Phenolic Compounds in Mesoamerican Fruits—Characterization, Health Potential and Processing with Innovative Technologies
Abstract
1. Introduction
2. Nutritional Composition, Phenolic Compounds and Health Potential of Mesoamerican Fruits
2.1. Description and Geographical Region
2.2. Macronutrient Composition
2.3. Micronutrient Composition
2.4. Phenolic Compounds
2.4.1. Phenolic Acids
2.4.2. Flavonoids
2.4.3. Tannins
2.4.4. Lignans
2.4.5. Stilbenes
2.5. Health Benefits of Mesoamerican Fruits
3. Effects of Innovative Technologies on Phenolic Compounds in Fruits
3.1. High Hydrostic Pressure (HHP)
3.2. Pulsed Electric Fields (PEF)
3.3. Ultrasound (US)
3.4. Microwave (MW)
3.5. Cold Plasma (CP)
3.6. Ultraviolet Light (UV)
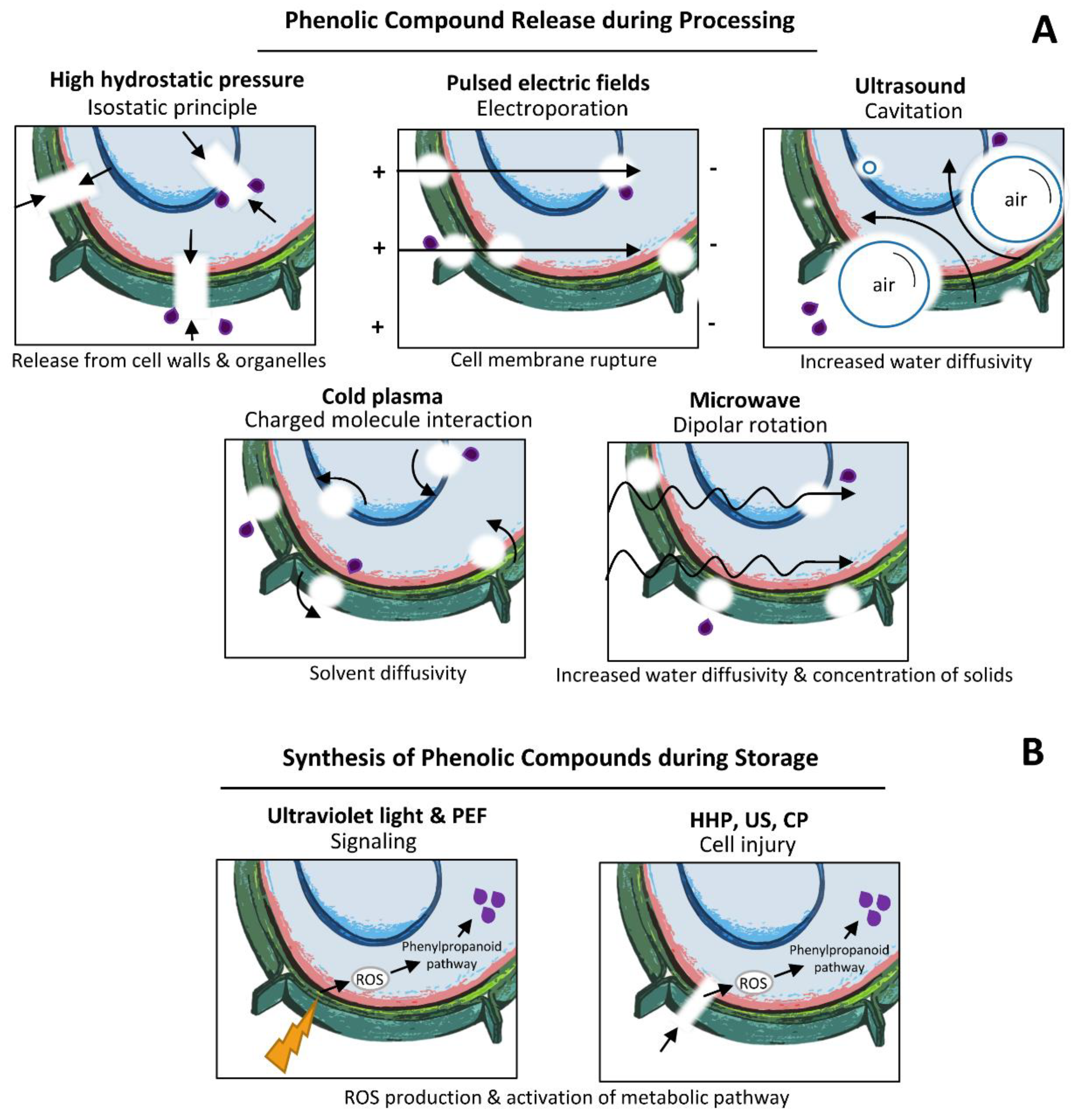
3.7. Mechanisms of Innovative Technologies on Phenolic Compounds
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| PPO | Polyphenol oxidase |
| PME | Pectin methyl esterase |
| POD | Peroxidase |
| HHP | High Hydrostatic Pressure |
| PEF | Pulsed Electric Fields |
| US | Ultrasound |
| MW | Microwave |
| CP | Cold Plasma |
| UV light | Ultraviolet light |
References
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. Origin of agriculture and plant domestication in West Mesoamerica. Genet. Resour. Crop Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Escobedo-Avellaneda, Z.; Cano, M.P.; Welti-Chanes, J. Phenolic Compounds in Food. In Phenolic Compounds in Food: Characterization and Analysis, 1st ed.; Nollet, L.M.L., Gutierrez-Uribe, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–60. [Google Scholar]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds–a review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2009, 30, 1–7. [Google Scholar] [CrossRef]
- De Paiva, J.R.; Barros, L.D.M.; Cavalcanti, J.J.V. Cashew (Anacardium occidentale L.) breeding: A global perspective. In Breeding Plantation Tree Crops: Tropical Species; Springer: New York, NY, USA, 2009; pp. 287–324. [Google Scholar]
- Larranaga, N.; Albertazzi, F.J.; Fontecha, G.; Palmieri, M.; Rainer, H.; Van Zonneveld, M.; Hormaza, J.I. A Mesoamerican origin of cherimoya (Annona cherimola Mill.): Implications for the conservation of plant genetic resources. Mol. Ecol. 2017, 26, 4116–4130. [Google Scholar] [CrossRef]
- Bermejo, J.E.H.; León, J. Neglected Crops: 1492 from a Different Perspective; Food & Agriculture Org: Rome, Italy, 1994; Volume 26. [Google Scholar]
- Ibarra-Manríquez, G.; Cornejo-Tenorio, G. Diversidad de frutos de los árboles del bosque tropical perennifolio de México. Acta Bot. Mex. 2010, 90, 51–104. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Moura, E.F.; Ramos, G.K.S.; Oliveira, M.S.P. Genetic variability analysis of Byrsonima crassifolia germplasm collected in Pará State using ISSR markers. Genet. Mol. Res. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; Ruiz, J.J.L.; d’Eeckenbrugge, G.C.; Hijmans, R.J.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Meléndez, A.; Morrell, P.L.; Roose, M.L.; Kim, S.C. Genetic diversity and structure in semiwild and domesticated chiles (Capsicum annuum; Solanaceae) from Mexico. Am. J. Bot. 2009, 96, 1190–1202. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sonoyama, T.; Muraga, Y.; Koeda, S.; Goto, T.; Yoshida, Y.; Yasuba, K. Multiple loss-of-function putative aminotransferase alleles contribute to low pungency and capsinoid biosynthesis in Capsicum chinense. Mol. Breed. 2015, 35, 142. [Google Scholar] [CrossRef]
- Ibarra-Torres, P.; Valadez-Moctezuma, E.; Pérez-Grajales, M.; Rodríguez-Campos, J.; Jaramillo-Flores, M.E. Inter-and intraspecific differentiation of Capsicum annuum and Capsicum pubescens using ISSR and SSR markers. Sci. Hortic. 2015, 181, 137–146. [Google Scholar] [CrossRef]
- Chan, Y.K. Breeding papaya (Carica papaya L.). In Breeding Plantation Tree Crops: Tropical Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 121–159. [Google Scholar]
- Petersen, J.J.; Parker, I.M.; Potter, D. Origins and close relatives of a semi-domesticated neotropical fruit tree: Chrysophyllum cainito (Sapotaceae). Am. J. Bot. 2012, 99, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Angel, R.; Borys, M.W. Germoplasma y usos del tejocote en México. In Enfoques Tecnológicos en la Fruticultura; Un tributo a Raúl Mosqueda: UACh, Mexico, 2008. [Google Scholar]
- Smith, B.D. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 1997, 276, 932–934. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutierrez-Orozco, F. Black sapote (Diospyros digyna Jacq.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 244–251. [Google Scholar]
- Solano, J.P.L.; Cano, M.E.A.; Hernández, R.G. Diversidad genética en pitahaya (Hylocereus undatus Haworth. Britton y Rose). Rev. Fitotec. Mex. 2005, 28, 179–185. [Google Scholar]
- Thompson, K.M.; Culley, T.M.; Zumberger, A.M.; Lentz, D.L. Genetic variation and structure in the neotropical tree, Manilkara zapota (L) P. Royen (Sapotaceae) used by the ancient Maya. Tree Genet. Genomes 2015, 11, 40. [Google Scholar] [CrossRef]
- Francis, J.K. Melicoccus Bijugatus Jacq. Quenepa. Sapindaceae. Soapberry Family; USDA Forest Service, Southern Forest Experiment Station, Institute of Tropical Forestry: New Orleans, LA, USA, 1992.
- Guzmán-Maldonado, S.H.; Herrera-Hernández, G.; Hernández-López, D.; Reynoso-Camacho, R.; Guzmán-Tovar, A.; Vaillant, F.; Brat, P. Physicochemical, nutritional and functional characteristics of two underutilised fruit cactus species (Myrtillocactus) produced in central Mexico. Food Chem. 2010, 121, 381–386. [Google Scholar] [CrossRef]
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Samah, S.; Pardo, C.V.D.T.; Cruz, M.A.S.; Valadez-Moctezuma, E. Genetic diversity, genotype discrimination, and population structure of Mexican Opuntia sp., determined by SSR markers. Plant Mol. Biol. Rep. 2016, 34, 146–159. [Google Scholar] [CrossRef]
- Chen, H.; Morrell, P.L.; Ashworth, V.E.; De La Cruz, M.; Clegg, M.T. Tracing the geographic origins of major avocado cultivars. J. Heredity 2009, 100, 56–65. [Google Scholar] [CrossRef]
- Awang-Kanak, F.; Bakar, M.F.A. Canistel—Pouteria campechiana (Kunth) Baehni. In Exotic Fruits Reference Guide; Rodrigues, S., Silva, E., de Brito, E., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2018; pp. 107–111. [Google Scholar]
- Marquis, D.A. Prunus serotina Ehrh. black cherry. Silv. N. Am. 1990, 2, 594–604. [Google Scholar]
- Pommer, C.V.; Murakami, K.R. Breeding guava (Psidium guajava L.). In Breeding Plantation Tree Crops: Tropical Species; Mohan, J.S., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 83–120. [Google Scholar]
- Jenkins, J.A. The origin of the cultivated tomato. Econ. Bot. 1948, 2, 379–392. [Google Scholar] [CrossRef]
- Casas, A.; Caballero, J.; Valiente-Banuet, A.; Soriano, J.A.; Da’ vila, P. Morphological variation and the process of domestication of Stenocereus stellatus (Cactaceae) in Central Mexico. Am. J. Bot. 1999, 86, 522–533. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Slavin, J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non—Insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Stampfer, M.J.; Rosner, B.; Willett, W.C. Dietary fiber and the risk of colorectal cancer and adenoma in women. N. Engl. J. Med. 1999, 340, 169–176. [Google Scholar] [CrossRef]
- Runjala, S.; Kella, L. Cashew apple (Anacardium occidentale L.) therapeutic benefits, processing and product development: An overview. Pharma Innov. 2017, 6, 260–264. [Google Scholar]
- U.S. Department of Agriculture (USDA). FoodData Central. Available online: https://ndb.nal.usda.gov/ (accessed on 25 August 2020).
- Julián-Loaeza, A.P.; Santos-Sánchez, N.F.; Valadez-Blanco, R.; Sánchez-Guzmán, B.S.; Salas-Coronado, R. Chemical composition, color, and antioxidant activity of three varieties of Annona diversifolia Safford fruits. Ind. Crop Prod. 2011, 34, 1262–1268. [Google Scholar] [CrossRef]
- Flores-García, A.; Márquez-Meléndez, R.; Salas, E.; Ayala-Soto, G.; Salmerón, I.; Hernández-Ochoa, L. Physicochemical and Sensory Characteristics of a Chagalapoli Fruit (Ardisia compressa) Beverage Fermented Using Saccharomyces cerevisiae. Int. J. Food Sci. 2019, 2019, 9687281. [Google Scholar] [CrossRef]
- Morales de León, J.; Bourges Rodríguez, H.; Camacho Parra, M.E. Tables of Composition of Food and Food Products (Condensed Version); INCMNSZ: Mexico City, Mexico, 2015. [Google Scholar]
- Contreras, L.E.; Jaimez, O.J.; Villanueva, R.S. Sensory profile and chemical composition of Opuntia joconostle from Hidalgo, Mexico. J. Stored Prod. 2011, 2, 37–39. [Google Scholar]
- Menchú, M.T.; Méndez, H. Tabla de Composición de Alimentos de Centroamérica, 2nd ed.; Serviprensa, S.A., Ed.; Instituto de Nutrición de Centro América y Panamá: Guatemala City, Guatemala, 2012. [Google Scholar]
- Pérez-Loredo, M.G.; García-Ochoa, F.; Barragán-Huerta, B.E. Comparative analysis of betalain content in Stenocereus stellatus fruits and other cactus fruits using principal component analysis. Int. J. Food Prop. 2016, 19, 326–338. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/home (accessed on 26 August 2020).
- Muñoz de Chávez, M. Valor Nutritivo de los Alimentos de Mayor Consumo, 2nd ed.; Mc Graw Hill: Mexico, Mexico, 2010; pp. 84–95. [Google Scholar]
- de Brito, E.S.; de Araújo, M.C.P.; Lin, L.Z.; Harnly, J. Determination of the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chem. 2007, 105, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Michodjehoun-Mestres, L.; Souquet, J.M.; Fulcrand, H.; Meudec, E.; Reynes, M.; Brillouet, J.M. Characterisation of highly polymerised prodelphinidins from skin and flesh of four cashew apple (Anacardium occidentale L.) genotypes. Food Chem. 2009, 114, 989–995. [Google Scholar] [CrossRef]
- Gordon, A.; Friedrich, M.; da Matta, V.M.; Moura, C.F.H.; Marx, F. Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 2012, 67, 267–276. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; Vargas, M.D.L.V.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, A.P.; de Carvalho-Silva, L.B.; Vieira, N.M.; de Souza Goes, T.; Wurlitzer, N.J.; de Fatima Borges, M.; de Brito, E.S.; Ionta, M.; de Figueiredo, R.W. Cashew-apple (Anacardium occidentale L.) and yacon (Smallanthus sonchifolius) functional beverage improve the diabetic state in rats. Int. Food Res. J. 2015, 77, 171–176. [Google Scholar] [CrossRef]
- Abdullahi, S.; Olatunji, G.A. Antidiabetic activity of Anacardium occidentale in alloxan–diabetic rats. JST 2010, 30. [Google Scholar] [CrossRef]
- Jhansyrani, T.; Sujatha, D.; Bharathi, K.; Prasad, K.V.S.R.G. Ethanolic extract of cashew apple inhibits lipid metabolism and ameliorates obesity in atherogenic diet-induced obese rats. Asian Pac. J. Trop. Biomed. 2019, 9, 405. [Google Scholar]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic and other polar compounds in the edible part of Annona cherimola and its by-products by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2015, 78, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Brindis, F.; González-Trujano, M.E.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, R. Aqueous extract of Annona macroprophyllata: A potential α-glucosidase inhibitor. BioMed Res. Int. 2013, 2013, 591313. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.M.; Gruschwitz, M.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Identification of phenolic compounds in soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res. Int. 2014, 65, 42–46. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010, 123, 77–84. [Google Scholar] [CrossRef]
- Baskaran, R.; Pullencheri, D.; Somasundaram, R. Characterization of free, esterified and bound phenolics in custard apple (Annona squamosa L) fruit pulp by UPLC-ESI-MS/MS. Food Res. Int. 2016, 82, 121–127. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Watal, G.; Murthy, P.S.; Chandra, R.; Tandon, V. Nutritional and hypoglycemic effect of fruit pulp of Annona squamosa in normal healthy and alloxan-induced diabetic rabbits. Ann. Nutr. Metab. 2005, 49, 407–413. [Google Scholar] [CrossRef]
- Kaleem, M.; Asif, M.; Ahmed, Q.U.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singap. Med. J. 2006, 47, 670. [Google Scholar]
- Joaquín-Cruz, E.; Dueñas, M.; García-Cruz, L.; Salinas-Moreno, Y.; Santos-Buelga, C.; García-Salinas, C. Anthocyanin and phenolic characterization, chemical composition and antioxidant activity of chagalapoli (Ardisia compressa K.) fruit: A tropical source of natural pigments. Food Res. Int. 2015, 70, 151–157. [Google Scholar] [CrossRef]
- Mariutti, L.R.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Reyes-Chilpa, R.; León, E.; García, M.P.; Tortoriello, J.; Huerta-Reyes, M. Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonima crassifolia. Phytomedicine 2011, 18, 1255–1261. [Google Scholar] [CrossRef]
- Ramos Pinto Sampaio, C.; Crespo Anastácio, L.M.; Guimares de Francisco, T.M.; Hoffmann Ribani, R. Anthocyanins and phenolic compounds in five ripening stages of Byrsonima ligustrifolia after extraction optimization. J. Food Nutr. Res. 2015, 54, 365–378. [Google Scholar]
- Yoshihara, T.; Yamaguchi, K.; Takamatsu, S.; Sakamura, S. A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossurri). Agric. Biol. Chem. 1981, 45, 2593–2598. [Google Scholar]
- Zhang, D.; Hamauzu, Y. Phenolic compounds, ascorbic acid, carotenoids and antioxidant properties of green, red and yellow bell peppers. J. Food Agric. Environ. 2003, 1, 22–27. [Google Scholar]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Sora, G.T.S.; Haminiuk, C.W.I.; da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef]
- Lee, Y.; Howard, L.R.; Villalon, B. Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars. J. Food Sci. 1995, 60, 473–476. [Google Scholar] [CrossRef]
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vazquez-Flota, F.A.; Miranda-Ham, M.L. Antioxidant capacity and total phenolic content in fruit tissues from accessions of Capsicum chinense Jacq.(Habanero pepper) at different stages of ripening. Sci. World J. 2014, 2014, 809073. [Google Scholar] [CrossRef]
- De Jesús Ornelas-Paz, J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar] [CrossRef]
- Oboh, G.; Puntel, R.L.; Rocha, J.B.T. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain–in vitro. Food Chem. 2007, 102, 178–185. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant activity of fresh and processed Jalapeno and Serrano peppers. J Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Yang, H.J.; Jang, D.J.; Hwang, J.T. Anti-diabetic effects of Korean red pepper via AMPK and PPAR-γ activation in C2C12 myotubes. J. Funct. Foods 2012, 4, 552–558. [Google Scholar] [CrossRef]
- Magied, M.M.A.; Salama, N.A.R.; Ali, M.R. Hypoglycemic and hypocholesterolemia effects of intragastric administration of dried red chili pepper (Capsicum annum) in alloxan-induced diabetic male albino rats fed with high-fat-diet. J. Food Nutr. Res. 2014, 2, 850–856. [Google Scholar] [CrossRef]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kanthimathi, M.S. Inhibitory effects of fruit extracts on nitric oxide-induced proliferation in MCF-7 cells. Food Chem. 2011, 126, 956–960. [Google Scholar] [CrossRef]
- Sancho, L.E.G.G.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Luo, X.D.; Basile, M.J.; Kennelly, E.J. Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple). J. Agric. Food Chem. 2002, 50, 1379–1382. [Google Scholar] [CrossRef]
- Mao, L.M.; Qi, X.W.; Hao, J.H.; Liu, H.F.; Xu, Q.H.; Bu, P.L. In vitro, ex vivo and in vivo anti-hypertensive activity of Chrysophyllum cainito L. extract. Int. J. Clin. Exp. Med. 2015, 8, 17912. [Google Scholar]
- Li, L.B.; Lin, S.; Yan, J.; Wang, Q.L.; Fan, Z.Y.; Dong, Q.R.; Qin, J.Z.; Xie, Z.G. Poly-phenolic fraction of Chrysophyllum cainito extract induces cell death in osteosarcoma cells. Bangladesh J. Pharmacol. 2015, 10, 972–979. [Google Scholar] [CrossRef]
- da Rosa, R.L.; de Almeida, C.L.; Somensi, L.B.; Boeing, T.; Mariano, L.N.B.; Krueger, C.D.M.A.; de Souza, P.; Filho, V.C.; da Silva, L.M.; de Andrade, S.F. Chrysophyllum cainito (apple-star): A fruit with gastroprotective activity in experimental ulcer models. Inflammopharmacology 2019, 27, 985–996. [Google Scholar] [CrossRef]
- González-Jiménez, F.E.; Salazar-Montoya, J.A.; Calva-Calva, G.; Ramos-Ramírez, E.G. Phytochemical characterization, in vitro antioxidant activity, and quantitative analysis by micellar electrokinetic chromatography of hawthorn (Crataegus pubescens) fruit. J. Food Qual. 2018, 2018, 2154893. [Google Scholar] [CrossRef]
- Arrieta, J.; Siles-Barrios, D.; García-Sánchez, J.; Reyes-Trejo, B.; Sánchez-Mendoza, M.E. Relaxant effect of the extracts of Crataegus mexicana on guinea pig tracheal smooth muscle. Pharmacogn. J. 2010, 2, 40–46. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Baljeet, S.Y.; Roshanlal, Y.; Ritika, B.Y. Effect of cooking methods and extraction solvents on the antioxidant activity of summer squash (Cucurbita pepo) vegetable extracts. Int. Food Res. J. 2016, 23, 1531–1540. [Google Scholar]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef]
- García-Solís, P.; Yahia, E.M.; Morales-Tlalpan, V.; Díaz-Muñoz, M. Screening of antiproliferative effect of aqueous extracts of plant foods consumed in Mexico on the breast cancer cell line MCF-7. Int. J. Food Sci. Nutr. 2009, 60, 32–46. [Google Scholar] [CrossRef]
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White pitaya (Hylocereus undatus) juice attenuates insulin resistance and hepatic steatosis in diet-induced obese mice. PLoS ONE 2016, 11, e0149670. [Google Scholar] [CrossRef]
- García-Cruz, L.; Dueñas, M.; Santos-Buelgas, C.; Valle-Guadarrama, S.; Salinas-Moreno, Y. Betalains and phenolic compounds profiling and antioxidant capacity of pitaya (Stenocereus spp.) fruit from two species (S. Pruinosus and S. stellatus). Food Chem. 2017, 234, 111–118. [Google Scholar] [CrossRef]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z. Naturforsch. 2007, 62, 636–644. [Google Scholar] [CrossRef]
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Perez, G.R.M.; Vargas, S.R.; Ortiz, H.Y.D. Wound healing properties of Hylocereus undatus on diabetic rats. Phytother. Res. 2005, 19, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Swarup, K.R.A.; Sattar, M.A.; Abdullah, N.A.; Abdulla, M.H.; Salman, I.M.; Rathore, H.A.; Johns, E.J. Effect of dragon fruit extract on oxidative stress and aortic stiffness in streptozotocin-induced diabetes in rats. Pharmacogn. Res. 2010, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.J.; Kennelly, E.J. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno, P.C.D.S.; Delazari, D.S.; Guiguer, E.L.; Coqueiro, D.P.; Araújo, A.C.; de Souza, M.S.S.; Farinazzi-Manchado, F.M.C.; Mendes, C.G.; Groppo, M. Antidiabetic and antilipidemic effects of Manilkara zapota. J. Med. Food 2015, 18, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Aguilera, A.; Contreras-Esquivel, J.C.; Rodríguez, R.; Aguilar, C.N. Extraction of condensed tannins from Mexican plant sources. Z. Naturforsch. 2008, 63, 17–20. [Google Scholar] [CrossRef]
- Santiago-Mora, P.D.; Cardador-Martinez, A.; Tellez-Perez, C.; Montejano-Gaitan, J.G.; Martin del Campo, S.T. In-vitro antioxidant capacity and bioactive compounds preservation postdrying on Berry cacti (Myrtillocactus geometrizans). J. Food Res. 2017, 6, 121–133. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Martinez-Samayoa, P.; Ramos-Gomez, M.; Guzmán, H.; Salgado, L.M. Antidiabetic and renal protective properties of Berry cactus Fruit (Myrtillocactus geometrizans). J. Med. Food 2015, 18, 565–571. [Google Scholar] [CrossRef]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.; Cano, M.P. In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds of cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2020, 128087, in press. [Google Scholar]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef] [PubMed]
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute blood glucose lowering effects and long-term safety of OpunDia™ supplementation in pre-diabetic males and females. J. Ethnopharmacol. 2010, 130, 631–634. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef]
- Partovi, N.; Ebadzadeh, M.R.; Fatemi, S.J.; Khaksari, M. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat. Prod. Res. 2018, 32, 1180–1183. [Google Scholar] [CrossRef]
- Cortez-García, R.M.; Ortiz-Moreno, A.; Zepeda-Vallejo, L.G.; Necoechea-Mondragón, H. Effects of cooking methods on phenolic compounds in xoconostle (Opuntia joconostle). Plant Foods Hum. Nutr. 2015, 70, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Esquivel, O.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Herrera-Martínez, J.; Hernández-Navarro, M.D. Protective effect of phenolic-rich extracts from different parts of Opuntia joconostle fruit against carbon tetrachloride-induced oxidative stress in mice. J. Biomater. Nanobiotechnol. 2013, 4, 35–42. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E.; Méndez-Morán, L.; Ramírez-Hernández, B.C.; García de Alba-García, J.E.; Domínguez-Arias, R.M. Efecto de la ingestión del fruto de xoconostle (Opuntia joconostle Web.) sobre la glucosa y lípidos séricos. Agrociencia 2008, 42, 645–653. [Google Scholar]
- Martínez, C.R.L.; Mateos, R.G.; Vázquez, C.G.; Castellanos, J.S. Antioxidant components and nutritional quality of 15 genotypes of Xoconostle (Opuntia spp.). J. Prof. Assoc. Cactus 2015, 17, 33–49. [Google Scholar]
- Paiz, R.C.; Juárez-Flores, B.I.; Cecilia, J.R.A.R.N.; Ortega, C.; Aguuml, J.A.R.; Chávez, E.G.; Fuentes, G.Á. Glucose-lowering effect of xoconostle (Opuntia joconostle A. Web., Cactaceae) in diabetic rats. J. Med. Plant Res. 2010, 4, 2326–2333. [Google Scholar]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Shehata, M.M.S.M.; Soltan, S.S. Effects of bioactive component of kiwi fruit and avocado (fruit and seed) on hypercholesterolemic rats. World J. Dairy Food Sci. 2013, 8, 82–93. [Google Scholar]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208. [Google Scholar]
- Tabeshpour, J.; Razavi, B.M.; Hosseinzadeh, H. Effects of avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytother. Res. 2017, 31, 819–837. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Quantitative characterization of important metabolites of avocado fruit by gas chromatography coupled to different detectors (APCI-TOF MS and FID). Food Res. Int. 2014, 62, 801–811. [Google Scholar] [CrossRef]
- Chai, W.M.; Wei, M.K.; Wang, R.; Deng, R.G.; Zou, Z.R.; Peng, Y.Y. Avocado proanthocyanidins as a source of tyrosinase inhibitors: Structure characterization, inhibitory activity, and mechanism. J. Agric. Food Chem. 2015, 63, 7381–7387. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Ascencio-Martinez, D.; Hau-Poox, A.; Mendez-Trujillo, V.; Grimaldo-Juarez, O.; Santiaguillo-Hernández, J.F.; Cervantes Diaz, L.; Aviles-Marin, S.M. Phenolic compounds and physiochemical analysis of Physalis ixocarpa genotypes. Sci. Res. Essays 2011, 6, 3808–3814. [Google Scholar]
- Medina-Medrano, J.R.; Almaraz-Abarca, N.; González-Elizondo, M.S.; Uribe-Soto, J.N.; González-Valdez, L.S.; Herrera-Arrieta, Y. Phenolic constituents and antioxidant properties of five wild species of Physalis (Solanaceae). Bot. Stud. 2015, 56, 24. [Google Scholar] [CrossRef]
- Wen, X.; Erşan, S.; Li, M.; Wang, K.; Steingass, C.B.; Schweiggert, R.M.; Ni, Y.; Carle, R. Physicochemical characteristics and phytochemical profiles of yellow and red Physalis (Physalis alkekengi L. and P. pubescens L.) fruits cultivated in China. Food Res. Int. 2019, 120, 389–398. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Sivasudha, T.; Sasikumar, J.M.; Christabel, P.H.; Jeyadevi, R.; Ananth, D.A. Antioxidant and hepatoprotective potential of Pouteria campechiana on acetaminophen-induced hepatic toxicity in rats. J. Physiol. Biochem. 2014, 70, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, N.H.C.; Hamid, M.A.; Wahab, R.A. Assessment of fatty acid composition and response surface optimization of ultrasonic-assisted extraction of phenolic compounds from Pouteria campechiana pulp. Mal. J. Fund. Appl. Sci. 2018, 14, 269–277. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Basile, M.J.; Kennelly, E.J. Analysis of polyphenolic antioxidants from the fruits of three Pouteria species by selected ion monitoring liquid chromatography− mass spectrometry. J. Agric. Food Chem. 2004, 52, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; Torres-Trodriguez, A.; Valle-Guadarrama, S.; Soto-Hernández, R.M.; Alia-Tejacal, I. Proantocianidinas y actividad enzimática en fruto de mamey zapote (Pouteria sapota) durante su maduración. Rev. Bio Cienc. 2019, 6, 16. [Google Scholar]
- Ordaz-Galindo, A.; Wesche-Ebeling, P.; Wrolstad, R.E.; Rodriguez-Saona, L.; Argaiz-Jamet, A. Purification and identification of Capulin (Prunus serotina Ehrh) anthocyanins. Food Chem. 1999, 65, 201–206. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Phenolic compounds in Rosaceae fruits from Ecuador. J. Agric. Food Chem. 2009, 57, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, J.I.; Yahia, E.M.; Rivera-Pastrana, D.M.; Rojas-Molina, A.; Zavala-Sánchez, Á.M. Nutraceutical value of black cherry Prunus serotina Ehrh. fruits: Antioxidant and antihypertensive properties. Molecules 2013, 18, 14597–14612. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv.‘Criolla’) by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef]
- Dos Santos, W.N.L.; da Silva Sauthier, M.C.; dos Santos, A.M.P.; de Andrade Santana, D.; Azevedo, R.S.A.; da Cruz Caldas, J. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592. [Google Scholar] [CrossRef]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Fidrianny, I.; Ayu, D.; Hartati, R. Antioxidant capacities, phenolic, flavonoid and carotenoid content of various polarities extracts from three organs of Sechium edule (Jacq.) Swartz. J. Chem. Pharm. 2015, 7, 914–920. [Google Scholar]
- Gordon, E.A.; Guppy, L.J.; Nelson, M. The antihypertensive effects of the Jamaican Cho-Cho (Sechium edule). West Indian Med. J. 2000, 49, 27–31. [Google Scholar] [PubMed]
- Firdous, S.M.; Neeraja, K.; Debnath, R. Cardioprotective activity of fruits of Sechium edule. Bangladesh J. Pharmacol. 2015, 10, 125–130. [Google Scholar]
- Maity, S.; Firdous, S.M.; Debnath, R. Evaluation of antidiabetic activity of ethanolic extract of Sechium edule fruits in alloxan-induced diabetic rats. World J. Pharm. Pharm. Sci. 2013, 2, 3612–3621. [Google Scholar]
- Sateesh, G.; Hussaini, S.F.; Kumar, G.S.; Rao, B.S.S. Anti-ulcer activity of Sechium edule ethanolic fruit extract. Phrama Innov. 2012, 1, 77–81. [Google Scholar]
- Firdous, S.; Sravanthi, K.; Debnath, R.; Neeraja, K.A. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of Sechium edule fruits against carbon tetrachloride induced hepatic injury in rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 354–359. [Google Scholar]
- Díaz-de-Cerio, E.; Verardo, V.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. New insight into phenolic composition of chayote (Sechium edule (Jacq.) Sw.). Food Chem. 2019, 295, 514–519. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef] [PubMed]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in tejocote (Spondias purpurea L.) peels by ultrahigh-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Brito, S.A.; Barbosa, I.S.; de Almeida, C.L.; de Medeiros, J.W.; Silva Neto, J.C.; Rolim, L.A.; da Silva, T.G.; Ximenes, R.M.; de Menezes, I.R.A.; Caldas, G.F.R.; et al. Evaluation of gastroprotective and ulcer healing activities of yellow mombin juice from Spondias mombin L. PLoS ONE 2018, 13, e0201561. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, M.J.; Macía, M.J. Chemical composition, nutritional evaluation, and economic prospects of Spondias purpurea (Anacardiaceae). Econ. Bot. 1998, 52, 373–380. [Google Scholar] [CrossRef]
- Cervantes-Arista, C.; Roman-Guerrero, A.; Oidor-Chan, V.H.; Díaz de León-Sánchez, F.; Álvares-Ramírez, E.L.; Pelayo-Zaldívar, C.; Sierra Palacios, E.C.; Mendoza-Espinoza, J.A. Chemical characterization, antioxidant capacity, and anti-hyperglycemic effect of Stenocereus stellatus fruits from the arid Mixteca Baja region of Mexico. Food Chem. 2020, 328, 127076. [Google Scholar]
- García-Cruz, L.; Valle-Guadarrama, S.; Salinas-Moreno, Y.; Joaquín-Cruz, E. Physical, chemical, and antioxidant activity characterization of pitaya (Stenocereus pruinosus) fruits. Plant Foods Hum. Nutr. 2013, 68, 403–410. [Google Scholar] [CrossRef]
- Glass, A.D.; Dunlop, J. Influence of phenolic acids onion uptake IV. Depolarization of membrane potentials. Plant Physiol. 1974, 54, 855–858. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, 310–329. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Avellaneda, Z.; García-García, R.; Welti-Chanes, J. Exotic fruit juices: Traditional and emerging processes. In Innovative Technologies in Beverage Processing; (IFST Advances in Food Science Series); Aguiló, I., Plaza, L., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 107–130. [Google Scholar]
- Höhn, A.; Sun, D.; Nolle, F. Enzymes in the fruit juice and wine industry. In Processing Fruits, Science and Technology, 2nd ed.; Barret, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 97–112. [Google Scholar]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent advances in food processing using high hydrostatic pressure technology. Crit. Rev. Food Sci Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Biochemical changes during the storage of high hydrostatic pressure processed avocado paste. J. Food Sci. 2010, 75, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innov. Food Sci. Emerg. 2012, 16, 121–128. [Google Scholar] [CrossRef]
- López-Malo, A.; Palou, E.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color changes during storage of high hydrostatic pressure treated avocado puree. Food Res. Int. 1998, 31, 549–556. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Hernando, I.; Quiles, A. High hydrostatic pressure treatment as an alternative to pasteurization to maintain bioactive compound content and texture in red sweet pepper. Innov. Food Sci. Emerg. 2014, 26, 76–85. [Google Scholar] [CrossRef]
- Queiroz, C.; Moreira, C.F.F.; Lavinas, F.C.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Effect of high hydrostatic pressure on phenolic compounds, ascorbic acid and antioxidant activity in cashew apple juice. High Press. Res. 2010, 30, 507–513. [Google Scholar] [CrossRef]
- Yen, G.C.; Lin, H.T. Comparison of high pressure treatment and thermal pasteurization effects on the quality and shelf life of guava puree. Int. J. Food Sci. Technol. 1996, 31, 205–213. [Google Scholar] [CrossRef]
- Chen, D.; Pang, X.; Zhao, J.; Gao, L.; Liao, X.; Wu, J.; Li, Q. Comparing the effects of high hydrostatic pressure and high temperature short time on papaya beverage. Innov. Food Sci. Emerg. 2015, 32, 16–28. [Google Scholar] [CrossRef]
- Sandate-Flores, L.; Rostro-Alanis, M.D.J.; Mancera-Andrade, E.I.; Esquivel-Hernandez, D.A.; Brambila-Paz, C.; Parra-Saldívar, R.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.; Rodríguez-Rodríguez, J. Using high hydrostatic pressures to retain the antioxidant compounds and to reduce the enzymatic activity of a pitaya–pineapple (Stenocereus sp.–Fragaria ananassa) beverage. J. Food Sci. Technol. 2017, 54, 611–619. [Google Scholar] [CrossRef]
- Quiroz-González, B.; Ybarra-Moncada, M.C.; Rodriguez-Martinez, V.S.; Welti-Chanes, J.S.; García-Mateos, M.R.; Corrales-García, J.; Leyva-Ruelas, G.; Torres, J.A. Refrigerated storage of high hydrostatic pressure treated pitaya (Stenocereus pruinosus) juice. Rev. Mex. Ing. Quim. 2020, 19, 387–399. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Escobedo-Avellaneda, Z.; Martín-Belloso, O.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; García-García, R.; Torres, J.A.; Welti-Chanes, J. Effect of high hydrostatic pressure on the content of phytochemical compounds and antioxidant activity of prickly pears (Opuntia ficus-indica) beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of anti-inflammatory and antioxidant activities of prickly pear fruits by high hydrostatic pressure: A chemical and microstructural approach. Innov. Food Sci. Emerg. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Welti-Chanes, J.; Cano, M.P. Release mechanisms of bioactive compounds in fruits submitted to high hydrostatic pressure: A dynamic microstructural analysis based on prickly pear cells. Food Res. Int. 2020, 130, 108909. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Ortega-Hernández, É.; Serrano-Sandoval, S.N.; Jacobo-Velázquez, D.A.; García-Cayuela, T.; Cano, M.P.; Welti-Chanes, J. Addressing key features involved in bioactive extractability of vigor prickly pears submitted to high hydrostatic pressurization. J. Food Process Eng. 2020, 43, e13202. [Google Scholar] [CrossRef]
- Shinwari, K.J.; Rao, P.S. Rheological and physico-chemical properties of a reduced-sugar sapodilla (Manilkara zapota L.) jam processed under high-hydrostatic pressure. J. Food Process Eng. 2020, 43, e13388. [Google Scholar] [CrossRef]
- Dede, S.; Alpas, H.; Bayındırlı, A. High hydrostatic pressure treatment and storage of carrot and tomato juices: Antioxidant activity and microbial safety. J. Sci. Food Agric. 2007, 87, 773–782. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Sensory shelf-life limiting factor of high hydrostatic pressure processed avocado paste. J. Food Sci. 2011, 76, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Ramos-Parra, P.A.; Hernández-Brenes, C. Survival analysis applied to the sensory shelf-life dating of high hydrostatic pressure processed avocado and mango pulps. J. Food Sci. 2010, 75, 286–291. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martinez, P.; Martin-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventós, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013, 136, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14, 401–417. [Google Scholar] [CrossRef]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Islam, R.; Paek, K.Y. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process. Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Salleh-Mack, S.Z.; Roberts, J.S. Ultrasound pasteurization: The effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrason Sonochem 2007, 14, 323–329. [Google Scholar] [CrossRef]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrason. Sonochem. 2015, 27, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.; Cavalcanti, R.N.; Couto, S.M.; Moraes, J.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.L.; Gut, J.A.W.; Ramaswamy, H.S.; Tadini, C.C.; et al. Microwave processing: Current background and effects on the physicochemical and microbiological aspects of dairy products. Compr. Rev. Food Sci. Food 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Dibanda, R.F.; Akdowa, E.P.; Tongwa, Q.M. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020, 302, 125308. [Google Scholar] [CrossRef]
- Hihat, S.; Remini, H.; Madani, K. Effect of oven and microwave drying on phenolic compounds and antioxidant capacity of coriander leaves. Int. Food Res. J. 2017, 24, 503–509. [Google Scholar]
- Zhou, L.; Tey, C.Y.; Bingol, G.; Bi, J. Effect of microwave treatment on enzyme inactivation and quality change of defatted avocado puree during storage. Innov. Food Chem. Emerg. 2016, 37, 61–67. [Google Scholar] [CrossRef]
- Dorantes-Alvarez, L.; Jaramillo-Flores, E.; González, K.; Martinez, R.; Parada, L. Blanching peppers using microwaves. Procedia Food Sci. 2011, 1, 178–183. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Gimeno-Añó, V.; Martín-Belloso, O. Modeling changes in health-related compounds of tomato juice treated by high-intensity pulsed electric fields. J. Food Eng. 2008, 89, 210–216. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. 2008, 9, 272–279. [Google Scholar] [CrossRef]
- Vallverduú-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventos, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Effects of pulsed electric fields on the bioactive compound content and antioxidant capacity of tomato fruit. J. Agric. Food Chem. 2012, 60, 3126–3134. [Google Scholar] [CrossRef]
- Bi, X.; Hemar, Y.; Balaban, M.O.; Liao, X. The effect of ultrasound on particle size, color, viscosity and polyphenol oxidase activity of diluted avocado puree. Ultrason. Sonochem. 2015, 27, 567–575. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Leite, A.K.F.; da Silva, A.R.A.; Fernandes, F.A.N.; Rodrigues, S. Sonication effect on bioactive compounds of cashew apple bagasse. Food Bioprocess Technol. 2017, 10, 1854–1864. [Google Scholar] [CrossRef]
- Dabir, M.P.; Ananthanarayan, L. Effect of thermosonication on peroxidase, pectin methylesterase activities and on bioactive compounds in custard apple juice. J. Food Meas. Charact. 2017, 11, 1623–1629. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Le, T.T.; Le, V.V.M. Application of combined ultrasound and cellulase preparation to guava (Psidium guajava) mash treatment in juice processing: Optimization of biocatalytic conditions by response surface methodology. Int. Food Res. J. 2013, 20, 377. [Google Scholar]
- Cansino, N.C.; Carrera, G.P.; Rojas, Q.Z.; Olivares, L.D.; García, E.A.; Moreno, E.R. Ultrasound processing on green cactus pear (Opuntia ficus indica) juice: Physical, microbiological and antioxidant properties. J. Food Process. Technol. 2013, 4, 1–6. [Google Scholar]
- Martínez-Moreno, O.G.; Anaya-Esparza, L.M.; Sánchez-Burgos, J.A.; Meza-Espinoza, L.; Pérez-Larios, A.; Bojorquez-Quintal, J.E.; Montalvo-González, E. Effect of vacuum-thermosonication on the inactivation of Escherichia coli, Staphylococcus aureus, polyphenol oxidase and the quality parameters of soursop puree. Innov. Food Sci. Emerg. 2020, 59, 102255. [Google Scholar] [CrossRef]
- Mehta, D.; Sharma, N.; Bansal, V.; Sangwan, R.S.; Yadav, S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. 2019, 52, 343–349. [Google Scholar] [CrossRef]
- Salazar-González, C.; San Martín-González, M.F.; Vergara-Balderas, F.T.; López-Malo, A.; Sosa-Morales, M.E. Physical-chemical and microbiological stability during refrigerated storage of microwave-pasteurized guava nectar. Focus. Mod. Food Ind. 2014, 3, 43–51. [Google Scholar] [CrossRef]
- Palma-Orozco, G.; Sampedro, J.G.; Ortiz-Moreno, A.; Nájera, H. In situ inactivation of polyphenol oxidase in mamey fruit (Pouteria sapota) by microwave treatment. J. Food Sci. 2012, 77, 359–365. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Matan, N.; Puangjinda, K.; Phothisuwan, S.; Nisoa, M. Combined antibacterial activity of green tea extract with atmospheric radio-frequency plasma against pathogens on fresh-cut dragon fruit. Food Control 2015, 50, 291–296. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Beltrán, J.Á.G. Short-wave ultraviolet-C light effect on pitaya (Stenocereus griseus) juice inoculated with Zygosaccharomyces bailii. J. Food Eng. 2013, 117, 34–41. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Effects of UVB light, wounding stress, and storage time on the accumulation of betalains, phenolic compounds, and ascorbic acid in red prickly pear (Opuntia ficus-indica cv. Rojo Vigor). Food Bioprocess Technol. 2018, 11, 2265–2274. [Google Scholar] [CrossRef]
- Ryu, Y.H.; Kim, Y.H.; Lee, J.Y.; Shim, G.B.; Uhm, H.S.; Park, G.; Choi, E.H. Effects of background fluid on the efficiency of inactivating yeast with non-thermal atmospheric pressure plasma. PLoS ONE 2013, 8, e66231. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Keyser, M.; Műller, I.A.; Cilliers, F.P.; Nel, W.; Gouws, P.A. Ultraviolet radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juice. Innov. Food Sci. Emerg. 2008, 9, 348–354. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, J.; Zhang, Y.; Liao, X.; Chen, F.; Hu, X. Microstructural and morphological behaviors of asparagus lettuce cells subject to high pressure processing. Food Res. Int. 2015, 71, 174–183. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; del Rosario Cuéllar-Villarreal, M.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Ramos-Parra, P.A.; Hernández-Brenes, C. Nonthermal processing technologies as elicitors to induce the biosynthesis and accumulation of nutraceuticals in plant foods. Trends Food Sci. Technol. 2017, 60, 80–87. [Google Scholar] [CrossRef]
| Scientific Name | Fruit Name (Spanish Name) | Native Regions | |
|---|---|---|---|
| 1 | Anacardium occidentale L. | Cashew apple (Marañón) | Brazil and Central America [6]. |
| 2 | Annona cherimola Mill. | Cherimoya (Chirimoya) | Mesoamerica [7]. |
| 3 | Annona diversifolia Saff. | Annona (Ilama/Papausa) | Mesoamerica [8]. |
| 4 | Annona muricate L. | Soursop (Guanábana) | Central America and northern South America [8]. |
| 5 | Annona reticulata L. | Custard apple (Anona roja) | Mesoamerica (Guatemala and Belize) [8]. |
| 6 | Annona squamosa L. | Sugar apple (Saramuyo) | Southeast Mexico [8]. |
| 7 | Ardisia compressa Kunth | Chagalapoli | Tropical rain forests of Mexico [9]. |
| 8 | Byrsonima crassifolia (L.) Kunth | Nance | Amazon region and tropical America [10]. |
| 9 | Capsicum annuum L. | Bell pepper (Pimiento) | Capsicum annuum L. peppers (9–14): Domesticated species of Capsicum annuum var. Glabriusculum of Mesoamerican origin (Mexico) [11,12]. |
| 10 | Capsicum annuum L. | Jalapeño pepper | |
| 11 | Capsicum annuum L. | Poblano pepper | |
| 12 | Capsicum annuum L. | Serrano pepper | |
| 13 | Capsicum annuum L. | Yahualica pepper | |
| 14 | Capsicum annuum L. | Chilaca pepper | |
| 15 | Capsicum chinense Jacq. | Habanero pepper | Amazon region (domesticated in Mesoamerica) [13]. |
| 16 | Capsicum pubescens Ruiz et Pav. | Manzano pepper | Mesoamerica (Central and South America) [14]. |
| 17 | Carica papaya L. | Papaya | Mesoamerica (Mexico) [15]. |
| 18 | Chrysophyllum cainito L. | Cainito (Caimito) | Southern Mesoamerica (Panama) [16]. |
| 19 | Crataegus mexicana Moc. et Sessé | Mexican hawthorn (Tejocote) | Mesoamerica (Mexico) [17]. |
| 20 | Cucurbita pepo L. | Zucchini (Calabacita) | Mesoamerica (Mexico) [18]. |
| 21 | Diospyros digyna Jacq. | Black sapote (Zapote negro) | Mesoamerica [19]. |
| 22 | Hylocereus undatus (Haw.) Britton et Rose | Dragon fruit (Pitahaya) | Mesoamerica (central Mexico) [20]. |
| 23 | Manilkara zapota (L.) P. Royen | Sapodilla (Chicozapote) | Mesoamerica (Mexico, Guatemala and Belize) [21]. |
| 24 | Melicoccus bijugatus Jacq. | Mamoncillo (Guaya) | South America (Colombia and Venezuela) [22]. |
| 25 | Myrtillocactus geometrizans (Mart. ex Pfeiff) | Cactus berry (Garambullo) | Arid and semiarid regions of Mexico [23]. |
| 26 | Opuntia ficus-indica (L.) Mill. | Prickly pear (Tuna) | Mesoamerica (central and southern Mexico) [24]. |
| 27 | Opuntia joconostle Web. | Sour prickly pear (Xoconostle) | Mesoamerica [25]. |
| 28 | Persea americana Mill. | Avocado (Aguacate) | Mesoamerica (Mexico and Central America) [26]. |
| 29 | Physalis philadelphica Lam. | Tomatillo | Mesoamerica (Mexico) [8]. |
| 30 | Pouteria campechiana (Kunth) Baehni | Canistel (Zapote amarillo) | Mesoamérica (Bahamas, Belize, El Salvador, Guatemala and southern Mexico) [27]. |
| 31 | Pouteria sapota (Jacq.) H.E. Moore et Stearn | Mamey | Mesoamerica [8]. |
| 32 | Prunus serotina Ehrh. | Capulin | Mesoamerica (Mexico and Guatemala) [28]. |
| 33 | Psidium guajava L. | Guava (Guayaba) | Mesoamerica [29]. |
| 34 | Sechium edule (Jacq.) Swartz | Squash (Chayote) | Mesoamerica (southern Mexico and Guatemala) [8]. |
| 35 | Solanum lycopersicum L. | Tomato (Jitomate) | Peru-Ecuador (domesticated in Mexico) [30]. |
| 36 | Spondias mombin L. | Yellow mombin (Ciruela amarilla) | Mesoamerica [8]. |
| 37 | Spondias purpurea L. | Red mombin (Ciruela roja) | Mesoamerica (Yucatán in Mexico) [8]. |
| 38 | Stenocereus stellatus (Pfeiff.) Riccob. | Pitaya (Pitaya) | Mesoamérica (central Mexico) [31]. |
| Fruit | Water | Protein | Fat | Carbohydrate 1 | Fiber, Total Dietary | Ref. | |
|---|---|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (g) | |||
| 1 | Cashew apple | 86.3 | 0.2 | 0.1 | 11.1 | 3.2 | [36] |
| 2 | Cherimoya | 79.4 | 1.6 | 0.7 | 17.7 | 3.0 | [37] |
| 3 | White annona | 79.6 | 1.1 | 0.3 | 13.6 | 4.4 | [38] |
| Pink annona | 78.9 | 0.9 | 0.2 | 18.4 | 0.6 | [37] | |
| Deep Pink annona | 77.1 | 0.9 | 0.2 | 20.3 | 0.7 | [37] | |
| 4 | Soursop | 81.2 | 1.0 | 0.3 | 16.8 | 3.3 | [37] |
| 5 | Custard apple | 71.5 | 1.7 | 0.6 | 25.2 | 2.4 | [37] |
| 6 | Sugar apple | 73.2 | 2.1 | 0.3 | 23.6 | 4.4 | [37] |
| 7 | Chagalapoli | 80.5 | 8.6 | 0.6 | 11.9 | 3.6 | [39] |
| 8 | Nance | 80.6 | 0.7 | 1.2 | 17.0 | 7.5 | [37] |
| 9 | Bell pepper | 93.3 | 0.9 | 0.2 | 5.1 | 1.8 | [37] |
| 10 | Jalapeño pepper | 91.7 | 0.9 | 0.4 | 6.5 | 2.8 | [37] |
| 11 | Poblano pepper | 93.9 | 0.9 | 0.2 | 4.6 | 1.7 | [37] |
| 12 | Serrano pepper | 90.3 | 1.7 | 0.4 | 6.7 | 3.7 | [37] |
| 14 | Chilaca pepper | 89.4 | 1.5 | 0.3 | 7.4 | 0.9 4 | [40] |
| 15 | Habanero pepper | 91 | 2.3 | 0.8 | 3.6 | 1.6 4 | [40] |
| 17 | Papaya | 88.1 | 0.5 | 0.3 | 10.8 | 1.7 | [37] |
| 18 | Purple cainito | 84.5 | 0.6 | 1.7 | 12.7 | - | [40] |
| White cainito | 84.7 | 0.8 | 1.6 | 13.2 | - | [40] | |
| 19 | Mexican hawthorn | 74.7 | 0.8 | 0.6 | 22.0 | 2.7 4 | [40] |
| 20 | Zucchini | 92.7 | 2.7 | 0.4 | 3.1 | 1.1 | [37] |
| 21 | Black sapote | 83.6 | 0.6 | 1.1 | 14.5 | 5.3 | [37] |
| 22 | Dragon fruit 2 | 82.3 | 1.4 | 0.1 | 13.6 | 2.1 4 | [40] |
| 23 | Sapodilla | 78.0 | 0.4 | 1.1 | 20.0 | 5.3 | [37] |
| 26 | Prickly pear | 87.6 | 0.7 | 0.5 | 9.6 | 3.6 | [37] |
| 27 | Sour prickly pears | 87.6 | 1.1 | 0.1 | 6.7 | 4.0 4 | [41] |
| 28 | Avocado | 73.2 | 2.0 | 14.7 | 8.5 | 6.7 | [37] |
| 29 | Tomatillo | 91.6 | 1.0 | 1.0 | 5.8 | 1.9 | [37] |
| 30 | Canistel | 60.6 | 2.0 | 0.5 | 35.9 | - | [42] |
| 31 | Mamey | 64.9 | 1.5 | 0.5 | 32.1 | 5.4 | [37] |
| 33 | Guava | 80.8 | 2.6 | 1.0 | 14.3 | 5.4 | [37] |
| 34 | Squash | 94.2 | 0.8 | 0.1 | 4.5 | 1.7 | [37] |
| 35 | Tomato | 94.8 | 1.2 | 0.2 | 3.2 | 0.9 | [37] |
| 36 | Yellow mombin | 70.4 | 1.4 | 0.1 | 26.7 | - | [42] |
| 37 | Red mombin | 76.2 | 0.9 | 0.1 | 22.0 | - | [42] |
| 38 | White pitaya 3 | 86.6 | 1.1 | 0.5 | 9.8 | 1.6 4 | [43] |
| Yellow pitaya | 85.4 | 1.2 | 0.5 | 10.6 | 1.6 4 | [43] | |
| Purple pitaya | 86.6 | 1.3 | 0.5 | 9.6 | 1.4 4 | [43] | |
| Red pitaya | 86.4 | 1.3 | 0.4 | 9.8 | 1.6 4 | [43] |
| Minerals | Vitamins | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | Ca | Fe | Mg | P | K | Na | Zn | Cu | Mn | Se | Vit C | Thiamin (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic Acid (B5) | Pyridoxine (B6) | Folate, Total | Vit A 3 | Ref. | |
| mg | mg | mg | mg | mg | mg | mg | mg | mg | µg | mg | mg | mg | mg | mg | mg | µg | µg | |||
| 1 | Cashew apple | 37 | 6.68 | 292 | 593 | 660 | 12 | 5.78 | 2.20 | 1.66 | 19.9 | 0.5 | 0.42 | 0.06 | 1.06 | 0.86 | 0.42 | 25.0 | 0.0 | [36] |
| 2 | Cherimoya | 10 | 0.27 | 17 | 26 | 287 | 7 | 0.16 | 0.07 | 0.09 | - | 12.6 | 0.10 | 0.13 | 0.64 | 0.35 | 0.26 | 23.0 | 0.0 | [37] |
| 3 | White annona | 0.9 | - | 8 | - | 348 | 2 | 0.13 | - | - | - | 2.4 | - | - | - | - | - | - | - | [38] |
| Pink annona | 23 | - | 13 | - | 336 | 3 | 12.71 | - | - | - | 1.6 | - | - | - | - | - | - | - | [37] | |
| Deep pink annona | 14 | - | 14 | - | 347 | 3 | 14.01 | - | - | - | 1.5 | - | - | - | - | - | - | - | [37] | |
| 4 | Soursop | 14 | 0.6 | 21 | 27 | 278 | 14 | 0.10 | 0.09 | - | 0.6 | 20.6 | 0.07 | 0.05 | 0.90 | 0.25 | 0.06 | 14.0 | 0.0 | [37] |
| 5 | Custard apple | 30 | 0.71 | 18 | 21 | 382 | 4 | - | - | - | 19.2 | 0.08 | 0.10 | 0.50 | 0.14 | 0.22 | - | 2.0 | [37] | |
| 6 | Sugar apple | 24 | 0.6 | 21 | 32 | 247 | 9 | 0.10 | 0.09 | 0.6 | 36.3 | 0.11 | 0.11 | 0.88 | 0.23 | 0.20 | 14.0 | 0.0 | [37] | |
| 8 | Nance | 46 | 0.38 | 20 | 10 | 244 | 3 | 0.09 | 0.04 | 0.25 | 0.4 | 92.5 | 0.02 | 0.02 | 0.29 | 0.18 | 0.02 | 8.0 | 4.0 | [37] |
| 9 | Bell pepper | 9 | 0.37 | 11 | 22 | 188 | 3 | 0.17 | 0.05 | - | 0 | 97 | 0.06 | 0.05 | 0.66 | - | 0.25 | 23.0 | 67.0 | [37] |
| 10 | Jalapeño pepper | 12 | 0.25 | 15 | 26 | 248 | 3 | 0.14 | 0.05 | 0.10 | 0.4 | 118.6 | 0.04 | 0.07 | 1.28 | 0.32 | 0.42 | 27.0 | 54.0 | [37] |
| 11 | Poblano pepper | 10 | 0.34 | 10 | 20 | 175 | 3 | 0.13 | 0.07 | - | 0 | 80.4 | 0.06 | 0.03 | 0.48 | - | 0.22 | 10.0 | 18.0 | [37] |
| 12 | Serrano pepper | 11 | 0.86 | 22 | 40 | 305 | 10 | 0.26 | 0.13 | - | 0.4 | 44.9 | 0.05 | 0.08 | 1.54 | - | 0.51 | 23.0 | 47.0 | [37] |
| 14 | Chilaca pepper | 40 | 4.00 | - | 23 | 340 | - | - | - | - | 0.04 | 178.2 | 0.08 | 0.06 | 1.00 | - | - | - | 16.0 | [40,45] |
| 15 | Habanero pepper | 18 | 2.44 | - | 26 | - | - | - | - | - | - | 94 | 0.11 | 0.16 | 0.71 | - | - | - | - | [40] |
| 17 | Papaya | 20 | 0.25 | 21 | 10 | 182 | 8 | 0.08 | 0.05 | 0.04 | 0.6 | 60.9 | 0.02 | 0.03 | 0.36 | 0.19 | 0.04 | 37.0 | 47.0 | [40] |
| 18 | Purple cainito | 34 | 2.20 | - | 19 | - | - | - | - | - | - | 12.8 | 0.10 | 0.03 | 0.64 | - | - | - | - | [40] |
| White cainito | 25 | 0.94 | - | 15 | - | - | - | - | - | - | 19.0 | 0.03 | 0.04 | 0.66 | - | - | - | 2.0 | [40] | |
| 19 | Mexican hawthorn | 94 | 1.56 | - | 33 | - | - | - | - | - | - | 73.8 | 0.04 | 0.05 | 0.43 | - | - | - | - | [37] |
| 20 | Zucchini | 21 | 0.79 | 33 | 93 | 459 | 3 | 0.83 | 0.10 | 0.20 | 0.3 | 34.1 | 0.04 | 0.04 | 0.71 | 0.37 | 0.14 | 20.0 | 25.0 | [37] |
| 21 | Black zapote | 27 | 2.48 | 12 | 29 | 193 | 12 | 0.10 | - | - | - | 28.7 | 0.00 | 0.02 | 0.26 | - | - | 14.0 | 3.0 | [40] |
| 22 | Dragon fruit 1 | 5 | 0.75 | - | 15 | - | - | - | - | - | - | 25.8 | 0.11 | 0.13 | 0.37 | - | - | - | 0.0 | [40] |
| 23 | Sapodilla | 21 | 0.80 | 12 | 12 | 193 | 12 | 0.10 | 0.09 | - | 0.6 | 14.7 | 0.00 | 0.02 | 0.20 | 0.25 | 0.04 | 14.0 | 3.0 | [37] |
| 26 | Prickly pear | 56 | 0.30 | 85 | 24 | 220 | 5 | 0.12 | 0.08 | 0.6 | 14 | 0.01 | 0.06 | 0.46 | 0.06 | 6.0 | 2.0 | [37] | ||
| 28 | Avocado | 12 | 0.55 | 29 | 52 | 485 | 7 | 0.64 | 0.19 | 0.14 | 0.4 | 10 | 0.07 | 0.13 | 1.00 | 1.39 | 0.26 | 81.0 | 7.0 | [37] |
| 29 | Tomatillo | 7 | 0.62 | 20 | 39 | 268 | 1 | 0.22 | 0.08 | 0.15 | 0.5 | 11.7 | 0.04 | 0.04 | 1.85 | 0.15 | 0.06 | 7.0 | 6.0 | [37] |
| 30 | Canistel | 20 | 1.00 | - | 42 | - | - | - | - | - | - | 43 | 0.02 | 0.02 | 3.13 | - | - | - | - | [42] |
| 31 | Mamey | 18 | 0.78 | 11 | 26 | 454 | 7 | 0.19 | 0.21 | 0.20 | - | 23 | 0.01 | 0.12 | 1.43 | 0.40 | 0.72 | 7.0 | 7.0 | [37] |
| 33 | Guava | 18 | 0.26 | 22 | 40 | 417 | 2 | 0.23 | 0.23 | 0.15 | 0.6 | 228.3 | 0.07 | 0.04 | 1.08 | 0.45 | 0.11 | 49.0 | 31.0 | [37] |
| 34 | Squash | 17 | 0.34 | 12 | 18 | 125 | 2 | 0.74 | 0.12 | 0.19 | 0.2 | 7.7 | 0.03 | 0.03 | 0.47 | 0.25 | 0.08 | 93.0 | 0.0 | [37] |
| 35 | Tomato | 5 | 0.47 | 8 | 29 | 212 | 42 | 0.14 | 0.06 | 0.09 | 0.4 | 16 | 0.05 | 0.03 | 0.59 | 0.19 | 0.06 | 29.0 | 75.0 | [37] |
| 36 | Yellow mombin | 34 | 3.00 | 73 | - | - | - | - | - | - | 51 | 0.10 | 0.05 | 0.94 | - | - | - | - | [42] | |
| 37 | Red mombin | 22 | 0.60 | - | 40 | -- | - | - | - | - | - | 43 | 0.07 | 0.03 | 1.00 | - | - | - | - | [42] |
| 38 | White pitaya 2 | - | - | - | - | - | - | - | - | - | - | 55 | - | - | - | - | - | - | - | [43] |
| Yelllow pitaya | - | - | - | - | - | - | - | - | - | - | 44.5 | - | - | - | - | - | - | - | [43] | |
| Purple Pitaya | - | - | - | - | - | - | - | - | - | - | 41.8 | - | - | - | - | - | - | - | [43] | |
| Red pitaya | - | - | - | - | - | - | - | - | - | - | 35.5 | - | - | - | - | - | - | - | [43] | |
| Fruit | Total Phenolics a | Antioxidant Capacity | Phenolic Profile | Bioactivity | Ref | |
|---|---|---|---|---|---|---|
| 1 | Red cashew apple | 118–740 | 618 1 274 2 | Phenolic acids: ferulic, ellagic, caffeic, protocatechuic, gallic, gentisic, p-coumaric, salicylic and sinapic acid Flavonoids: 3-O-galactoside, 3-O-glucoside, 3-O-xylopyranoside, 3-O-arabinopyranoside, 3-O-arabinofuranoside, 3-O-rhamnoside of myricetin and quercetin Anthocyanins: 5-methylcyanidin 3-O-hexoside and hexosides of cyanidin, petunidin and peonidin Tannins: (−)-epigallocatechin, (−)-epigallocatechin-O-gallate and (−)-epicatechin-3-O-gallate | In vivo anti-diabetic, antioxidant, anti-obesity and anti-inflammatory activity In vitro antioxidant activity | [46,47,48,49,50,51,52] |
| Yellow cashew apple | 186–634 | 642 1 345 2 | ||||
| 2 | Cherimoya | 125–683 | 879 1 230 2 867 3 | Phenylethanoids: hydroxytyrosol hexoside Phenolic acids: 4-O-caffeyolquinic acid, caffeic acid-O-hexoside and sinapic acid Flavonoids: catechin, epicatechin and quercetin-3-O-glucoronide. Tannins: procyanidin dimers, trimers and tetramers types A and B Lignins | In vitro antioxidant and anticancer activity | [53,54,55] |
| 3 | Annona/Ilama | 129–246 | 675 1 358 2 | Not reported | In vitro antidiabetic and antioxidant activity | [38,49,56,57] |
| 4 | Soursop | 236–577 | 1451 3 | Phenolic acids: p-coumaric, coumaric acid hexose, 5-caffeoylquinic, caffeic acid derivative and dicaffeoylquinic acid Flavonoids: dihydrokaempferol-hexoside | In vitro antioxidant activity | [58] |
| 5 | Custard apple | 358 | 650 1 376 2 | Not reported | In vitro antioxidant activity | [49] |
| 6 | Green sugar apple | 208 | 646 1 369 2 | Phenolic acids: gallic, protocatechuic, caffeic, p-coumaric, sinapic and ferulic acid Flavonoids: catechin, epicatechin and epigallocatechin gallate Tannins: procyanidin B2 | In vivo antidiabetic and antioxidant activity In vitro antioxidant activity | [49,59,60,61] |
| Purple sugar apple | 82 | 656 1 3582 | ||||
| 7 | Chagalapoli | 1051 | 44501 | Phenolic acids: derivates of caffeic and p-coumaric acid (hydroxycinnamoyl compounds) Flavonoids: (+)-catechin, (−)-epicatechin, myricetin-O-hexoside, kaempferol di-deoxyhexosyl-hexoside, kaempferol di-deoxyhexosyl-hexoside, (epi)catechin-3-O-gallate, quercetin 3-O-rutinoside and isorhamnetin rutinoside Anthocyanins: delphinidin 3-O-galactoside, petunidin 3-O-galactoside, cyanidin 3-O-galactoside, peonidin 3-O-galactoside and malvidin 3-O-galactoside Tannins: procyanidin B2 | In vitro antioxidant activity | [62] |
| 8 | Green nance | 195 | 669 1 381 2 | Phenolic acids: gallic, tetragalloylquinic, ellagic acid galloyl hexoside, protocatechuic, p-hydroxybenzoic, caffeic and p-coumaric acid Flavonoids: (−) epicatechin, catechin, rutin, taxifolin, quercetin pentoside, kaempferol, hesperidin, quercetin-3-O-xyloside, quercetin and quercetin-3-glucoside Anthocyanins: cyanidin-3-glucoside, pelargonidin-3-glucoside, peonin-3-glucoside and delphinidin-3-glucoside Tannins: proanthocyanidin dimers | In vivo antidepressant activity In vitro antioxidant activity | [49,63,64,65] |
| Red nance | 266 | 662 1 3762 | ||||
| Yellow nance | 241 | 662 1 373 2 | ||||
| 9 | Green bell pepper | 48–120 | 856–1717 1 228–560 2 399 4 | Stilbenoids: resveratrol Phenolic acids: gallic, caffeic and chlorogenic Flavonoids: myricetin, quercetin, quercetin 3-rutinoside, quercetin-D-glucoside, luteolin and kaempferol Lignan amides: p-aminobenzaldehyde, N-cis-feruloyl tyramine, N-trans-feruloyl tyramine, grossamide, N-trans-p-coumaroyl tyramine, N-trans-feruloyl octopamine and N-trans-p-coumaroyl octopamine Phenolic amides: dihydrocapsaicin | In vitro antibacterial, anti-inflammatory and antioxidant activity | [66,67,68,69] |
| Red bell pepper | 64–414 | 696 1 6322 | ||||
| Yellow bell pepper | 55–260 | 504 1 472 2 | ||||
| 10 | Jalapeño pepper | 92–244 | 229–538 2 4368–12, 420 3 55–659 4 | Phenolic compounds in peppers (10–16): Phenolic acids: sinapic acid-O-hexoside, caffeic acid glycoside, p-hydroxybenzoic acid β-glucoside and vanillic acid 1-O-β-D-glucopyranoside Flavonoids: quercetin, luteolin, kaempferol, apigenin, quercetin dihexoside, quercetin 3,7-di-O-rhamnopyranoside, apigenin apiofuranosyl-glucopyranoside, quercetin glucopyranoside, luteloin-glucopyranoside, naringenin chalcone hexose and naringenin 7-O-glucoside Phenolic amides: capsaicin, dihydrocapsaicin and nordihydrocapsaicin Lignans: Lariciresinol glucopyranoside | Bioactivity in peppers (10–16): In vivo antidiabetic, hypocholesterolemic, cardioprotective and antiobesity activity In vitro antidiabetic, anti-inflammatory, anticancer and antioxidant activity | [69,70,71,72,73,74,75,76,77,78] |
| 11 | Poblano pepper | 188–305 | 48 2 0.5 5 62 6 | |||
| 12 | Serrano pepper | 69–296 | 242–476 2 6344–6844 3 487–5554 | See section above (compilation of phenolics and bioactivity in peppers 10–16) | ||
| 13 | Yahualica pepper | 180 | 70 6 | See section above (compilation of phenolics and bioactivity in peppers 10–16) | ||
| 14 | Chilaca pepper | 974 | 710 1 47–55 2 215 4 | See section above (compilation of phenolics and bioactivity in peppers 10–16) | ||
| 15 | Habanero pepper | 16–232 | 2027–2694 1 260 2 481–898 4 | See section above (compilation of phenolics and bioactivity in peppers 10–16) | ||
| 16 | Manzano pepper | 132 | 2 8900 | See section above (compilation of phenolics and bioactivity in peppers 10–16) | ||
| 17 | Papaya | 45–159 | 661 1 270–988 3 | Phenolic acids: caffeic acid-O-hexoside-O-rhamnoside, caffeic acid hexoside-O-pentoside, protocatechuic acid-O-hexoside, ferulic and p-coumaric acid Flavonoids: quercetin-3-O(2′rhamnosyl)-rutinoside, quercetin-3-O-glucuronide and apigenin-O-pentoside | In vitro antiproliferative, anti-inflammatory and antioxidant activity | [53,58,79,80] |
| 18 | Green cainito | 18–20 | 685 1 333 2 | Phenolic acids: gallic acid Flavonoids: (+)-catechin, (−)-epicatechin, (+)-galocatechin, (−)-epigallocatechin, quercetin, quercitrin, isoquercitrin and myricitrin Tannins | In vivo hypertensive and gastroprotective activity Ex vivo antihypertensive activity In vitro antihypertensive, anticancer and antioxidant activity | [49,81,82,83,84] |
| Purple cainito | 15–80 | 650 1 367 2 | ||||
| 19 | Mexican hawthorn | 50–550 | 1472 5 0.06–0.35 6 | Phenolic acids: chlorogenic acid Flavonoids: (+)-catechin, (−)-epicatechin, rutin, vitexin, hyperoside, quercetin and vitexin 2-O-rhamnoside Tannins: procyanidin dimer, procyanidin trimer and progyadinidin tetramer | In vitro antioxidant and relaxant activity | [85,86] |
| 20 | Zucchini | 519–867 | 12 6 370 5 | Phenolic acids: p-coumaric, ferulic, caftaric, chlorogenic, caffeic, 2-O-caffeoylmalic, chicoric, dicaffeic, sinapic acid hexoside, protocatechuic, p-hydroxybenzoic, benzoic, vanillic, vanillic acid glycoside and hydroxybenzoic acid hexose Flavonoids: quercetin 3-O-rhamnosyl-rhamnosyl-glucoside, luteolin O-glucoside, quercetin, isorhamentin, robinin, quercetin 3-rutinoside, quercetin O-glucoside, isorhamnetin O-rutinoside, kampeferol rutinoside, kaempferol O-glycoside, astragalin, myricetin and rutin Tannins | In vitro antioxidant activity | [87,88,89] |
| 21 | Black zapote | 158–247 | 560 1 118 2 | Phenolic acids: cinnamic acid, p-hydroxybenzoic acid, dicoumaroylhexose-deoxyhexose, caffeic acid, sinapic acid, ferulic acid, o-coumaric acid and protocatechuic acid Flavonoids: catechin, epicatechin, myricetin, diapigenin hexoside, isorhamnetin hexose-malonate and dimyricetin hexose-malonate Tannins | In vitro antioxidant and anticancer activity | [49,90,91] |
| 22 | Dragon fruit | 42–59 | 220–900 1 199 2 953 3 | Phenylethanoid: tyrosol Stilbene: coumarin Phenolic acids: gallic, ellagic, caffeoyl hexoside and p-coumaroyl quinic acid Flavonoids: quercetin 3-O-rutinoside, kaempferol hexoside, isorhamnetin hexoside, isorhamnetin 3-O-glucoside, eriodictyol hexoside, eriodictyol, naringenin acetylhexoside and taxifolin acetylhexoside Tannins | In vivo antidiabetic, wound healing and antihypertensive activity In vitro anticancer, anti-inflammatory and antioxidant activity | [49,58,79,92,93,94,95,96,97] |
| 23 | Sapodilla | 15–159 | 405 1 208 2 4847 3 | Phenolic acids: 4-O-galloylchlorogenic, gallic, 4-O-galloylchlorogenate and methyl chlorogenate acid Flavonoids: quercitrin, myricitrin, (+)-catechin and (+)-gallocatechin | In vivo antitumor, anti-obesity, and antidiabetic activity In vitro antioxidant activity | [49,58,98,99] |
| 24 | Mamoncillo | 295–647 | 665 1 322 2 | Stilbenes: resveratrol derivative Phenolic acid derivatives: p-coumaric acid derivative, caffeic acid derivative, ferulic acid derivative p-hydroxybenzoylhexose and p-coumaroylhexose acid | In vitro antioxidant activity | [49,100] |
| 25 | Cactus berry | 740–1046 | 17 1 171 2 320 3 47–3300 4 | Phenolic acids: caffeic, gallic, vanillin, ellagic, protocatechuic, p-hydroxybenzoic, quinic and ferulic acid hexoside Flavonoids: quercetin, (−)-epicatechin, epigallocatechin, queretin-3-O-rhamnosyl rutinoside-glucoside, kaempferol-7-O-neohesperiodoside and isorhamnetin rhamnosyl-rutinoside Tannins: proanthocyanidins | In vivo antidiabetic and renal protective activity In vitro antioxidant, anti-inflammatory, antidiabetic and anticancer activity | [101,102,103,104] |
| 26 | Green prickly pear | 38–62 | 2630 3 | Phenolic acids: piscidic, caffeic, ferulic, hydroxybenzoic, eucomic, protocatechuic, malic and succinic acid Flavonoids: isorhamnetin glucosyl-rhamnosyl-rhamnoside, isorhamnetin glucosyl-rhamnosyl-penstoside, isorhamnetin-hexosyl-hexosyl-pentoside, isorhamnetin glucosyl-pentoside, rutin, kaempferol-glucosyl-rhamnoside, isorhamnetin glucosyl-rhamnoside, isorhamnetin and isorhamnetin-3-O-robinobioside | In vivo antidiabetic, antioxidant and kidney protective activity In vitro anticancer, antioxidant, anti-inflammatory and antidiabetic activity. | [91,105,106,107,108,109,110] |
| Purple prickly pear | 282–350 | 308–630 2 2348–2378 3 | ||||
| Red prickly pear | 198–218 | 83–540 2 1988–2348 3 | ||||
| Yellow prickly pear | 62–158 | 23–345 2 1253–2115 3 | ||||
| 27 | Sour prickly pear | 132–260 | 6400 1 988 2 42 6 253–313 7 | Phenolic acids: gallic, vanillic, 4-hydroxybenzoic, syringic, ferulic and protocatechuic acid Flavonoids: epicatechin, catechin, rutin, vanillin, quercetin, quercitrin and kaempferol | In vivo antidiabetic and antioxidant activity In vitro antioxidant activity | [111,112,113,114,115,116] |
| 28 | Avocado | 11–490 | 130 2 1160 3 | Phenylethanoids: tyrosol-hexoside pentoside Phenolic acids: caffeic acid, α-resorcyclic acid, protocatechuic acid, p-coumaric acid glycoside, 5-feruloylquinic acid, ferulic acid, benzoic acid, trans-cinnamic acid, chlorogenic acid and sinapinic acid Flavonoids: catechin, epicatechin, epigallocatechin, rutin, quercetin, myricetin, kaempferol and isorhamnetin Proanthocyanidins: (epi)gallocatechin benzylthioether, catechin benzynthioether, epicatechin, benzylthioether and (epi)afzelchin benzylthioether and benzyl mercaptan | In vivo anti-obesity and antidiabetic activity In vitro anticancer, anti-inflammatory, antidiabetic, and antioxidant activity | [79,117,118,119,120,121,122,123] |
| 29 | Tomatillo | 78–970 | 15–90 6 | Phenolic acids: chlorogenic, caffeoyl hexoside, coumaroyl hexoside, coumaroyl dihexoside, feruloyl dihexoside, sinapoyl hexoside and cinnamoyl dihexoside acid Flavonoids: quercetin, epicatechin, kaemperol-3-O-glycoside, quercetin-3-O-glycoside and dihydroflavonol Anthocyanins (in purple varieties) | In vitro antioxidant activity | [124,125,126] |
| 30 | Canistel | 98 | 54 5 | Phenolic acids: gallic acid Flavonoids: (+)-gallocatechin, (+)-catechin and myricitrin Tannins | In vivo hepatoprotective and antioxidant activity In vitro antioxidant and anti-inflammatory activity | [127,128,129] |
| 31 | Mamey | 14–29 | 394 1 113 2 | Phenolic acids: gallic, syringic, p-coumaric, protocatechuic hexose-malonate, hydroxybenzoic acid derivative, p-hydroxybenzoic acid dimer and p-hydroxybenzoic acid Flavonoids: epicatechin dimer, epicatechin, gallocatechin and catechin 3-O-gallate Proanthocyanidins | In vitro antioxidant activity | [49,130] |
| 32 | Capulin | 243–331 | 130 2 | Phenolic acids: chlorogenic acid Flavonoids: (+)-catechin, quercetin hexoside, quercetin dipentoside, kaempferol hexoside, quercetin 3-O-glucoronide, rutin and quercetin-3-O-arabinoside Anthocyanins: cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside and cyanidin Proanthocyanidins: procyanidin dimer B and procyanidin trimer B | In vivo antihypertensive and vasorelaxant activity In vitro antioxidant activity | [54,131,132,133] |
| 33 | Guava | 175–462 | 3 1 1–300 2 1305 3 | Phenolic acids: gallic, chlorogenic, caffeic, p-coumaric, syringic, vanilic, ferulic and ellagic acid Flavonoids: catechin gallate, quercetin hexoside, quercetin pentoside, quercetin, (+) catequin, rutin and kaempferol Proanthocyanidins: PAC B-Type (E)GC-(E)C and PAC B-Type (E)Cg-(E)GC Ellagitannins: bilactone of valoneic acid | In vitro anticancer and antioxidant activity | [54,58,134,135] |
| 34 | Squash | 80–494 | 76 2 34 4 | Phenolic acids: cinnamic, protocatechuic acid hexoside and coumaric acid Flavonoids: apigenin glucoside pentoside, luteolin-7-O-rutinoside, lutein-7-O-glucoside, muricitrin, apigenin 7-O-rutinoside, chrysoeriol 7-O-rutinoside, diosmetin and luteolin | In vivo antihypertensive, cardioprotective, antidiabetic Antiulcer and hepatic injury protective activity In vitro antioxidant activity | [136,137,138,139,140,141,142,143] |
| 35 | Tomato | 11–142 | 80 2 10–27 6 | Phenolic acids: chlorogenic, caffeic, p-coumaric, ferulic, hydroxybenzoic acid hexose, protocatechuic, gentisic, dihydroxybenzoic acid pentose, benzoic acid, coumaric acid hexose, sinapic acid hexose, feruloylquinic, isoferulic, dicaffeoylquinic, caffeoyl-hexose, coumaroyl-hexose and tricaffeoylquinic acid Flavonoids: quercetin, kaempferol, naringenin, naringenin dihexose, rutin hexoside, apigenin acetylhexoside, quercetin 3,7-dihexoside, rutin pentoside, kaempferol 3,7-dihexoside, isorhamnetin 3-sophoroside, rutin, kaempferol-3-rutinoside and naringenin chalcone | In vitro anticancer and antioxidant activity | [55,91,144,145] |
| 36 | Yellow mombin | 131–260 | 625 1 349 2 | Flavonoids: epicatechin and quercetin | In vivo gastroprotective and ulcer healing activity In vitro antioxidant activity | [49,146,147,148] |
| 37 | Red mombin | 116–249 | 663 1 170–333 2 | Phenolic acids: 3-caffeoylquinic, dihydroxybenzoic acid hexoside and gallic acid Flavonoids: quercetin 3-O-pentosylhexoside, quercetin-3-O-pentosylrutinoside, quercetin pentoside, quercetin deoxyhexoside, rutin, quercetin-3-O-glucopyranoside, kaempferol-3-O-rutinoside, kaempferol-3-O-hexosyl-pentoside, astragalin and rhamnetin hexosyl pentoside | In vivo and in vitro antioxidant activity | [49,54,149] |
| 38 | Pitaya | 18–160 | 250–900 1 2268–3369 8 | Phenylethanoids: tyrosol Phenolic acids: caffeoyl hexoside, feruloyl dihexoside and p-coumaroyl quinic acid Flavonoids: quercetin 3-O-rutinoside, kaempferol hexoside, isorhamentin hexoside, isorhamnetin 3-O-glucoside, eriodictyol hexoside, eriodictyol acetylhexoside, naringenin acetylhexoside and taxifolin acetylhexoside | In vitro antioxidant activity | [150,151] |
| Fruit | Intensity (MPa) | Parameters Related to the Stability of Phenolic Compounds | Effect on Phenolic Content | Ref |
|---|---|---|---|---|
| Avocado slices | 200 |
| Not reported. | [163] |
| >300 |
| Not reported. | ||
| Avocado puree | 600 |
| Degradation (processing) Enhanced extractability (storage). | [164,165] |
| 345–689 |
| Not reported. | [166] | |
| Bell pepper slices | 100 |
| Degradation (processing). | [167] |
| 500 |
| Retention (processing). | ||
| Cashew apple juice | 250–400 |
| Enhanced extractability (processing). | [168] |
| Guava puree | 400 |
| Not reported. | [169] |
| 600 |
| Not reported. | ||
| Papaya beverage | 550 |
| Degradation (processing) Retention (storage). | [170] |
| Pitaya beverage | 400 |
| Degradation (processing). | [171] |
| 550–600 |
| Retention (processing and storage). | [172] | |
| 600 |
| Degradation (processing). | [171] | |
| Prickly pear beverage | 550 |
| Enhanced extractability (processing). | [173] |
| Prickly pear slices | 100 |
| Enhanced extractability (processing). | [174,175] |
| 350 |
| Enhanced extractability (processing). | [174,175] | |
| 600 |
| Enhanced extractability (processing). | [174,175] | |
| 400–600 |
| Enhanced extractability (processing) | [176] | |
| Sapodilla jam | 400 |
| Enhanced extractability (processing). | [177] |
| Tomato juice | 250 |
| Retention (processing and storage). | [178] |
| Fruit | Intensity | Parameters Related to the Stability of Phenolic Compounds | Effects on Phenolic Content | Ref |
|---|---|---|---|---|
| Pulsed Electric Fields | ||||
| Tomato juice | 20 kV/cm |
| Not reported. | [193] |
| 35 kV/cm |
| Retention (processing and storage). | [194] | |
| Tomato fruit | 1 kV/cm |
| Synthesis (storage). | [181] |
| 1.2 kV/cm |
| Synthesis (storage). | [182] | |
| 1.2 kV/cm |
| Synthesis (storage). | [195] | |
| Ultrasound | ||||
| Avocado puree | 20 kHz |
| Not reported. | [196] |
| Cashew apple puree | 226 W/cm2 |
| Enhanced extractability (processing). | [197] |
| Custard apple juice | 20 kHz |
| Enhanced extractability (processing). | [198] |
| Guava juice | 20 kHz+cellulase |
| Enhanced extractability (processing). | [199] |
| Prickly pear juice | 20 kHz |
| Enhanced extractability (processing). | [200] |
| Soursop puree | 24 kHz 50 °C +vacuum |
| Not reported. | [201] |
| Tomato fruit | 45 kHz |
| Synthesis (storage). | [187] |
| Tomato beverage | 37 kHz |
| Retention (processing). | [202] |
| Microwave | ||||
| Avocado puree | 11 W/g |
| Enhanced extractability (processing). | [191] |
| Guava nectar | 500–950 W |
| Not reported. | [203] |
| Jalapeño pepper | Not reported |
| Degradation (processing). | [192] |
| Mamey pulp | 937 W |
| Not reported. | [204] |
| Cold Plasma | ||||
| Cashew apple juice | nitrogen 80 kHz |
| Enhanced extractability (processing). | [205] |
| Pitaya fruit | 60 kV |
| Enhanced extractability (processing) Synthesis (storage). | [206] |
| Dragon fruit | argon 40 W |
| Retention (processing). | [207] |
| Tomato beverage | 50 kHz |
| Enhanced extractability (processing). | [202] |
| Ultraviolet light | ||||
| Pitaya juice | UV-C 57 µW/cm2 |
| Retention (processing). | [208] |
| Prickly pear fruit | UV-B 6.4 W/m2 |
| Retention (processing)Synthesis (storage). | [209] |
| Tomato beverage | UV-C Not reported |
| Retention (processing). | [202] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Maqueo, A.; Escobedo-Avellaneda, Z.; Welti-Chanes, J. Phenolic Compounds in Mesoamerican Fruits—Characterization, Health Potential and Processing with Innovative Technologies. Int. J. Mol. Sci. 2020, 21, 8357. https://doi.org/10.3390/ijms21218357
Gómez-Maqueo A, Escobedo-Avellaneda Z, Welti-Chanes J. Phenolic Compounds in Mesoamerican Fruits—Characterization, Health Potential and Processing with Innovative Technologies. International Journal of Molecular Sciences. 2020; 21(21):8357. https://doi.org/10.3390/ijms21218357
Chicago/Turabian StyleGómez-Maqueo, Andrea, Zamantha Escobedo-Avellaneda, and Jorge Welti-Chanes. 2020. "Phenolic Compounds in Mesoamerican Fruits—Characterization, Health Potential and Processing with Innovative Technologies" International Journal of Molecular Sciences 21, no. 21: 8357. https://doi.org/10.3390/ijms21218357
APA StyleGómez-Maqueo, A., Escobedo-Avellaneda, Z., & Welti-Chanes, J. (2020). Phenolic Compounds in Mesoamerican Fruits—Characterization, Health Potential and Processing with Innovative Technologies. International Journal of Molecular Sciences, 21(21), 8357. https://doi.org/10.3390/ijms21218357







