Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy
Abstract
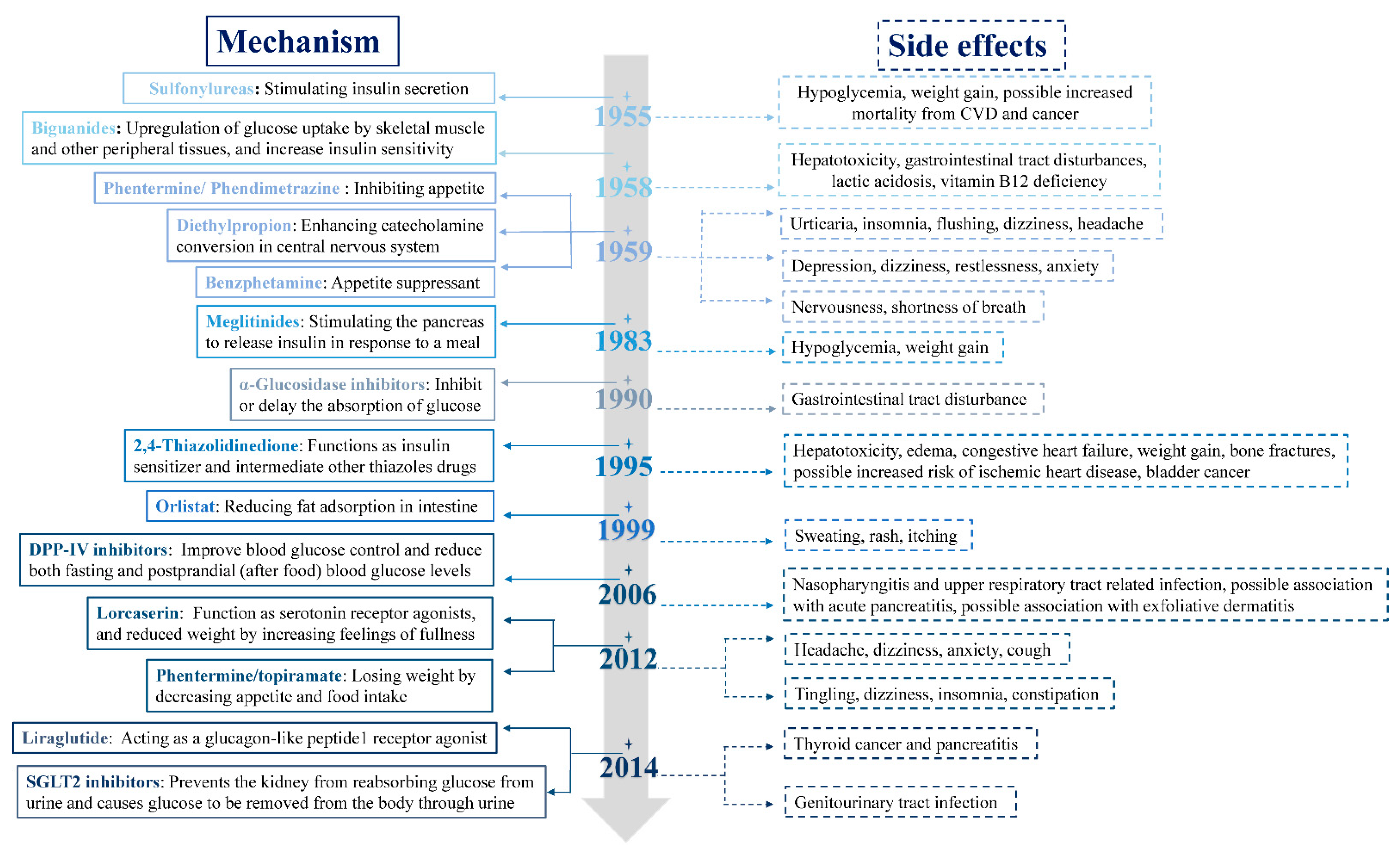
:1. Introduction
2. Current Progress and Advances on miRNAs in the Context of MetS
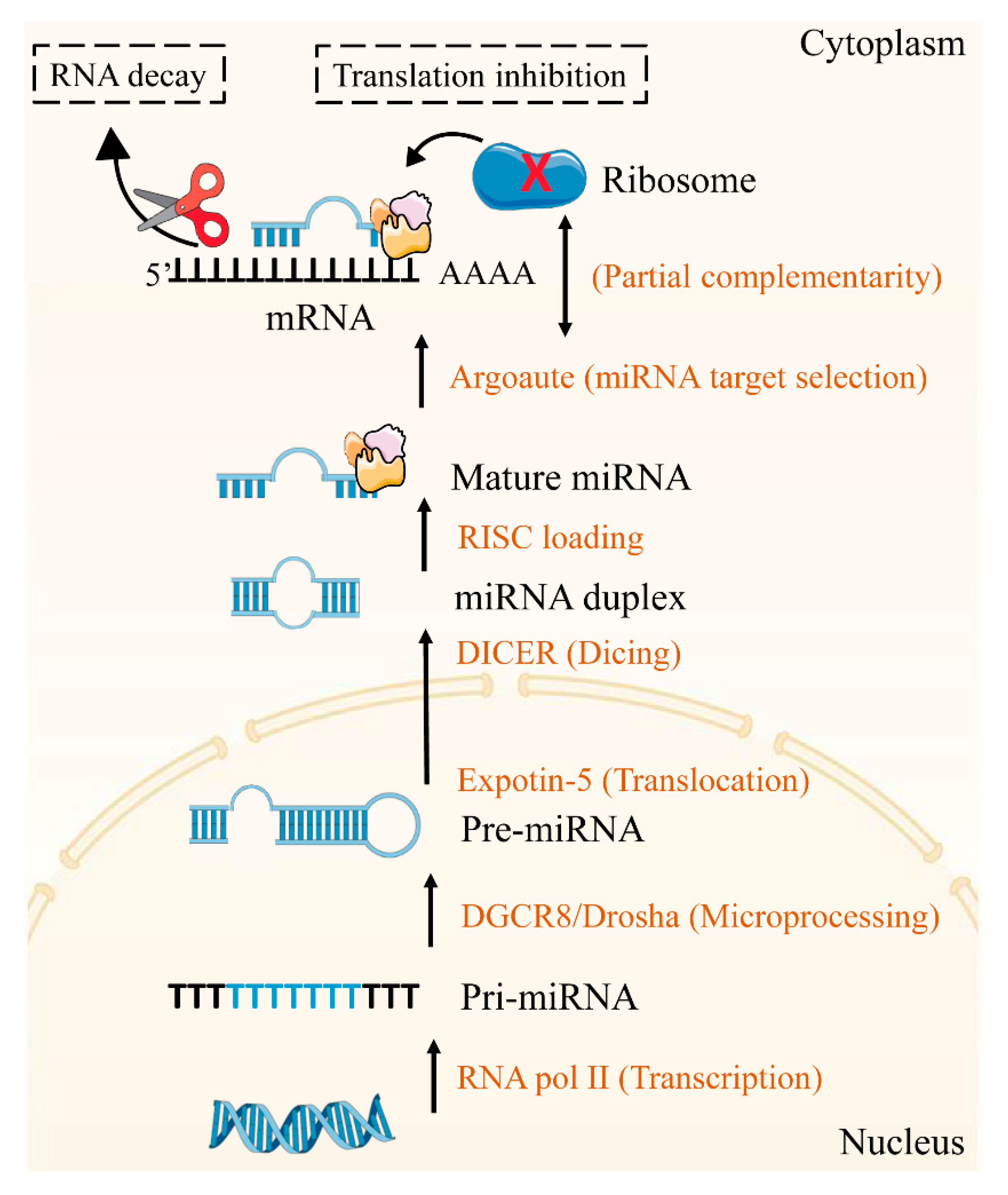
2.1. The Mechanisms of miRNAs
2.2. Therapeutic Target of miRNAs
| Metabolic Tissues | MicroRNAs | Pathway/Process | Targets | References |
|---|---|---|---|---|
| Pancreas | miR-375 | Islet functions | PDX1, HNF6, INSM1, Ngn3 | [20,21] |
| miR-15a/b, miR-16, miR-195 | Islet functions | Ngn3 | [22] | |
| miR-7 | Islet cells differentiation | Pax6 | [23,24] | |
| miR-96 | Insulin secretion | Stx-1α | [25] | |
| miR-124a | Insulin secretion | Stx-1α | [25] | |
| miR-124a | Insulin release | SNAP25, Rab3A, Synapin-1A, Rab27A, Noc2 | [25] | |
| miR-124a | Islet functions | Foxa2, Pdx1, Creb1 | [26] | |
| miR-9 | Insulin secretion | Stx-1α | [25] | |
| miR-9 | Insulin release | Onecut-2, Granuphilin/slp4 | [27] | |
| miR-29 | Insulin secretion | Stx-1α, Mct1 | [28] | |
| miR-192-5p | Islet β-cell functions | Clock | [29,30,31,32] | |
| miR-192-5p | Insulin sensitivity | CaV1 | [29,30,31,32] | |
| miR-103, miR-107 | Insulin sensitivity | CaV1 | [33] | |
| Liver | miR-122 | Liver functions | LETFS, HNF6, HNF4a | [34,35] |
| miR-122 | Lipid metabolism | SREBF1 | [34,35] | |
| miR-26a | Lipid metabolism | ACSL3, ACSL4, PKC, GSK3β, SREBF2 | [36] | |
| miR-33b | Lipid metabolism | SREBF1 | [37] | |
| miR-34a | Fatty acid metabolism | SIRT1 | [38] | |
| miR-34a | Lipid metabolism | SREBP1 | [38] | |
| miR-370 | Lipid metabolism | MECPT | [39] | |
| miR-96, miR-183 | Lipid metabolism | SREBP | [40] | |
| miR-30c | Lipid metabolism | RARB, LPGAT1, MTP | [41,42,43,44] | |
| miR-30c | Glucose metabolism | IDH1, LIN28B | [41,42,43,44] | |
| miR-192-5p | De novo lipogenesis | SREBF1, SCD-1 | [30,31,32,33] | |
| miR-192-5p | Inflammation | FoxO1 | [30,31,32,33] | |
| miR-192-5p | Cholesterol homeostasis | ABCG4 | [30,31,32,33] | |
| miR-192-5p | Lipid metabolism | ElOVL1, ElOVL5, PPARA, VLDLR, FABP3, ATF1, CAV2, CRTc2, DBT, IGF1 | [30,31,32,33] | |
| miR-33a-3p | Cholesterol efflux | ABCA1, ABCG1 | [45] | |
| miR-33a-3p | Insulin signaling | IRS2, SIRT6 | [45] | |
| miR-33a-3p | Lipid metabolism | SREBP2, SREBF1 | [45] | |
| miR-223 | Cholesterol efflux | ABCA1 | [45] | |
| miR-223 | Cholesterol biosynthesis | HMG-CoA, SC4MOL | [46] | |
| miR-206 | Lipid metabolism | LXR2 | [46] | |
| miR-200 | Liver cell growth and proliferation | PI3K | [47] | |
| miR-27a-3p | Cholesterol metabolism | LDLRAP1, LRP6 | [48,49,50,51] | |
| miR-27a-3p | De novo lipogenesis | SCD1, RXRα | [48,49,50,51] | |
| miR-27a-3p | Inflammation | Nrf2, NF-κB | [48,49,50,51] | |
| miR-27a-3p | Lipid metabolism | PPARA, FASN, SREBF1, FAS | [48,49,50,51] | |
| miR-128 | Cholesterol metabolism | LDLR | [52] | |
| miR-128 | Cholesterol efflux | ABCA1 | [52] | |
| miR-128 | Inflammation | Nrf2 | [52] | |
| miR-130b, miR-301b | Cholesterol metabolism | LDLR | [53,54,55] | |
| miR-130b, miR-301b | Cholesterol efflux | ABCA1 | [53,54,55] | |
| miR-27b | Cholesterol efflux | ABCA1 | [48,49,50,51] | |
| miR-27b | Lipid metabolism | LDLR | [48,49,50,51] | |
| miR-140-5p | Cholesterol metabolism | LDLR | [10,55,56] | |
| miR-140-5p | Inflammation | Nrf2 | [10,55,56] | |
| miR-140-5p | AMPK/SREBP1 pathway | NEAT1 | [10,55,56] | |
| miR-2, miR-148a-3p, miR-185 | Cholesterol metabolism | LDLR | [57,58] | |
| miR-2, miR-148a-3p, miR-185 | Lipid metabolism | SERBP2 | [57,58] | |
| miR-21 | Lipid metabolism | HMGCR, HOMER1, Smad7 | [59,60,61,62,63] | |
| miR-344 | Lipid metabolism (Wnt/β-catenin signaling pathway) | GSK3β | [49] | |
| Muscle | miR-29a | IR | PPARδ, | [64] |
| miR-29a | Glucose uptake | IRS-1 | [65] | |
| miR-106b | Mitochondrial dysfunction, IR | mitofusin-2 | [66] | |
| miR-208 | Glucose metabolism | MED13 | [67,68] | |
| miR-199a-3p, miR-590-3p | p/Akt pathway | HOMER1, CLIC5 | [55,58,69,70,71] | |
| AT | miR-14 | Lipid metabolism | p38, MAPK | [72] |
| miR-143 | Adipocyte differentiation | ERK5 signaling | [73] | |
| miR-27a, miR-130a | Adipocyte differentiation | PPARγ | [55] | |
| miR-27b, miR-363 | Adipocyte differentiation | C/EBPα, PPARγ | [74] | |
| Let-7 | Cell functions | RAS, HMGA2 | [75,76,77] | |
| Let-7 | Glucose metabolism | INSR, IGF1R | [75,76,77] | |
| Let-7 | Adipogenesis | AT-hook2, PPARγ, FABP4 | [75,76,77] | |
| miR-375 | Adipocyte differentiation | C/EBPα, PPARγ2 | [78] | |
| miR-206 | Lipid accumulation | PPARγ, PTEN, FAS, C/EBPα | [79] | |
| miR-146b | Metabolic homeostasis | SIRT1 | [80] | |
| miR-8 | Adipogenesis | FABP4 | [47,81] | |
| miR-8 | Fat body growth and differentiation | PI3K | [47,81] | |
| miR-210 | Adipocyte differentiation (PI3K/Akt pathway) | SHIPI | [82] | |
| miR-21 | Adipocyte differentiation | AP-1, TGF-β receptor 2 | [83] | |
| miR-30c | Adipocyte differentiation | SERPINE1, ACVR1 | [41,42,43,44] | |
| miR-142-5p | Inflammation | Nrf2 | [55,56,84] | |
| miR-26a | Inflammation | IL-6, IL-17 | [78,85,86,87] | |
| miR-26a | Autophagy | BECN1, LC3 | [78,85,86,87] | |
| miR-370 | Metabolic homeostasis | CPTIA | [39] | |
| miR-22 | Adipogenesis | HDAC6 | [88] | |
| miR-31 | Lipid accumulation | C/EBPα | [89] | |
| miR-33b | Lipogenesis | EBF1 | [37] | |
| miR-93 | Adipogenesis | Sirt7, Tbx3 | [90] | |
| miR-125a | Adipogenesis | ERRα | [91] | |
| miR-155 | Adipocyte differentiation | PPARγ | [92] | |
| miR-145 | Preadipocyte differentiation | IRS1 | [93] | |
| miR-155 | Lipid metabolism | C/EBPβ | [94] | |
| miR-194 | Stimulates osteogenesis and inhibits adipogenesis | COUP-TFII | [95] | |
| miR-224 | Fatty acid metabolism | EGR2 | [96] | |
| miR-363 | Adipocyte differentiation | E2F3, C/EBPα, PPARγ | [74] | |
| miR-369 | Adipogenic differentiation | FABP4 | [97] | |
| miR-448 | Lipid metabolism | KLF5, 5-HT2AR, 5-HT2CR | [98] | |
| miR-709 | Lipid metabolism (Wnt/ß-catenin signaling) | GSK3β | [99] | |
| miR-637 | Adipogenesis | Sp7 | [100] | |
| miR-320 | Adipogenesis | RUNX2 | [101] | |
| miR-199a | Adipogenesis | Smad1 | [70] | |
| miR-103 | Adipogenesis (AKT/mTOR signal pathway) | MEF2D | [102] | |
| miR-26b | Adipogenic differentiation | PTEN | [79,87] |
2.3. Therapeutic Potential of miRNAs
2.4. Challenges in miRNA-Based Treatment
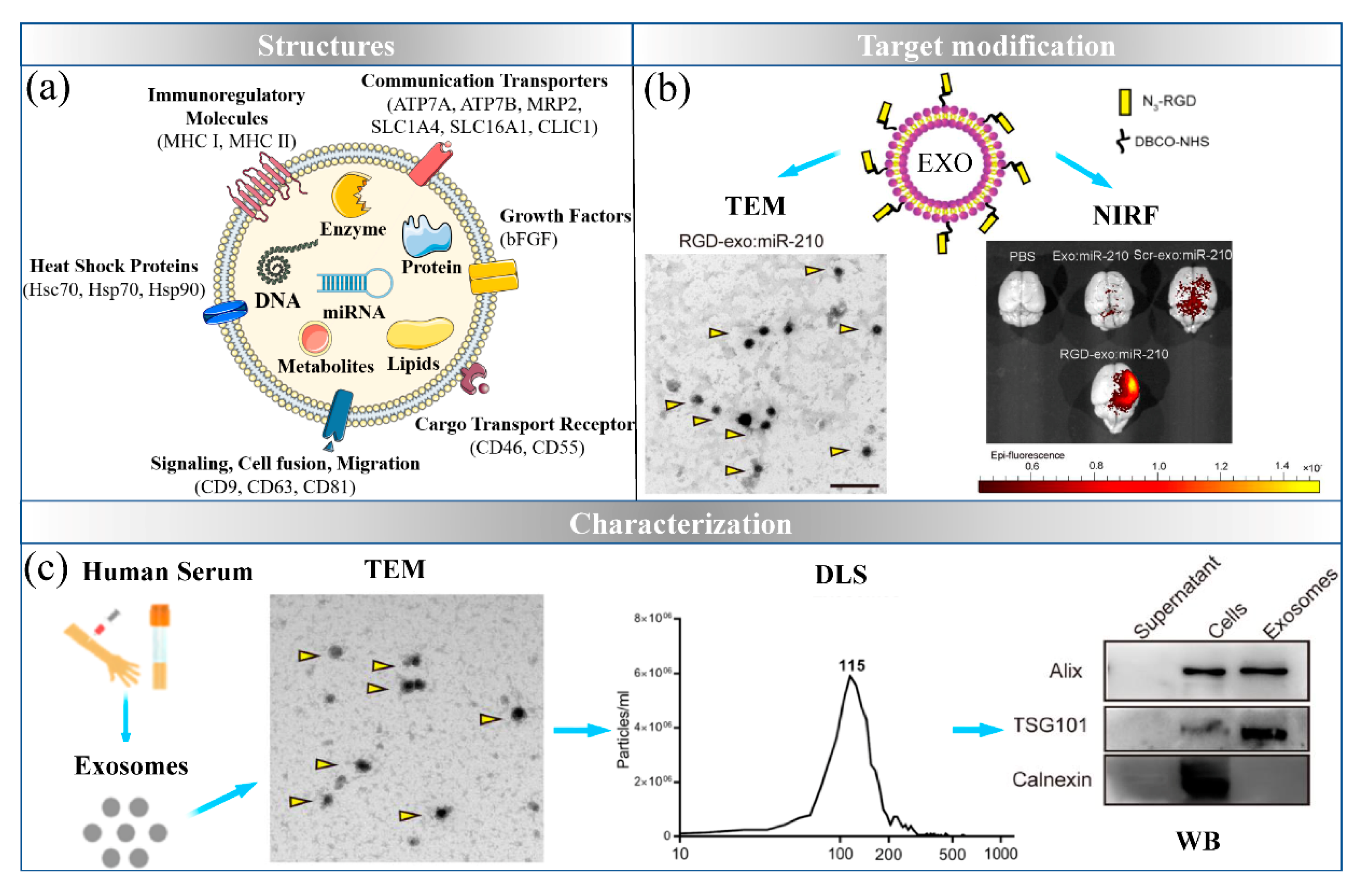
3. Exosomes: The Natural Nanocarrier
3.1. Sources and Targets for Engineered Exosomes
3.1.1. Peripheral Blood
3.1.2. Mesenchymal Stem Cells (MSCs)
3.1.3. Adipocytes
3.1.4. Immune Cells
3.1.5. Milk
3.2. Exosomes Delivery of miRNAs for MetS Treatment
3.3. Challenges in Exosomes-Based Delivery System
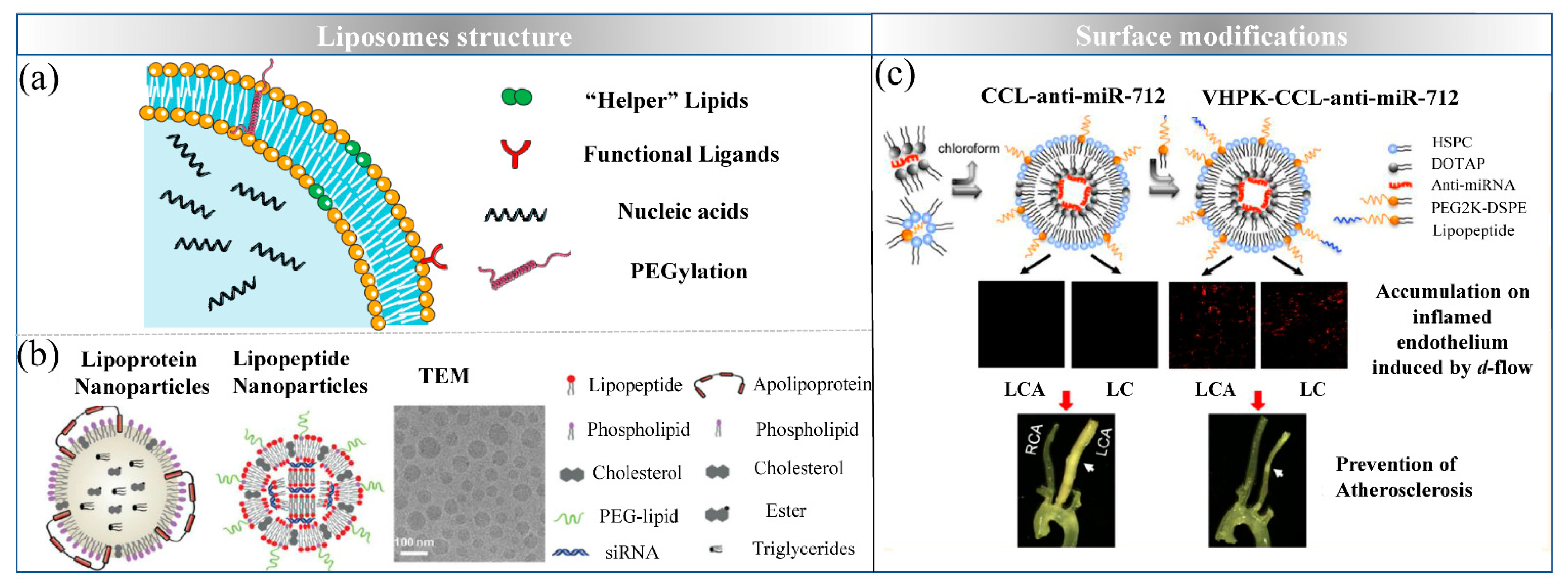
4. Liposomes: Simulating Biological Membrane
4.1. Design Strategies of Lipid-Based Delivery Systems
4.2. Preparation of Lipid-Based Delivery Systems
4.3. Liposomes Delivering miRNAs for MetS Treatment
4.4. Challenges in Lipid-Based Delivery System
5. Polymeric Nanoparticles: Biodegradable Polymer Based Nanoformulations
5.1. Polymeric Materials
5.2. Functionalized Strategies of Polymeric Nanoparticles
5.3. Polymeric Delivery of miRNAs for MetS Therapy
5.4. Challenges in Polymeric Delivery System
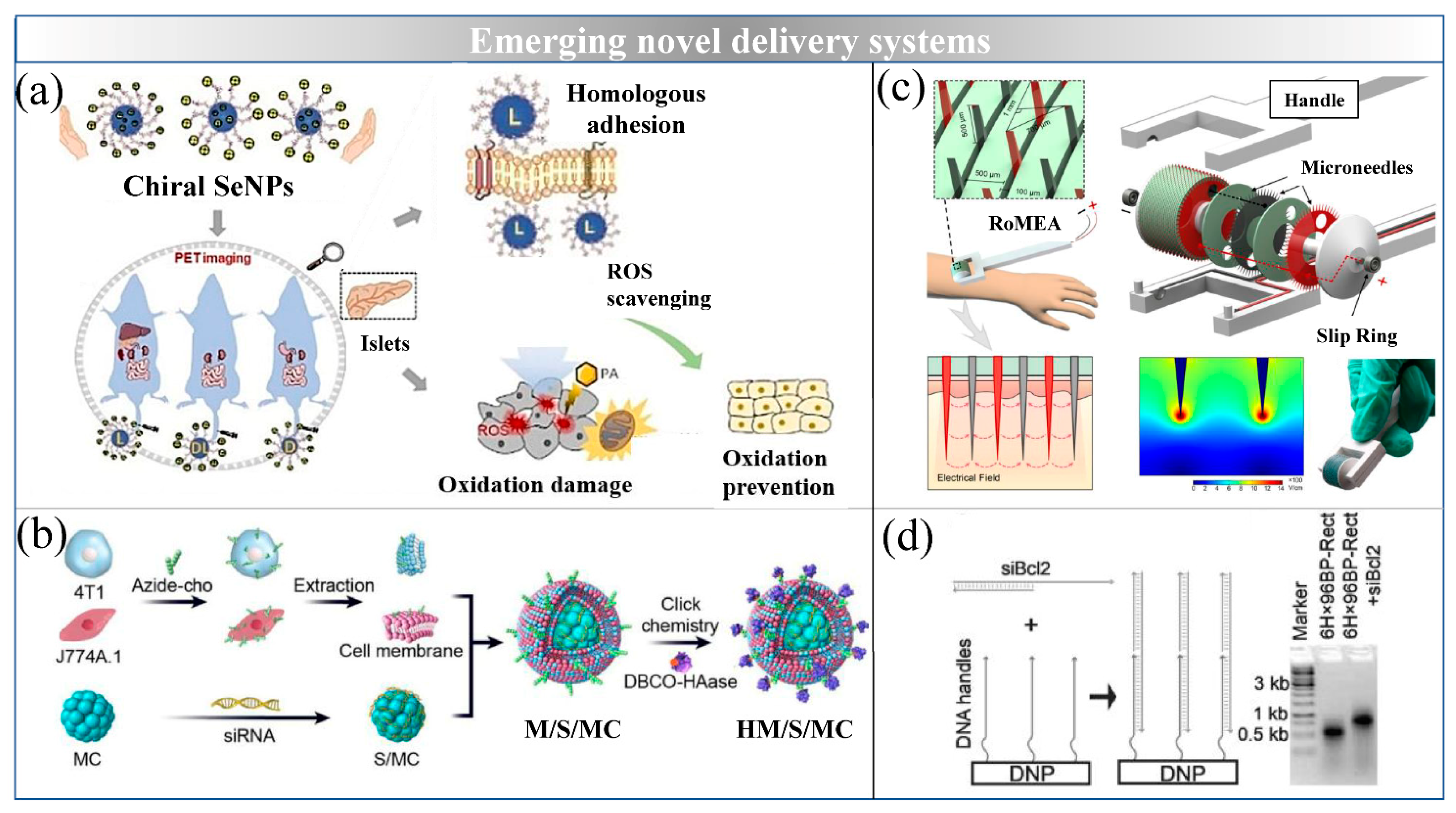
6. Emerging Novel Delivery Systems
6.1. Inorganic NPs
6.2. Magnetosomes
6.3. DNA Origami
6.4. Microneedles
7. Nanosystem-Based Delivery of miRNAs for MetS Treatment in Clinical Transformation
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Part 1: Diagnosis and Classification of of Diabetes Mellitus. Provisional Report of a WHO Consultation. Diabetic. Med. 1999, 15, 539–553. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Kokil, G.R.; Veedu, R.N.; Ramm, G.A.; Prins, J.B.; Parekh, H.S. Type 2 Diabetes Mellitus: Limitations of Conventional Therapies and Intervention with Nucleic Acid-Based Therapeutics. Chem. Rev. 2015, 115, 4719–4743. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The Impact of MicroRNAs on Protein Output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Yu, J.T.; Hu, N.; Tan, L. Non-coding RNAs in Alzheimer’s Disease. Mol. Neurobiol. 2013, 135, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz-Rokah, O.; Modai, S.; Pasmanik-Chor, M.; Toren, A.; Shomron, N.; Raanani, P.; Shpilberg, O.; Granot, G. MiR-30e Induces Apoptosis and Sensitizes K562 Cells to Imatinib Treatment via Regulation of the BCR-ABL Protein. Cancer Lett. 2015, 356, 597–605. [Google Scholar] [CrossRef]
- Khan, M.; Khurram, A.A.; Li, T.; Zhao, T.; Subhani, T.; Gul, I.H.; Ali, Z.; Patel, V. Synergistic Effect of Organic and Inorganic Nano Fillers on the Dielectric and Mechanical Properties of Epoxy Composites. J. Mater. Sci. Technol. 2018, 34, 2424–2430. [Google Scholar] [CrossRef]
- Ash, G.I.; Kim, D.; Choudhury, M. Promises of Nanotherapeutics in Obesity. Trends Endocrinol. Metab. 2019, 30, 369–383. [Google Scholar] [CrossRef]
- Chong, M.; Zhang, G.; Cheloufi, S.; Neubert, T.A.; Hannon, G.J.; Littman, D.R. Canonical and Alternate Functions of the Microrna Biogenesis Machinery. Gene Dev. 2010, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.E.; Dhawan, S. β-cell Identity in Type 2 Diabetes: Lost or found? Diabetes 2015, 64, 2698–2700. [Google Scholar] [CrossRef] [Green Version]
- Ouaamari, A.E.; Baroukh, N.; Martens, G.A.; Lebrun, P.; Pipeleers, D.; Van Obberghen, E. MiR-375 Targets 3′l-Phosphoinositide-Dependent Protein Kinase-1 and Regulates Glucose-Induced Biological Responses in Pancreatic β-Cells. Diabetes 2008, 57, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. MiR-122 Regulation of Lipid Metabolism Revealed by in vivo Antisense Targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; Perle, K.L.; Chivukula, R.R.; et al. Essential Metabolic, Anti-inflammatory, and Anti-tumorigenic Functions of MiR-122 in Liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted MicroRNAs: A New Form of Intercellular Communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic Steatohepatitis is Associated with Altered Hepatic MicroRNA Expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, J.J. The MyomiR Network in Skeletal Muscle Plasticity. Exerc. Sport Sci. Rev. 2011, 39, 150. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, S.; George, B.; Feng, Q.; Chakrabarti, S. MiR133a Regulates Cardiomyocyte Hypertrophy in Diabetes. Diabetes. Metab. Res. Rev. 2010, 26, 40–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, P.; Shi, W.; Li, J.; Hao, Q.; Cao, X.; Lu, Q.; Zeng, Y. MicroRNA-29a Induces Insulin Resistance by Targeting PPARδ in Skeletal Muscle Cells. Int. J. Mol. Med. 2016, 37, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Lee, M.J.; Abdelmohsen, K.; Kim, W.; Kim, M.M.; Srikantan, S.; Martindale, J.L.; Hutchison, E.R.; Kim, H.H.; Marasa, B.S.; et al. MiR-130 Suppresses Adipogenesis by Inhibiting Peroxisome Proliferator-Activated Receptor Expression. Mol. Cell. Biol. 2011, 31, 626–638. [Google Scholar] [CrossRef] [Green Version]
- Hilton, C.; Neville, M.J.; Karpe, F. MicroRNAs in Adipose Tissue: Their Role in Adipogenesis and Obesity. Int. J. Obes. 2013, 37, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Shaer, A.; Azarpira, N.; Vahdati, A.; Karimi, M.H.; Shariati, M. MiR-375 Induces Human Decidua Basalis-derived Stromal Cells to become Insulin-producing Cells. Cell. Mol. Biol. 2014, 19, 483–499. [Google Scholar] [CrossRef] [Green Version]
- Kredo-Russo, S.; Mandelbaum, A.D.; Ness, A.; Alon, I.; Lennox, K.A.; Behlke, M.A.; Hornstein, E. Pancreas-enriched MiRNA Refines Endocrine Cell Differentiation. Development 2012, 139, 3021–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaragosi, L.E.; Wdziekonski, B.; Brigand, K.L.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA Sequencing Reveals MiR-642a-3p as a Novel Adipocyte-specific MicroRNA and MiR-30 as a Key Regulator of Human Adipogenesis. Genome Biol. 2011, 12, R64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Guan, X.; Guo, F.; Zhou, J.; Chang, A.; Sun, B.; Cai, Y.; Ma, Z.; Dai, C.; Li, X.; et al. MiR-30e Reciprocally Regulates the Differentiation of Adipocytes and Osteoblasts by Directly Targeting Low-density Lipoprotein Receptor-related Protein 6. Cell Death Dis. 2013, 4, e845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Landstreet, S.R.; Levin, M.G.; Shoucri, B.M.; Toth, C.L.; Taylor, R.C.; Palmisano, B.T.; Tabet, F.; Cui, H.L.; Rye, K.A.; et al. MicroRNA-223 Coordinates Cholesterol Homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 14518–14523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, D.; Huang, G.; Zhang, Y.; Zeng, Y.; Xu, Z.; Zhao, Y.; He, X.; He, F. MicroRNA-1 and MicroRNA-206 Suppress LXRα-induced Lipogenesis in Hepatocytes. Cell. Signal. 2013, 25, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Lee, J.H.; Jin, H.; Nam, J.W.; Namkoong, B.; Lee, G.; Chung, J.; Kim, V.N. Conserved MicroRNA MiR-8/miR-200 and its Target USH/FOG2 Control Growth by Regulating PI3K. Cell 2009, 139, 1096–1108. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b is a Regulatory Hub in Lipid Metabolism and is Altered in Dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Chen, Y.; Niu, Y.; Chen, W.; Wang, Q.; Xiao, S.; Li, A.; Xie, Y.; Li, J.; Zhao, X.; et al. A Deep Investigation into the Adipogenesis Mechanism: Profile of MicroRNAs Regulating Adipogenesis by Modulating the Canonical Wnt/β-catenin Signaling Pathway. BMC Genom. 2010, 11, 320. [Google Scholar] [CrossRef] [Green Version]
- Chu, X.L.; Wang, Y.Q.; Pang, L.W.; Huang, J.C.; Sun, X.T.; Chen, X.F. MiR-130 Aggravates Acute Myocardial Infarction-induced Myocardial Injury by Targeting PPAR-γ. J. Cell. Biochem. 2018, 119, 7235–7244. [Google Scholar] [CrossRef]
- Singaravelu, R.; Chen, R.; Lyn, R.K.; Jones, D.M.; O’Hara, S.; Rouleau, Y.; Cheng, J.; Srinivasan, P.; Nasheri, N.; Russell, R.S.; et al. Hepatitis C Virus Induced Up-regulation of MicroRNA-27: A Novel Mechanism for Hepatic Steatosis. Hepatology 2014, 59, 98–108. [Google Scholar] [CrossRef]
- Al-Rawaf, H.A. Circulating MicroRNAs and Adipokines as Markers of Metabolic Syndrome in Adolescents with Obesity. Clin. Nutr. 2019, 38, 2231–2238. [Google Scholar] [CrossRef]
- Ortega, F.J.; Mercader, J.M.; Catalán, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gómez-Ambrosi, J.; Anglada, R.; Fernández-Formoso, J.A.; Ricart, W.; et al. Targeting the Circulating MicroRNA Signature of Obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Villard, A.; Marchand, L. Diagnostic Value of Cell-free Circulating Micrornas for Obesity and Type 2 Diabetes: A Meta-analysis. J. Mol. Biomark. Diagn. 2015, 6, 251. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, M.; Tong, M.; Yang, L.; Pang, L.; Chen, L.; Xu, G.; Chi, X.; Hong, Q.; Ni, Y.; et al. MiR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci. Rep. 2015, 5, 9930. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Moreno-Navarrete, J.M.; Pardo, G.; Sabater, M.; Hummel, M.; Ferrer, A.; Rodriguez-Hermosa, J.I.; Ruiz, B.; Ricart, W.; Peral, B.; et al. MiRNA Expression Profile of Human Subcutaneous Adipose and During Adipocyte Differentiation. PLoS ONE 2010, 2, e9022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, S.; Mahdavi, R.; Alipoor, B.; Panahi, G.; Nasli Esfahani, E.; Razi, F.; Taghikhani, M.; Meshkani, R. Decreased Serum MicroRNA-21 Level is Associated with Obesity in Healthy and Type 2 Diabetic Subjects. Arch. Physiol. Biochem. 2018, 124, 300–305. [Google Scholar] [CrossRef]
- Donghui, T.; Shuang, B.; Xulong, L.; Meng, Y.; Yujing, G.; Yujie, H.; Juan, L.; Dongsheng, Y. Improvement of Microvascular Endothelial Dysfunction Induced by Exercise and Diet is Associated with MicroRNA-126 in Obese Adolescents. Microvasc. Res. 2019, 123, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B. Essentials of Personality Disorders. Psychiatr. Serv. 2010, 33. [Google Scholar] [CrossRef]
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) Post-transcriptionally Downregulates Tumor Suppressor Pdcd4 and Stimulates Invasion, Intravasation and Metastasis in Colorectal Cancer. Oncogene 2008, 27, 2128–2136. [Google Scholar] [CrossRef] [Green Version]
- Rotllan, N.; Price, N.; Pati, P.; Goedeke, L.; Fernández-Hernando, C. MicroRNAs in Lipoprotein Metabolism and Cardiometabolic Disorders. Atherosclerosis 2016, 246, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbiener, M.; Neuhold, C.; Opriessnig, P.; Prokesch, A.; Scheideler, M. Microrna-30c Promotes Human Adipocyte Differentiation and Co-represses PAI-1 and ALK2. RNA Biol. 2011, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.M.; Jeong, H.J.; Park, S.Y.; Lee, W. Induction of MiR-29a by Saturated Fatty Acids Impairs Insulin Signaling and Glucose Uptake through Translational Repression of IRS-1 in Myocytes. FEBS Lett. 2014, 588, 2170–2176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, L.; Gao, Y.F.; Fan, Z.M.; Cai, X.Y.; Liu, M.Y.; Guo, X.R.; Gao, C.L.; Xia, Z.K. MicroRNA-106b Induces Mitochondrial Dysfunction and Insulin Resistance in C2C12 Myotubes by Targeting Mitofusin-2. Mol. Cell. Endocrinol. 2013, 381, 230–240. [Google Scholar] [CrossRef]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martínez, C.; Ricart, W.; Rieusset, J.; et al. Profiling of Circulating MicroRNAs Reveals Common MicroRNAs Linked to Type 2 Diabetes that Change with Insulin Sensitization. Diabetes Care 2014, 37, 1375–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, K.K.; Grueter, C.E.; Kusminski, C.M.; Holland, W.L.; Bookout, A.L.; Satapati, S.; Kong, Y.M.; Burgess, S.C.; Malloy, C.R.; Scherer, P.E. MED 13-dependent Signaling from the Heart Confers Leanness by Enhancing Metabolism in Adipose Tissue and Liver. EMBO Mol. Med. 2014, 6, 1610–1621. [Google Scholar] [CrossRef]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “Mediator” of Systemic Metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.D.; Cismowski, M.J.; Serpico, M.; Pusateri, A.; Brigstock, D.R. Elevation of Circulating MicroRNA Levels in Obese Children Compared to Healthy Controls. Clin. Obes. 2017, 7, 216–221. [Google Scholar] [CrossRef]
- Shi, X.E.; Li, Y.F.; Jia, L.; Ji, H.L.; Song, Z.Y.; Cheng, J.; Wu, G.F.; Song, C.C.; Zhang, Q.L.; Zhu, J.Y.; et al. MicroRNA-199a-5p Affects Porcine Preadipocyte Proliferation and Differentiation. Int. J. Mol. Sci. 2014, 15, 8526–8538. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Zheng, Y.; Zhao, Y.; Xiu, X.; Wang, J. MiR-590-3p Suppresses Cancer Cell Migration, Invasion and Epithelial-mesenchymal Transition in Glioblastoma Multiforme by Targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015, 468, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila MicroRNA MiR-14 Suppresses Cell Death and is Required for Normal Fat Metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.D.; Krüger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Brönneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Böttger, T.; et al. Obesity-induced Overexpression of MiRNA-143 Inhibits Insulin-stimulated AKT Activation and Impairs Glucose Metabolism. Nat. Cell Biol. 2011, 13, 434–446. [Google Scholar] [CrossRef]
- Zaiou, M.; El Amri, H.; Bakillah, A. The Clinical Potential of Adipogenesis and Obesity-related MicroRNAs. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 91–111. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Pfeffer, S.; Baumert, T.F.; Perle, L.K.; J, G.K. MiR-122 Acts as a Tumor Suppressor in Hepatocarcinogenesis in vivo. J. Hepatol. 2013, 58, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, J.; Hou., J.; Long, J.; Li, C.; Liu, L. A Novel Negative Regulator of Adipogenesis: MicroRNA-363. Stem Cells 2014, 32, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, L.M.; Lu, Y.; Zhang, M.X.; Zhang, Z.N.; Wang, K.; Lv, J.R. Down-regulation of MicroRNA-142-5p Attenuates Oxygen-glucose Deprivation and Reoxygenation-induced Neuron Injury through Up-regulating Nrf2/ARE Signaling Pathway. Biomed. Pharmacother. 2017, 89, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ng, S.C.; Segr, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 Axis Regulates Glucose Metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.J.A.; Olson, E.N. Control of Glucose Homeostasis and Insulin Sensitivity by the Let-7 Family of MicroRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.Y.; Wen, G.B.; Feng, S.D.; Tuo, Q.H.; Ou, H.S.; Yao, C.H.; Zhu, B.Y.; Gao, Z.P.; Zhang, L.; Liao, D.F. MicroRNA-375 Promotes 3T3-L1 Adipocyte Differentiation through Modulation of Extracellular Signal-regulated Kinase Signalling. Clin. Exp. Pharmacol. Physiol. 2011, 38, 239. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wang, Y.; Xue, J.; Ma, Q.; Zhang, J.; Chen, Y.F.; Shang, Z.Z.; Li, Q.Q.; Zhao, S.L.; Zhao, L. Effect of Corilagin on the MiR-21/smad7/ERK Signaling Pathway in a Schistosomiasis-induced Hepatic Fibrosis Mouse Model. Parasitol. Int. 2016, 65, 308–315. [Google Scholar] [CrossRef]
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 Regulates Adipocyte Differentiation through FoxO1 Acetylation/Deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennell, J.A.; Gerin, I.; MacDougald, O.A.; Cadigan, K.M. The MicroRNA MiR-8 is a Conserved Negative Regulator of Wnt Signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 15417–15422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.C.; Wang, Y.; Wan, D.C.C.; Yeung, V.S.Y.; Waye, M.M.Y. Characterization of MiR-210 in 3T3-L1 Adipogenesis. J. Cell. Biochem. 2013, 114, 2699–2707. [Google Scholar] [CrossRef]
- Jeong Kim, Y.; Jin Hwang, S.; Chan Bae, Y.; Sup Jung, J. MiR-21 Regulates Adipogenic Differentiation through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Cui, X.; You, L.; Zhu, L.; Wang, X.; Zhou, Y.; Li, Y.; Wen, J.; Xia, Y.; Wang, X.; Ji, C.; et al. Change in Circulating MicroRNA Profile of Obese Children Indicates Future Risk of Adult Diabetes. Metabolism 2018, 78, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Karbiener, M.; Pisani, D.F.; Frontini, A.; Oberreiter, L.M.; Lang, E.; Vegiopoulos, A.; Mössenböck, K.; Bernhardt, G.A.; Mayr, T.; Hildner, F.; et al. MicroRNA-26 Family is Required for Human Adipogenesis and Drives Characteristics of Brown Adipocytes. Stem Cells 2014, 32, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, X. MiR-375, a microRNA related to diabetes. Gene 2014, 533, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, S.; Bian, C.; Yang, Z.; Zhou, H.; Zeng, Y.; Li, H.; Han, Q.; Zhao, R.C. Upregulation of MiR-22 Promotes Osteogenic Differentiation and Inhibits Adipogenic Differentiation of Human Adipose Tissue-derived Mesenchymal Stem Cells by Repressing HDAC6 Protein Expression. Stem Cells Dev. 2012, 21, 2531–2540. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.F.; Zhang, Y.; Li, X.Y.; Li, C.; Tian, W.; Liu, L. Expression of MiR-31, MiR-125b-5p, and MiR-326 in the Adipogenic Differentiation Process of Adipose-derived Stem Cells. Omics 2009, 13, 331–336. [Google Scholar] [CrossRef]
- Cioffi, M.; Vallespinos-Serrano, M.; Trabulo, S.M.; Fernandez-Marcos, P.J.; Firment, A.N.; Vazquez, B.N.; Vieira, C.R.; Mulero, F.; Camara, J.A.; Cronin, U.P.; et al. MiR-93 Controls Adiposity via Inhibition of Sirt7 and Tbx3. Cell Rep. 2015, 12, 1594–1605. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.L.; Song, C.C.; Li, Y.F.; He, J.J.; Li, Y.L.; Zheng, X.L.; Yang, G.S. MiR-125a Inhibits Porcine Preadipocytes Differentiation by Targeting ERRα. Mol. Cell. Biochem. 2014, 395, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bian, C.; Zhou, H.; Huang, S.; Wang, S.; Liao, L.; Zhao, R.C. MicroRNA Hsa-miR-138 Inhibits Adipogenic Differentiation of Human Adipose Tissue-derived Mesenchymal Stem Cells through Adenovirus EID-1. Stem Cells Dev. 2011, 20, 259–267. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhang, Y.; Zhang, Y.; Chen, L.; Mo, D. Up-regulated MiR-145 Expression Inhibits Porcine Preadipocytes Differentiation by Targeting IRS1. Int. J. Biol. Sci. 2012, 8, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Skårn, M.; Namløs, H.M.; Noordhuis, P.; Wang, M.Y.; Meza-Zepeda, L.A.; Myklebost, O. Adipocyte Differentiation of Human Bone Marrow-derived Stromal Cells is Modulated by MicroRNA-155, MicroRNA-221, and MicroRNA-222. Stem Cells Dev. 2012, 21, 873–883. [Google Scholar] [CrossRef]
- Belarbi, Y.; Mejhert, N.; Lorente-Cebrián, S.; Dahlman, I.; Arner, P.; Rydén, M.; Kulyté, A. MicroRNA-193b Controls Adiponectin Production in Human White Adipose Tissue. J. Clin. Endocrinol. Metab. 2015, 100, E1084–E1088. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.C.; Kang, I.H.; Hwang, Y.C.; Kim, S.H.; Koh, J.T. MicroRNA-194 Reciprocally Stimulates Osteogenesis and Inhibits Adipogenesis via Regulating COUP-TFII Expression. Cell Death Dis. 2014, 5, e1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Xiang, H.; Chen, C.; Zheng, R.; Chai, J.; Peng, J.; Jiang, S. MiR-224 Impairs Adipocyte Early Differentiation and Regulatesfatty Acid Metabolism. Int. J. Biochem. Cell Biol. 2013, 8, 1585–1593. [Google Scholar] [CrossRef]
- Yamada, Y.; Enokida, H.; Kojima, S.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. MiR-96 and MiR-183 Detection in Urine Serve as Potential Tumor Markers of Urothelial Carcinoma: Correlation with Stage and Grade, and Comparison with Urinary Cytology. Cancer Sci. 2011, 102, 522–529. [Google Scholar] [CrossRef]
- Kinoshita, M.; Ono, K.; Horie, T.; Nagao, K.; Nishi, H.; Kuwabara, Y.; Takanabe-Mori, R.; Hasegawa, K.; Kita, T.; Kimura, T. Regulation of Adipocyte Differentiation by Activation of Serotonin (5-HT) Receptors 5-HT2AR and 5-HT2CR and Involvement of MicroRNA-448-mediated Repression of KLF5. Mol. Endocrinol. 2010, 24, 1978–1987. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Mo, D.; Li, M.; Zhang, Y.; Chen, L.; Zhang, X.; Li, M.; Zhou, X.; Chen, Y. MiR-709 Inhibits 3T3-L1 Cell Differentiation by Targeting GSK3β of Wnt/β-catenin Signaling. Cell. Signal. 2014, 26, 2583–2589. [Google Scholar] [CrossRef]
- Zhang, J.F.; Fu, W.M.; He, M.L.; Wang, H.; Wang, W.M.; Yu, S.C.; Bian, X.W.; Zhou, J.; Lin, M.C.M.; Lu, G.; et al. MiR-637 Maintains the Balance Between Adipocytes and Osteoblasts by Directly Targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, Z.; Zhang, Z.; Liu, G.; Sun, S.; Sun, C. MiR-103 Promotes 3T3-L1 Cell Adipogenesis through AKT/mTOR Signal Pathway with its Target being MEF2D. Biol. Chem. 2015, 396, 235–244. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Norman Salem, J.; Heine, U. Exfoliation of Membrane Ecto-enzymes in the Form of Micro-vesicles. BBA Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Maryam, T.; Hossein., H.; Maryam., S.; Mehdi., K.; Farshid., N.; Reza., M. Inhibiting MiR-27a and MiR-142-5p Attenuate Nonalcoholic Fatty Liver Disease by Regulating Nrf2 Signaling Pathway. IUBMB Life 2020, 72, 361–372. [Google Scholar] [CrossRef]
- Sun, L.; Xu, R.; Sun, X.; Duan, Y.; Han, Y.; Zhao, Y.; Qian, H.; Zhu, W.; Xu, W. Safety Evaluation of Exosomes Derived from Human Umbilical Cord Mesenchymal Stromal Cell. Cytotherapy 2016, 18, 413–422. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Ning., C.Y.; Ma., Y.; Jin., L.; Tang., Q.Z.; Li., X.W.; Li., M.Z.; Liu., H.F. MiR-26b Promotes 3T3-L1 Adipocyte Differentiation Through Targeting PTEN. DNA Cell Biol. 2017, 36, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal Stem Cells Release Exosomes that Transfer MiRNAs to Endothelial Cells and Promote Angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk MiRNAs Encapsulated in Exosomes are Stable to Human Digestion and Permeable to Intestinal Barrier in vitro. J. Funct. Foods 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated Targeted Delivery of MiR-210 for Angiogenic Therapy after Cerebral Ischemia in Mice. J. Nanobiotechnol. 2019, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human Peripheral Blood-derived Exosomes for MicroRNA Delivery. Int. J. Mol. Med. 2019, 43, 2319–2328. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaño, C.; Kalko, S.; Novials, A.; Párrizas, M. Obesity-associated Exosomal MiRNAs Modulate Glucose and Lipid Metabolism in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12158–12163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maja, L.; Željko, K.; Mateja, P. Sustainable Technologies for Liposome Preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, X.; Hao, J.; Li, Y.; Sha, X. Triolein-based Polycation Lipid Nanocarrier for Efficient Gene Delivery: Characteristics and Mechanism. Int. J. Nanomed. 2011, 6, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D.; Bartucci, R.; Sportelli, L. Lipid Membranes with Grafted Polymers: Physicochemical Aspects. BBA-Biomembr. 2003, 1615, 33–59. [Google Scholar] [CrossRef] [Green Version]
- Koide, H.; Asai, T.; Hatanaka, K.; Akai, S.; Ishii, T.; Kenjo, E.; Ishida, T.; Kiwada, H.; Tsukada, H.; Oku, N. T cell-independent B Cell Response is Responsible for ABC Phenomenon Induced by Repeated Injection of PEGylated Liposomes. Int. J. Pharm. 2010, 392, 218–223. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Sun, S.; Li, B.; Du, D.; Sun, J.; Cao, F.; Li, H.; Jia, F.; Wang, T.; et al. The Use of Antibody Modified Liposomes Loaded with AMO-1 to Deliver Oligonucleotides to Ischemic Myocardium for Arrhythmia Therapy. Biomaterials 2014, 35, 3697–3707. [Google Scholar] [CrossRef]
- Dong, Y.; Love, K.T.; Dorkin, J.R.; Sirirungruang, S.; Zhang, Y.; Chen, D.; Bogorad, R.L.; Yin, H.; Chen, Y.; Vegas, A.J.; et al. Lipopeptide Nanoparticles for Potent and Selective SiRNA Delivery in Rodents and Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2014, 111, 3955–3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, Y.; Xu, C.; Sui, D.; Du, J.; Xu, F.J.; Li, Y. Effective Delivery of Hypertrophic MiRNA Inhibitor by Cholesterol-Containing Nanocarriers for Preventing Pressure Overload Induced Cardiac Hypertrophy. Adv. Sci. 2019, 6, 1900023. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Sun, J.; Li, H.; Liu, W.; Zhang, Y.; Li, B.; Qian, W.; Wang, H.; Chen, J.; Guo, Y. Lyophilized HER2-specific PEGylated Immunoliposomes for Active SiRNA Gene Silencing. Biomaterials 2010, 31, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.; Larsen, T.B.; Jølck, R.I.; Eliasen, R.; Holm, R.; Gjetting, T.; Andresen, T.L. Investigation of Enzyme-Sensitive Lipid Nanoparticles for Delivery of SiRNA to Blood–brain Barrier and Glioma Cells. Int. J. Nanomed. 2015, 10, 5995–6008. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tam, Y.Y.C.; Chen, S.; Zaifman, J.; Van Der Meel, R.; Ciufolini, M.A.; Cullis, P.R. The Niemann-Pick C1 Inhibitor NP3.47 Enhances Gene Silencing Potency of Lipid Nanoparticles Containing SiRNA. Mol. Ther. 2016, 24, 2100–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Huang, L. Exploring the Tumor Microenvironment with Nanoparticles. Cancer Treat. Res. 2015, 166, 193–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, R.J.; Yu, K.; Bi, Y.; Qi, Y.; Sun, Y.; Li, Y.; Xie, J.; Teng, L. Delivery of SiRNA Using Lipid Nanoparticles Modified with Cell Penetrating Peptide. ACS Appl. Mater. Interfaces 2016, 14, 1878. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, F.; Oki, E.; Tokunaga, E.; Maehara, Y.; Kuroki, M.; Miyamoto, S. HB-EGF Orchestrates the Complex Signals Involved in Triple-negative and Trastuzumab-resistant Breast Cancer. Int. J. Cancer 2010, 127, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, Y.; Li, H.; Peng, Q.; Wang, P.; Yang, L.; Chen, S.; Li, M.; Fu, J.J.; Yu, X.Y.; et al. Artificial Platelets for Efficient siRNA Delivery to Clear “Bad Cholesterol”. ACS Appl. Mater. Interfaces 2020, 12, 28034–28046. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Zhou, Y.; Ge, Q.; Chang, J.; Chen, Y.; Zhang, K.; Peng, D.; Chen, W. Co-delivery of Gambogenic Acid and VEGF-siRNA with Anionic Liposome and Polyethylenimine Complexes to HepG2 Cells. J. Liposome Res. 2019, 29, 322–331. [Google Scholar] [CrossRef]
- Li, F.; Yu, W.; Zhang, J.; Dong, Y.; Ding, X.; Ruan, X.; Gu, Z.; Yang, D. Spatiotemporally Programmable Cascade Hybridization of Hairpin DNA in Polymeric Nanoframework for Precise SiRNA Delivery. Nat. Commun. 2021, 12, 1138. [Google Scholar] [CrossRef]
- Li, C.; Dou, Y.; Chen, Y.; Qi, Y.; Li, L.; Han, S.; Jin, T.; Guo, J.; Chen, J.; Zhang, J. Site-Specific MicroRNA-33 Antagonism by pH-Responsive Nanotherapies for Treatment of Atherosclerosis via Regulating Cholesterol Efflux and Adaptive Immunity. Adv. Funct. Mater. 2020, 30, 1–17. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qin, X.; Wang, H.; Zhao, X.; Liu, Y.; Wo, H.T.; Liu, C.; Nishiga, M.; Chen, H.; Ge, J.; et al. An in Vivo MiRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano 2019, 13, 9880–9894. [Google Scholar] [CrossRef]
- Liu, X.L.; Pan, Q.; Cao, H.X.; Xin, F.Z.; Zhao, Z.H.; Yang, R.X.; Zeng, J.; Zhou, H.; Fan, J.G. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 Regulate Insulin Sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Brown, M.E.; Zhang, H.; Martinez, M.; Zhao, Z.; Bhutani, S.; Yin, S.; Trac, D.; Xi, J.J.; Davis, M.E. High-throughput Screening Identifies MicroRNAs that Target Nox2 and Improve Function after Acute Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1002–H1012. [Google Scholar] [CrossRef] [Green Version]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.J.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and MiRNA Delivery to Inhibit Atherosclerosis in ApoE-/- Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics with Endogenous and Exogenous Ligand-based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Ouimet, M.; Ediriweera, H.N.; Gundra, U.M.; Sheedy, F.J.; Ramkhelawon, B.; Hutchison, S.B.; Rinehold, K.; van Solingen, C.; Fullerton, M.D.; Cecchini, K.; et al. MicroRNA-33-dependent Regulation of Macrophage Metabolism Directs Immune Cell Polarization in Atherosclerosis. J. Clin. Investig. 2015, 125, 4334–4348. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.X.; Xiong, M.H.; Wang, Y.C.; Zhu, J.; Wang, J. N-acetylgalactosamine Functionalized Mixed Micellar Nanoparticles for Targeted Delivery of SiRNA to Liver. J. Control Release 2013, 166, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, V.; Iafisco, M.; Salvarani, N.; Vacchiano, M.; Carullo, P.; Ramírez-Rodríguez, G.B.; Patrício, T.; Tampieri, A.; Miragoli, M.; Catalucci, D. Bioinspired Negatively Charged Calcium Phosphate Nanocarriers for Cardiac Delivery of MicroRNAs. Nanomedicine 2016, 11, 891–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Gao, F.; Huang, J.; Wu, Y.; Wu, S.; Lei, X.G. Regulation and Function of Avian Selenogenome. BBA-Gen. Subjects 2018, 1862, 2473–2479. [Google Scholar] [CrossRef]
- Zheng, W.; Yin, T.; Chen, Q.; Qin, X.; Huang, X.; Zhao, S.; Xu, T.; Chen, L.; Liu, J. Co-delivery of Se Nanoparticles and Pooled SiRNAs for Overcoming Drug Resistance Mediated by P-glycoprotein and Class III β-tubulin in Drug-resistant Breast Cancers. Acta Biomater. 2016, 31, 197–210. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, Y.; Li, M.; Jiang, D.; Kutyreff, C.J.; Engle, J.W.; Lan, X.; Cai, W.; Chen, T. Chirality-driven Transportation and Oxidation Prevention by Chiral Selenium Nanoparticles. Angew. Chem. Int. Edit. 2020, 132, 1. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.; Zhang, S.; López, V.M.; Wang, W. Bacterial Magnetosome and its Potential Application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Lyu, C.; Lu, G.; Bao, W.; Li, F.; Wang, S.; Zhang, F.; Gao, X.; Kamiya, H.; Ma, G.; Wei, W. Engineering Magnetosomes with Chimeric Membrane and Hyaluronidase for Efficient Delivery of HIF-1 SiRNA into Deep Hypoxic Tumors. Chem. Eng. J. 2020, 398, 125453. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Wang, P.; Zhao, Z.; Wang, D.; Nannapaneni, S.; Zhang, C.; Chen, Z.; Griffith, C.C.; Hurwitz, S.J.; Chen, Z.G.; et al. Systemic Delivery of Bc12-targeting SiRNA by DNA Nanoparticles Suppresses Cancer Cell Growth. Angew. Chem. Int. Edit. 2017, 132, 12675. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Wen, D.; Chen, G.; Gu, Z. The Potential of a Microneedle Patch for Reducing Obesity. Expert Opin. Drug Deliv. 2018, 15, 431–433. [Google Scholar] [CrossRef]
- Than, A.; Liang, K.; Xu, S.; Sun, L.; Duan, H.; Xi, F.; Xu, C.; Chen, P. Transdermal Delivery of Anti-obesity Compounds to Subcutaneous Adipose Tissue with Polymeric Microneedle Patches. Small Methods 2017, 1, 1700269. [Google Scholar] [CrossRef]
- Yang, T.; Huang, D.; Li, C.; Zhao, D.; Li, J.; Zhang, M.; Chen, Y.; Wang, Q.; Liang, Z.; Liang, X.J.; et al. Rolling Microneedle Electrode Array (RoMEA) Empowered Nucleic Acid Delivery and Cancer Immunotherapy. Nano Today 2021, 36, 101017. [Google Scholar] [CrossRef]
- Kawasaki, A.M.; Casper, M.D.; Freier, S.M.; Lesnik, E.A.; Zounes, M.C.; Cummins, L.L.; Gonzalez, C.; Dan Cook, P. Uniformly Modified 2′-Deoxy-2′-fluoro Phosphorothioate Oligonucleotides as Nuclease-resistant Antisense Compounds with High Affinity and Specificity for RNA Targets. J. Med. Chem. 1993, 36, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Vester, B.; Lundberg, L.B.; Sørensen, M.D.; Babu, B.R.; Douthwaite, S.; Wengel, J. LNAzymes: Incorporation of LNA-type Monomers into DNAzymes Markedly Increases RNA Cleavage. J. Am. Chem. Soc. 2002, 124, 13682–13683. [Google Scholar] [CrossRef] [PubMed]
- Rozners, E. Recent Advances in Chemical Modification of Peptide Nucleic Acids. J. Nucleic Acids 2012, 2012, 518162. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Wang, W.; Wang, C.; Wang, Y.; Zhou, J.; Ding, Y.; Wang, X.; Jin, Y. A Chitosan-graft-PEI-candesartan Conjugate for Targeted Co-delivery of Drug and Gene in Anti-angiogenesis Cancer Therapy. Biomaterials 2014, 35, 8450–8466. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Bai, X.; Cao, X.; Jiao, X.; Huang, Y.; Li, Y.; Qin, Y.; Wen, Y. Stimuli-responsive Nanocarrier for Co-delivery of MiR-31 and Doxorubicin to Suppress High MtEF4 Cancer. ACS Appl. Mater. Interfaces 2018, 10, 22767–22775. [Google Scholar] [CrossRef]
- Manninen, V.; Tenkanen, L.; Koskinen, P.; Huttunen, J.K.; Mänttäri, M.; Heinonen, O.P.; Frick, M.H. Joint Effects of Serum Triglyceride and LDL Cholesterol and HDL Cholesterol Concentrations on Coronary Heart Disease Risk in the Helsinki Heart Study: Implications for Treatment. Circulation 1992, 85, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Brautbar, A.; Ballantyne, C.M. Pharmacological Strategies for Lowering LDL Cholesterol: Statins and Beyond. Nat. Rev. Cardiol. 2011, 8, 253–265. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C. Oral Delivery of ShRNA and SiRNA via Multifunctional Polymeric Nanoparticles for Synergistic Cancer Therapy. Biomaterials 2014, 35, 4589–4600. [Google Scholar] [CrossRef] [PubMed]
- Castanotto, D.; Rossi, J.J. The Promises and Pitfalls of RNA-interference-based Therapeutics. Nature 2009, 37, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhang, F.; Dong, L.; Wu, H.; Xu, J.; Li, H.; Wang, J.; Zhou, Z.; Liu, C.; Wang, Y.; et al. SIDT1-dependent Absorption in the Stomach Mediates Host Uptake of Dietary and Orally Administered MicroRNAs. Cell Res. 2020, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]

| NA | Nanocarrier Classification | Composition and Feature | Disease | Target Strategies | References |
|---|---|---|---|---|---|
| siPPARA | Exosomes | Exosomes isolated from plasma | Obesity | - | [93] |
| miR-21 mimic and inhibitor | Exosomes | Peripheral blood-derived exosomes | MI | - | [91] |
| cholesterol-modified miR-210 | Exosomes | Conjugated c(RGDyK) peptide on mesenchymal stromal cell (MSC)-derived exosomes | Cerebral ischemia | Peptide target Ischemia part | [90] |
| miR-155 mimic | Exosomes | ATM-derived exosomes | IR | - | [92] |
| miR-192-5p inhibitor | Exosomes | Exosomes isolated from serum | NAFLD | - | [117] |
| miR-103/107 antagomirs | Liposomes | Liposomes were composed of DLin-KC2-DMA, distearoyl phosphatidylcholine, cholesterol and mPEG2000-DMG, used at the molar ratio 50:10:38.5:1.5 | T2DM | - | [118] |
| miR-106b, miR-148b, and miR-204 mimics | Liposomes | Cationic lipid: DOTAP | MI | - | [119] |
| anti-miR-712 | Liposomes | DOTAP: DSPE-PEG2k:HSPC:chol (9.3:3.1:52.6:35, molar ratio) | AS | - | [120] |
| anti-miR-1 antisense oligonucleotides | Liposomes | anti-cardiac troponin I (cTnI) antibody modified liposomes (EPC, CHO and DSPE-PEG2000, with molar ratio of 49/50/1) | Ischemic myocardium | - | [100] |
| miR-182 inhibitor | Liposomes | CHO/PGEA | Cardiac hypertrophy | - | [102] |
| siFVII | Liposomes | DSPC, PEG-lipid, CHO | Liver disease | - | [101] |
| siFVII | Liposomes | N-acetylgalactosamine (GalNAc)–PEG–lipid | Liver disease | Receptor target liver | [121] |
| miR-33 mimic | Polymers | Chitosan via the ionic gelation method using TPP as a cross-linker | AS | Inflammation | [114] |
| anti-miR33 | Polymers | pH-responsive polymers synthesized by acetylation of cyclo-dextrins (CDs) and DSPE-PEG | AS | pH-response | [122] |
| miR-199a-3p mimic | Polymers | a core−shell structure: PFBT core, PEG shell, and TAT conjugate on the surface of NPs | MI | - | [116] |
| miR-21 mimic | Polymers | spontaneously assembled due to the complexation of hyaluronan-sulfate with the NA mediated by calcium ion bridges | MI | - | [115] |
| sipcsk9 | Polymers | PLGA: DOTAP (ratio of 20:3), platelets membrane coating | Hypercholesterolemia | - | [109] |
| siapoB | Polymers | The micellar NPs assembled in aqueous solution from mixed N-acetylgalactosamine (GalNAc) functionalized PCL-b-PPEEA | Liver disease | Receptor target liver | [123] |
| miR-133a | Inorganic nanomaterials | negatively charged calcium phosphate nanoparticles | CVD | - | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhu, L.; Zhu, L.; Wang, P.; Xu, W.; Huang, J. Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy. Int. J. Mol. Sci. 2021, 22, 7855. https://doi.org/10.3390/ijms22157855
Li T, Zhu L, Zhu L, Wang P, Xu W, Huang J. Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy. International Journal of Molecular Sciences. 2021; 22(15):7855. https://doi.org/10.3390/ijms22157855
Chicago/Turabian StyleLi, Tong, Liye Zhu, Longjiao Zhu, Pengjie Wang, Wentao Xu, and Jiaqiang Huang. 2021. "Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy" International Journal of Molecular Sciences 22, no. 15: 7855. https://doi.org/10.3390/ijms22157855






