; Nelson, W.G.; Isaacs, W.B.; De Marzo, A.M. Cyclooxygenases in cancer: Progress and perspective. Cancer Lett. 2004, 215, 1–20. [Google Scholar] [CrossRef]
- Hoellen, F.; Kelling, K.; Dittmer, C.; Diedrich, K.; Friedrich, M.; Thill, M. Impact of cyclooxygenase-2 in breast cancer. Anticancer Res. 2011, 31, 4359–4367. [Google Scholar] [PubMed]
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.V.; Feldman, D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef] [Green Version]
- Karmali, R.A.; Welt, S.; Thaler, H.T.; Lefevre, F. Prostaglandins in breast cancer: Relationship to disease stage and hormone status. Br. J. Cancer 1983, 48, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Bennett, A.; Stamford, I.F.; Berstock, D.A.; Dische, F.; Singh, L.; A’Hern, R.P. Breast cancer, prostaglandins and patient survival. Br. J. Cancer 1989, 59, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Bennett, A.; Berstock, D.A.; Carroll, M.A.; Stamford, I.F.; Wilson, A.J. Breast cancer, its recurrence, and patient survival in relation to tumor prostaglandins. Adv. Prostaglandin Thromboxane Leukot. Res. 1983, 12, 299–302. [Google Scholar] [PubMed]
- Friedrich, M.; Reichert, K.; Woeste, A.; Polack, S.; Fischer, D.; Hoellen, F.; Rody, A.; Koster, F.; Thill, M. Effects of Combined Treatment with Vitamin D and COX2 Inhibitors on Breast Cancer Cell Lines. Anticancer Res. 2018, 38, 1201–1207. [Google Scholar]
- Cordes, T.; Hoellen, F.; Dittmer, C.; Salehin, D.; Kümmel, S.; Friedrich, M.; Köster, F.; Becker, S.; Diedrich, K.; Thill, M. Correlation of prostaglandin metabolizing enzymes and serum PGE2 levels with vitamin D receptor and serum 25(OH)2D3 levels in breast and ovarian cancer. Anticancer. Res. 2012, 32, 351–357. [Google Scholar]
- Thill, M.; Becker, S.; Fischer, D.; Cordes, T.; Hoellen, F.; Friedrich, M.; Diedrich, K.; Dittmer, C. Is the combination of COX-2 inhibitor and calcitriol a new chemopreventive approach to decrease the incidence of breast cancer? J. Clin. Oncol. 2011, 29, e11103. [Google Scholar] [CrossRef]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-Dihydroxyvitamin D 3 Inhibits Angiogenesis In Vitro and In Vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Trump, D.L.; Potter, D.M.; Muindi, J.; Brufsky, A.; Johnson, C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006, 106, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-S.; Choi, K.-C.; Jeung, E.-B. Glucocorticoids differentially regulate expression of duodenal and renal calbindin-D9k through glucocorticoid receptor-mediated pathway in mouse model. Am. J. Physiol. Metab. 2006, 290, E299–E307. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.A.; Deeb, K.K.; Pike, J.W.; Johnson, C.S.; Trump, D.L. Dexamethasone Enhances 1α,25-Dihydroxyvitamin D3 Effects by Increasing Vitamin D Receptor Transcription. J. Biol. Chem. 2011, 286, 36228–36237. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.A.; Trump, D.L.; Johnson, C.S. Glucocorticoid regulation of the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2010, 121, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.J.; Smaletz, Ò.; Solit, D.; Kelly, W.K.; Slovin, S.; Flombaum, C.; Curley, T.; DeLaCruz, A.; Schwartz, L.; Fleisher, M.; et al. High-dose calcitriol, zoledronate, and dexamethasone for the treatment of progressive prostate carcinoma. Cancer 2004, 100, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; Von Essen, M.R. The vitamin d receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [Green Version]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listì, A.; Maragliano, R.; Vincenzi, B.; Calò, V.; Iovanna, J.L.; Bazan, V.; et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell. Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef]
- Karkeni, E.; Morin, S.O.; Tayeh, B.B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Papaioannou, G.; Zhao, H.; Luderer, H.F.; Miller, C.; Dall’Osso, C.; Nazarian, R.M.; Wagers, A.J.; DeMay, M.B. The Vitamin D Receptor Regulates Tissue Resident Macrophage Response to Injury. Endocrinology 2016, 157, 4066–4075. [Google Scholar] [CrossRef] [Green Version]
- Yip, K.H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.; Anderson, P.; Samuel, M.; et al. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364. [Google Scholar] [CrossRef] [Green Version]
- Weeres, M.A.; Robien, K.; Ahn, Y.-O.; Neulen, M.-L.; Bergerson, R.; Miller, J.S.; Verneris, M.R. The Effects of 1,25-Dihydroxyvitamin D3on In Vitro Human NK Cell Development from Hematopoietic Stem Cells. J. Immunol. 2014, 193, 3456–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersanelli, M.; Vaglio, A.; Sverzellati, N.; Galetti, M.; Incerti, M.; Parziale, R.; Corrado, M.; Cosenza, A.; Ferri, L.; Leonardi, F.; et al. Potential role of hypovitaminosis D in renal cell carcinoma patients treated with immune-checkpoint inhibitors. J. Clin. Oncol. 2017, 35, 50. [Google Scholar] [CrossRef]
- Pirianov, G.; Colston, K.W. Interactions of vitamin D analogue CB1093, TNFalpha and ceramide on breast cancer cell apoptosis. Mol. Cell. Endocrinol. 2001, 172, 69–78. [Google Scholar] [CrossRef]
- Martínez-Reza, I.; Díaz, L.; García-Becerra, R. Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. J. Biomed. Sci. 2017, 24, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustino-Rocha, A.I.; Ferreira, R.; Gama, A.; Oliveira, P.A.; Ginja, M. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017, 172, 27–41. [Google Scholar] [CrossRef]
- Ramírez, A.; García-Quiroz, J.; Aguilar-Eslava, L.; Sánchez-Pérez, Y.; Camacho, J. Novel Therapeutic Approaches of Ion Channels and Transporters in Cancer. Rev. Physiol. Biochem. Pharmacol. 2020, 1–57. [Google Scholar] [CrossRef]
- García-Quiroz, J.; García-Becerra, R.; Santos-Martínez, N.; Barrera, D.; Ordaz-Rosado, D.; Avila, E.; Halhali, A.; Villanueva, O.; Ibarra-Sánchez, M.J.; Esparza-López, J.; et al. In vivo dual targeting of the oncogenic Ether-à-go-go-1 potassium channel by calcitriol and astemizole results in enhanced antineoplastic effects in breast tumors. BMC Cancer 2014, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Colston, K.W.; Chander, S.K.; Mackay, A.G.; Coombes, R. Effects of synthetic vitamin d analogues on breast cancer cell proliferation in vivo and in vitro. Biochem. Pharmacol. 1992, 44, 693–702. [Google Scholar] [CrossRef]
- VanWeelden, K.; Flanagan, L.; Binderup, L.; Tenniswood, M.; Welsh, J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology 1998, 139, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Packman, K.; Juba, B.; O’Neill, S.; Tenniswood, M.; Welsh, J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J. Steroid Biochem. Mol. Biol. 2003, 84, 181–192. [Google Scholar] [CrossRef]
- Anisiewicz, A.; Pawlik, A.; Filip-Psurska, B.; Turlej, E.; Dzimira, S.; Milczarek, M.; Gdesz, K.; Papiernik, D.; Jarosz, J.; Klopotowska, D.; et al. Unfavorable effect of calcitriol and its low-calcemic analogs on metastasis of 4T1 mouse mammary gland cancer. Int. J. Oncol. 2018, 52, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Johnson, C.S.; Freeman, C.C.; Muindi, J.; Wilson, J.W.; Trump, D.L. A Phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin. Cancer Res. 1999, 5, 1339–1345. [Google Scholar] [PubMed]
- Beer, T.M.; Munar, M.; Henner, W.D. A Phase I trial of pulse calcitriol in patients with refractory malignancies: Pulse dosing permits substantial dose escalation. Cancer 2001, 91, 2431–2439. [Google Scholar] [CrossRef]
- Muindi, J.; Peng, Y.; Potter, U.M.; Hershberger, P.A.; Tauch, J.S.; Capozzoli, M.J.; Egorin, M.J.; Johnson, C.S.; Trump, D.L. Pharmacokinetics of high-dose oral calcitriol: Results from a phase 1 trial of calcitriol and paclitaxel. Clin. Pharmacol. Ther. 2002, 72, 648–659. [Google Scholar] [CrossRef]
- Muindi, J.R.; Potter, D.M.; Peng, Y.; Johnson, C.S.; Trump, D.L. Pharmacokinetics of liquid calcitriol formulation in advanced solid tumor patients: Comparison with caplet formulation. Cancer Chemother. Pharmacol. 2005, 56, 492–496. [Google Scholar] [CrossRef]
- Beer, T.M.; Javle, M.M.; Ryan, C.W.; Garzotto, M.; Lam, G.N.; Wong, A.; Henner, W.D.; Johnson, C.S.; Trump, D.L. Phase I study of weekly DN-101, a new formulation of calcitriol, in patients with cancer. Cancer Chemother. Pharmacol. 2007, 59, 581–587. [Google Scholar] [CrossRef]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Atoum, M.; Alzoughool, F. Vitamin D and Breast Cancer: Latest Evidence and Future Steps. Breast Cancer: Basic Clin. Res. 2017, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mohr, S.B.; Gorham, E.D.; Alcaraz, J.E.; Kane, C.J.; Macera, C.A.; Parsons, J.K.; Wingard, D.L.; Garland, C.F. Serum 25-hydroxyvitamin D and prevention of breast cancer: Pooled analysis. Anticancer. Res. 2011, 31, 2939–2948. [Google Scholar] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.; Giovannucci, E.L.; Lipkin, M.; Newmark, H.; Holick, M.; Garland, F.C. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.N.; de Lyra, E.C.; Katayama, M.L.H.; Basso, R.A.; de Assis, P.E.Z.; Cardoso, A.P.T.; Roela, R.A.; Nonogaki, S.; Góes, J.C.G.S.; Brentani, M.M.; et al. Calcitriol supplementation effects on Ki67 expression and transcriptional profile of breast cancer specimens from post-menopausal patients. Clin. Nutr. 2014, 33, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Calcium Plus Vitamin D Supplementation Is Not Associated with a Reduced Breast Cancer Risk. JNCI J. Natl. Cancer Inst. 2008, 100, 1561. [CrossRef] [Green Version]
- Chukir, T.; Liu, Y.; Hoffman, K.; Bilezikian, J.; Farooki, A. Calcitriol Elevation Is Associated with a Higher Risk of Refractory Hypercalcemia of Malignancy in Solid Tumors. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Rhee, Y.; Song, K.; Park, S.; Park, H.S.; Lim, S.K.; Park, B.W. Efficacy of a combined alendronate and calcitriol agent (Maxmarvil(R)) in Korean postmenopausal women with early breast cancer receiving aromatase inhibitor: A double-blind, randomized, placebo-controlled study. Endocr. J. 2013, 60, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.A.; Serene, M.; Durling, F.C.; Weilbaecher, K. Aromatase inhibitors and bone loss. Oncology 2006, 20, 1029–1048. [Google Scholar]
- Love, R.R.; Mazess, R.B.; Barden, H.S.; Epstein, S.; Newcomb, P.A.; Jordan, V.C.; Carbone, P.P.; DeMets, D.L. Effects of Tamoxifen on Bone Mineral Density in Postmenopausal Women with Breast Cancer. N. Engl. J. Med. 1992, 326, 852–856. [Google Scholar] [CrossRef]
- Zidan, J.; Keidar, Z.; Basher, W.; Israel, O. Effects of Tamoxifen on Bone Mineral Density and Metabolism in Postmenopausal Women with Early-Stage Breast Cancer. Med. Oncol. 2004, 21, 117–122. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the Prevention of Breast Cancer: Current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Vehmanen, L.; Elomaa, I.; Blomqvist, C.; Saarto, T. Tamoxifen Treatment After Adjuvant Chemotherapy Has Opposite Effects on Bone Mineral Density in Premenopausal Patients Depending on Menstrual Status. J. Clin. Oncol. 2006, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocana, A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastell, R.; Adams, J.E.; Coleman, R.E.; Howell, A.; Hannon, R.A.; Cuzick, J.; Mackey, J.R.; Beckmann, M.W.; Clack, G. Effect of Anastrozole on Bone Mineral Density: 5-Year Results From the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J. Clin. Oncol. 2008, 26, 1051–1057. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, K.; Cho, Y.; Han, S.; Kim, H.; Lim, H. Disease Progression Modeling Analysis of the Change of Bone Mineral Density by Postoperative Hormone Therapies in Postmenopausal Patients With Early Breast Cancer. J. Clin. Pharmacol. 2019, 59, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Body, J.-J.; Aapro, M.S.; Brufsky, A.; Coleman, R.E.; Guise, T.; Lipton, A.; Tubiana-Hulin, M. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann. Oncol. 2008, 19, 1407–1416. [Google Scholar] [CrossRef]
- Drocourt, L.; Ourlin, J.-C.; Pascussi, J.M.; Maurel, P.; Vilarem, M.-J. Expression of CYP3A4, CYP2B6, andCYP2C9 Is Regulated by the Vitamin D Receptor Pathway in Primary Human Hepatocytes. J. Biol. Chem. 2002, 277, 25125–25132. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.J.; Ennis, M.; Pritchard, K.I.; Townsley, C.; Warr, D.; Elser, C.; Amir, E.; Bedard, P.L.; Rao, L.; Stambolic, V.; et al. Association between BMI, vitamin D, and estrogen levels in postmenopausal women using adjuvant letrozole: A prospective study. NPJ Breast Cancer 2020, 6, 22. [Google Scholar] [CrossRef]
- Teft, W.A.; Gong, I.Y.; Dingle, B.; Potvin, K.; Younus, J.; Vandenberg, T.A.; Brackstone, M.; Perera, F.E.; Choi, Y.-H.; Zou, G.; et al. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res. Treat. 2013, 139, 95–105. [Google Scholar] [CrossRef]
- Antunes, M.V.; Timm, T.A.D.F.; de Oliveira, V.; Staudt, D.E.; Raymundo, S.; Gössling, G.; Biazús, J.V.; Cavalheiro, J.A.; Rosa, D.D.; Wallemacq, P.; et al. Influence of CYP2D6 and CYP3A4 Phenotypes, Drug Interactions, and Vitamin D Status on Tamoxifen Biotransformation. Ther. Drug Monit. 2015, 37, 733–744. [Google Scholar] [CrossRef]
- Kim, H.J.; Koh, B.S.; Yu, J.H.; Lee, J.W.; Son, B.H.; Kim, S.B.; Ahn, S.H. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur. J. Cancer 2014, 50, 1403–1411. [Google Scholar] [CrossRef]
- VanderWalde, A.; Hurria, A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J. Clin. 2011, 61, 139–156. [Google Scholar] [CrossRef]
- Khan, Q.J.; O’Dea, A.P.; Sharma, P. Musculoskeletal Adverse Events Associated with Adjuvant Aromatase Inhibitors. J. Oncol. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2009, 119, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altundag, K. Combined use of vitamin D and omega-3 fatty acid in breast cancer patients might be more beneficial for reducing aromatase inhibitors-associated arthralgia. J. Balk. Union Oncol. 2019, 24, 862. [Google Scholar]
- Grossmann, M.; Ramchand, S.K.; Milat, F.; Vincent, A.; Lim, E.; Kotowicz, M.A.; Hicks, J.; Teede, H. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: Position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone & Mineral Society, the Australasian Menopause Society and the Clinical Oncology Society of Australia. Clin. Endocrinol. 2018, 89, 280–296. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Janghorban, R.; Alipour, S.; Tahmasebi, S.; Jokar, A. The effect of vitamin D and E vaginal suppositories on tamoxifen-induced vaginal atrophy in women with breast cancer. Support. Care Cancer 2019, 27, 1325–1334. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, J.; Huang, X.; Thabane, L.; Li, G. Effect of Vitamin D Supplementation on Risk of Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2021, 8, 655727. [Google Scholar] [CrossRef]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Bersanelli, M.; Leonetti, A.; Buti, S. The link between calcitriol and anticancer immunotherapy: Vitamin D as the possible balance between inflammation and autoimmunity in the immune-checkpoint blockade. Immunotherapy 2017, 9, 1127–1131. [Google Scholar] [CrossRef] [Green Version]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goins, B.; Phillips, W.T.; Bao, A. Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opin. Drug Deliv. 2016, 13, 873–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonor, J.C.; Schaefer, R.J.; Menegazzo, N.; Booksh, K.; Nohe, A.G. Design of 1,25 dihydroxyvitamin D3 coupled quantum dots, a novel imaging tool. J. Nanosci. Nanotechnol. 2012, 12, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
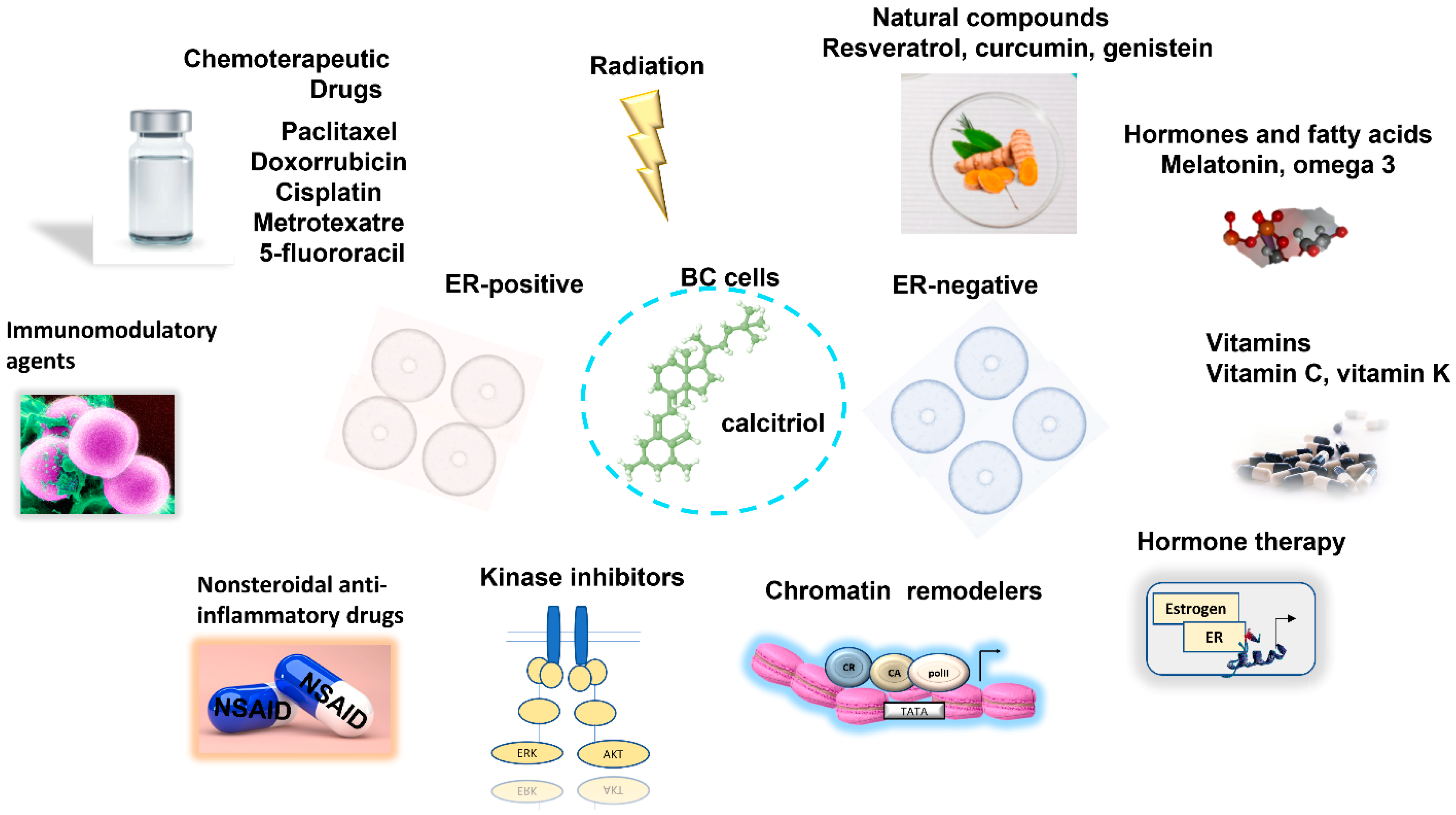
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segovia-Mendoza, M.; García-Quiroz, J.; Díaz, L.; García-Becerra, R. Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update. Int. J. Mol. Sci. 2021, 22, 12741. https://doi.org/10.3390/ijms222312741
Segovia-Mendoza M, García-Quiroz J, Díaz L, García-Becerra R. Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update. International Journal of Molecular Sciences. 2021; 22(23):12741. https://doi.org/10.3390/ijms222312741
Chicago/Turabian StyleSegovia-Mendoza, Mariana, Janice García-Quiroz, Lorenza Díaz, and Rocío García-Becerra. 2021. "Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update" International Journal of Molecular Sciences 22, no. 23: 12741. https://doi.org/10.3390/ijms222312741