Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins
Abstract
1. Introduction
2. Results and Discussion
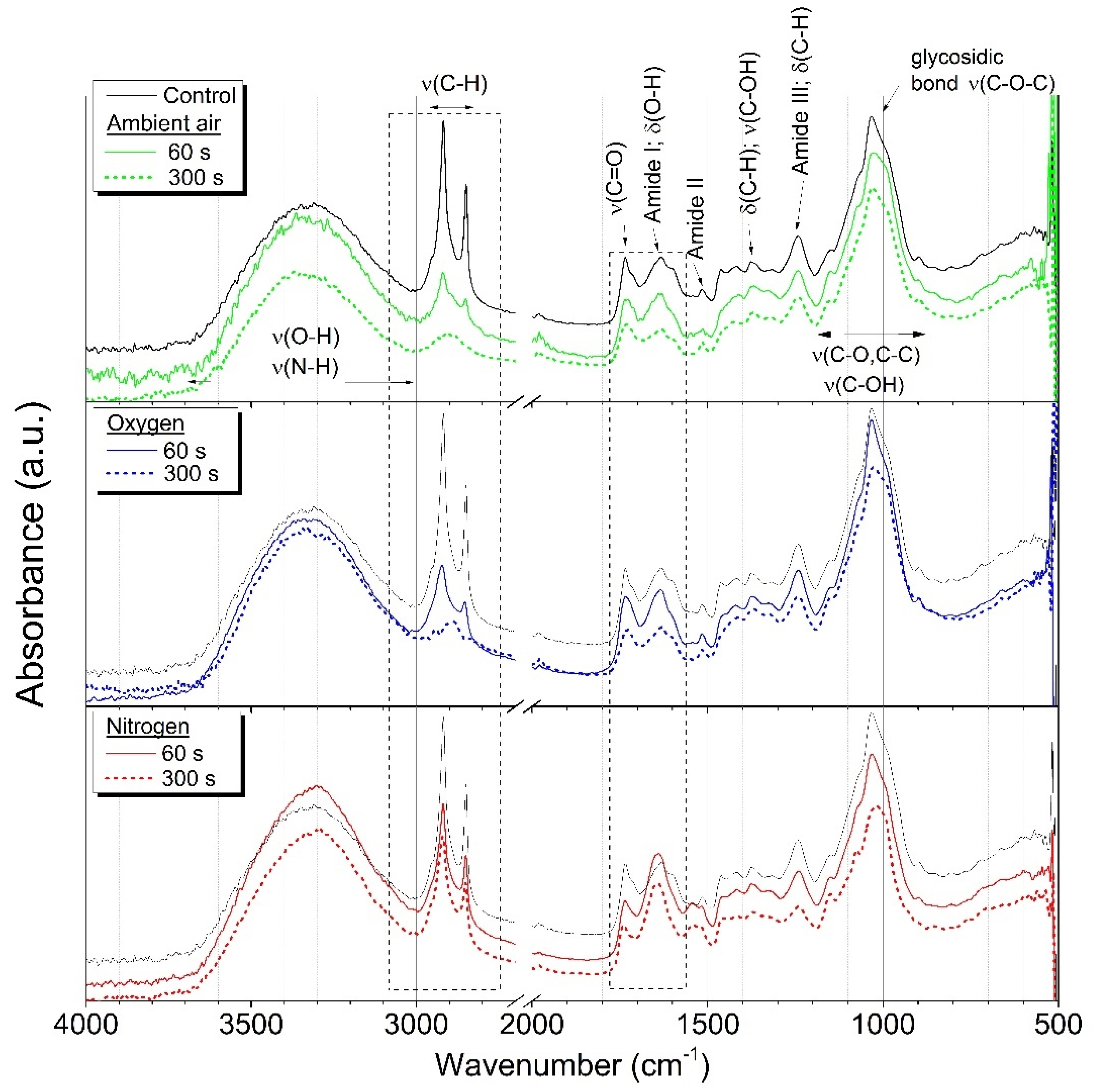
2.1. ATR-FTIR
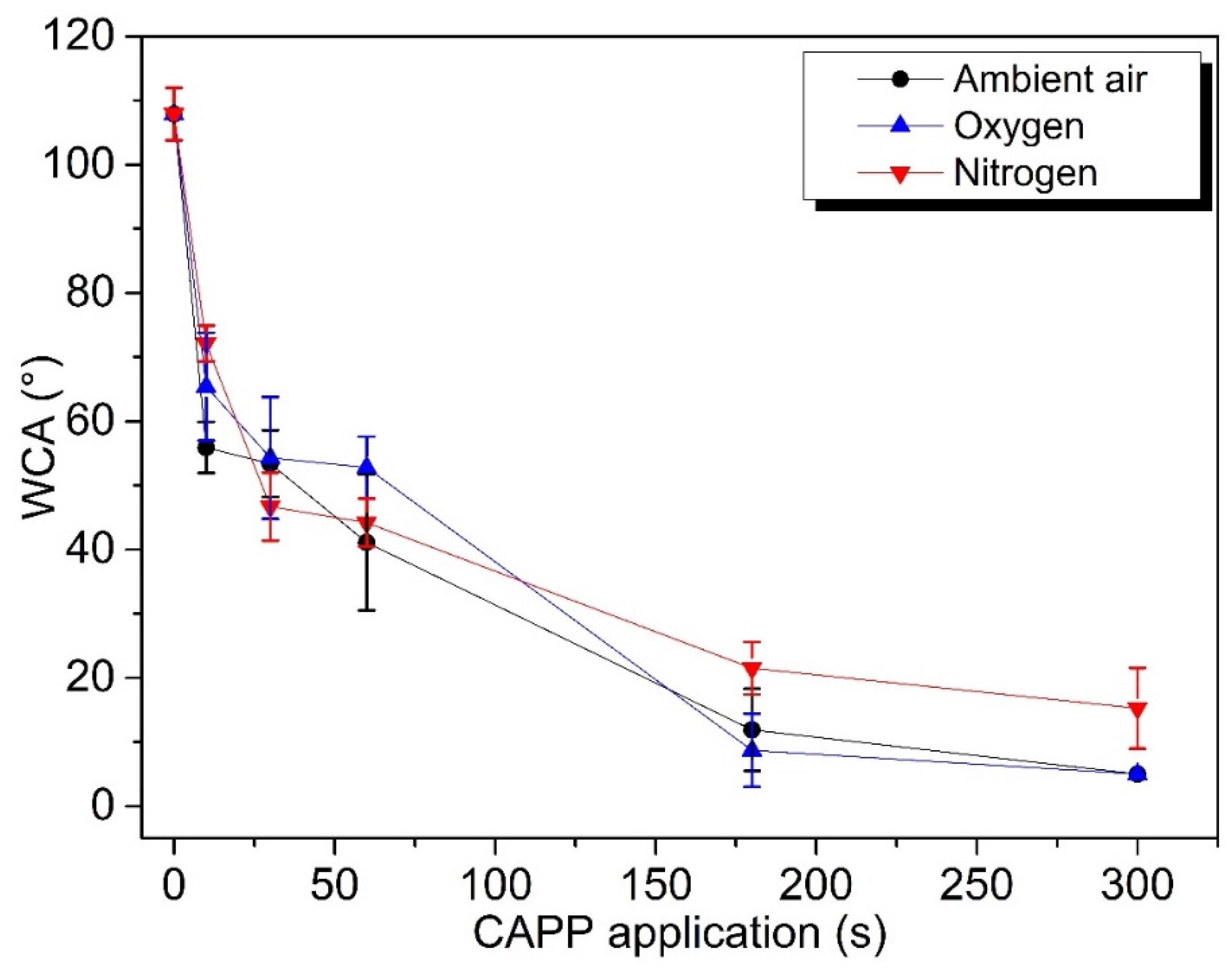
2.2. Wettability
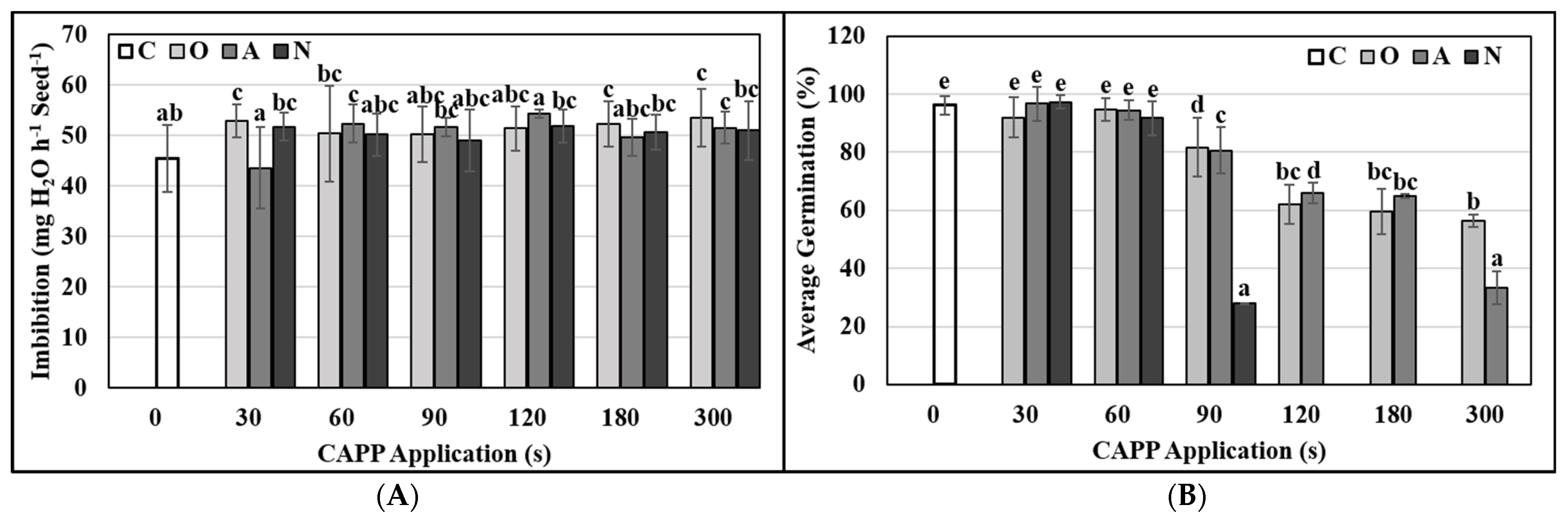
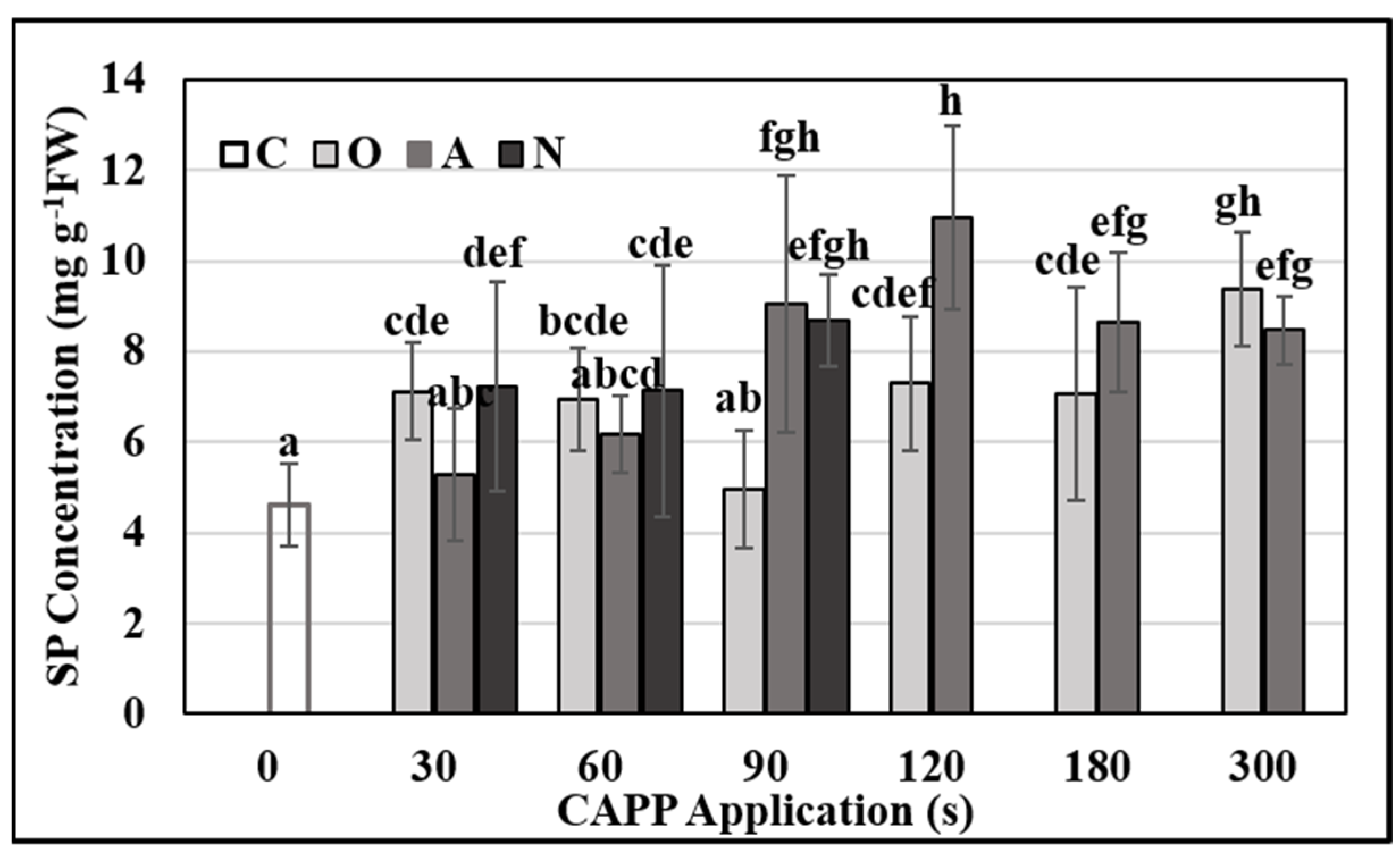
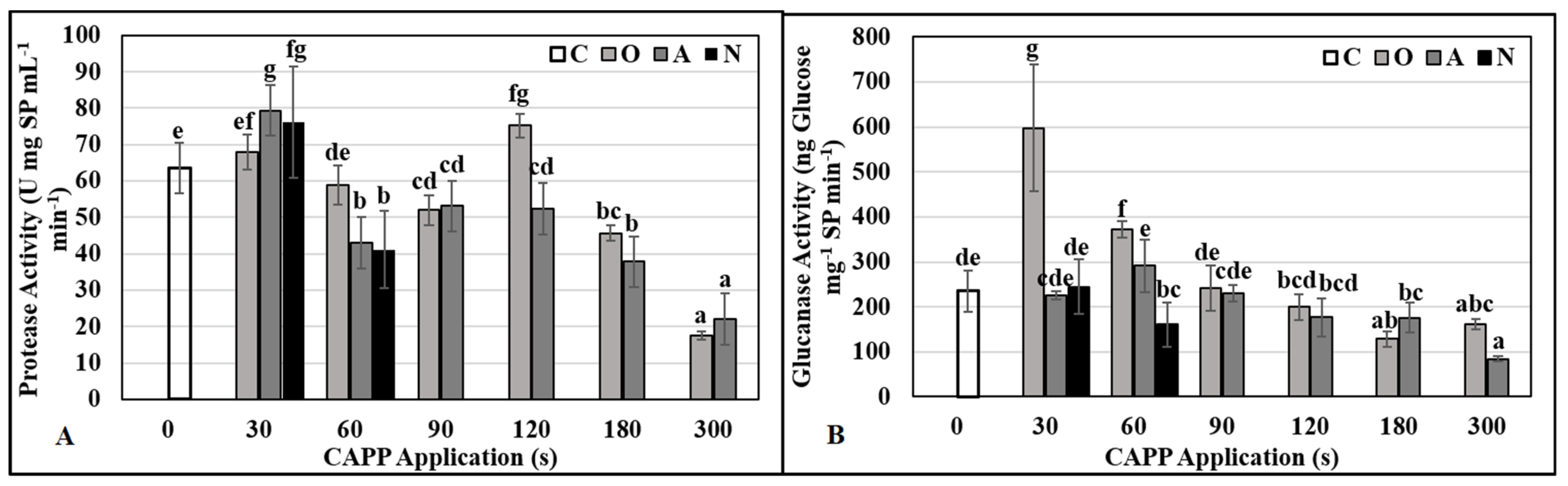
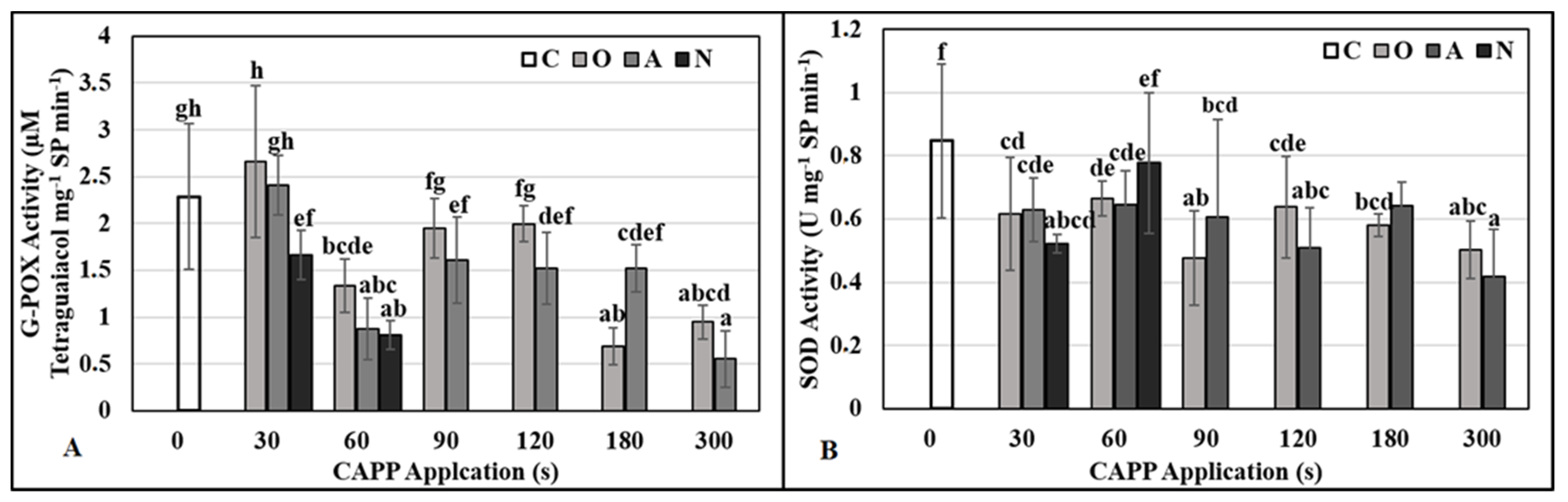
2.3. Physiological and Biochemical Parameters
2.4. Comet Assay
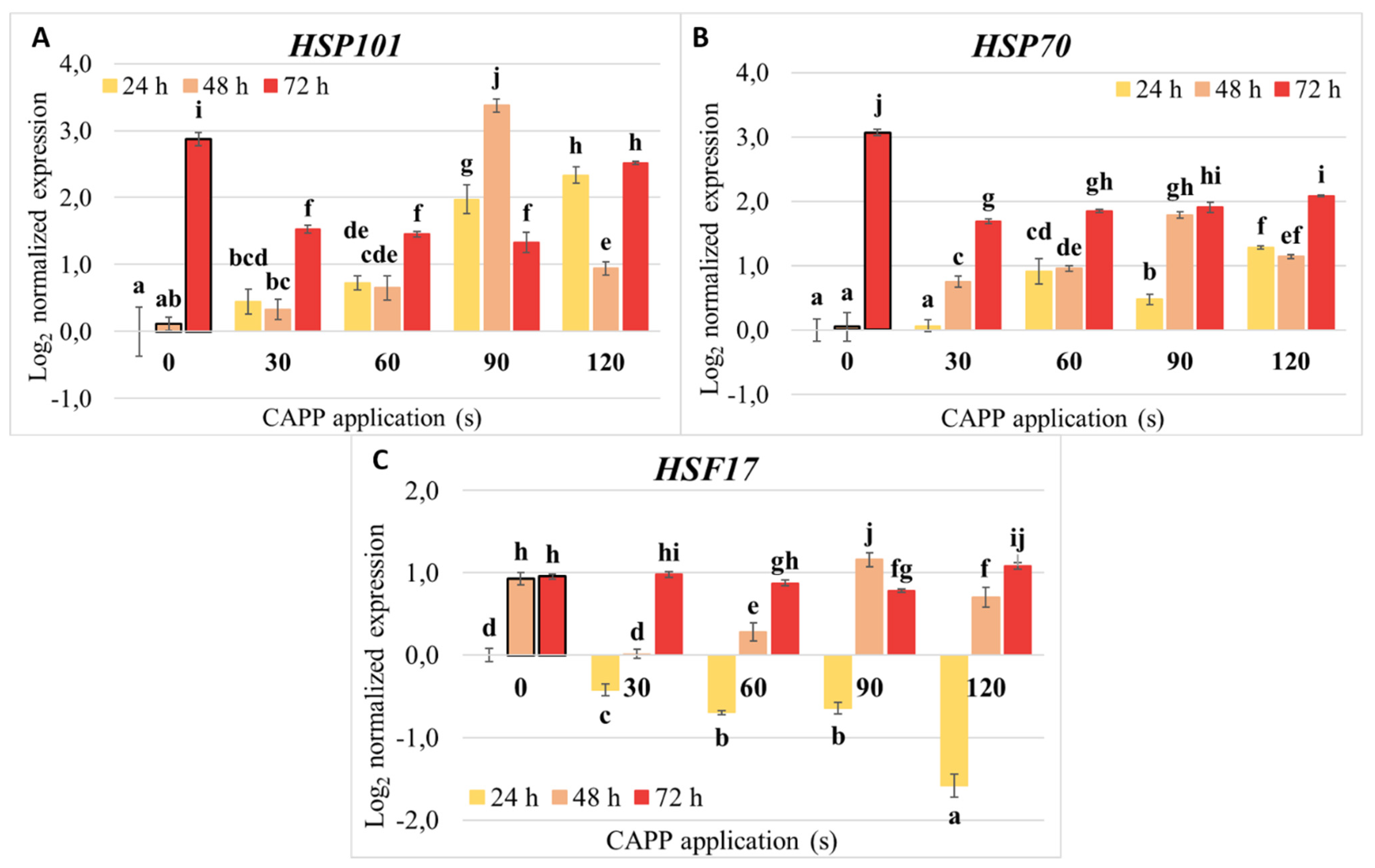
2.5. Expression of Heat Shock Proteins
3. Materials and Methods
3.1. Plant Material
3.2. Plasma Source and Treatment of Maize Grains
3.3. ATR-FTIR
3.4. Wettability Properties
3.5. Imbibition, Germination and Growth Conditions
3.6. Total Soluble Proteins Content
3.7. Assay on Lytic Enzymes Assessment
3.8. Assay on Antioxidant Enzymes Assessment
3.9. Comet Assay
3.10. RT-PCR
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO, Ed.; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef] [PubMed]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem. Plasma Process. 2020, 40, 1571–1584. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, e01495. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.Y.; Kim, Y.S.; Balaraju, K.; Mok, Y.S.; Yoo, S.J.; Jeon, Y. Enhancement of seed germination and microbial disinfection on ginseng by cold plasma treatment. J. Ginseng Res. 2020, in press. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.I.; Gupta, D.; Jacob, M.V. Afterglow of atmospheric non-thermal plasma for disinfection of lentil seeds from botrytis grey mould. Innov. Food Sci. Emerg. Technol. 2020, 66, 102488. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effects of nonthermal plasma treatment on decontamination and sprouting of radish (Raphanus sativus L.) seeds. Food Bioprocess. Technol. 2017, 10, 1093–1102. [Google Scholar] [CrossRef]
- Pechanova, O.; Pechan, T. Maize-pathogen interactions: An ongoing combat from a proteomics perspective. Int. J. Mol. Sci. 2015, 16, 28429–28448. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef]
- Volkov, A.G.; Hairston, J.S.; Patel, D.; Gott, R.P.; Xu, K.G. Cold Plasma poration and corrugation of pumpkin seed coats. Bioelectrochemistry 2019, 128, 175–185. [Google Scholar] [CrossRef]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Vajpayee, M.; Singh, M.; Ledwani, L. Non-thermal plasma treatment of cellulosic biopolymer to enhance its surface property for various applications: A Review. Mater. Today Proc. 2021, 43, 3250–3255. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Švubová, R.; Slováková, L.; Holubová, L.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the impact of cold atmospheric pressure plasma on soybean seed germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef]
- Pet’ková, M.; Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, L.; Ševčovičová, A.; Gálová, E. The effects of cold atmospheric pressure plasma on germination parameters, enzyme activities and induction of DNA damage in barley. Int. J. Mol. Sci. 2021, 22, 2833. [Google Scholar] [CrossRef]
- Cui, D.; Yin, Y.; Wang, J.; Wang, Z.; Ding, H.; Ma, R.; Jiao, Z. Research on the physio-biochemical mechanism of non-thermal plasma-regulated seed germination and early seedling development in Arabidopsis. Front. Plant. Sci. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo l.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Puač, N.; Petrović, Z.L.; Živković, S.; Giba, Z.; Grubbišić, D.; Dordević, A.R. Low-temperature plasma treatment of dry empress-tree seeds. In Plasma Processes and Polymers; d’Agostino, R., Favia, P., Oehr, C., Wertheimer, M.R., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 193–203. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Banzet, N.; Richaud, C.; Deveaux, Y.; Kazmaier, M.; Gagnon, J.; Triantaphylidés, C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant. J. 1998, 13, 519–527. [Google Scholar] [CrossRef]
- Banti, V.; Mafessoni, F.; Loreti, E.; Alpi, A.; Perata, P. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant. Physiol. 2010, 152, 1471–1483. [Google Scholar] [CrossRef]
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A Hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant. Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein. Plant. Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Cardi, M.; Lentini, M.; Castiglia, D.; Cianciullo, P.; Conte, B.; Loppi, S.; Esposito, S. Effects of heavy metals on ultrastructure and Hsp70 induction in Lemna minor L. exposed to water along the Sarno river, Italy. Ecotoxicol. Environ. Saf. 2015, 114, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Vasques, C.T.; Domenech, S.C.; Severgnini, V.L.S.; Belmontte, L.A.O.; Barreto, P.L.M.; Soldi, V. Effect of thermal treatment on the stability and structure of maize starch cast films. Starch/Stärke 2007, 59, 161–170. [Google Scholar] [CrossRef]
- Kuhnen, S.; Ogliari, J.B.; Dias, P.F.; Boffo, E.F.; Correia, I.; Ferreira, G.; Delgadillo, I.; Maraschin, M. ATR-FTIR spectroscopy and chemometric analysis applied to discrimination of landrace maize flours produced in southern Brazil. Int. J. Food Sci. Technol. 2010, 45, 1673–1681. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Huang, C.; Jiang, B.; Zhang, T. Impact of B-amylase degradation on properties of sugary maize soluble starch particles. Food Chem. 2015, 177, 1–7. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR—ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Jayaram, S.; Kapoor, S.; Dharmesh, S.M. Pectic polysaccharide from corn (Zea mays L.) effectively inhibited multi-step mediated cancer cell growth and metastasis. Chem. Biol. Interact. 2015, 235, 63–75. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Chapter 4. Imbibition, germination, and growth. In Physiology and Biochemistry of Seeds in Relation to Germination; Springer: Berlin/Heidelberg, Germany, 1978; pp. 106–131. [Google Scholar] [CrossRef]
- Miano, A.C.; Augusto, P.E.D. The hydration of grains: A critical review from description of phenomena to process improvements. Compr. Rev. Food Sci. Food Saf. 2018, 17, 352–370. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds—Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Hou, J.Q.; Kendall, E.J.; Simpson, G.M. Water uptake and distribution in non-dormant and dormant wild oat (Avena fatua L.) caryopses. J. Exp. Bot. 1997, 48, 683–692. [Google Scholar] [CrossRef]
- Yin, M.; Huang, M.; Ma, B.; Ma, T. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar]
- Dubinov, A.E.; Lazarenko, E.M.; Selemir, V.D. Effect of glow discharge air plasma on grain crops seed. IEEE Trans. Plasma Sci. 2000, 28, 180–183. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess. Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, R.; De Groot, G.; Bazaka, K.; Murphy, A.B.; Ostrikov, K.K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 5601:1–5601:9. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Characterization of water distribution and activities of enzymes during germination in magnetically-exposed maize (Zea mays L) Seeds. Indian J. Biochem. Biophys. 2010, 47, 311–318. [Google Scholar]
- Computer and computing technologies in agriculture VIII. In Proceedings of the 8th IFIP WG 5.14 International Conference, CCTA 2014, Beijing, China, 16–19 September 2014; Volume 258, p. 756. [CrossRef]
- Feng, J.; Pang, A.; Wang, D.; Wang, G.; Liu, L.; Shao, C.; He, C. Effect of different time and power of low-temperature helium plasma on germination characteristics of maize (Zea mays L.). In Proceedings of the 2016 American society of agricultural and biological engineers annual international meeting, Orlando, FL, USA, 17–20 July 2016; pp. 2–7. [Google Scholar] [CrossRef]
- Karmakar, S.; Billah, M.; Hasan, M.; Sohan, S.R.; Hossain, M.F.; Faisal Hoque, K.M.; Kabir, A.H.; Rashid, M.M.; Talukder, M.R.; Reza, M.A. Impact of LFGD (Ar+O2) plasma on seed surface, germination, plant growth, productivity and nutritional composition of maize (Zea mays L.). Heliyon 2021, 7, e06458. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop. Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Ashraf, M. Protease activity and associated changes during germination and early seedling stages of cotton grown under saline conditions. Int. J. Bot. 2005, 1, 103–107. [Google Scholar] [CrossRef]
- Tsuji, A.; Tsukamoto, K.; Iwamoto, K.; Ito, Y.; Yuasa, K. Enzymatic characterization of germination-specific cysteine protease-1 expressed transiently in cotyledons during the early phase of germination. J. Biochem. 2013, 153, 73–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müntz, K.; Belozersky, M.A.; Dunaevsky, Y.E.; Schlereth, A.; Tiedemann, J. Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, H.T.; Choi, U.; Ryu, H.S.; Lee, S.J.; Kwon, O. Mobilization of storage proteins in soybean seed (Glycine max L.) during germination and seedling growth. BBA Proteins Proteom. 2011, 1814, 1178–1187. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiate). LWT Food Sci. Technol. 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Su, L.; Lan, Q.; Pritchard, H.W.; Xue, H.; Wang, X. Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant. Physiol. Biochem. 2016, 109, 406–415. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Zahoranová, A.; Ševčovičová, A.; Gálová, E. Genotoxic effect of low temperature plasma treatment on plant seeds. Toxicol. Lett. 2017, 280S, S119. [Google Scholar] [CrossRef]
- Asada, K. The water- water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant. Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Felzer, B.S.; Cronin, T.; Reilly, J.M.; Melillo, J.M.; Wang, X. Impacts of ozone on trees and crops. C. R. Geosci. 2007, 339, 784–798. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Jha, M.; Tuteja, N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci. World J. 2015, 2015, 250158. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitric oxide from a “green” perspective. Nitric Oxide Biol. Chem. 2015, 45, 15–19. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant. Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- McLoughlin, F.; Kim, M.; Marshall, R.S.; Vierstra, R.D.; Vierling, E. HSP101 interacts with the proteasome and promotes the clearance of ubiquitylated protein aggregates. Plant. Physiol. 2019, 180, 1829–1847. [Google Scholar] [CrossRef]
- Hong, S.W.; Vierling, E. Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant. J. 2001, 27, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sotelo, J.; Martínez, L.M.; Ponce, G.; Cassab, G.I.; Alagón, A.; Meeley, R.B.; Ribaut, J.M.; Yang, R. Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant. Cell 2002, 14, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Katiyar-Agarwal, S.; Grover, A. Plant Hsp100 proteins: Structure, function and regulation. Plant. Sci. 2002, 163, 397–405. [Google Scholar] [CrossRef]
- Kumar, R.; Khungar, L.; Shimphrui, R.; Tiwari, L.D.; Tripathi, G.; Sarkar, N.K.; Agarwal, S.K.; Agarwal, M.; Grover, A. AtHsp101 research sets course of action for the genetic improvement of crops against heat stress. J. Plant Biochem. Biotechnol. 2020, 29, 715–732. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ardebili, N.O.; Ardebili, Z.O.; Shafaati, M.; Ghoranneviss, M. Non-thermal plasma induced expression of heat shock factor A4A and improved wheat (Triticum aestivum L.) growth and resistance against salt stress. Plasma Chem. Plasma Process. 2018, 38, 29–44. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, Ľ.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. EDP Sci. 2009, 47, 1–6. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1951, 195, 19–23. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Matušíková, I.; Salaj, J.; Moravčíková, J.; Mlynárová, L.; Nap, J.P.; Libantová, J. Tentacles of in vitro-grown round-leaf sundew (Drosera rotundifolia L.) show induction of chitinase activity upon mimicking the presence of prey. Planta 2005, 222, 1020–1027. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Frič, F.; Fuchs, W.H. Veranderungen der Aktivität Einiger Enzyme im Weizenblatt in Abhängigkeit von der Temperaturlabilen Verträglichkeit für Puccinia graminis tritici. J. Phytopathol. 1970, 67, 161–174. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Horváthová, E.; Kozics, K.; Melusová, M.; Galová, E.; Ševčovičová, A.; Kusznierewicz, B.; Chmiel, T.; Slamenová, D. Chromatographic analyses of Lavandula angustifolia and Rosmarinus officinalis extracts and their biological effects in mammalian cells and cell-free systems. Neoplasma 2017, 64, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Aranda, P.S.; LaJoie, D.M.; Jorcyk, C.L. Bleach Gel: A simple agarose gel for analyzing RNA quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]

| CAPP Application (s) | Germination Potential (%) | Germination Index (%) | Grain Vitality Index (%) | Seedling Vitality Index (%) | Seedling Length Index (%) |
|---|---|---|---|---|---|
| C (0) | 96.9 ± 3.52 f | 10.84 ± 1.64 jk | 158.1 ± 23.9 fgh | 0.34 ± 0.08 ef | 172.8 ± 20.5 ef |
| O30 | 94.0 ± 8.48 f | 8.66 ± 1.88 ghi | 205.4 ± 18.5 i | 0.34 ± 0.09 ef | 189.5 ± 41.2 f |
| O60 | 92.3 ± 6.32 f | 11.66 ± 0.38 k | 124.4 ±2 9.7 cde | 0.29 ± 0.11 def | 154.2 ± 34.9 def |
| O90 | 78.7 ± 12.3 ef | 7.00 ± 0.05 efgh | 152.9 ± 24.0 efgh | 0.27 ± 0.04 cde | 136.0 ± 1.0 cde |
| O120 | 64.2 ± 5.83 de | 7.56 ± 0.79 fgh | 92.3 ± 4.7 bc | 0.23 ± 0.01 bcde | 107.3 ± 11.3 bc |
| O180 | 59.5 ± 7.77 bcde | 7.00 ± 0.35 efgh | 78.5 ± 26.8 b | 0.14 ± 0.03 ab | 98.1 ± 33.5 bc |
| O300 | 58.0 ± 2.82 bcde | 4.83 ± 0.23 bcde | 73.9 ± 21.2 ab | 0.08 ± 0.02 a | 90.8 ± 23.6 bc |
| A30 | 95.0 ± 7.07 f | 8.66 ± 0.47 ghi | 189. 6 ± 14.1 hi | 0.29 ± 0.06 def | 163.8 ± 17.3 ef |
| A60 | 93.0 ± 4.35 f | 10.75 ± 1.75 ijk | 145.6 ± 28.8 ef | 0.31 ± 0.09 def | 182.1 ± 36.0 f |
| A90 | 67.0 ± 4.24 abc | 6.16 ± 1.17 cdef | 139.1 ± 8.8 def | 0.29 ± 0.02 def | 113.3 ± 3.7 bcd |
| A120 | 68.0 ± 2.82 cde | 5.50 ± 0.71 abcd | 96.6 ± 4.1 bcd | 0.22 ± 0.01 bcd | 96.3 ± 5.1 bc |
| A180 | 56.7 ± 14.52 abcd | 4.40 ± 1.12 abc | 94.6 ± 2.3 bc | 0.21 ± 0.07 bcd | 87.0 ± 12.1 ab |
| A300 | 36.2 ± 5.44 ab | 2.91 ± 0.87 ab | 38.0 ± 5.2 a | 0.17 ± 0.01 abc | 49.3 ± 6.9 a |
| N30 | 98.0 ± 2.82 f | 9.00 ± 1.41 hij | 188.0 ± 5.4 ghi | 0.35 ± 0.05 ef | 172.7 ± 27.1 ef |
| N60 | 87.2 ± 6.31 f | 6.66 ± 1.52 defg | 148.4 ± 28.3 efg | 0.39 ± 0.11 f | 182.5 ± 30.2 f |
| N90 | 28.0 ± 0.50 a | 2.33 ± 0.23 a | – | – | – |
| N120 | – | – | – | – | – |
| N180 | – | – | – | – | – |
| N300 | – | – | – | – | – |
| Gene | Primer | Primer Sequence (5′->3′) |
|---|---|---|
| HSP101 (NM_001111465.2) | HSP101_F | CGAGGTACATCATGGGTCGG |
| HSP101_R | GGCTTTACTGGCCTTGTCCT | |
| HSP70 (NM_001196236.1) | HSP70_F | AGCTTGAGGGGATCTGCAAC |
| HSP70_R | CCTCACCAAACCAAACCTCG | |
| HSF17 (MK736813.1) | HSF17_F | GCCTGATTGGGCCACATGAT |
| HSF17_R | CAGGGCTATCGTTCTCCTCG |
| Gene | Primer | Primer Sequence (5′->3′) |
|---|---|---|
| EF1A (NM_001112117.2) | EF1α_F | TGGGCCTACTGGTCTTACTACTGA |
| EF1α_R | ACATACCCACGCTTCAGATCCT | |
| TUB B (NM_001112218.1) | TUB β_F | CTACCTCACGGCATCTGCTATGT |
| TUB β_R | GTCACACACACTCGACTTCACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubová, Ľ.; Švubová, R.; Slováková, Ľ.; Bokor, B.; Chobotová Kročková, V.; Renčko, J.; Uhrin, F.; Medvecká, V.; Zahoranová, A.; Gálová, E. Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins. Int. J. Mol. Sci. 2021, 22, 8509. https://doi.org/10.3390/ijms22168509
Holubová Ľ, Švubová R, Slováková Ľ, Bokor B, Chobotová Kročková V, Renčko J, Uhrin F, Medvecká V, Zahoranová A, Gálová E. Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins. International Journal of Molecular Sciences. 2021; 22(16):8509. https://doi.org/10.3390/ijms22168509
Chicago/Turabian StyleHolubová, Ľudmila, Renáta Švubová, Ľudmila Slováková, Boris Bokor, Valéria Chobotová Kročková, Ján Renčko, Filip Uhrin, Veronika Medvecká, Anna Zahoranová, and Eliška Gálová. 2021. "Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins" International Journal of Molecular Sciences 22, no. 16: 8509. https://doi.org/10.3390/ijms22168509
APA StyleHolubová, Ľ., Švubová, R., Slováková, Ľ., Bokor, B., Chobotová Kročková, V., Renčko, J., Uhrin, F., Medvecká, V., Zahoranová, A., & Gálová, E. (2021). Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins. International Journal of Molecular Sciences, 22(16), 8509. https://doi.org/10.3390/ijms22168509






