Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice
Abstract
:1. Introduction
2. QTL Analysis of Salt Tolerance in Rice
2.1. QTL Mapping Population for Salt Tolerance
2.2. Period and Method of Salt Tolerance Identification
2.3. Salt Tolerance Evaluation Parameter
2.4. Salt Tolerance QTL
2.5. Fine Mapping and Map-Based Cloning of QTLs for Salt Tolerance in Rice
3. Association Analysis of Rice Salt Tolerance
3.1. Association Analysis of Salt Tolerance Candidate Genes
3.2. SSR Association Analysis of Salt Tolerance
3.3. Salt Tolerance Genome-Wide Association Analysis
4. Issues and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and Improving Salt Tolerance in Plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Shannon, M.C.; Grieve, C.M. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica 2002, 127, 235–245. [Google Scholar] [CrossRef]
- Ponce, K.S.; Meng, L.; Guo, L.; Leng, Y.; Ye, G. Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, N.; Oakey, H.; Tester, M.; Negrão, S. Assessing Rice Salinity Tolerance: From Phenomics to Association Mapping. In Rice Genome Engineering and Gene Editing: Methods and Protocols; Bandyopadhyay, A., Thilmony, R., Eds.; Springer: New York, NY, USA, 2021; pp. 339–375. [Google Scholar]
- Quan, R.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of Salt Tolerance Using Wild Rice Genes. Front. Plant Sci. 2017, 8, 2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel-dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelm, E.v.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Shan, T.; Pang, S.; Wang, X.; Li, J.; Li, Q.; Su, L.; Li, X. A method to establish an “immortalized F-2” sporophyte population in the economic brown alga Undaria pinnatifida (Laminariales: Alariaceae). J. Phycol. 2020, 56, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Rohilla, M.; Bisht, D.S.; Krishnamurthy, S.L.; Barman, M.; Sarma, R.N.; Sharma, T.R.; Mondal, T.K. Identification and mapping of quantitative trait loci (QTL) and epistatic QTL for salinity tolerance at seedling stage in traditional aromatic short grain rice landrace Kolajoha (Oryza sativa L.) of Assam, India. Euphytica 2020, 216, 1211–1226. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Bao, Y.; Wu, Y.; Zhang, H. Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 2010, 178, 297–307. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Chen, Z.; Huang, J.; Bao, Y.; Wang, J.; Zhang, H. Identification of QTLs with main, epistatic and QTL x environment interaction effects for salt tolerance in rice seedlings under different salinity conditions. Theor. Appl. Genet. 2012, 125, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhao, H.; Liu, H.; Wang, J.; Zou, D. QTL analysis of Na+ and K+ concentrations in shoots and roots under NaCl stress based on linkage and association analysis in japonica rice. Euphytica 2014, 201, 109–121. [Google Scholar] [CrossRef]
- Flowers, T.J.; Koyama, M.L.; Flowers, S.A.; Sudhakar, C.; Singh, K.P.; Yeo, A.R. QTL: Their place in engineering tolerance of rice to salinity. J. Exp. Bot. 2000, 51, 99–106. [Google Scholar] [CrossRef]
- Koyama, M.L.; Levesley, A.; Koebner, R.; Flowers, T.; Yeo, A.R. Quantitative Trait Loci for Component Physiological Traits Determining Salt Tolerance in Rice. Plant Physiol. 2001, 125, 406–422. [Google Scholar] [CrossRef] [Green Version]
- Haq, U.T.; Gorham, J.; Akhtar, J.; Akhtar, N.; Steele, K.A. Dynamic quantitative trait loci for salt stress components on chromosome 1 of rice. Funct. Plant Biol. 2010, 37, 634–645. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, J.; Cha, Y.; Yun, D.; Lee, M.; Ko, J.; Lee, K.; Eun, M. Mapping of quantitative trait loci for salt tolerance at the seedling stage in rice. Mol. Cells 2006, 21, 192–196. [Google Scholar] [PubMed]
- Lee, S.Y.; Ahn, J.H.; Cha, Y.S.; Yun, D.W.; Lee, M.C.; Ko, J.C.; Lee, K.S.; Eun, M.Y. Mapping QTLs related to salinity tolerance of rice at the young seedling stage. Plant Breed. 2007, 126, 43–46. [Google Scholar] [CrossRef]
- Wang, B.; Lan, T.; Wu, W. Mapping of QTLs for Na+ content in rice seedlings under salt stress. Chin. J. Rice Sci. 2007, 21, 585–590. [Google Scholar]
- Thomson, M.J.; de Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K.; et al. Characterizing the Saltol Quantitative Trait Locus for Salinity Tolerance in Rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Qu, Y.; Yang, C.; Ma, X.; Cao, G.; Zhao, Z.; Zhang, S.; Zhang, T.; Han, L. Identification of QTLs associated with salt or alkaline tolerance at the seedling stage in rice under salt or alkaline stress. Euphytica 2014, 201, 441–452. [Google Scholar] [CrossRef]
- Tiwari, S.; Sl, K.; Kumar, V.; Singh, B.; Rao, A.R.; Mithra SV, A.; Rai, V.; Singh, A.K.; Singh, N.K. Mapping QTLs for Salt Tolerance in Rice (Oryza sativa L.) by Bulked Segregant Analysis of Recombinant Inbred Lines Using 50K SNP Chip. PLoS ONE 2016, 11, e0153610. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.; Rai, V.; Bal, S.; Sinha, S.; Kumar, V.; Chauhan, M.; Gautam, R.K.; Singh, R.; Sharma, P.C.; Singh, A.K.; et al. Combining QTL mapping and transcriptome profiling of bulked RILs for identification of functional polymorphism for salt tolerance genes in rice (Oryza sativa L.). Mol. Genet. Genom. 2010, 284, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zang, J.; Wang, Y.; Zhu, L.; Mohammadhosein, F.; Xu, J.; Li, Z. Mining Favorable Salt-tolerant QTL from Rice Germplasm Using a Backcrossing Introgression Line Population. Acta Agron. Sin. 2007, 33, 1611–1617. [Google Scholar]
- Kim, D.M.; Ju, H.G.; Kwon, T.R.; Oh, C.S.; Ahn, S.N. Mapping QTLs for salt tolerance in an introgression line population between Japonica cultivars in rice. J. Crop Sci. Biotech. 2009, 12, 121–128. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, H.; Chen, M.; Zhang, L.; Chen, B.; Cui, J.; Liu, H.; Zhu, L.; Shi, Y.; Gao, Y.; et al. Detection of Salt-tolerant QTL Using BC2F3 Yield Selected Introgression Lines of Rice (Oryza sativa L.). Mol. Plant Breed. 2009, 7, 224–232. [Google Scholar]
- Yang, J.; Sun, Y.; Cheng, L.R.; Zhou, Z.; Wang, Y.; Zhu, L.H.; Cang, J.; Xu, J.L.; Li, Z.K. Genetic Background Effect on QTL Mapping for Salt Tolerance Revealed by a Set of Reciprocal Introgression Line Populations in Rice. Acta Agron. Sin. 2009, 35, 974–982. [Google Scholar] [CrossRef]
- Alam, R.; Rahman, M.S.; Seraj, Z.I.; Thomson, M.J.; Ismail, A.M.; Tumimbang-Raiz, E.; Gregorio, G.B. Investigation of seedling-stage salinity tolerance QTLs using backcross lines derived from Oryza sativa L. Pokkali. Plant Breed. 2011, 130, 430–437. [Google Scholar] [CrossRef]
- Li, Y.F.; Zheng, Y.; Vemireddy, L.R.; Panda, S.K.; Jose, S.; Ranjan, A.; Panda, P.; Govindan, G.; Cui, J.; Wei, K.; et al. Comparative transcriptome and translatome analysis in contrasting rice genotypes reveals differential mRNA translation in salt-tolerant Pokkali under salt stress. BMC Genom. 2018, 19, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Tan, L.; Liu, F.; Cai, H.; Sun, C. Identification of quantitative trait loci associated with salt tolerance at seedling stage from Oryza rufipogon. J. Genet. Genom. 2011, 38, 593–601. [Google Scholar] [CrossRef]
- Qiu, X.; Yuan, Z.; Liu, H.; Xiang, X.; Yang, L.; He, W.; Du, B.; Ye, G.; Xu, J.; Xing, D.; et al. Identification of salt tolerance-improving quantitative trait loci alleles from a salt-susceptible rice breeding line by introgression breeding. Plant Breed. 2015, 134, 653–660. [Google Scholar] [CrossRef]
- Ahmadi, J.; Fotokian, M.H. Identification and mapping of quantitative trait loci associated with salinity tolerance in rice (Oryza Sativa) using SSR markers. Iran. J. Biotechnol. 2011, 9, 21–30. [Google Scholar]
- Cheng, L.; Wang, Y.; Meng, L.; Hu, X.; Cui, Y.; Sun, Y.; Zhu, L.; Ali, J.; Xu, J.; Li, Z. Identification of salt-tolerant QTLs with strong genetic background effect using two sets of reciprocal introgression lines in rice. Genome 2012, 55, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Sun, Y.; Wang, Y.; Yang, J.; Li, F.; Zhou, Y.; Zhu, L.; Jessica, R.; Mohammadhosein, F.; Xu, J.; et al. Dissection of genetic overlap of salt tolerance QTLs at the seedling and tillering stages using backcross introgression lines in rice. Sci. China C Life Sci. 2008, 51, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Takehisa, H.; Shimodate, T.; Fukuta, Y.; Ueda, T.; Yano, M.; Yamaya, T.; Kameya, T.; Sato, T. Identification of quantitative trait loci for plant growth of rice in paddy field flooded with salt water. Field Crop. Res. 2004, 89, 85–95. [Google Scholar] [CrossRef]
- Prasad, S.; Bagali, P.; Hittalmani, S.; Shashidhar, H. Molecular mapping of quantitative trait loci associated with seedling tolerance to salt stress in rice (Oryza sativa L). Curr. Sci. 2000, 78, 162–164. [Google Scholar]
- Gong, J.; He, P.; Qian, Q.; Shen, L.; Zhu, L.; Chen, S. Identification of salt-tolerance QTL in rice (Oryza sativa L.). Chin. Sci. Bull. 1999, 41, 68–71. [Google Scholar] [CrossRef]
- Gong, J.; Zheng, X.; Du, B.; Qian, Q.; Chen, S.; Zhu, L.; He, P. Comparative study of QTLs for agronomic traits of rice (Oryza sativa L.) between salt stress and nonstress environment. Sci. China C Life Sci. 2001, 44, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, S.; Zhao, Q.; Zhou, L.; Zhao, L.; Yao, S.; Zhang, Y.; Wang, C. Mapping of QTLs for bud-stage salinity tolerance based on chromosome segment substitution line in rice. Acta Agric. Boreali-Sin. 2017, 32, 106–111. [Google Scholar]
- Mardani, Z.; Rabiei, B.; Sabouri, H.; Sabouri, A.; Virk, P. Identification of molecular markers linked to salt-tolerant genes at germination stage of rice. Plant Breed. 2014, 133, 196–202. [Google Scholar] [CrossRef]
- Ghomi, K.; Rabiei, B.; Sabouri, H.; Sabouri, A. Mapping QTLs for traits related to salinity tolerance at seedling stage of rice (Oryza sativa L.): An agrigenomics study of an Iranian rice population. OMICS 2013, 17, 242–251. [Google Scholar] [CrossRef]
- Lin, H.X.; Zhu, M.Z.; Yano, M.; Gao, J.P.; Liang, Z.W.; Su, W.A.; Hu, X.H.; Ren, Z.H.; Chao, D.Y. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 2004, 108, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, H.; Sabouri, A. New evidence of QTLs attributed to salinity tolerance in rice. Afr. J. Biotechnol. 2008, 7, 4376–4383. [Google Scholar]
- Sabouri, H.; Rezai, A.; Moumeni, A.; Kavousi, A.; Katouzi, M.; Sabouri, A. QTLs mapping of physiological traits related to salt tolerance in young rice seedlings. Biol. Plant. 2009, 53, 657–662. [Google Scholar] [CrossRef]
- Javed, M.A.; Huyop, F.Z.; Wagiran, A.; Salleh, F.M. Identification of QTLs for Morph-Physiological Traits Related to Salinity Tolerance at Seedling Stage in Indica Rice. Procedia Environ. Sci. 2011, 8, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Salam, M.; Hassan, L.; Collard, B.Y.; Singh, R.; Gregorio, G. QTL mapping for salinity tolerance at seedling stage in rice. Emir. J. Food Agric. 2011, 23, 137. [Google Scholar] [CrossRef]
- Sun, J.; Zou, D.T.; Luan, F.S.; Zhao, H.W.; Wang, J.G.; Liu, H.L.; Xie, D.W.; Su, D.Q.; Ma, J.; Liu, Z.L. Dynamic QTL analysis of the Na+ content, K+ content, and Na+ /K+ ratio in rice roots during the field growth under salt stress. Biol. Plant. 2014, 58, 689–696. [Google Scholar] [CrossRef]
- Yao, M.; Wang, J.; Chen, H.; Zhai, H.; Zhang, H. Inheritance and QTL mapping of salt tolerance in rice. Rice Sci. 2005, 12, 25–32. [Google Scholar]
- Mohammadi, R.; Mendioro, M.; Diaz, G.; Gregorio, G.; Singh, R. Mapping quantitative trait loci associated with yield and yield components under reproductive stage salinity stress in rice (Oryza sativa L.). J. Genet. 2013, 93, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Bimpong, I.K.; Manneh, B.; Diop, B.; Ghislain, K.; Sow, A.; Amoah, N.K.A.; Gregorio, G.; Singh, R.K.; Ortiz, R.; Wopereis, M. New quantitative trait loci for enhancing adaptation to salinity in rice from Hasawi, a Saudi landrace into three African cultivars at the reproductive stage. Euphytica 2014, 200, 45–60. [Google Scholar] [CrossRef]
- Khan, M.S.K.; Saeed, M.; Iqbal, J. Identification of quantitative trait loci for Na+, K+ and Ca++ accumulation traits in rice grown under saline conditions using F2 mapping population. Braz. J. Bot. 2015, 38, 555–565. [Google Scholar] [CrossRef]
- Khan, M.S.K.; Saeed, M.; Iqbal, J. Quantitative trait locus mapping for salt tolerance at maturity stage in indica rice using replicated F2 population. Braz. J. Bot. 2016, 39, 641–650. [Google Scholar] [CrossRef]
- Ammar, M.; Pandit, A.; Singh, R.; Sameena, S.; Chauhan, M.; Singh, A.; Sharma, P.; Gaikwad, K.; Sharma, T.; Mohapatra, T.; et al. Mapping of QTLs controlling Na+, K+ and Cl− ion concentrations in salt tolerant indica rice variety. J. Plant Biochem. Biot. 2009, 18, 139–150. [Google Scholar] [CrossRef]
- Hossain, H.; Rahman, M.A.; Alam, M.S.; Singh, R.K. Mapping of Quantitative Trait Loci Associated with Reproductive-Stage Salt Tolerance in Rice. J. Agron. Crop Sci. 2015, 201, 17–31. [Google Scholar] [CrossRef]
- Gu, X.; Mei, M.; Yan, X.; Zheng, S.; Lu, Y. Preliminary detection of quantitative trait loci for salt tolerance in rice. Chin. J. Rice Sci. 2000, 14, 65–70. [Google Scholar] [CrossRef]
- Negrão, S.; Courtois, B.; Ahmadi, N.; Abreu, I.; Saibo, N.; Oliveira, M.M. Recent Updates on Salinity Stress in Rice: From Physiological to Molecular Responses. Crit. Rev. Plant Sci. 2011, 30, 329–377. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Cheng, J.; Lai, Y.; Wang, J.; Bao, Y.; Huang, J.; Zhang, H. QTL analysis of Na+ and K+ concentrations in roots and shoots under different levels of NaCl stress in rice (Oryza sativa L.). PLoS ONE 2012, 7, e51202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IRRI. Standard Evaluation System for Rice, 4th ed.; International Rice Research Institute: Manila, Philippines, 1996; Volume 52. [Google Scholar]
- Lin, H.; Yanagihara, S.; Zhuang, J.; Senboku, T.; Yashima, S. Identification of QTL for salt tolerance in rice via molecular markers. Chin. J. Rice Sci. 1998, 12, 72–78. [Google Scholar] [CrossRef]
- Masood, M.; Seiji, Y.; Shinwari, Z.; Anwar, R. Mapping quantitative trait loci (QTLs) for salt tolerance in rice (Oryza sativa) using RFLPs. Pak. J. Bot. 2004, 36, 825–834. [Google Scholar]
- Zheng, H.; Wang, J.; Zhao, H.; Liu, H.; Sun, J.; Guo, L.; Zou, D. Genetic structure, linkage disequilibrium and association mapping of salt tolerance in japonica rice germplasm at the seedling stage. Mol. Breed. 2015, 35, 152. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Ma, X.; Chen, W.; Zhao, J.; Sun, C.; Tan, L.; Liu, F. Integrated RNA Sequencing and QTL Mapping to Identify Candidate Genes from Oryza rufipogon Associated with Salt Tolerance at the Seedling Stage. Front. Plant Sci. 2017, 8, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonam, R.; Sunita, J.; Sheetal, Y.; Navinder, S.; Jain, R.K. Identification of SSR Markers for Salt-tolerance in Rice Variety CSR10 by Selective Genotyping. J. Plant Biochem. Biotechnol. 2009, 18, 87–91. [Google Scholar]
- Chai, L.; Zhang, J.; Pan, X.; Zhang, F.; Zheng, T.; Zhao, X.; Wang, W.; Jauhar, A.; Xu, J.; Li, Z. Advanced Backcross QTL Analysis for the Whole Plant Growth Duration Salt Tolerance in Rice (Oryza sativa L.). J. Integr. Agric. 2014, 13, 1609–1620. [Google Scholar] [CrossRef]
- Ren, Z.H.; Gao, J.P.; Li, L.G.; Cai, X.L.; Huang, W.; Chao, D.Y.; Zhu, M.Z.; Wang, Z.Y.; Luan, S.; Lin, H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Riedelsberger, J.; Miller, J.K.; Valdebenito-Maturana, B.; Pineros, M.A.; Gonzalez, W.; Dreyer, I. Plant HKT Channels: An Updated View on Structure, Function and Gene Regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, G.B. Tagging salinity tolerance genes in rice using amplified fragment length polymorphism (AFLP). Ph.D. Thesis, University of the Philippines, Los Banos, CA, USA, 1997; 118p. [Google Scholar]
- Bonilla, P.; Dvorak, J.; Mackill, D.; Deal, K.; Gregorio, G. RFLP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp. Agric. Sci. 2002, 65, 68–76. [Google Scholar]
- Niones, J.; Gregorio, G.; Tumimbang, E. Fine mapping of the salinity tolerance gene on chromosome 1 of rice (Oryza sativa L.) using near isogenic lines. Crop Sci. Soc. Philipp. 2004, 31, 46. [Google Scholar]
- Lan, T.; Zhang, S.; Liu, T.; Wang, B.; Guan, H.; Zhou, Y.; Duan, Y.; Wu, W. Fine mapping and candidate identification of SST, a gene controlling seedling salt tolerance in rice (Oryza sativa L.). Euphytica 2015, 205, 269–274. [Google Scholar] [CrossRef]
- Ogawa, D.; Abe, K.; Miyao, A.; Kojima, M.; Sakakibara, H.; Mizutani, M.; Morita, H.; Toda, Y.; Hobo, T.; Sato, Y.; et al. RSS1 regulates the cell cycle and maintains meristematic activity under stress conditions in rice. Nat. Commun. 2011, 2, 278. [Google Scholar] [CrossRef] [Green Version]
- Toda, Y.; Tanaka, M.; Ogawa, D.; Kurata, K.; Kurotani, K.; Habu, Y.; Ando, T.; Sugimoto, K.; Mitsuda, N.; Katoh, E.; et al. RICE SALT SENSITIVE3 forms a ternary complex with JAZ and class-C bHLH factors and regulates jasmonate-induced gene expression and root cell elongation. Plant Cell 2013, 25, 1709–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, P.; Jiang, D.; Dong, Y.; Shi, X.; Jing, W.; Zhang, W. Physiological characterisation and fine mapping of a salt-tolerant mutant in rice (Oryza sativa). Funct. Plant Biol. 2015, 42, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Deng, P.; Shi, X.; Zhou, J.; Wang, F.; Dong, Y.; Jing, W.; Zhang, W. Identification and Fine Mapping of a Mutation Conferring Salt-Sensitivity in Rice (Oryza sativa L.). Crop Sci. 2015, 55, 219–228. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Q.; Qian, Q.; Zhao, Q.; Wang, L.; Wang, A.; Guan, J.; Fan, D.; Weng, Q.; Huang, T.; et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, K.; Yamamoto, E.; Aya, K.; Takeuchi, H.; Lo, P.C.; Hu, L.; Yamasaki, M.; Yoshida, S.; Kitano, H.; Hirano, K.; et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 2016, 48, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Negrão, S.; Katsantonis, D.; Frouin, J.; Ploux, J.; Letourmy, P.; Droc, G.; Babo, P.; Trindade, H.; Bruschi, G.; et al. Targeted association analysis identified japonica rice varieties achieving Na+/K+ homeostasis without the allelic make-up of the salt tolerant indica variety Nona Bokra. Theor. Appl. Genet. 2011, 123, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Negrao, S.; Almadanim, M.C.; Pires, I.S.; Abreu, I.A.; Maroco, J.; Courtois, B.; Gregorio, G.B.; McNally, K.L.; Oliveira, M.M. New allelic variants found in key rice salt-tolerance genes: An association study. Plant Biotechnol. J. 2013, 11, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Xu, C.; Yang, C.; Zhang, Q.; Zhang, J.; Ma, X.; Qiao, Y.; Cao, G.; Zhang, S.; Han, L. Association mapping of salinity and alkalinity tolerance in improved japonica rice (Oryza sativa L. subsp. japonica Kato) germplasm. Genet. Resour. Crop Evol. 2014, 62, 539–550. [Google Scholar] [CrossRef]
- Li, B. Identification of Genes Conferring Plant Salt Tolerance using GWAS: Current Success and Perspectives. Plant Cell Physiol. 2020, 61, 1419–1426. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; Meyer, A.J.; Haring, M.A.; Julkowska, M.M.; Testerink, C. Genetic Loci Associated with Early Salt Stress Responses of Roots. iScience 2019, 21, 458–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Zhang, M.; Liang, X.; Li, F.; Shi, Y.; Yang, X.; Jiang, C. Natural variation of an EF-hand Ca(2+)-binding-protein coding gene confers saline-alkaline tolerance in maize. Nat. Commun. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Liang, X.; Wang, L.; Cao, Y.; Song, W.; Shi, J.; Lai, J.; Jiang, C. A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 2019, 5, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, A.; Mithra, S.V.A.; Krishnamurthy, S.L.; Parida, S.K.; Jain, S.; Tiwari, K.K.; Kumar, P.; Rao, A.R.; Sharma, S.K.; et al. Genome-wide association mapping of salinity tolerance in rice (Oryza sativa). DNA Res. 2015, 22, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ponce, K.S.; Meng, L.; Chakraborty, P.; Zhao, Q.; Guo, L.; Gao, Z.; Leng, Y.; Ye, G. QTL identification for salt tolerance related traits at the seedling stage in indica rice using a multi-parent advanced generation intercross (MAGIC) population. Plant Growth Regul. 2020, 92, 365–373. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Zhao, X.; Wang, X.; Shen, C.; Zhu, Y.; Dai, M.; Qiu, X.; Yang, R.; Xing, D.; et al. Identification of genes for salt tolerance and yield-related traits in rice plants grown hydroponically and under saline field conditions by genome-wide association study. Rice (NY) 2019, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Batayeva, D.; Labaco, B.; Ye, C.; Li, X.; Usenbekov, B.; Rysbekova, A.; Dyuskalieva, G.; Vergara, G.; Reinke, R.; Leung, H. Genome-wide association study of seedling stage salinity tolerance in temperate japonica rice germplasm. BMC Genet. 2018, 19, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neang, S.; de Ocampo, M.; Egdane, J.A.; Platten, J.D.; Ismail, A.M.; Seki, M.; Suzuki, Y.; Skoulding, N.S.; Kano-Nakata, M.; Yamauchi, A.; et al. A GWAS approach to find SNPs associated with salt removal in rice leaf sheath. Ann. Bot. 2020, 126, 1193–1202. [Google Scholar] [CrossRef]
- Yu, J.; Zao, W.; He, Q.; Kim, T.S.; Park, Y.J. Genome-wide association study and gene set analysis for understanding candidate genes involved in salt tolerance at the rice seedling stage. Mol. Genet. Genom. 2017, 292, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, F.; Zhou, Y. The Application of Multi-Locus GWAS for the Detection of Salt-Tolerance Loci in Rice. Front. Plant Sci. 2018, 9, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekklar, C.; Pongpanich, M.; Suriya-Arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhao, W.; Tong, W.; He, Q.; Yoon, M.Y.; Li, F.P.; Choi, B.; Heo, E.B.; Kim, K.W.; Park, Y.J. A Genome-Wide Association Study Reveals Candidate Genes Related to Salt Tolerance in Rice (Oryza sativa) at the Germination Stage. Int. J. Mol. Sci. 2018, 19, 3145. [Google Scholar] [CrossRef] [Green Version]
- Rohila, J.S.; Edwards, J.D.; Tran, G.D.; Jackson, A.K.; McClung, A.M. Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection. Plants 2019, 8, 472. [Google Scholar] [CrossRef] [Green Version]
- Warraich, A.S.; Krishnamurthy, S.L.; Sooch, B.S.; Vinaykumar, N.M.; Dushyanthkumar, B.M.; Bose, J.; Sharma, P.C. Rice GWAS reveals key genomic regions essential for salinity tolerance at reproductive stage. Acta Physiol. Plant. 2020, 42, 134. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.; Liu, K.; Wang, B.; Tian, Y.; Ge, Y.; Zhang, Y.; Tang, W.; Chen, G.; Yu, J.; Wu, W.; et al. Genome-wide association study identifies QTLs conferring salt tolerance in rice. Plant Breed. 2019, 139, 73–82. [Google Scholar] [CrossRef]
- Le, T.D.; Gathignol, F.; Vu, H.T.; Nguyen, K.L.; Tran, L.H.; Vu, H.T.T.; Dinh, T.X.; Lazennec, F.; Pham, X.H.; Very, A.A.; et al. Genome-Wide Association Mapping of Salinity Tolerance at the Seedling Stage in a Panel of Vietnamese Landraces Reveals New Valuable QTLs for Salinity Stress Tolerance Breeding in Rice. Plants (Basel) 2021, 10, 1088. [Google Scholar] [CrossRef]
- Li, N.; Zheng, H.; Cui, J.; Wang, J.; Liu, H.; Sun, J.; Liu, T.; Zhao, H.; Lai, Y.; Zou, D. Genome-wide association study and candidate gene analysis of alkalinity tolerance in japonica rice germplasm at the seedling stage. Rice (NY) 2019, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, H.; Wu, W.; Liu, H.; Wang, J.; Jia, Y.; Li, J.; Yang, L.; Lei, L.; Zou, D.; et al. QTL mapping and candidate gene analysis for alkali tolerance in Japonica rice at the bud stage based on linkage mapping and genome-wide association study. Rice (NY) 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.A.; Zhang, F.; Zhang, J.; Zheng, T.Q.; Meng, L.J.; Pang, Y.L.; Xu, J.L.; Li, Z.K. Identification of QTN and candidate genes for salinity tolerance at the germination and seedling stages in rice by genome-wide association analyses. Sci. Rep. 2018, 8, 6505. [Google Scholar] [CrossRef] [Green Version]
- Patishtan, J.; Hartley, T.N.; De Carvalho, R.F.; Maathuis, F.J.M. Genome-wide association studies to identify rice salt-tolerance markers. Plant Cell Environ. 2018, 41, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Bollinedi, H.; Krishnan, S.G.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Singh, A.K. Genome-Wide association study reveals marker-trait associations for early vegetative stage salinity tolerance in rice. Plants (Basel) 2021, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, X.; Zhao, Y.; Khan, N.U.; Zhao, Z.; Zhang, Y.; Wen, X.; Tang, F.; Wang, F.; Li, Z. Genetic basis and identification of candidate genes for salt tolerance in rice by GWAS. Sci. Rep. 2020, 10, 9958. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; de Boer, G.J.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeraja, C.N.; Mishra, B.; Rao, K.S.; Singh, R.K.; Padmavati, G.; Shenoy, V.V. Linkage disequilibrium in salt tolerant genotypes of rice (Oryza sativaL). J. Plant Biochem. Biotechnol. 2008, 17, 65–68. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Brien, C.; Oakey, H.; Berger, B.; Saade, S.; Ho, Y.S.; Schmockel, S.M.; Tester, M.; Negrao, S. Salinity tolerance loci revealed in rice using high-throughput non-invasive phenotyping. Nat. Commun. 2016, 7, 13342. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zheng, H.; Zhao, H.; Wang, J.; Liu, H.; Sun, J.; Li, N.; Lei, L.; Zou, D. Association analysis of saline-alkali related traits and SSR markers in Japonica rice under soda saline-alkali stress. Acta Agric. Boreali-Sin. 2018, 33, 139–148. [Google Scholar]
- Huang, X.; Wei, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef]
- Xie, L.; Zheng, C.; Li, W.; Pu, M.; Zhou, G.; Sun, W.; Wu, X.; Zhao, X.; Xie, X. Mapping and identification a salt-tolerant QTL in a salt-resistant rice landrace, Haidao86. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front. Plant Sci. 2015, 6, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimpong, I.K.; Manneh, B.; Sock, M.; Diaw, F.; Amoah, N.K.A.; Ismail, A.M.; Gregorio, G.; Singh, R.K.; Wopereis, M. Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci. 2016, 242, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Lakra, N.; Nutan, K.K.; Das, P.; Anwar, K.; Singla-Pareek, S.L.; Pareek, A. A nuclear-localized histone-gene binding protein from rice (OsHBP1b) functions in salinity and drought stress tolerance by maintaining chlorophyll content and improving the antioxidant machinery. J. Plant Physiol. 2015, 176, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Soda, N.; Kushwaha, H.R.; Soni, P.; Singla-Pareek, S.L.; Pareek, A. A suite of new genes defining salinity stress tolerance in seedlings of contrasting rice genotypes. Funct. Integr. Genom. 2013, 13, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nee Sabharwal, V.P.; Kushwaha, H.R.; Sopory, S.K.; Singla-Pareek, S.L.; Pareek, A. Transcriptome map for seedling stage specific salinity stress response indicates a specific set of genes as candidate for saline tolerance in Oryza sativa L. Funct. Integr. Genom. 2008, 9, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, Y.; Xalaxo, S.; Verulkar, S.; Yadav, N.; Singh, S.; Singh, N.; Prasad, K.S.N.; Kondayya, K.; Rao, P.V.R.; et al. From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016, 242, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gopalakrishnan, S.; Singh, V.P.; Prabhu, K.V.; Mohapatra, T.; Singh, N.K.; Sharma, T.R.; Nagarajan, M.; Vinod, K.K.; Singh, D.; et al. Marker Assisted Selection: A paradigm shift in Basmati breeding. Indian J. Genet. Plant Breed. 2011, 71, 120–128. [Google Scholar]
- Linh le, H.; Linh, T.H.; Xuan, T.D.; Ham le, H.; Ismail, A.M.; Khanh, T.D. Molecular breeding to improve salt tolerance of rice (Oryza sativa L.) in the red river delta of Vietnam. Int. J. Plant Genom. 2012, 2012, 949038. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, G.; Islam, R.; Vergara, G.; Thirumeni, S. Recent advances in rice science to design salinity and other abiotic stress tolerant rice varietie. SABRAO J. Breed. Genet. 2013, 45, 31–41. [Google Scholar]
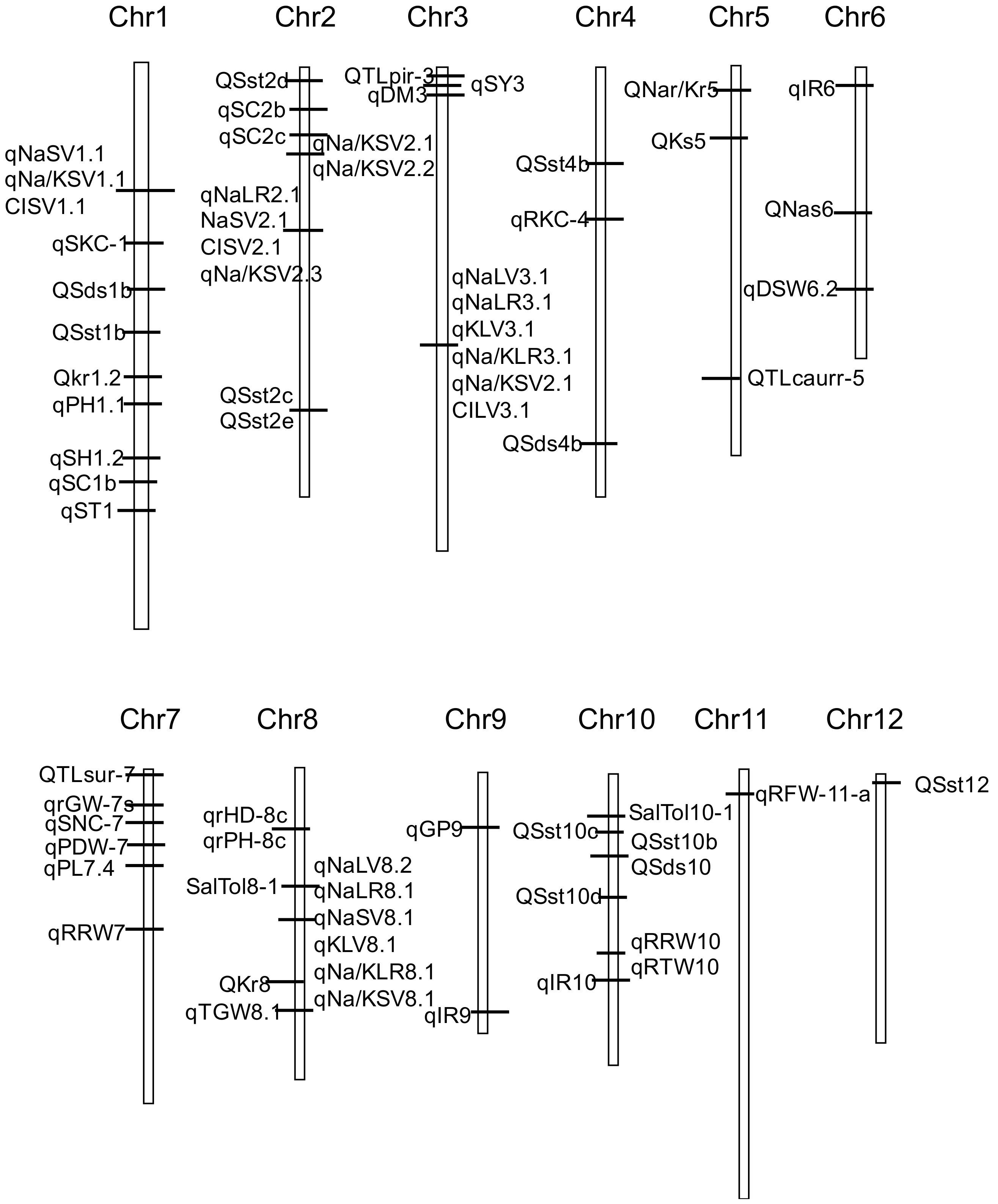
| Stage | Parents for Cross | Population Type | Evaluation Parameter for Salt Tolerance | PVE% | QTL | High-PVE QTL | Reference |
|---|---|---|---|---|---|---|---|
| Germination stage | IR64×Azucena | DH | GR, seedling root length, seedling dry mass, seedling vigor | 13.5–19.5 | 7 | 0 | [38] |
| Jiucaiqing×IR26 | RIL | GR, RL, SH | 6.5–43.7 | 7 | 4 | [13] | |
| Gharib×Sepidroud | F2/F2:4 | GR, germination percentage, radicle length, plumule length, coleoptile length, radicle fresh weight, plumule fresh weigh, radicle dry weight, plumule dry weight, coleoptile fresh weight, coleoptile dry weight | 10.0–21.9 | 17 | 2 | [42] | |
| 9311×japonica | CSSL | Survival rate | 5.1–93.2 | 4 | - | [41] | |
| Seedling stage | Dongnong425×Changbai10 | BC2F2/BC2F2:3 | SST, SNC, SKC, RNC, RKC | 6.45–17.95 | 13 | 0 | [63] |
| O. rufipogon×O. Sative | ILs | SDS, STT | 2–8 | 10 | - | [64] | |
| (Nona Bokra×Pokkali)×(IR4630-22-2-5-1-3×IR10167-129-3-4) | RIL | SNC, SKC, SNKR | - | 4 | - | [16] | |
| IR4630×IR15324 | RIL | SNC, SKC, SNKR, total Na+ and K+, SDW | 6.4–19.6 | 11 | 0 | [17] | |
| Milyang 23×Gihobyeo | RIL | SST | 9.2–27.8 | 2 | 1 | [19] | |
| Milyang 23×Gihobyeo | RIL | SST | 9.1–27.8 | 2 | 1 | [20] | |
| H359×Acc 8558 | RIL | SNC | 1.68–45.39 | 13 | 3 | [21] | |
| IR29×Pokkali | RIL | SNC, SKC, RKC, RNKC, SH, chlorophyll content, seedling survival rate, initial and final SST | 6–67 | 27 | 16 | [22] | |
| Yiai1×Lishuinuo | RIL | Dead rate of leaf and seedling | 8.65–27.20 | 6 | 1 | [23] | |
| IR64×Tarom Molaii | IL | SST, SDS, SKC, SNC, RKC, RNC | - | 23 | - | [26] | |
| Ilpumbyeo×Moroberekan | IL | The reduction rate of fresh and dry weight, leaf area and SH | 10.2–13.9 | 8 | 0 | [27] | |
| Shuhui527×ZDZ057, Minghui86×Teqing, Minghui86×ZDZ057, Shuhui527×Teqing | IL | SST, SDS | 8.17–42.18 | 43 | 12 | [28] | |
| Lemont×Teqing | IL | SST, SDS, SKC, SNC | - | 36 | - | [29] | |
| Pokkali×IR29 | IL | SST | 4.00-18.42 | 6 | 0 | [30] | |
| Ce258×IR75862, ZGX1×IR75862 | IL | SST, SDS, SKC, SNC | 5.13–13.75/3.73–8.26 * | 18/2 * | 0 | [33] | |
| Tarome-Molaei×Tiqing | IL | SNC, SKC, SNKR, RNC, RKC, RNKR | 9.0–30.0 | 14 | 5 | [34] | |
| Xiushui 09×IR2061-520-6-9 | IL | SST, SDS, SKC, SNC, SKNR | 5.14–18.89/2.60–14.30 * | 26/21 * | 0 | [35] | |
| Zaiyeqing8×Jingxi17 | DH | SDS | 10.2–38.4 | 10 | 2 | [39] | |
| Nona Bokra×Koshihikari | F2/F3 | SDS, SNC, SKC, RNC, RKC, Na+ and K+ in root, SDW | 12.4–48.5 | 11 | 3 | [44] | |
| Tarommahalli×Khazar | F2/F3 | Survival rate, chlorophyll content, SH, RL, leaf area, the weight of stem and root, total Na+ and K+ in shoot, SNKR | 9.03–38.22 | 32 | 14 | [45] | |
| Tarommahali×Khazar | F2/F3 | STR, DM, Na+ content, K+ content, Na+/K+ | 9.03–20.90 | 14 | 1 | [46] | |
| Pokkali×Shaheen Basmati | F2/F3 | SST, SH, SDW, SFW, SNC, SKC, SNKR, RNC, RKC, RNKR | 4.89–10.55 | 22 | 0 | [47] | |
| BRRI Dhan40×IR61920-3B-22-2-l | F2 | SST | 12.5–29.0 | 3 | 2 | [48] | |
| Jiucaiqing×IR26 | RIL | RNKR, SH, SDW, RDW | 7.8–23.9/- * | 15/5 * | 2 | [14] | |
| Jiucaiqing×IR26 | RIL | RKC, SNC, SKC, SST | 8.5–18.9/- * | 13/9 * | 0 | [59] | |
| Tesanai 2×CB | RIL | SDS | 1.5–11.6 | 4 | 0 | [61] | |
| Tesanai 2×CB | RIL | SDS, SDW, RDW, SNC, SKC, SKNR | 4.4–15.0 | 31 | 0 | [62] | |
| Teqing×Oryza rufipogon | IL | SST, relative SDW, RDW and total plant dry weight | 8–26 | 15 | 3 | [32] | |
| Vegetative growth stage | Co39×Moroberekan | RIL | Content of Na+ in shoot, SNKR, fresh weight of stem, moisture content of leaf | 11.0–26.3 | 14 | 3 | [18] |
| Nipponbare×Kasalath | IL | SH, SDW, number of tillers | 12–41 | 31 | 11 | [37] | |
| Dongnong425×Changbai10 | BC1F2/BC1F2:3, F2/F3 | RNC, RKC, RNKR, relative RNC, relative RKC, relative RNKR | 3.61–27.9 | 50 | 4 | [49] | |
| CSR10×Taraori Basmati | F3 | Relative growth rate, SNKR, visual salt-injury symptoms | 25.6–31.3 | 14 | - | [65] | |
| Jiucaiqing×IR26 | F2 | SST, SNKR, SDW | 6.7–19.3 | 7 | 0 | [50] | |
| Reproductive growth stage | CSRll×MI48, CSR27×MI48 | RIL | Sensitivity index of grain yield stress | - | 55 | - | [24] |
| Zhaiyeqing 8×Jingxi 17 | DH | Effective tiller number, thousand-grain weight, PH, heading date, number of grains per panicle | 7.9–40.1 | 24 | 3 | [40] | |
| Sadri×FL478 | F2 | Heading date, PH, length and number of panicles, dry weight of straw, number of fertile and sterile spikelets per plant, total number of spikelets per plant, yield per plant, spikelet fertility, thousand grain weight | 4.2–30.0 | 37 | 1 | [51] | |
| NERICA-L-19×Hasawi, Sahel108×Hasawi, BG90-2×Hasawi | F2 | SST, PH, TN, heading date, panicle number per plant, panicle sterility rate, grain number per ear, thousand-grain weight, yield per plant | 6.5–49.5 | 75 | 37 | [52] | |
| IR36×Pokkali | F2 | Content of Na+ and Ca2+, absorption rate of Ca2+, relative content of Na+, K+ and Ca2+, relative ion content, relative absorption rate of Na+, K+, Ca2+ and Na+/K+ | 7.69–26.33 | 14 | 3 | [53] | |
| IR36×Pokkali | F2 | PH, TN, number of effective tillers, panicle weight, panicle length, number of spikelets panicle, number of unfilled grains panicle, number of grains panicle, panicle fertility, days of 50% flowering, days to maturity, grain length, grain width, grain length–width ratio, grain yield, thousand-grain weight, straw yield, harvest index | 11.52–81.56 | 6 | 1 | [54] | |
| Cheriviruppu×Pusa Basmati 1 | F2 | PH, TN, panicle length, yield, biomass, pollen fertility, Na+ content in flag leaf, Na+/K+ | 3.8–48.7 | 24 | 5 | [56] | |
| HHZ×Budda, HHZ×Gang46B | BC2F5 | Grain weight, spikelet number, thousand-grain weight, seed fertility | 4.7–90.6 | 22 | 1 | [66] | |
| Sahel108×Hasawi, NERICA-L-19×Hasawi, BG90-2×Hasawi | F2 | Days to flowering/heading, PH, TN, panicle sterility, grain yield, yield per plant, yield-component data for each plot, salt tolerance score | 7.3–31.9 | 75 | - | [52] | |
| Tarommahalli×Khazar | F2/F3 | PH, TN, number of full grains, number of empty grains, length and number of panicle, biomass | 8.76–26.83 | 12 | 3 | [56] | |
| Multiple growth stages | CSR27×MI48 | RIL | Vegetative growth period: content of Na+ in stem, content of K+ and Cl− content in leaf | 5.86–8.55 | 4 | 0 | [25] |
| Reproductive growth period: content of Na+, K+ in straw, Na+/K+ in straw, spikelet fertility stress sensitivity index | 7.22–14.05 | 5 | 0 | ||||
| IR64×Binam | IL | Seedling stage: SST, SDS, SKC, SNC | - | 13 | - | [36] | |
| Vegetative growth period: PH, panicle number, fresh weight | - | 22 | - | ||||
| CSR27×MI48 | F2/F3 | Seedling stage: SST | 14.38 | 1 | 0 | [55] | |
| F2 | Vegetative growth period: Na+, Cl− content in leaf and stem, K+ content in stem, Na+/K+ in leaf and stem | 11.13–55.72 | 17 | 15 | |||
| F2 | Reproductive growth period: content of Na+, K+ and Cl− in leaf, Na+/K+ in leaf | 26.26–52.63 | 7 | 7 | |||
| Peta×Pokkali | BC1F1 | Vegetative growth stage: SST, SFW, SDW, Na+ content | - | 4 | - | [57] | |
| Reproductive growth stage: weight of stem and leaf, PH, TL, effective panicle, number, panicle weight, main panicle length, grain weight, seed setting rate | - | 11 | - |
| Stage | Population Size | Maker Type | QTL | Traits | Reference |
|---|---|---|---|---|---|
| Generation stage | 478 | SNP (6.36M) | 11 | GR, germination index, vigor index, germination time, and imbibition rate | [97] |
| 295 | SNP (1.65M) | 12 | GR, germination energy, germination index, SH, RL | [94] | |
| 184 | SNP (788K) | 8 | RL under control condition, alkaline stress and relative RL | [101] | |
| Seedling stage | 32 | SSR (64) | 28 | Salt tolerance level | [107] |
| 342 | SSR (160) | 12 | SST, SNC, SKC, RNC and RKC | [15] | |
| 533 | SNP (700K) | 20 | Relative growth rate, transpiration use efficiency and transpiration rates | [108] | |
| 295 | SNP (1.65M) | 25 | Leaf width, SH, RL, total dry weight | [91] | |
| 235 | SNP (30K) | 27 | Tiller number, SH, RL, SDW, RDW, RDW/SDW, leaf area, SNKR | [79] | |
| 306 | SNP (200K) | 58 | SNC, SKC | [103] | |
| 203 | SNP (68K) | 26 | Shoot Na+ and K+ content, standard evaluation score, percentage of damage, SDW | [89] | |
| 708 | SNP (3.45M) | 41 | SDS, SSI | [88] | |
| 162 | SNP (3.2M) | 9 | SSI, SDW, RDW, SDS | [95] | |
| 176 | SSR (154) | 13 | Salinization damage grade, SNC, SKC, SNKR | [109] | |
| 221 | SNP (55K) | 7 | SES, SDS, SH and RL under salt treatment, relative SH and RL, SDW and RDW after salt treatment, relative SDW and RDW, relative biomass. | [87] | |
| 181 | SNP (32K) | 54 | SH, SFW and SDW under control, salt stress conditions, relative SH, SFW and SDW | [98] | |
| 295 | SNP (788K) | 8 | SST, SNC, SKC, SNKR | [100] | |
| 664 | SNP (3M) | 21 | SH, RL, SFW, SDW, RFW, RDW, salt tolerance level | [105] | |
| Vegetative growth stage | 104 | SNP (112K) | 200 | Photosynthetic parameters and cell membrane stability | [93] |
| 296 | SNP (44K) | 11 | Na+ and Cl− of leaf blades, Na+ and Cl− sheath:blade ratios, SES | [90] | |
| 179 | SNP (21K) | 26 | SES, chlorophyll content, water content, Na+ and K+ contents, SNKR | [99] | |
| 96 | SNP (50K) | 23 | SH, RL, SFW, SDW, RFW, RDW, RNC, SNC, RKC, SKC, RNK, SNKR, RNKR | [104] | |
| Reproductive growth stage | 220 | SNP (6K) | 64 | SNKR, PH, TN, spikelet fertility, unfilled or filled grains, yield | [86] |
| 347 | SSR (148) | 25 | Salinity tolerance index | [81] | |
| Multiple growth stages | 180 | SSR (150) | 28 | Na+, K+, Ca2+, Mg2+ content in stem and leaves, grain yield and SSI | [96] |
| 208 | SNP (395K) | 20 | Generation stage: GR.Seedling stage: SH, RL | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Jiang, H.; Meng, L.; Chen, J. Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 11674. https://doi.org/10.3390/ijms222111674
Fan X, Jiang H, Meng L, Chen J. Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice. International Journal of Molecular Sciences. 2021; 22(21):11674. https://doi.org/10.3390/ijms222111674
Chicago/Turabian StyleFan, Xiaoru, Hongzhen Jiang, Lijun Meng, and Jingguang Chen. 2021. "Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice" International Journal of Molecular Sciences 22, no. 21: 11674. https://doi.org/10.3390/ijms222111674
APA StyleFan, X., Jiang, H., Meng, L., & Chen, J. (2021). Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice. International Journal of Molecular Sciences, 22(21), 11674. https://doi.org/10.3390/ijms222111674







