Heterologous Expression of the Melatonin-Related Gene HIOMT Improves Salt Tolerance in Malus domestica
Abstract
:1. Introduction
2. Results
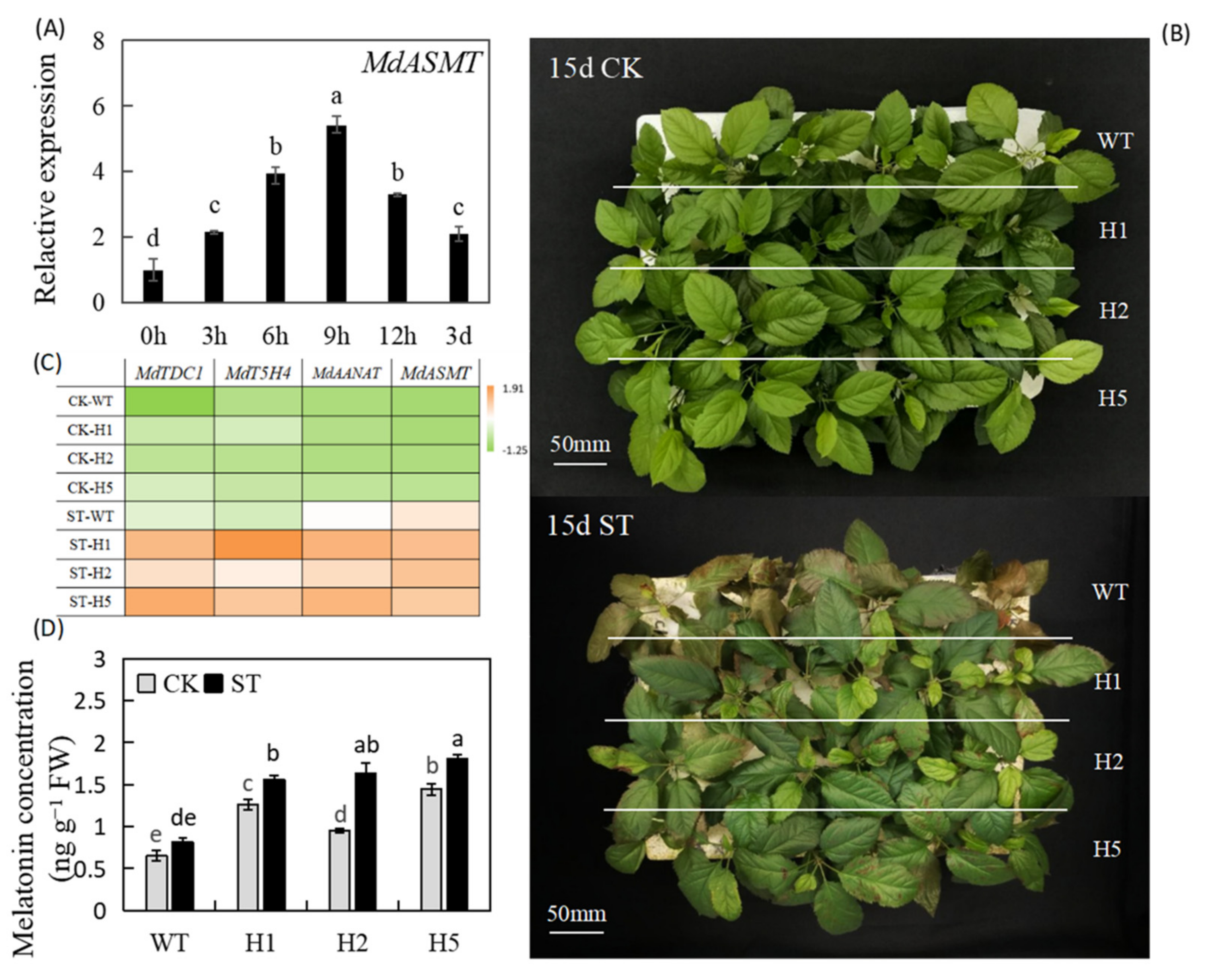
2.1. Heterologous Expression of HIOMT Improved Apple Salt Stress Tolerance and Increased Melatonin Concentration in Apple Leaves
2.2. Heterologous Expression of HIOMT Promoted Better Growth and Development of Plants under Salinity Stress
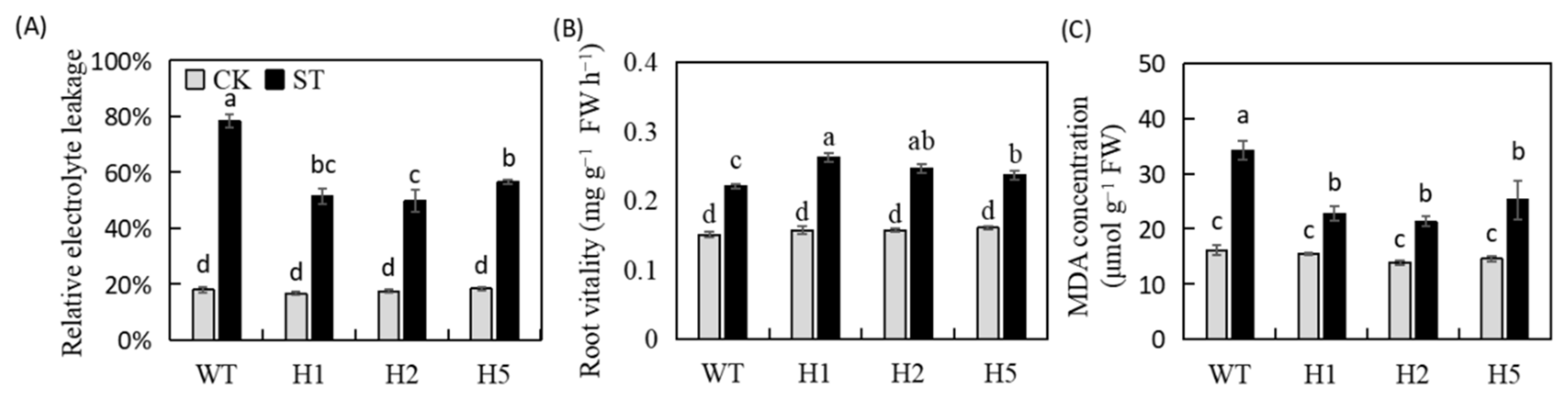
2.3. Heterologous Expression of HIOMT Changed REL, Root Vitality, and MDA Concentration in Apple
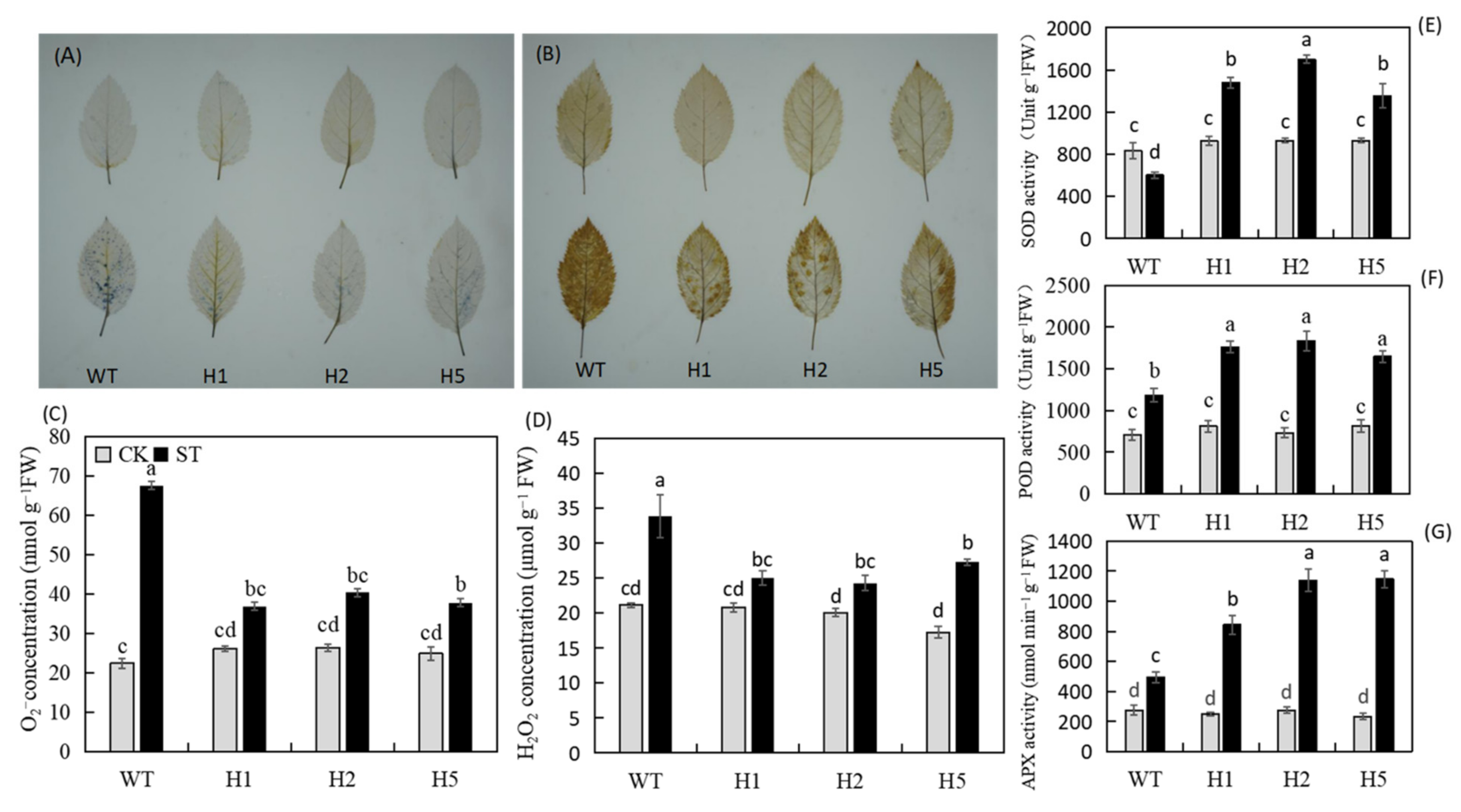
2.4. Heterologous Expression of HIOMT Enhanced the Antioxidant Activity of Apple Plants under Salinity Stress
2.5. Heterologous Expression of HIOMT Enabled Apple Plants to Maintain Higher Photosynthetic Capacity under Salinity Stress
2.6. Heterologous Expression of HIOMT Alleviated Stomatal Closure in Apple under Salinity Stress
2.7. Heterologous Expression of HIOMT Reduced the Na+/K+ Ratio in Apple Plants under Salinity Stress
2.8. Heterologous Expression of HIOMT Mediated Amino Acid Metabolism under Salinity Stress
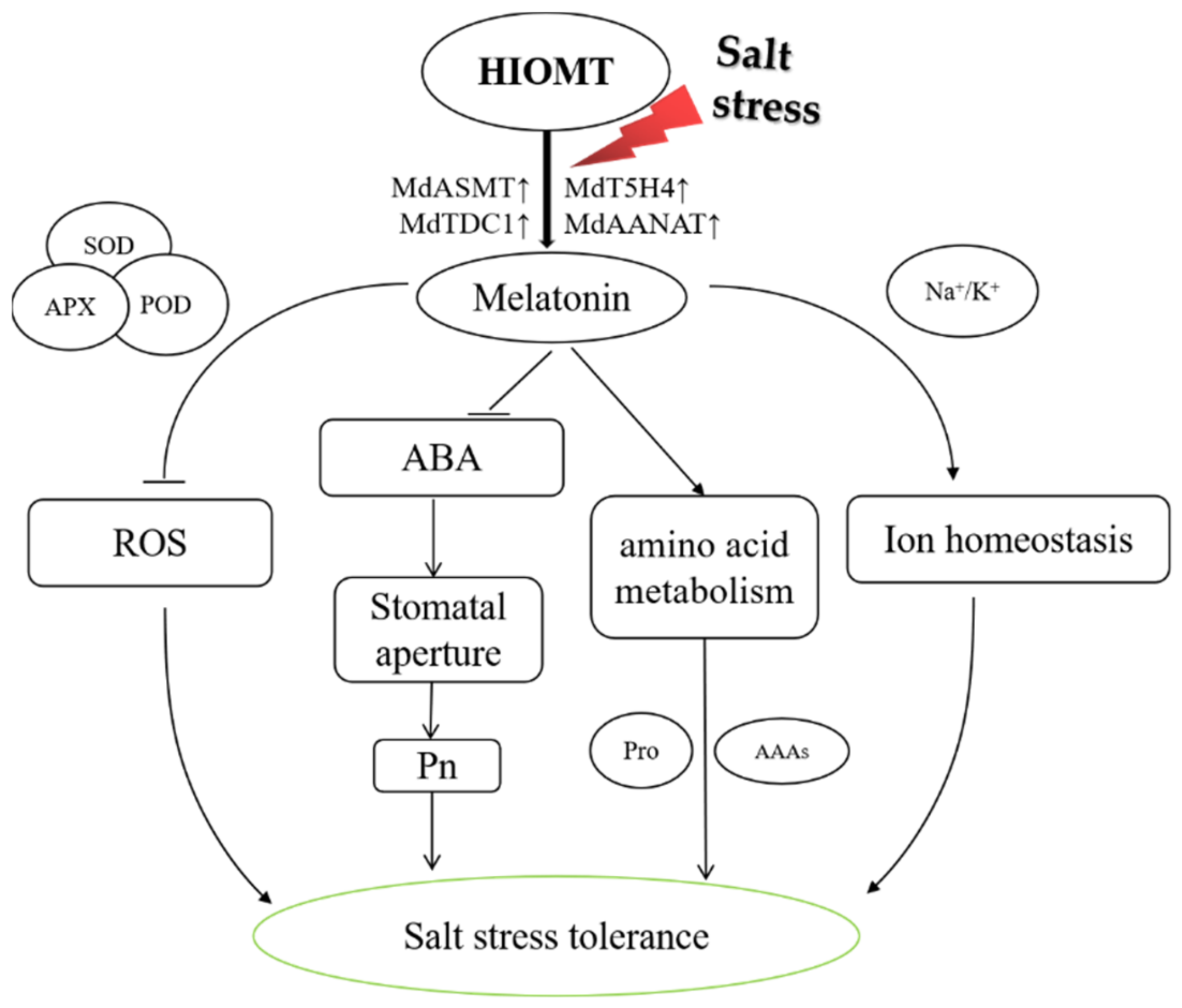
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Melatonin
4.3. Growth Measurements
4.4. Measurement of Relative Electrolyte Leakage, Root Vitality, and MDA Concentration
4.5. Qualitative and Quantitative Determination of H2O2 and O2−
4.6. Determination of Antioxidant Enzyme Activity
4.7. Quantification of Photosynthetic Parameters
4.8. Chlorophyll Concentration and Fv/Fm Measurements
4.9. Stomatal Observations by Scanning Electron Microscopy (SEM)
4.10. Determination of ABA Concentration
4.11. Measurements of Sodium and Potassium Ions
4.12. Measurements of Amino Acids
4.13. qRT–PCR Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, M.; Li, C.; Ma, F. Physiological responses and tolerance to NaCl stress in different biotypes of Malus prunifolia. Euphytica 2013, 189, 101–109. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, M.; Gong, X.; Liu, J. The transcription factor FcWRKY40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS2 and P5CS1 homologs. New Phytol. 2018, 219, 972–989. [Google Scholar] [CrossRef] [Green Version]
- Crawford, T.; Lehotai, N.; Strand, A. The role of retrograde signals during plant stress responses. J. Exp. Bot. 2018, 69, 83–95. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Z.; Wen, X.; Lu, C. Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Mol. Biol. 2007, 66, 73–86. [Google Scholar] [CrossRef]
- Van Zelm, E.V.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Barragan, V.; Leidi, E.; Andres, Z.; Rubio, L.; Luca, A.D.; Fernandez, J.; Cubero, B.; Pardo, J. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Wang, X.; Liu, L.; Lin, X.; Wang, W.; Qi, C.; Cao, Y.; Li, S.; Ren, S.; et al. PvNAC1 increases biomass and enhances salt tolerance by decreasing Na+ accumulation and promoting ROS scavenging in switchgrass (Panicum virgatum L.). Plant Sci. 2019, 280, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabala, L.; Huang, Y.; Bie, Z. An early ABA induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wei, Z.; Liang, D.; Zhou, S.; Li, Y.; Liu, C.; Ma, F. Enhanced salt resistance in apple plants overexpressing a malus vacuolar Na+/H+ antiporter gene is associated with differences in stomatal behavior and photosynthesis. Plant Physiol. Biochem. 2013, 70, 164–173. [Google Scholar] [CrossRef]
- Mahi, H.E.; Pérez-Hormaeche, J.; Luca, A.D.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.M.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A Critical Role of Sodium Flux via the Plasma Membrane Na+/H+ Exchanger SOS1 in the Salt Tolerance of Rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Salt and drought stress signal transduction in plants. Annu Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Quintero, F.; Pardo, J.; Zhu, J. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002, 14, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Zheng, X.; Tian, Y.; Wang, C. Exogenous Brassinolide Alleviates Salt Stress in Malus hupehensis Rehd. by Regulating the Transcription of NHX-Type Na+(K+)/H+ Antiporters. Front. Plant Sci. 2020, 11, 00038. [Google Scholar] [CrossRef]
- Ren, X.; Qi, G.; Feng, H.; Zhao, S.; Wu, W. Calcineurinb-like protein CBl10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013, 74, 258–266. [Google Scholar] [CrossRef]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front. Plant Sci. 2020, 11, 2110. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ping, W.; Wei, Z.; Dong, L.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.; Wang, J. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2021, 1–14. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2020, 105, 376–391. [Google Scholar] [CrossRef]
- Dhole, A.; Shelat, H. Phytomelatonin: A plant hormone for management of stress. J. Anal. Pharm. Res. 2018, 7, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, S.; Yuan, S.; Guan, C.; Tian, D.; Cui, X.; Zhang, Y.; Yang, F. Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Tan, D.; Hardeland, R.; Back, K.; Manchester, L.; Alatorre-Jiménez, M.; Reiter, R. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wei, Z.; Gao, T.; Zhang, Z.; Tan, K.; Li, C.; Feng, M. Ectopic expression of AANAT or HIOMT improves melatonin production and enhances UV-B. Fruit Res. 2021, 1, 4–13. [Google Scholar]
- Li, C.; Liang, B.; Chang, C.; Wei, Z.; Zhou, S.; Ma, F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016, 61, 218–229. [Google Scholar] [CrossRef]
- Li, J.; Yuan, F.; Liu, Y.; Zhang, M.; Chen, M. Exogenous melatonin enhances salt secretion from salt glands by upregulating the expression of ion transporter and vesicle transport genes in Limonium bicolor. BMC Plant Biol. 2020, 20, 493. [Google Scholar] [CrossRef]
- Gong, X.; Shi, S.; Dou, F.; Song, Y.; Ma, F. Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by Regulating the Biosynthesis of Polyamines. Molecules 2017, 22, 1542. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Sun, S.; Zhao, N.; Yang, W.; Shi, Q.; Gong, B. COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ. Pollut. 2019, 252, 51–61. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Li, T.; Marcelis, L. Salt Stress Slows Down Dynamic Photosynthesis Mainly through Osmotic Effects on Dynamic Stomatal Behavior. Chemistry 2021. Available online: https://europepmc.org/article/ppr/ppr351620 (accessed on 1 October 2021).
- Harrison, E.L.; Cubas, L.A.; Gray, J.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2019, 101, 768–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Gao, T.; Liang, B.; Zhao, Q.; Ma, F.; Li, C. Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int. J. Mol. Sci. 2018, 19, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178–E11187. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.F.; Ma, F.W. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Z.K.; Mobin, M. Comparative growth and physiological responses of two wheat (Triticum aestivum L.) cultivars differing in salt tolerance to salinity and cyclic drought stress. Arch. Agron. Soil Sci. 2016, 62, 745–758. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, X.; Cen, H.; Wang, K.; Zhang, Y. Transcriptomic analysis reveals vacuolar Na+ (K+)/H+ antiporter gene contributing to growth, development, and defense in switchgrass (Panicum virgatum L.). BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Ma, Q.; Zhang, J.; Hu, J.; Bao, A.; Wei, L.; Wang, Q.; Luan, S.; Wang, S. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 2015, 395, 173–187. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Kanawapee, N.; Sanitchon, J.; Lontom, W.; Threerakulpisut, P. Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil 2012, 358, 235–249. [Google Scholar] [CrossRef]
- Lynch, J.H.; Dudareva, N. Aromatic amino acids: A complex network ripe for future exploration. Trends Plant Sci. 2020, 25, 670–681. [Google Scholar] [CrossRef]
- Michard, E.; Simon, A. Melatonin’s antioxidant properties protect plants under salt stress. Plant Cell Environ. 2020, 43, 2587–2590. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Tan, K.; Zheng, J.; Gao, T.; Zhang, Z.; Zhao, Y.; Ma, F.; Li, C. MdTyDc overexpression improves alkalinity tolerance in Malus domestica. Front. Plant Sci. 2021, 12, 213. [Google Scholar] [CrossRef]
- Pothinuch, P.; Tongchitpakdee, S. Melatonin contents in mulberry (morus spp.) leaves: Effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011, 128, 415–419. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, D.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef]
- Liang, B.; Gao, T.; Zhao, Q.; Ma, C.; Chen, Q.; Wei, Z.; Ma, F. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Li, Y.; Chen, X.; Zhang, Z.; Liu, B.; Li, P.; Gong, X.; Ma, F. MdUGT88F1-mediated phloridzin biosynthesis regulates apple development and valsa canker resistance. Plant Physiol. 2019, 180, 2290–2305. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.; Guo, Z.; Wang, P.; Zhang, Z.; Jia, X.; Sun, Y.; Sun, X.; Gong, X.; Ma, F. MdATG8i functions positively in apple salt tolerance by maintaining photosynthetic ability and increasing the accumulation of arginine and polyamines. Environ. Exp. Bot. 2020, 172, 103989. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Zhang, D.; Pan, X.; Chang, F.; Wang, S. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B Biol. 2013, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, C.; Chao, L.; Dong, L.; Ma, F. Contrasting hypoxia tolerance and adaptation in malus species is linked to differences in stomatal behavior and photosynthesis. Physiol. Plant. 2013, 147, 514–523. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhao, Y.B.; Wang, G.P.; Chang, R.F.; Li, C.M.; Shu, H.R. Dynamics of endogenous cytokinins during phase change in Malus domestica Borkh. Acta Hort. 2008, 774, 29–33. [Google Scholar] [CrossRef]
- Perini, P.; Pasquali, G.; Margis-Pinheiro, M.; Oliviera, P.; Revers, L. Erratum to: Reference genes for transcriptional analysis of flowering and fruit ripening stages in apple (Malus domestica Borkh.). Mol. Breed. 2014, 34, 829–842. [Google Scholar] [CrossRef]





| Treatment | SH (cm) | RL (cm) | LN (No. Plant−1) | LFW (g) | LDW (g) | TFW (g) | TDW (g) | Relative |
|---|---|---|---|---|---|---|---|---|
| Growth | ||||||||
| CK-WT | 13.97 ± 0.91 a | 7.50 ± 1.78 c | 12.50 ± 1.05 a | 1.32 ± 0.10 a | 0.45 ± 0.05 a | 2.11 ± 0.16 a | 0.62 ± 0.05 a | 47.9% |
| ST-WT | 9.84 ± 0.26 b | 8.60 ± 1.36 b,c | 8.17 ± 0.75 c | 0.51 ± 0.11 c | 0.21 ± 0.05 c | 1.01 ± 0.22 c | 0.31 ± 0.06 c | |
| CK-H1 | 13.27 ± 1.07 a | 9.05 ± 0.82 b,c | 13.00 ± 0.89 a | 1.34 ± 0.16 a | 0.43 ± 0.07 a | 2.11 ± 0.14 a | 0.61 ± 0.08 a | 65.9% |
| ST-H1 | 10.27 ± 0.42 b | 10.86 ± 2.04 a | 10.00 ± 1.41 b | 0.82 ± 0.21 b | 0.32 ± 0.08 b | 1.39 ± 0.22 b | 0.45 ± 0.08 b | |
| CK-H2 | 13.32 ± 1.28 a | 8.68 ± 1.39 b,c | 12.50 ± 1.05 a | 1.36 ± 0.13 a | 0.48 ± 0.06 a | 2.20 ± 0.25 a | 0.64 ± 0.08 a | 68.2% |
| ST-H2 | 9.97 ± 0.33 b | 10.30 ± 0.97 a,b | 9.83 ± 1.47 b | 0.89 ± 0.13 b | 0.35 ± 0.07 b | 1.50 ± 0.15 b | 0.48 ± 0.08 b | |
| CK-H5 | 13.90 ± 1.45 a | 8.48 ± 1.53 b,c | 13.67 ± 1.63 a | 1.40 ± 0.17 a | 0.47 ± 0.06 a | 2.26 ± 0.24 a | 0.64 ± 0.08 a | 65.0% |
| ST-H5 | 10.41 ± 0.56 b | 9.67 ± 0.53 a, b | 9.50 ± 1.05 b | 0.85 ± 0.12 b | 0.34 ± 0.07 b | 1.47 ± 0.19 b | 0.48 ± 0.09 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, K.; Zheng, J.; Liu, C.; Liu, X.; Liu, X.; Gao, T.; Song, X.; Wei, Z.; Ma, F.; Li, C. Heterologous Expression of the Melatonin-Related Gene HIOMT Improves Salt Tolerance in Malus domestica. Int. J. Mol. Sci. 2021, 22, 12425. https://doi.org/10.3390/ijms222212425
Tan K, Zheng J, Liu C, Liu X, Liu X, Gao T, Song X, Wei Z, Ma F, Li C. Heterologous Expression of the Melatonin-Related Gene HIOMT Improves Salt Tolerance in Malus domestica. International Journal of Molecular Sciences. 2021; 22(22):12425. https://doi.org/10.3390/ijms222212425
Chicago/Turabian StyleTan, Kexin, Jiangzhu Zheng, Cheng Liu, Xianghan Liu, Xiaomin Liu, Tengteng Gao, Xinyang Song, Zhiwei Wei, Fengwang Ma, and Chao Li. 2021. "Heterologous Expression of the Melatonin-Related Gene HIOMT Improves Salt Tolerance in Malus domestica" International Journal of Molecular Sciences 22, no. 22: 12425. https://doi.org/10.3390/ijms222212425
APA StyleTan, K., Zheng, J., Liu, C., Liu, X., Liu, X., Gao, T., Song, X., Wei, Z., Ma, F., & Li, C. (2021). Heterologous Expression of the Melatonin-Related Gene HIOMT Improves Salt Tolerance in Malus domestica. International Journal of Molecular Sciences, 22(22), 12425. https://doi.org/10.3390/ijms222212425






