USP48 Governs Cell Cycle Progression by Regulating the Protein Level of Aurora B
Abstract
1. Introduction
2. Results
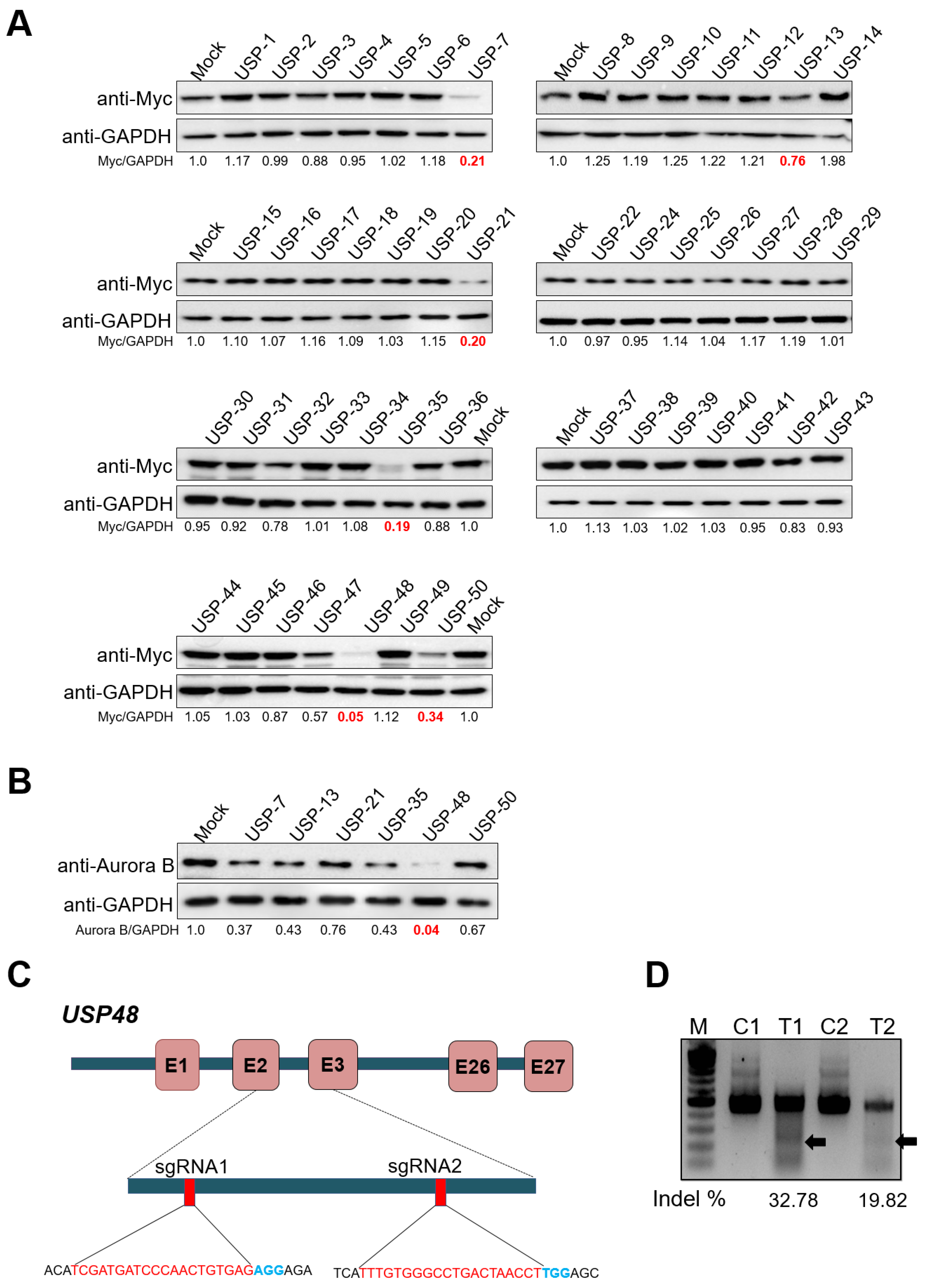
2.1. CRISPR-Based Genome-Scale Screening of USP Sub-Family Proteins Exhibiting Reduction of Aurora B Protein Levels
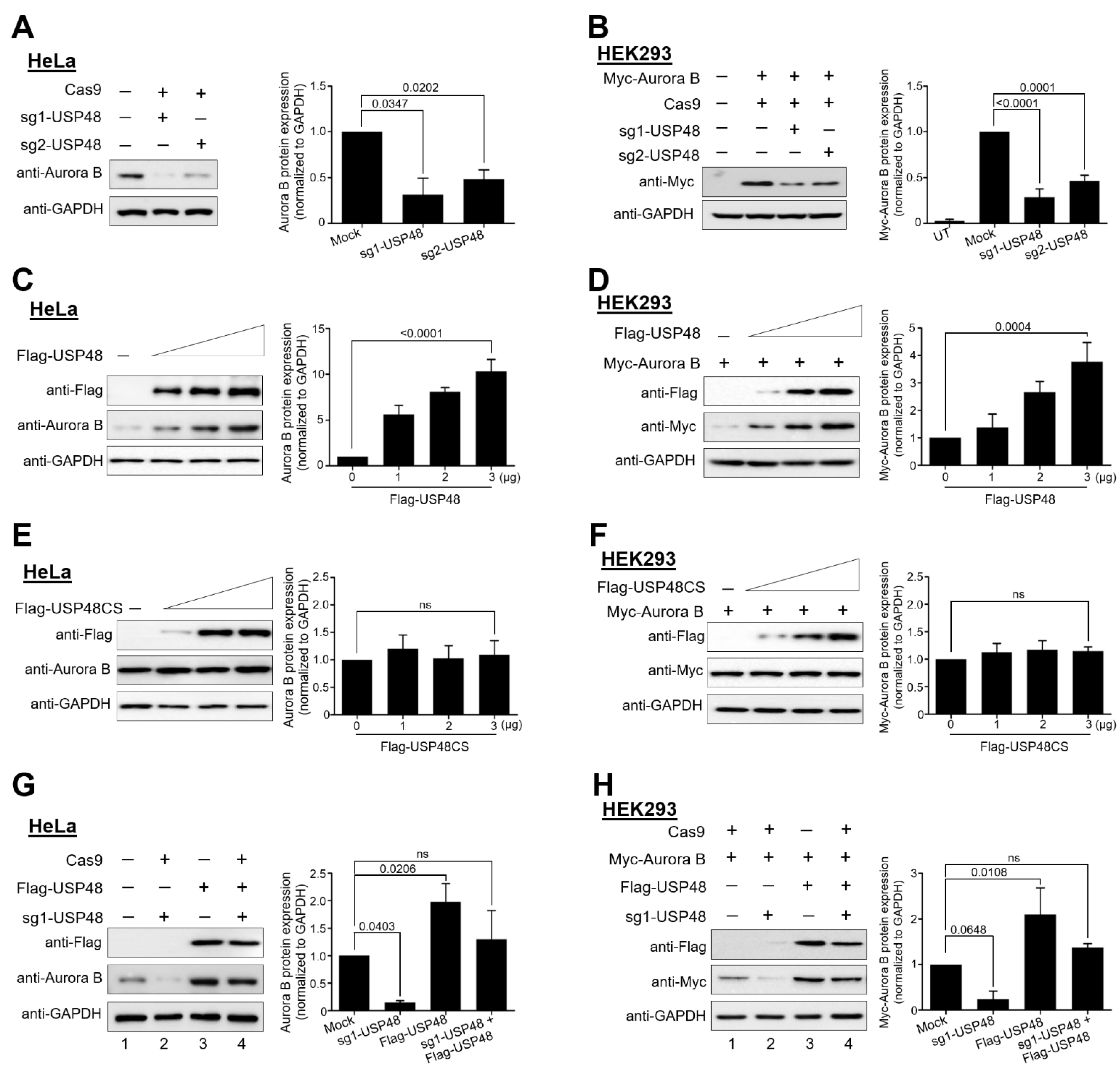
2.2. USP48 Regulates Aurora B Protein Stability
2.3. USP48 Deubiquitinates Aurora B Protein and Extends Its Half-Life
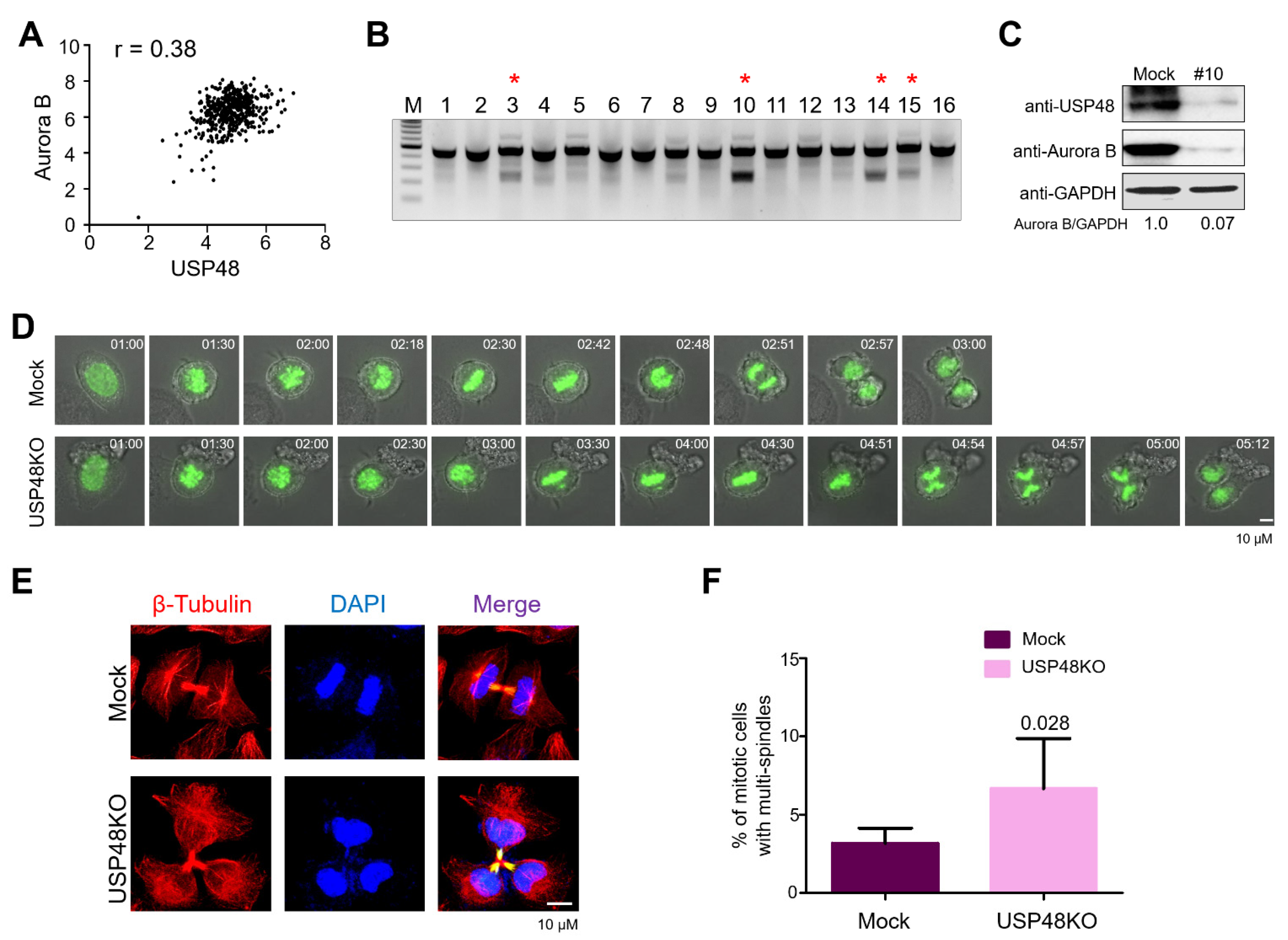
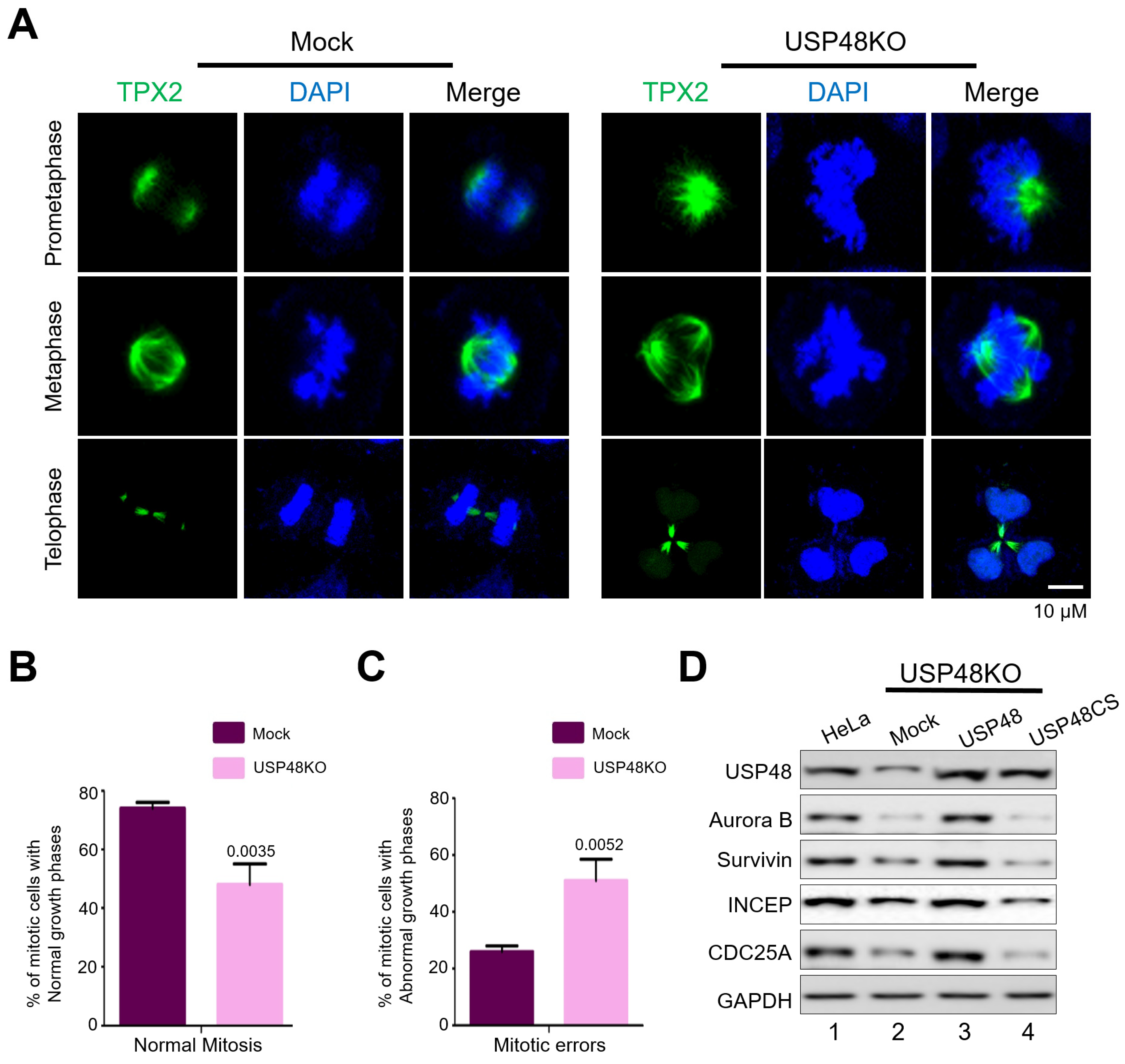
2.4. USP48 Influences Cell Cycle Progression
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Antibodies and Reagents
4.3. Cas9 and sgRNA Constructs
4.4. Cell Culture and Treatments
4.5. Generation of Single-Cell-Derived USP48 Knockout Clones
4.6. T7 Endonuclease I (T7E1) Assay
4.7. Immunoprecipitation and Immunoblotting
4.8. Time-Lapse Microscopy
4.9. Immunofluorescence Microscopy
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, F.; Nie, L.; Wei, W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021, 28, 427–438. [Google Scholar] [CrossRef]
- Carmena, M.; Earnshaw, W.C. The cellular geography of Aurora kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Descamps, S.; Prigent, C. On the role of aurora-A in centrosome function. Oncogene 2002, 21, 6175–6183. [Google Scholar] [CrossRef]
- Quartuccio, S.M.; Schindler, K. Functions of Aurora kinase C in meiosis and cancer. Front. Cell Dev. Biol. 2015, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Van De Werken, C.; De Vries, M.; Jahr, H.; Vromans, M.J.; Laven, J.S.; Fauser, B.C.; Kops, G.; Lens, S.M.; Baart, E. A role for Aurora C in the chromosomal passenger complex during human preimplantation embryo development. Hum. Reprod. 2011, 26, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Slattery, S.D.; Moore, R.V.; Brinkley, B.R.; Hall, R.M. Aurora-C and Aurora-B share phosphorylation and regulation of cenp-A and borealin during mitosis. Cell Cycle 2008, 7, 787–795. [Google Scholar] [CrossRef]
- Liu, D.; Vader, G.; Vromans, M.J.M.; Lampson, M.A.; Lens, S.M.A. Sensing Chromosome Bi-Orientation by Spatial Separation of Aurora B Kinase from Kinetochore Substrates. Science 2009, 323, 1350–1353. [Google Scholar] [CrossRef]
- van der Waal, M.S.; Hengeveld, R.C.; van der Horst, A.; Lens, S.M. Cell division control by the Chromosomal Passenger Complex. Exp. Cell Res. 2012, 318, 1407–1420. [Google Scholar] [CrossRef]
- Munoz-Barrera, M.; Monje-Casas, F. Increased Aurora B activity causes continuous disruption of kinetochore-microtubule attachments and spindle instability. Proc. Natl. Acad. Sci. USA 2014, 111, E3996–E4005. [Google Scholar] [CrossRef]
- Yeung, S.-C.; Gully, C.; Lee, M.-H. Aurora-B Kinase Inhibitors for Cancer Chemotherapy. Mini-Rev. Med. Chem. 2008, 8, 1514–1525. [Google Scholar] [CrossRef]
- Keen, N.; Taylor, S. Aurora-kinase inhibitors as anticancer agents. Nat. Rev. Cancer 2004, 4, 927–936. [Google Scholar] [CrossRef]
- Sorrentino, R.; Libertini, S.; Pallante, P.L.; Troncone, G.; Palombini, L.; Bavetsias, V.; Spalletti-Cernia, D.; Laccetti, P.; Linardopoulos, S.; Chieffi, P.; et al. Aurora B Overexpression Associates with the Thyroid Carcinoma Undifferentiated Phenotype and Is Required for Thyroid Carcinoma Cell Proliferation. J. Clin. Endocrinol. Metab. 2005, 90, 928–935. [Google Scholar] [CrossRef]
- Smith, S.L.; Bowers, N.L.; Betticher, D.C.; Gautschi, O.; Ratschiller, D.; Hoban, P.R.; Booton, R.; Santibáñez-Koref, M.F.; Heighway, J. Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br. J. Cancer 2005, 93, 719–729. [Google Scholar] [CrossRef]
- Huang, D.; Huang, Y.; Huang, Z.; Weng, J.; Zhang, S.; Gu, W. Relation of AURKB over-expression to low survival rate in BCRA and reversine-modulated aurora B kinase in breast cancer cell lines. Cancer Cell Int. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Esposito, F.; Libertini, S.; Franco, R.; Abagnale, A.; Marra, L.; Portella, G.; Chieffi, P. Aurora B expression in post-puberal testicular germ cell tumours. J. Cell. Physiol. 2009, 221, 435–439. [Google Scholar] [CrossRef]
- Chieffi, P.; Troncone, G.; Caleo, A.; Libertini, S.; Linardopoulos, S.; Tramontano, D.; Portella, G. Aurora B expression in normal testis and seminomas. J. Endocrinol. 2004, 181, 263–270. [Google Scholar] [CrossRef] [PubMed]
- López-Ríos, F.; Chuai, S.; Flores, R.; Shimizu, S.; Ohno, T.; Wakahara, K.; Illei, P.B.; Hussain, S.; Krug, L.; Zakowski, M.F.; et al. Global Gene Expression Profiling of Pleural Mesotheliomas: Overexpression of Aurora Kinases and P16/CDKN2A Deletion as Prognostic Factors and Critical Evaluation of Microarray-Based Prognostic Prediction. Cancer Res. 2006, 66, 2970–2979. [Google Scholar] [CrossRef]
- Zeng, W.F.; Navaratne, K.; Prayson, R.A.; Weil, R.J. Aurora B expression correlates with aggressive behaviour in glioblastoma multiforme. J. Clin. Pathol. 2006, 60, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.; Cozzolino, L.; Kisslinger, A.; Libertini, S.; Staibano, S.; Mansueto, G.; DE Rosa, G.; Villacci, A.; Vitale, M.; Linardopoulos, S.; et al. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate 2006, 66, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Tatsuka, M.; Katayama, H.; Ota, T.; Tanaka, T.; Odashima, S.; Suzuki, F.; Terada, Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998, 58, 4811–4816. [Google Scholar]
- Fernández-Miranda, G.; de Castro, I.P.; Carmena, M.; Aguirre-Portolés, C.; Ruchaud, S.; Fant, X.; Montoya, G.; Earnshaw, W.; Malumbres, M. SUMOylation modulates the function of Aurora-B kinase. J. Cell Sci. 2010, 123, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Fadri-Moskwik, M.; Weiderhold, K.N.; Deeraksa, A.; Chuang, C.; Pan, J.; Lin, S.; Yu-Lee, L. Aurora B is regulated by acetylation/deacetylation during mitosis in prostate cancer cells. FASEB J. 2012, 26, 4057–4067. [Google Scholar] [CrossRef][Green Version]
- Petsalaki, E.; Akoumianaki, T.; Black, E.J.; Gillespie, D.; Zachos, G. Phosphorylation at serine 331 is required for Aurora B activation. J. Cell Biol. 2011, 195, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Monaco, L.; Kolthur-Seetharam, U.; Loury, R.; Murcia, J.M.-D.; de Murcia, G.; Sassone-Corsi, P. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc. Natl. Acad. Sci. USA 2005, 102, 14244–14248. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, J.; Kim, E.E.; Song, E.J. Deubiquitinating Enzymes: A Critical Regulator of Mitosis. Int. J. Mol. Sci. 2019, 20, 5997. [Google Scholar] [CrossRef]
- Lindon, C.; Grant, R.; Min, M. Ubiquitin-Mediated Degradation of Aurora Kinases. Front. Oncol. 2016, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Sumara, I.; Quadroni, M.; Frei, C.; Olma, M.H.; Sumara, G.; Ricci, R.; Peter, M. A Cul3-Based E3 Ligase Removes Aurora B from Mitotic Chromosomes, Regulating Mitotic Progression and Completion of Cytokinesis in Human Cells. Dev. Cell 2007, 12, 887–900. [Google Scholar] [CrossRef]
- Maerki, S.; Olma, M.H.; Staubli, T.; Steigemann, P.; Gerlich, D.W.; Quadroni, M.; Sumara, I.; Peter, M. The Cul3–KLHL21 E3 ubiquitin ligase targets Aurora B to midzone microtubules in anaphase and is required for cytokinesis. J. Cell Biol. 2009, 187, 791–800. [Google Scholar] [CrossRef]
- Teng, C.-L.; Hsieh, Y.-C.; Phan, L.; Shin, J.; Gully, C.; Velazquez-Torres, G.; Skerl, S.; Yeung, S.-C.J.; Hsu, S.-L.; Lee, M.-H. FBXW7 is involved in Aurora B degradation. Cell Cycle 2012, 11, 4059–4068. [Google Scholar] [CrossRef]
- Chen, B.B.; Glasser, J.R.; Coon, T.A.; Mallampalli, R.K. Skp-cullin-F box E3 ligase component FBXL2 ubiquitinates Aurora B to inhibit tumorigenesis. Cell Death Dis. 2013, 4, e759. [Google Scholar] [CrossRef] [PubMed]
- Floyd, S.; Whiffin, N.; Gavilan, M.P.; Kutscheidt, S.; DE Luca, M.; Marcozzi, C.; Min, M.; Watkins, J.; Chung, K.; Fackler, O.; et al. Spatiotemporal organization of Aurora-B by APC/CCdh1 after mitosis coordinates cell spreading via FHOD1. J. Cell Sci. 2013, 126, 2845–2856. [Google Scholar] [CrossRef]
- Kaushal, K.; Antao, A.M.; Kim, K.-S.; Ramakrishna, S. Deubiquitinating enzymes in cancer stem cells: Functions and targeted inhibition for cancer therapy. Drug Discov. Today 2018, 23, 1974–1982. [Google Scholar] [CrossRef]
- Esposito, M.; Akman, H.B.; Giron, P.; Ceregido, M.A.; Schepers, R.; Paez, L.C.R.; La Monaca, E.; De Greve, J.; Coux, O.; De Trez, C.; et al. USP13 controls the stability of Aurora B impacting progression through the cell cycle. Oncogene 2020, 39, 6009–6023. [Google Scholar] [CrossRef]
- Song, C.; Ma, R.; Yang, X.; Pang, S. The Deubiquitinating Enzyme USP14 Regulates Leukemic Chemotherapy Drugs-Induced Cell Apoptosis by Suppressing Ubiquitination of Aurora Kinase B. Cell. Physiol. Biochem. 2017, 42, 965–973. [Google Scholar] [CrossRef]
- Park, J.; Kwon, M.-S.; Kim, E.E.; Lee, H.; Song, E.J. USP35 regulates mitotic progression by modulating the stability of Aurora B. Nat. Commun. 2018, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chandrasekaran, A.P.; Suresh, B.; Haq, S.; Kang, J.-H.; Lee, S.-J.; Kim, J.; Kim, J.; Lee, S.; Kim, H.H.; et al. Genome-scale screening of deubiquitinase subfamily identifies USP3 as a stabilizer of Cdc25A regulating cell cycle in cancer. Cell Death Differ. 2020, 27, 3004–3020. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Tatsuka, M.; Suzuki, F.; Yasuda, Y.; Fujita, S.; Otsu, M. AIM-1: A mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998, 17, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-C.; Chen, Y.-J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead Box M1 Regulates the Transcriptional Network of Genes Essential for Mitotic Progression and Genes Encoding the SCF (Skp2-Cks1) Ubiquitin Ligase. Mol. Cell. Biol. 2005, 25, 10875–10894. [Google Scholar] [CrossRef] [PubMed]
- Vong, Q.P.; Cao, K.; Li, H.Y.; Iglesias, P.A.; Zheng, Y. Chromosome Alignment and Segregation Regulated by Ubiquitination of Survivin. Science 2005, 310, 1499–1504. [Google Scholar] [CrossRef]
- Van Leuken, R.J.; Luna-Vargas, M.P.; Sixma, T.K.; Wolthuis, R.; Medema, R.H. Usp39 is essential for mitotic spindle checkpoint integrity and controls mRNA-levels of Aurora B. Cell Cycle 2008, 7, 2710–2719. [Google Scholar] [CrossRef]
- Schweitzer, K.; Naumann, M. CSN-associated USP48 confers stability to nuclear NF-κB/RelA by trimming K48-linked Ub-chains. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ankers, J.M.; Awais, R.; Jones, N.A.; Boyd, J.; Ryan, S.; Adamson, A.D.; Harper, C.; Bridge, L.; Spiller, D.; Jackson, D.A.; et al. Dynamic NF-κB and E2F interactions control the priority and timing of inflammatory signalling and cell proliferation. eLife 2016, 5, e10473. [Google Scholar] [CrossRef]
- Mistry, P.; Deacon, K.; Mistry, S.; Blank, J.; Patel, R. NF-κB Promotes Survival during Mitotic Cell Cycle Arrest. J. Biol. Chem. 2004, 279, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, D.; Zhao, J.; Weathington, N.M.; Shang, D.; Zhao, Y. The deubiquitinating enzyme USP48 stabilizes TRAF2 and reduces E-cadherin-mediated adherens junctions. FASEB J. 2018, 32, 230–242. [Google Scholar] [CrossRef]
- Cetkovská, K.; Šustová, H.; Uldrijan, S. Ubiquitin-specific peptidase 48 regulates Mdm2 protein levels independent of its deubiquitinase activity. Sci. Rep. 2017, 7, srep43180. [Google Scholar] [CrossRef] [PubMed]
- Uckelmann, M.; Densham, R.; Baas, R.; Winterwerp, H.H.K.; Fish, A.; Sixma, T.K.; Morris, J.R. USP48 restrains resection by site-specific cleavage of the BRCA1 ubiquitin mark from H2A. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Lin, K.; Zhang, S.; Ma, L.; Xue, J.; Morris, S.; Aldape, K.D.; Huang, S. Gli1-induced deubiquitinase USP 48 aids glioblastoma tumorigenesis by stabilizing Gli1. EMBO Rep. 2017, 18, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Luise, C.; Capra, M.; Donzelli, M.; Mazzarol, G.; Jodice, M.G.; Nuciforo, P.; Viale, G.; Di Fiore, P.P.; Confalonieri, S. An Atlas of Altered Expression of Deubiquitinating Enzymes in Human Cancer. PLoS ONE 2011, 6, e15891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ding, Y. LINC00467 enhances head and neck squamous cell carcinoma progression and the epithelial–mesenchymal transition process via miR-299-5p/ubiquitin specific protease-48 axis. J. Gene Med. 2020, 22, e3184. [Google Scholar] [CrossRef]
- Trakala, M.; Fernández-Miranda, G.; De Castro, I.P.; Heeschen, C.; Malumbres, M. Aurora B prevents delayed DNA replication and premature mitotic exit by repressing p21(Cip1). Cell Cycle 2013, 12, 1030–1041. [Google Scholar] [CrossRef]
- Amaral, N.; Vendrell, A.; Funaya, C.; Idrissi, F.-Z.; Maier, M.; Kumar, A.; Neurohr, G.; Colomina, N.; Torres-Rosell, J.; Geli, M.-I.; et al. The Aurora-B-dependent NoCut checkpoint prevents damage of anaphase bridges after DNA replication stress. Nat. Cell Biol. 2016, 18, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Vader, G.; Medema, R.; Lens, S.M. The chromosomal passenger complex: Guiding Aurora-B through mitosis. J. Cell Biol. 2006, 173, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Aressy, B.; Ducommun, B. Cell cycle control by the CDC25 phosphatases. Anti-Cancer Agents Med. Chem. 2008, 8, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Dad, A.-B.K.; Beloor, J.; Gopalappa, R.; Lee, S.-K.; Kim, H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

| Gene | sgRNA | Step | Direction | Sequence (5′–3′) |
|---|---|---|---|---|
| USP48 | sgRNA1 | I PCR | FP | CATTTGGGTGGCTTCCAATA |
| I PCR | RP | TAAAACAGGCAGCTGCGTAA | ||
| II PCR | FP | TCCACACCCTCACAAACTGA | ||
| II PCR | RP | TAAAACAGGCAGCTGCGTAA | ||
| sgRNA2 | I PCR | FP | CGCTTGTTCAAAACCGATCT | |
| I PCR | RP | TCTGTTTCCCAAGCCAGAGT | ||
| II PCR | FP | TCCTGTGGTCAACCCAAAAT | ||
| II PCR | RP | TCTGTTTCCCAAGCCAGAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antao, A.M.; Kaushal, K.; Das, S.; Singh, V.; Suresh, B.; Kim, K.-S.; Ramakrishna, S. USP48 Governs Cell Cycle Progression by Regulating the Protein Level of Aurora B. Int. J. Mol. Sci. 2021, 22, 8508. https://doi.org/10.3390/ijms22168508
Antao AM, Kaushal K, Das S, Singh V, Suresh B, Kim K-S, Ramakrishna S. USP48 Governs Cell Cycle Progression by Regulating the Protein Level of Aurora B. International Journal of Molecular Sciences. 2021; 22(16):8508. https://doi.org/10.3390/ijms22168508
Chicago/Turabian StyleAntao, Ainsley Mike, Kamini Kaushal, Soumyadip Das, Vijai Singh, Bharathi Suresh, Kye-Seong Kim, and Suresh Ramakrishna. 2021. "USP48 Governs Cell Cycle Progression by Regulating the Protein Level of Aurora B" International Journal of Molecular Sciences 22, no. 16: 8508. https://doi.org/10.3390/ijms22168508
APA StyleAntao, A. M., Kaushal, K., Das, S., Singh, V., Suresh, B., Kim, K.-S., & Ramakrishna, S. (2021). USP48 Governs Cell Cycle Progression by Regulating the Protein Level of Aurora B. International Journal of Molecular Sciences, 22(16), 8508. https://doi.org/10.3390/ijms22168508








